Abstract
Regulation of synaptic transmission by modulation of the calcium influx that triggers transmitter release underlies different forms of synaptic plasticity, and thus could contribute to learning. In the mollusk Aplysia, the neuromodulator serotonin (5-HT) increases evoked transmitter release from sensory neurons and thereby contributes to dishabituation and sensitization of defensive reflexes. We combined electrophysiological recording with fluorescence measurements of intracellular calcium in sensory neuron synapses in culture to test whether direct up-modulation by 5-HT of calcium influx triggered by single action potentials contributes to facilitation of transmitter release. We observe increases in a previously undescribed calcium influx that are strongly correlated with increases in the amplitude of the evoked postsynaptic potentials and which cannot be accounted for by action potential prolongation. Our results suggest that direct modulation of a presynaptic calcium conductance that controls neurotransmitter release contributes to the presynaptic facilitation that underlies a simple form of learning.
Introduction
Regulation of synaptic transmission by modulation of the calcium influx that triggers transmitter release is an attractive candidate mechanism for different forms of synaptic plasticity, including those involved in learning. Many examples of inhibition of voltage-dependent calcium conductance by neuromodulatory substances have been documented, and in some cases this inhibition contributes to inhibition of transmitter release (Chen and van den Pol 1998; Czesnik et al. 2001; Dunlap and Fischbach 1978; Edmonds et al. 1990; Isaacson 1998; Pisani et al. 2002; Wu and Saggau 1997). By contrast, there have been few studies demonstrating the enhancement of calcium conductance in neurons by modulators (Braha et al. 1993; Edmonds et al. 1990; Fossier et al. 1994; Huang et al. 1998; Wang et al. 2006), and no reports linking this enhancement to the enhancement of transmitter release involved in any form of learning. We demonstrate here a direct up-modulation of calcium influx by an endogenous modulatory transmitter in the mollusk Aplysia that is strongly correlated with the facilitation of transmitter release that underlies a simple form of learning and which is likely to contribute to this facilitation.
Facilitation of transmitter release from sensory neurons by the endogenous modulatory transmitter serotonin (5-HT) contributes to behavioral sensitization and dishabituation of defensive withdrawal reflexes of Aplysia (Byrne and Kandel 1996; Marinesco and Carew 2002). One of the actions of 5-HT is to increase the voltage-gated calcium influx triggered by depolarization of the sensory neurons (Blumenfeld et al. 1990; Boyle et al. 1984; Braha et al. 1993). A calcium conductance that is enhanced by 5-HT is blocked by dihydropyridines, but this dihydropyridine-sensitive conductance does not contribute to transmitter release, implying that direct modulation of calcium conductance is not a mechanism of facilitation (Edmonds et al. 1990).
Subsequently, it was found that 5-HT increases the calcium influx caused by trains of spikes in sensory neurons in co-culture with motor neurons even in the presence of the dihydropyridine nitrendipine (Eliot et al. 1993). This paradoxical result was explained by suggesting that prolongation of action potentials by 5-HT, caused by down-modulation of potassium conductance that contributes to repolarization (Baxter and Byrne 1989; Hochner and Kandel 1992; Klein and Kandel 1980), is responsible for the increased calcium influx and the facilitation of transmitter release. However, further experiments demonstrated that action potential broadening contributes little, if at all, to facilitation by 5-HT (Klein 1994).
We have now resolved the question of the role of enhanced calcium influx by showing that 5-HT increases calcium entry through a different pathway. Modulation of calcium conductance changes when sensory neurons are isolated from their normal synaptic environment: 5-HT increases calcium entry through one conductance in isolated sensory neurons, but enhances calcium entry in a different manner—most likely through a different conductance—in sensory neurons with synaptic connections.
The increase in calcium entry at synapses cannot be accounted for by spike broadening, and there is a strong correlation between the magnitude of presynaptic calcium transients triggered by single action potentials and the amplitude of the evoked postsynaptic potentials (PSPs). Taken together, our results imply that direct modulation of presynaptic calcium influx contributes to the facilitation of transmitter release that underlies a simple form of learning.
Results
Application of 5-HT increases presynaptic calcium influx and postsynaptic potentials
Pairing a sensory neuron of Aplysia with a motor neuron in culture leads to the formation of synaptic connections that display the same forms of synaptic plasticity as sensory neuron-motor neuron synapses in situ (Coulson and Klein 1997; Schacher et al. 1990). An action potential triggered in a sensory neuron in such a co-culture elicits a PSP in the motor neuron and, if the sensory neuron has been filled with a calcium indicator, the calcium influx caused by the action potential can be recorded at the same time (Armitage and Siegelbaum 1998; Eliot et al. 1993). In this way it is possible to examine the contribution of changes in presynaptic calcium influx to changes in synaptic transmission resulting from application of neuromodulatory substances such as 5-HT.
Application of 5-HT to a co-culture of a sensory neuron with a motor neuron increases the amplitude of the PSP evoked by an action potential (Schacher et al. 1990), and, in addition, increases the presynaptic calcium influx triggered by action potentials (Blumenfeld et al. 1990; Boyle et al. 1984) (see example in Fig. 1). Voltage-clamp studies showed that 5-HT increases calcium influx through the action of protein kinase C on calcium channels sensitive to the dihydropyridines nifedipine and nitrendipine (Braha et al. 1993; Edmonds et al. 1990). However, the dihydropyridine-sensitive calcium conductance does not contribute to the transmitter release evoked by an action potential (Edmonds et al. 1990), while calcium influx is increased even in the presence of nitrendipine (Eliot et al. 1993). Because an increase in calcium influx due to action potential prolongation is not a candidate mechanism since facilitation can occur in the absence of a change in the action potential (Klein 1993, 1994), we decided to reexamine the basis for the increase in calcium influx caused by 5-HT and its relation to synaptic facilitation.
Figure 1. Concurrent recording of presynaptic action potentials and the calcium transients and PSPs triggered by those action potentials.
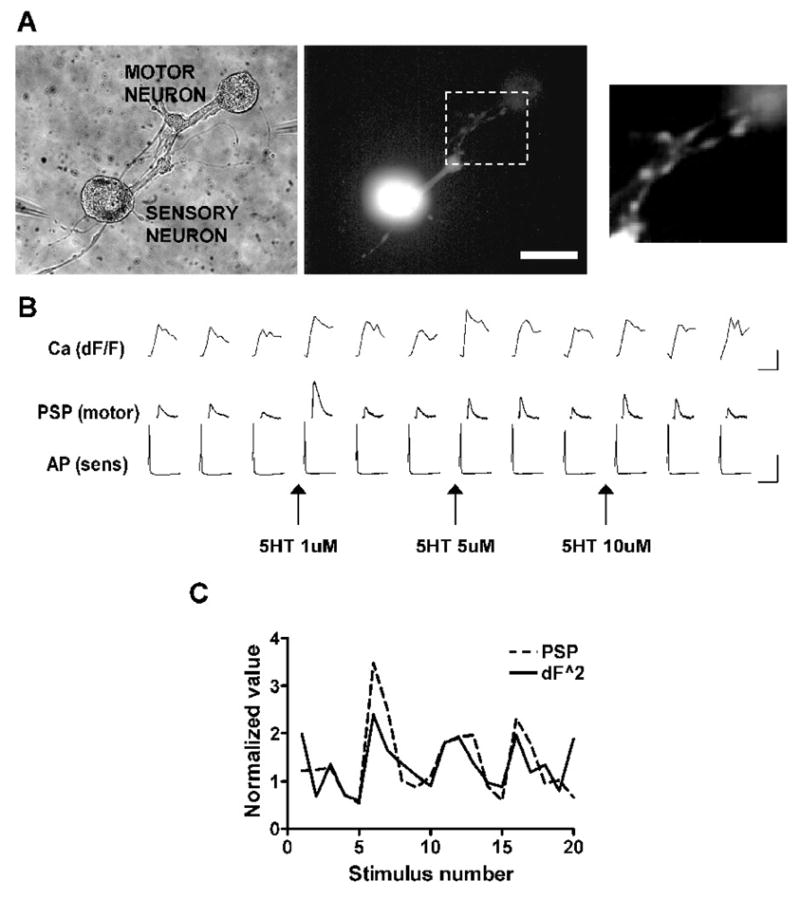
A. A co-culture of an Aplysia sensory neuron and motor neuron in which the sensory neuron has been filled with the fluorescent calcium indicator fluo-4. The rightmost panel shows the varicosities of the sensory neuron from which calcium transients in response to single action potentials were recorded. Scale bar is 60 μm for 2 left panels and 30 μm for rightmost panel. B. Intracellular stimulation of the sensory neuron at 60 second intervals resulting in the firing of single action potentials (AP) and the corresponding calcium transients in the sensory neuron varicosities (Ca) and postsynaptic potentials (PSP) recorded in the motor neuron. Progressively higher concentrations of 5-HT were applied at the arrows. Application of 5-HT resulted in increases in the PSP in the motor neuron and in the presynaptic calcium transient. There was no change in the resting membrane potential or in the shape of the action potential. Traces show 3 stimuli of a total of 5 for each condition (see 1C). Calibrations are 15% dF/F and 500 ms for the calcium transients, 20 mV and 20 ms for the PSPs and 50 mV and 20 ms for the action potentials. C. Correspondence between the amplitudes of PSPs and peak amplitudes of calcium transients in the same experiment as A and B. Calcium transients raised to the 2.0 power gave the best match to the postsynaptic reponses (see Fig. 6).
The increase in calcium influx in cell bodies of sensory neurons cannot be accounted for by prolongation of the action potential
We investigated the relationship between the calcium influx evoked by an action potential and the duration of the action potential by monitoring calcium transients in the cell bodies of sensory neurons with the fluorescent calcium indicator fluo-4 while concurrently recording action potentials with an intracellular microelectrode. We increased the duration of action potentials by applying a blocker of potassium conductance, 3,4-diaminopyridine (3,4-DAP), and examined the resulting changes in the calcium transients (Fig. 2A1).
Figure 2. Action potential broadening cannot account for the increase in calcium transients caused by 5-HT.
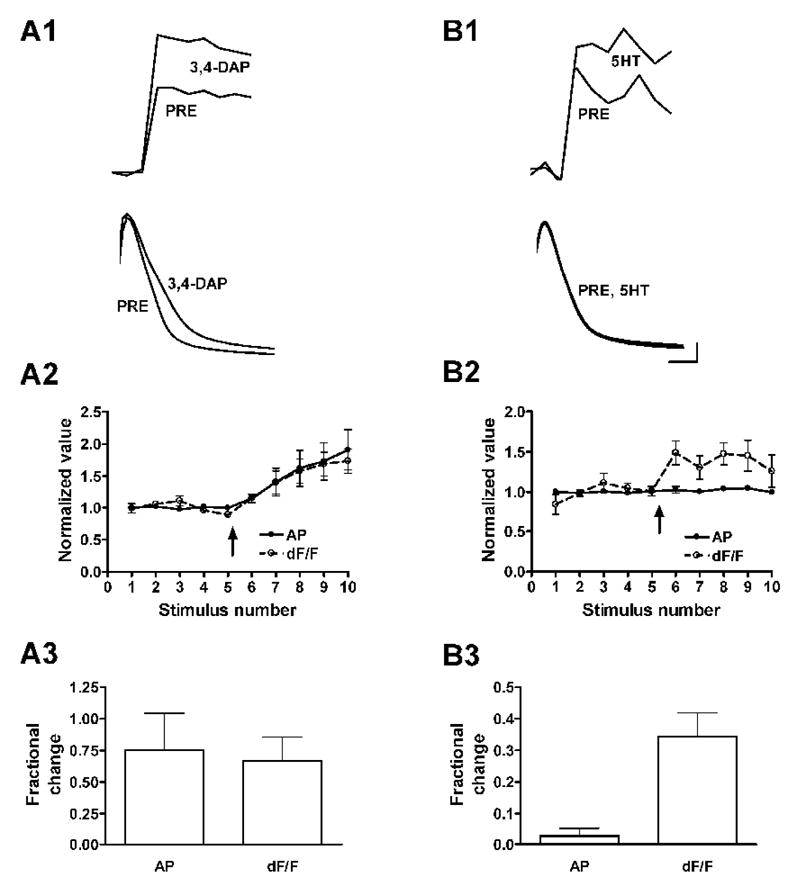
A. Application of the potassium channel blocker 3,4-diaminopyridine prolonged the action potential and increased the calcium transient in the cell body of the sensory neuron. A1, average of 3 calcium transients (top) and action potentials (bottom) before and after application of 3,4-DAP. A2, plots of normalized action potential duration (AP) and peak calcium transients (dF/F) from 5 experiments; 3,4-DAP was applied at the arrow. A3, average fractional changes in spike duration and calcium transients from responses to stimuli 8–10. B. Application of 5-HT at 1 μM increased the calcium transient in the cell body without prolonging the action potential. B1, average of 3 calcium transients before and after 5-HT application (top), and superimposed records of 3 action potentials before and 3 action potentials after application of 5-HT (bottom). B2 and B3 as for part A; n = 6. Calibration is 15 mV and 1.5 ms for the action potentials and 100 ms for the calcium transients; amplitude of calcium transients is in arbitrary units. Error bars in this and all subsequent figures represent standard errors.
Calcium transients (dF/F) increased in parallel with the increases in duration of the spike induced by 3,4-DAP (Fig. 2A2; n = 5). The peak increase in duration of the action potential was 75.4 ± 29.1% and the corresponding increase in the calcium transient was 66.7 ± 18.7%, (Fig. 2A3, not significantly different; p = 0.515, paired t-test). The increases were strongly correlated (r2 = 0.926; p = 0.0087). If the increase in calcium transients caused by 5-HT were the result of spike broadening, then the increases in the calcium transient and in the action potential duration should also be similar with application of 5-HT. This was not the case: Application of 1 μM 5-HT, which facilitated the PSP by about 100% (see Figure 3B), increased the duration of action potentials by 2.8 ± 2.5%, while the calcium transients increased by 34.3 ± 7.6% (Fig. 2B; p = 0.0034, n=6). A hundredfold higher concentration of 5-HT, which approximately tripled the amplitude of the PSP (not shown), increased the action potential duration by 9.7 ± 1.5% and the calcium transients by 53.9 ± 14.4% (p = 0.0161, n = 8; not shown). Thus, the increase in calcium influx caused by 5-HT in cell bodies cannot be accounted for by an increase in the duration of action potentials, but must involve direct modulation of calcium entry.
Figure 3. PSPs facilitated by 5-HT show no change in shape, unlike PSPs increased by spike broadening.
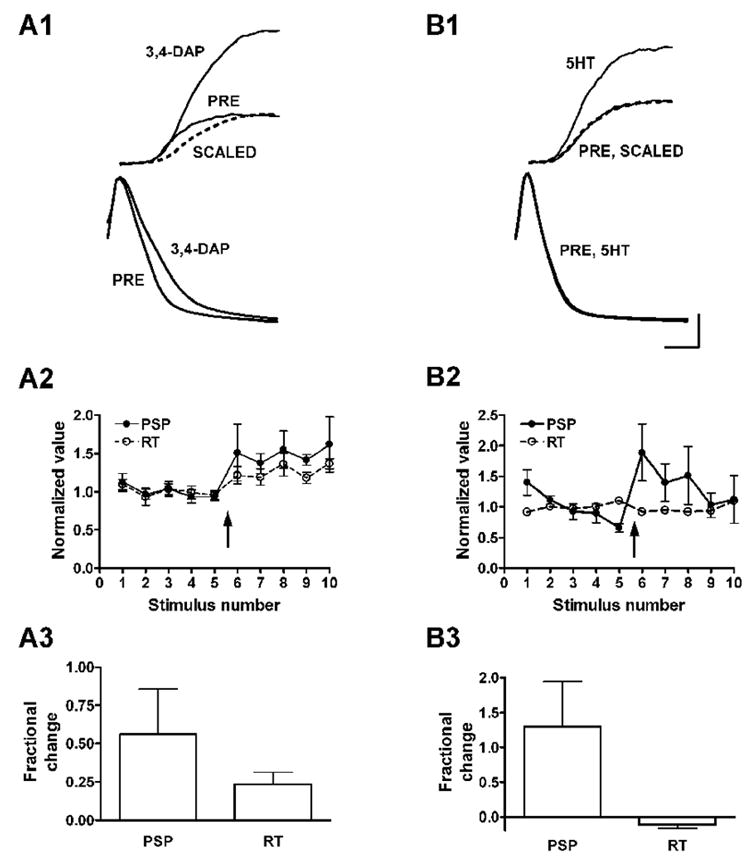
A. Prolongation of presynaptic action potentials is accompanied by an increase in the rise time of PSPs. A1. Application of 3,4-DAP broadens action potentials (bottom) and increases the time to peak of PSPs (top). Each trace is the average of 2 action potentials or PSPs before and after application of 3,4-DAP. Trace labeled SCALED is the PSP in 3,4-DAP reduced to match the amplitude of the control trace (PRE) in order to demonstrate prolongation of the rise time by 3,4-DAP. A2 and A3, summary data from 6 experiments; 3,4-DAP added at the arrow. PSP and RT are amplitude and rise time of PSP, respectively. B. Facilitation by 5-HT is not accompanied by an increase in PSP rise time. Summary data in B2 and B3 are from 5 experiments (error bars for RT in B2 are smaller than the symbols). Calibration: 5 mV and 15 mV for PSPs and spikes, respectively, and 1.5 ms for both.
Prolongation of action potentials can account neither for increased calcium transients at synaptic terminals nor for synaptic facilitation
Action potentials monitored at the site of intracellular recording in the cell body may not reflect changes occurring at the synaptic terminals. Although prolongation of action potentials by 5-HT in the cell body is insufficient to account for the increase in calcium transients, it is possible that the action potentials at the presynaptic terminals are prolonged to a greater extent, and that prolongation at these sites is responsible for both the increase in calcium transients at the terminals and the facilitation of synaptic potentials.
Increases in calcium transients resulting from application of 5-HT can be seen in synaptic varicosities (Fig. 1), but action potentials at these sites of transmitter release cannot be monitored directly using electrophysiological techniques. In order to determine whether the increases in the calcium transients and in the accompanying synaptic potentials result from a change in duration of the action potential at the synaptic sites, we examined the shape of the PSPs triggered by these action potentials. If presynaptic action potentials were prolonged to a significant degree by 5-HT in synaptic facilitation, this might be reflected in an increase in the rise times of the resulting synaptic potentials. To test this possibility, we first examined the effects of 3,4-DAP on the shape of PSPs.
Application of 3,4-DAP, in addition to prolonging presynaptic action potentials, caused an increase in the amplitudes of PSPs and a corresponding increase in their rise times (Fig. 3A1). By comparing the increase in the amplitudes of the PSPs with the increase in their rise times we could estimate the contribution of broadening of the presynaptic action potential to the increase in amplitude of the postsynaptic response.
There was a good correlation between the amplitudes of the PSPs and their rise times with application of 3,4-DAP (Fig. 3A2; r2 = 0.826, p = 0.033, n = 5), with the fractional increase in the PSP rise time amounting to about half the fractional increase in the amplitude (Fig. 3A2, 3). Thus, although we still could not determine directly the shape of the presynaptic action potential, we now had a measure of the contribution of spike broadening to the increase in amplitude of the PSPs. If 5-HT acts like 3,4-DAP and causes synaptic facilitation by prolonging the presynaptic action potential, then facilitation by 5-HT should be accompanied by a proportional increase in the rise time of the PSP.
Application of 5-HT at 1μM approximately doubled the amplitude of the synaptic potential, but caused no increase at all in its rise time (Fig. 3B1-3). The calcium transients recorded at the cell body in the same experiments increased by about 30%. At a concentration of 100 μM, 5-HT increased the PSP amplitude by about 200%, and the rise time by approximately 5% (not shown). Extrapolating from the experiments with 3,4-DAP, this increase in rise time indicates that prolongation of the presynaptic action potential could account for no more than 5% of the synaptic facilitation using the highest concentration of 5-HT--consistent with the results of Klein, 1994. The absence of any change at all in the rise time with 5-HT at 1 μM suggests no contribution whatsoever of action potential broadening at the lower concentration. The lower concentration of 5-HT is likely to be of physiological relevance: Stimulation of a nerve pathway that causes facilitation is accompanied by a rise in the concentration of 5-HT in the neuropil to 1 μM or less, as estimated from direct measurements with a carbon fiber electrode (Marinesco and Carew 2002).
Synapse formation changes the modulation of calcium conductance by 5-HT
The above experiments indicate that prolongation of the presynaptic spike is unlikely to contribute significantly to the enhancement of calcium transients or of PSPs by 5-HT. Since 5-HT directly increases a dihydropyridine-sensitive calcium conductance in the sensory neurons (Braha et al. 1993; Edmonds et al. 1990), it is possible that the enhancement of this conductance—which does not play a role in synaptic transmission (Edmonds et al. 1990)—underlies the increase in calcium transients in the experiments reported above. If this were the case, then it would follow that enhancement of calcium influx is unrelated to the facilitation of transmitter release.
We tested this possibility by examining the effect of the dihydropyridine nitrendipine on the modulation of calcium transients by 5-HT (Fig. 4). Consistent with the earlier reports, nitrendipine at 100 μM completely blocked the increase in calcium transients in isolated sensory neurons (−5.1 ± 5.7%, n = 7 in nitrendipine versus 50.1 ± 13.5%, n = 4 for controls; p < 0.002).
Figure 4. Nitrendipine blocks the enhancement of calcium transients by 5-HT in isolated sensory neurons (top), but not in sensory neurons with synaptic connections (middle).
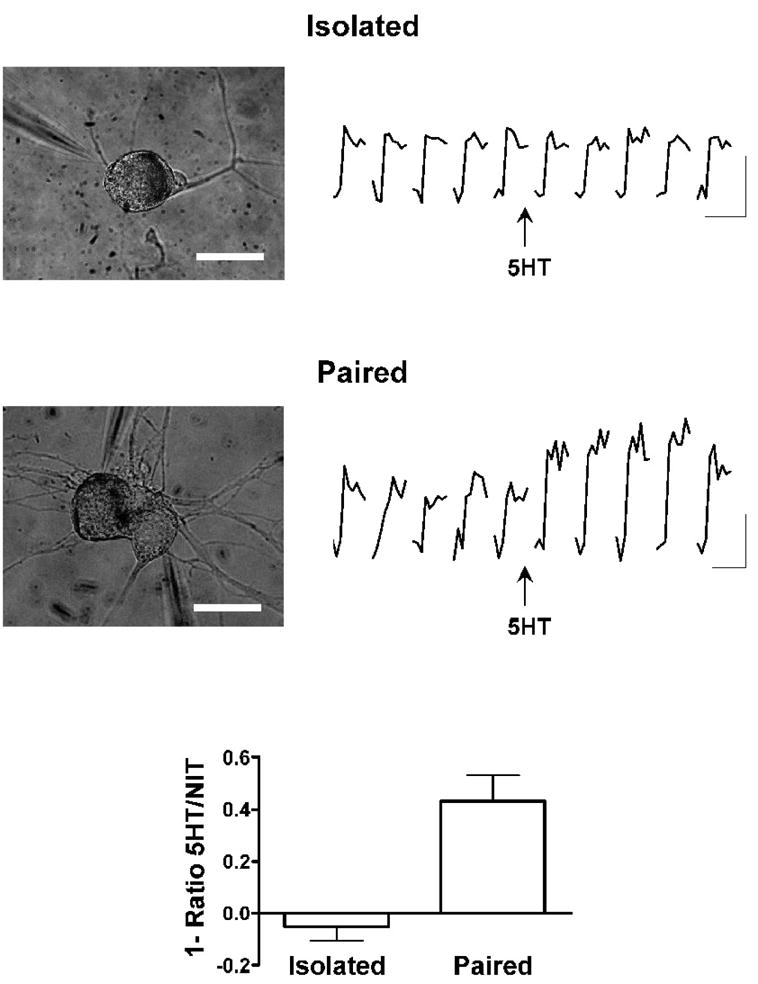
Bottom graph shows summary of fractional increases in calcium transients after application of 5-HT, in the presence of nitrendipine 10 μM, in isolated (n = 7) and paired (n = 6) sensory neurons. Scale bars are 100 μm and 50 μm for the top and middle micrographs, respectively; calibrations are 1.5% and 2.5% dF/F for top and bottom sets of traces, respectively, and 500 ms for both.
At the sensory neuron-motor neuron synapses of Aplysia, dihydropyridine-sensitive calcium conductance is not involved in the release of neurotransmitter to a single action potential (Edmonds et al. 1990). Because other calcium conductances are responsible for transmitter release, it is possible that these conductances might be absent or diminished in isolated sensory neurons, or that, even if they were present, the mechanism for their modulation might not be in place. Since most of the earlier experiments had been done on isolated sensory neurons, we now repeated the nitrendipine experiment with sensory neurons that had formed synapses in culture.
Application of 5-HT to co-cultures of sensory neurons paired with motor neurons resulted in an increase of 29.5 ± 8.2% in the calcium transient (n = 13). In contrast to the results with isolated neurons, the increase in calcium transients caused by 5-HT in co-cultures was not blocked by nitrendipine (Fig. 4; increase of 43 ± 10%, n = 6; p = 0.345 compared to controls). This result implies that 5-HT modulates a different pathway for calcium entry in sensory neurons with synapses than it does in isolated sensory neurons. Since the calcium influx that is enhanced by 5-HT at synapses is insensitive to nitrendipine, the experiments reported in the earlier studies do not bear on the role of this influx and its modulation in neurotransmitter release or in synaptic plasticity. The possibility remains, therefore, that calcium influx that is subject to up-modulation by 5-HT contributes to synaptic transmission, and that enhancement of this influx by 5-HT results in facilitation of transmitter release. In order to address this possibility, we examined the relationship between calcium transients and synaptic potentials in greater detail.
Changes in the amplitude of PSPs are well correlated with changes in the amplitude of calcium transients in specific regions of the sensory neurons
We monitored calcium transients and baseline calcium levels in experiments in which we concurrently recorded postsynaptic potentials. Of 23 experiments in which 5-HT facilitated postsynaptic potentials, 19 showed increases in calcium transients and 4 showed increases in the resting calcium. The changes in the transients or in the resting calcium generally paralleled the changes in the PSPs (see Fig. 1 and Fig. 5). However, because release of neurotransmitter is restricted to specialized sites in neurons, we attempted to improve the resolution of the experiments by examining the calcium in different regions of the sensory neurons in co-culture with postsynaptic targets.
Figure 5. Correspondence between calcium transients at hot spots and PSP amplitudes.
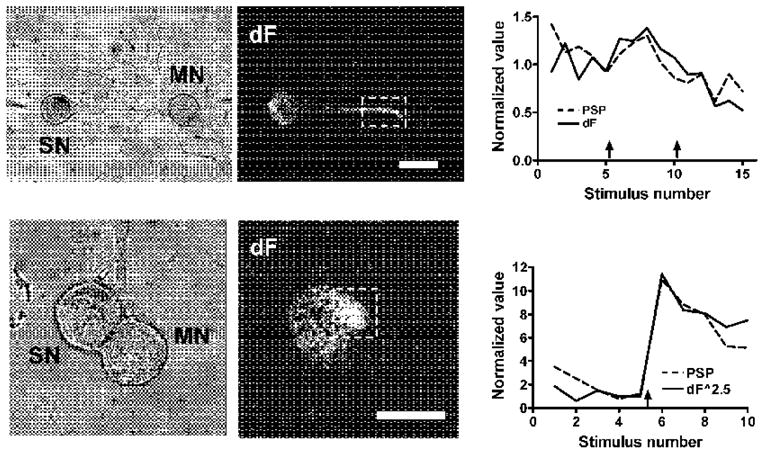
Dark field images show differences between peak of calcium transients and baseline calcium (dF); regions of greater fluorescence intensity are “hot spots” of calcium entry. Graphs to the right of the images are plots of PSP amplitude and peak of calcium transients; 5-HT applications at arrows. The upper plot shows a linear relation between the PSP and the calcium, while in the lower plot the PSP is best matched by the calcium transient raised to the 2.5 power. Scale bars are 75 μm and 50 μm for top and bottom micrographs, respectively.
Different types of calcium channels are found in different functional parts of a neuron (Christie et al. 1995; Westenbroek et al. 1998). For example, calcium channels that control transmitter release can be restricted to the membranes of synaptic terminals, while different channel types can be involved in the regulation of transcription of DNA or translation of RNA in the cell body or neurites (Ahn et al. 1999; Deisseroth et al. 1996; Graef et al. 1999). Moreover, receptors for modulatory transmitters and the downstream targets of receptor activation are not distributed uniformly over the cell surface (Sherff and Carew 2002; Sun et al. 1996), so that the same type of channel in different parts of the cell could be affected differently by modulatory inputs. For both these reasons, it would be expected that calcium conductances in different regions of the sensory neurons would not be affected in the same way by 5-HT. We therefore attempted to examine calcium transients at sites of transmitter release in order to see how facilitation of synaptic transmission and modulation of calcium influx by 5-HT are related.
In some experiments we were able to examine calcium transients in varicosities of sensory neuron neurites in contact with postsynaptic neurons. These boutons are sites of synaptic interactions in Aplysia, as they are in vertebrates (Bailey et al. 1979). Figure 1 shows a co-culture with a sensory neuron whose well-defined varicosities overlap the neurites of a target postsynaptic cell. Enhancement by 5-HT of calcium transients triggered by single action potentials was tightly correlated with the facilitation of the corresponding PSPs (Fig. 1B,C). In this experiment we measured the transients in the whole region delineated by the box. The large majority of the fluorescence signal came from the boutons, and the relative changes throughout the region were similar. Sequential application of 1, 5 and 10 μM 5-HT facilitated the PSP by 376, 91 and 177% respectively, while the calcium transients increased by 77, 36 and 29%. The diminishing increases caused by higher concentrations of 5-HT may be due to receptor desensitization.
Transmitter release is usually related to the calcium influx by a power function, for two different reasons. First, the calcium sensor molecule, thought to be synaptotagmin (Fernandez-Chacon et al. 2001), requires the binding of 4 or 5 calcium ions for its activation. Therefore, when release depends on a relatively homogeneous average calcium concentration, as would be the case if the calcium was contributed by many open channels that are not close together, release will vary with approximately the 4th power of the calcium current (Augustine and Charlton 1986; Borst and Sakmann 1996; Dodge, Jr. and Rahamimoff 1967; Lando and Zucker 1994; Sakaba and Neher 2001). In this situation, the increase in calcium can increase the probability of release of transmitter at release sites that had been active previously.
In contrast, if the calcium sensor is situated in close proximity to a calcium channel, the opening of a single channel can raise the local calcium concentration sufficiently to activate the sensor and trigger release (Augustine 1990; Borst and Sakmann 1999; Shahrezaei et al. 2006; Stanley 1993). If additional channels open, release will be triggered at new sites that are closely coupled to the newly recruited channels, resulting in a linear dependence of release on calcium influx.
In the intermediate situation—if the calcium channels and the calcium sensors are somewhat further apart so that the calcium entering through a single channel is not enough to trigger release—the opening of a small number of channels will be required in order to activate the calcium sensor. Transmitter release will then be related to the calcium influx by a power function that reflects the number of channels that must open in order to activate one release site (Fedchyshyn and Wang 2005; Shahrezaei et al. 2006). In order to further characterize the relationship between the increases in calcium influx and transmitter release induced by 5-HT, we plotted the normalized PSP against the normalized calcium transients on log-log coordinates (see Fig. 6 for examples).
Figure 6. Log-log plots of PSP amplitude to fluorescence transients in experiments with application of 5-HT.
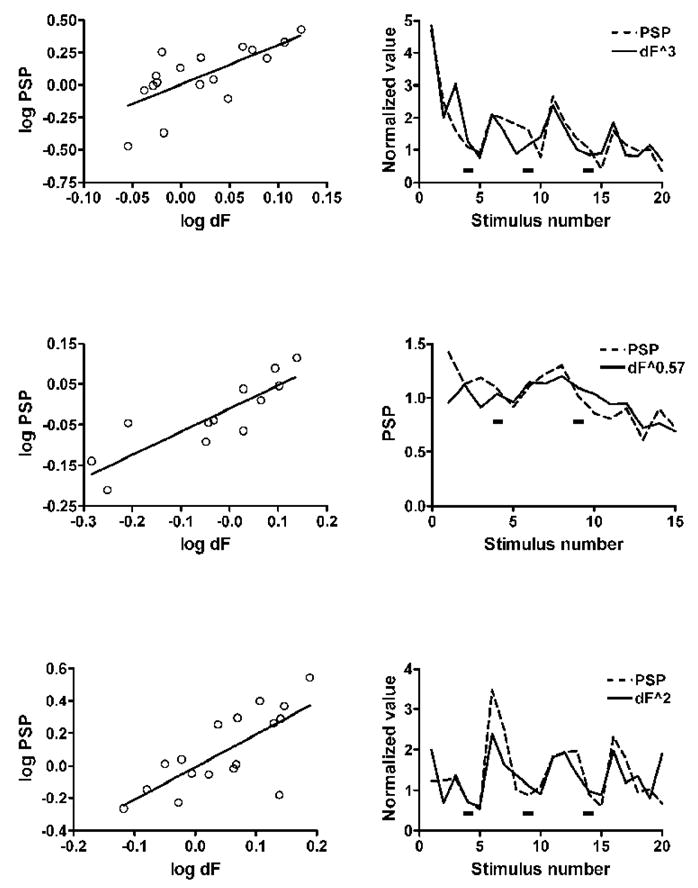
In these three examples, each pair of graphs shows the log-log plot on the left and the plot for the same experiment of the PSP and the calcium transient raised to the power that gave the best fit in the log-log plot on the right. Increasing concentrations of 5-HT (1, 5 and 10 μM in the top and bottom examples, and 1 and 5 μM in the middle example) were added at the black rectangles in the graphs on the right. The first two examples are from experiments with the calcium transient recorded at hot spots of calcium entry, and the bottom graphs are from the experiment of Figure 1 with transients recorded at varicosities. The slopes of the lines that gave the best fit were 3.0, 0.57 and 2.0, respectively.
In the experiment shown in Figure 1, the log-log plot of 17 successive responses had a slope of 2.0 with a correlation coefficient of 0.735 (p < 0.001), and the plot of calcium transients raised to the second power was strikingly parallel to the plot of the PSPs (Fig. 1C and Fig. 6). This relationship between the calcium influx and transmitter release suggests that in this experiment calcium entering through a small number of channels is sufficient to trigger release of a quantum of transmitter. The good correspondence between the calcium transients and the PSPs combined with the specialized morphology support the likelihood that these varicosities represent sites of transmitter release. The parallel between the calcium transients and transmitter release is consistent with the idea that the increase in transmitter release caused by 5-HT is a consequence of the increase in the calcium influx triggered by single action potentials. In two other experiments in which calcium transients could be measured at varicosities the slopes of the log-log plots were 0.5 (correlation coefficient 0.822, p = 0.023) and 2.8 (correlation coefficient 0.977, p = 0.0002), respectively.
In many experiments synaptic varicosities were not well-defined, and several experiments were done using sensory neuron-motor neuron cultures in a soma-soma configuration in which the cell bodies of the two neurons are apposed and in which individual varicosities cannot be discerned (Fig. 5). Earlier ultrastructural examination of soma-soma co-cultures showed that synapses form on neurites that are embedded in the cell bodies (Klein 1994). Nonetheless, we were able to locate likely sites of transmitter release in many experiments, based on the following considerations:
Work in several preparations has shown that presynaptic terminals are often distinguished by a high density of calcium channels relative to other regions, permitting synaptic regions to be detected as “hot spots” of calcium entry (Haydon et al. 1994; Llinas et al. 1992; Robitaille et al. 1990; Smith et al. 1993). We looked for such hot spots in the co-cultures where we could not resolve synaptic varicosities in order to see whether calcium transients in these specialized regions paralleled the synaptic potentials, as was the case for varicosities.
In 10 of 16 experiments with 5-HT in which we did not see obvious varicosities hot spots of calcium entry showed strong correlations between calcium transients and PSPs (Fig. 5 and Table 1). The slopes of the log-log plots in these experiments ranged from 0.6 to 5.8, similar to the exponents relating calcium entry to transmitter release at a variety of synapses in other organisms, where the slopes range from 1 to 5 (Lando and Zucker 1994; Sakaba and Neher 2001; Shahrezaei et al. 2006). The striking parallel between the magnitude of the calcium transients raised to a power and the postsynaptic responses indicates that the power relationship holds throughout the experiment, both in the absence and in the presence of 5-HT (see graphs in Fig. 6 for additional examples).
Table 1.
Slopes of log-log plots of PSPs vs. calcium transients, correlation coefficients and p-values for calcium transients recorded at calcium “hot spots”.
| Slope | r | p |
|---|---|---|
| 5.8 | 0.815 | 0.0256 |
| 1.7 | 0.951 | 0.001 |
| 1.5 | 0.933 | 0.0022 |
| 0.8 | 0.952 | 0.001 |
| 3.2 | 0.980 | 0.0001 |
| 2.4 | 0.979 | 0.0001 |
| 4.0 | 0.977 | 0.0001 |
| 1.4 | 0.748 | 0.0006 |
| 3.0 | 0.698 | 0.0019 |
| 0.6 | 0.863 | 0.0003 |
In experiments where we could not detect hot spots of calcium entry, we were still usually able to find a region of the cell in which the calcium transients showed a good correspondence with the PSPs (Table 2), suggesting that the synapses might be distributed across a larger area in these cultures rather than being localized in hot spots. The slopes of log-log plots in these experiments ranged from 1 to 4.2. However, the post-hoc localization of regions of correspondence between the calcium transients and the synaptic responses limits the significance of these results.
Table 2.
Slopes of log-log plots of PSPs vs. calcium transients, correlation coefficients and p-values for calcium transients in experiments without hot spots.
| Slope | r | p |
|---|---|---|
| 2.2 | 0.774 | 0.0411 |
| 1.0 | 0.886 | 0.0079 |
| 2.3 | 0.913 | 0.0041 |
| 4.2 | 0.858 | <0.0001 |
| *3.0 | 0.927 | 0.0077 |
| **1.8 | 0.704 | 0.0777 |
In the experiment marked with an asterisk a point was omitted where a jump in the baseline calcium occurred. The double asterisk denotes an experiment in which the correlation was not statistically significant.
Changes in baseline calcium with 5-HT
In 4 of 23 experiments we observed no change in the calcium transients after application of 5-HT, despite significant facilitation of the PSPs. It is possible that in some of these experiments the transients increased in restricted regions of the cell that were either out of the field of view or out of the plane of focus. However, when we examined these experiments more closely we found that facilitation of the PSP was accompanied by an increase in the resting calcium concentration and that the changes in the baseline calcium paralleled the PSP amplitude (Fig. 7). Thus, the total intracellular calcium in response to an action potential, that is, the sum of the resting calcium and the transient, increased in parallel with the PSP. In most of these experiments the increase in resting calcium was uniform throughout the cell body rather than being restricted to a particular region, and the slopes of the log-log plots of PSP amplitude against total calcium were close to one.
Figure 7. Example of an experiment in which application of 5-HT caused an increase in the baseline calcium but not in the calcium transient.
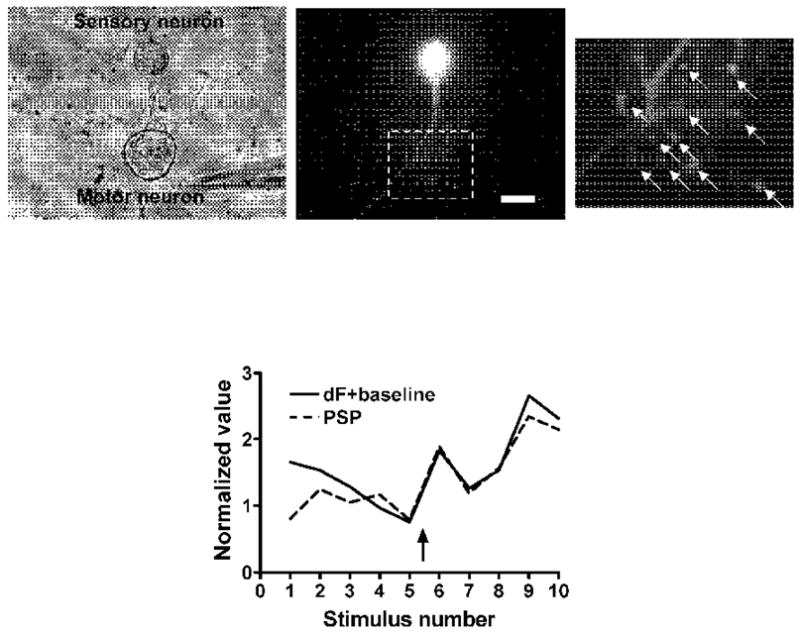
Rightmost image shows the 11 varicosities at which the calcium baseline and transients were recorded; the plot at the bottom shows the average calcium values (baseline plus transient) for all 11 varicosities. Calcium and PSP amplitudes are linearly related. 5-HT was added at the arrow. Scale bar represents 50 μm for the left and center micrographs and 20 μm for the right micrograph.
Discussion
Mechanisms of facilitation at sensory neuron-motor neuron synapses of Aplysia
Our results indicate that the modulatory neurotransmitter 5-HT directly enhances calcium influx distinct from the previously described increase in a calcium conductance that does not contribute to transmitter release or plasticity. We find that the up-modulation of calcium influx by 5-HT is well correlated with the facilitation of transmitter release that underlies behavioral sensitization in Aplysia, suggesting that the enhancement of this influx is a mechanism for synaptic and behavioral plasticity. Edmonds et al. (1990) reported down-modulation of a rapidly-inactivating calcium current by an inhibitory transmitter at these synapses, but did not find any increase in this current with application of 5-HT. Their failure to detect an increase in this current may have been the result of their whole-cell recording technique in which the cell is filled with an artificial solution. In particular, the presence of a high concentration of cesium ions and other components of the solution in the micropipette has been shown to cause activation of protein kinase A (Brette et al. 2003; Vargas et al. 1999), aside from other possible effects. Alternatively, a different calcium conductance contributing to transmitter release or a different route of calcium entry might be the target of 5-HT.
We showed earlier, using a modified quantal analysis, that facilitation of transmitter release by 5-HT is largely due to the switching on of release sites that had previously been silent (Royer et al. 2000). In the present report, the quantitative relationship between transmitter release and the calcium transients triggered by single action potentials implies a tight coupling between calcium channels and release sites, such that in some cases the opening of even a single channel is sufficient to trigger the release of a quantum of neurotransmitter. By making additional calcium channels available for opening by action potentials, 5-HT permits additional release sites to be recruited—consistent with our earlier analysis. The opening of new calcium channels can thus account for the switching on of previously silent release sites.
Facilitation by 5-HT of Aplysia sensory neuron-motor neuron synapses differs depending on the amount of prior stimulation: Facilitation of rested or minimally activated synapses is mediated primarily by protein kinase A (PKA), while facilitation of synapses that had undergone synaptic depression caused by extensive low-frequency stimulation is mediated mainly by protein kinase C (Braha et al. 1990; Ghirardi et al. 1992; Klein 1993). The experiments described here examined the effects of 5-HT after a small number of stimuli, usually 5, and so are likely to be of relevance to the PKA-mediated facilitation, although the involvement of a particular biochemical pathway remains to be demonstrated directly.
Although it is in principle possible that the increase in the calcium transients might result from an increase in release of calcium from intracellular stores or from slowing of the clearance of calcium, there are two reasons that suggest that these possibilities may be unlikely—although they cannot be excluded definitively without further experiments. First, the tight correlation between the amplitude of the synaptic potentials and the calcium transients strongly suggests a causal relationship (although it does not prove it); the brevity of the interval between the presynaptic action potential and the postsynaptic potential—on the order of 5 ms or less—makes it difficult to attribute the increase in the early part of the transient to an increase in calcium release from a site that is not in immediate proximity to the site of transmitter release, or to a slowing of calcium clearance from the cytoplasm. Second, a change in the intraterminal release or clearance of calcium might be expected to change the kinetics of the calcium transient—but application of 5-HT leads to no such change in kinetics. On the other hand, the correlated increases in the baseline calcium that we observed in a small minority of the experiments could be the result of intraterminal changes in handling of calcium, leading to the conclusion that there might be more than one mechanism contributing to the increased calcium levels in the terminals.
Modulation of presynaptic calcium channels in synaptic plasticity
Modulation of calcium conductance by electrical activity and by modulatory substances has been extensively documented, and in some cases has been implicated in the alteration of transmitter release. The vast majority of the studies of modulation by neurotransmitters and hormones report inhibition of calcium influx and the consequent inhibition of transmitter release, with many fewer reports of enhancement of calcium currents and transmitter release. The present study is the first report suggesting that modulation of calcium conductance by a neurotransmitter contributes to plasticity, and possibly to learning, at a synapse with a well defined behavioral role.
It is not known which of the calcium conductances that contribute to transmitter release is up-modulated by 5-HT at the sensory neuron-motor neuron synapses. We have had no consistent results with toxins used to target vertebrate channels of the N, P/Q and R types, each of which has been reported to participate in transmitter release at one or more vertebrate synapses. A study of a different synapse of Aplysia has shown up-regulation by a peptide of a conductance that appears to be related to the vertebrate N-type calcium channel (Fossier et al. 1994), and studies of vertebrate synapses have shown inhibition or enhancement of transmitter release by modulation of both N-type and P/Q type channels (Chen and van den Pol 1998; Fossier et al. 1994; Huang et al. 1998; Pisani et al. 2002; Wang et al. 2006). In the mollusk Lymnaea, there has been reported to be only one calcium channel involved in transmitter release, related to both vertebrate N-type and P/Q-type channels (Spafford et al. 2003), and this may be the case for Aplysia as well. The calcium conductances involved in transmitter release that are the final targets of 5-HT in the defensive withdrawal pathways of Aplysia thus remain to be identified.
Our results showing the direct enhancement of calcium influx in this model system are likely to be of relevance to associative learning as well. An activity-dependent form of the presynaptic facilitation induced by 5-HT at these sensory neuron-motor neuron synapses is thought to contribute to classical conditioning of defensive withdrawal reflexes of Aplysia (Hawkins et al. 1983). In addition, the implication of forms of presynaptic facilitation induced by 5-HT or by other agents in several brain regions (Beique et al. 2004; Nair and Gudelsky 2004; Wang et al. 2006), including those involved in fear conditioning (Huang et al. 1998; Huang and Kandel 2007; Mckernan and Shinnickgallagher 1997) and in working memory (Williams et al. 2002), suggests that mechanisms similar to the modulation of calcium influx reported here might play a role in short-term learning and memory in mammals as well.
Experimental Methods
Culture preparation
Cultures were prepared following published procedures (Klein 1993; Schacher and Proshansky 1983). Adult Aplysia californica (76–100 gm; RSMAS University of Miami, Miami, FL) were anesthetized by injection of 50–100 ml of 400 mM (isotonic) MgCl2. Pleural-pedal and abdominal ganglia were removed and digested in artificial seawater (ASW; see below for composition) containing 1% protease type IX (Sigma). Tail sensory neurons and siphon (LFS) motor neurons were isolated and plated in modified L15 containing 10% Aplysia hemolymph on dishes (Glass Bottom Mircowell Dish; MatTek Corporation) pretreated with poly-L-lysine (molecular weight, >300,000; Sigma). Dishes were prepared with isolated sensory neurons or co-cultures of sensory neurons and motor neurons for synapse formation.
Electrophysiology
An Axoclamp 2A or 2B amplifier (Molecular Devices, Palo Alto, CA) and glass micropipettes (tip resistance, 10–20 MΩ) filled with 0.5 M potassium chloride and 2 M potassium acetate were used for intracellular recordings. All recordings were done in ASW (in mM: 460 NaCl, 10 KCl, 11 CaCl2, 55 MgCl2, and 10 HEPES, pH 7.5). The membrane potential of sensory neurons was held at −55 mV. In co-cultures, the motor neuron was impaled first, and its membrane potential was maintained at −80 mV. To elicit a postsynaptic potential (PSP) in the motor neuron, an action potential was evoked in the sensory neuron with a short (5 ms) depolarizing current pulse at an interstimulus interval of 30 or 60 seconds. In order to ensure accurate measurements of action potentials, the amplitude of the depolarizing current pulse was adjusted so that the action potential was triggered after the end of the current pulse. Action potentials and PSPs were recorded and measured with Axograph X software (AxoGraph Scientific, Sydney, Australia).
Action potential duration was measured from the peak of the action potential to the point at which the falling limb crossed −10 mV, at approximately 40% of the peak amplitude of the spike, which is at about the peak of the current-voltage curve for calcium current in the sensory neurons (Klein, 1994). We chose to measure duration at a fixed membrane potential rather than at a fraction of the peak amplitude because the variable of interest is the calcium influx triggered by the action potential, and the calcium current is determined by the absolute membrane potential, not by a fraction of the peak amplitude of the spike. If the peak amplitude of the action potential does not change, it would make no difference which way the duration was measured. However, any change in the peak amplitude of the action potential would change the point at which the membrane potential reached any given fraction of the peak, without any actual broadening or narrowing of the spike. Measurement of the duration at a fraction of the peak could nonetheless indicate a change in duration, whereas measurement of duration at a fixed membrane potential far from the peak would show no change.
Peak PSP amplitudes were measured for PSPs smaller than 20–30 mV, while for larger PSPs the maximal slope of the PSP, corresponding to the peak of the underlying synaptic current, was recorded in order to avoid the complications of non-linear summation and the recruitment of voltage-gated conductances by large PSPs. The maximal slope and the amplitude of the PSP are closely correlated, and are linearly related over a large range of amplitudes (unpublished). Rise times of PSPs were measured from 20% to 50% of the peak amplitude except for larger PSPs that triggered action potentials. For these PSPs the time from 20% to 80% of the peak of the derivative of the PSP was taken as the rise time (i.e., from 20% to 80% of the peak synaptic current). In any given experiment all parameters were measured in the same manner. Facilitation of the PSP and the calcium transient was calculated as the average of the 2 responses immediately following drug application divided by the average of the 2 responses immediately prior to the application, except where noted otherwise.
Serotonin (creatinine sulfate; Sigma) was delivered as a bolus of 100 μl (1 μM, 5 μM, 10 μM or 100 μM in ASW) by a hand-held pipette directly into the bath, ~5 mm from the cells. The concentration to which the cells were exposed was estimated to be about half the concentration in the pipette (Klein, 1994). The potassium channel blocker 3,4-diaminopyridine (3,4-DAP; 1 mM), and the calcium channel blocker nitrendipine (100 μM in DMSO 0.5%) (both from Sigma) were applied in the same way, except for some experiments in which 3,4-DAP (500 μM) was perfused into the bath.
Microinjection of fluo-4
To monitor calcium transients, sensory neurons were loaded with the fluorescent calcium indicator fluo-4 (Molecular Probes, Eugene, OR; 10 mM in HEPES 100 mM, pH 7.6) by microinjection from back-filled glass micropipettes. The tip of the micropipette was inserted into the cell body, and short pressure pulses (10 ms duration, 20 psi) were delivered until the cell body became uniformly fluorescent. The cells were incubated in the dark at room temperature until the indicator diffused into neurites, usually about 10 minutes.
Fluorescence microscopy
Cultures were viewed at 20X or 40X with a Nikon Diaphot microscope attached to a xenon lamp for fluorescence excitation. A combination of optical filters and dichroic mirror (Omega Optical filter set XF23) was used for excitation centered at 485 nm; emission measurements were centered at 535 nm. Images were acquired with a cooled CCD camera (Retiga EXi) controlled by IPLab software (version 3.65; Scanalytics, Fairfax, VA). Image acquisition was begun just prior to triggering of an action potential in the sensory neuron. Twenty frames were captured at exposures of 50 or 100 ms. Values for peak calcium transients (dF) were obtained by subtracting the average of the 2 or 3 frames immediately preceding the transient from the average of 2 or 3 frames at the peak. In some experiments the dF values were divided by the baseline fluorescence (with background subtracted) to adjust for bleaching; these values are given as dF/F. Relative changes in fluorescence with experimental treatments within an experiment were generally unchanged by this correction, so either dF or dF/F values were used. Saturation of the indicator by high calcium concentrations would be detected as a relative flattening of the relationship between the PSPs and the calcium transients with increasing values. As this was not the case, saturation of the indicator was not considered to be a confounding factor in the experiments. The relationship between changes in fluorescence and in calcium concentration was presumed to be linear because the measured changes in fluorescence were on the order of 2-fold or less, which is less than 5% of the dynamic range of fluo-4 reported by the manufacturer.
Linear regressions for log-log plots of PSP vs. calcium transients started from the 4th PSP in all experiments because PSPs undergo homosynaptic depression, which is not reflected in a decrease in the calcium transients (Armitage and Siegelbaum 1998).
In control experiments strong depolarization and firing of the postsynaptic neuron had no effect on the fluorescence of the presynaptic neuron, confirming that the observed changes in fluorescence were not due to postsynaptic depolarization being transmitted to the presynaptic cell by electrical coupling or to a change in fluorescence in the postsynaptic neuron resulting from leakage of fluo-4 into the postsynaptic neuron.
All measurements are given as means ± SEM.
All experiments were performed at room temperature (20–23° C).
Acknowledgments
We thank Alan Grinnell and Wayne Sossin for helpful comments on an earlier version of the manuscript. Supported by NIH grant R01 NS047646 to MK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ahn S, Ginty DD, Linden DJ. A late phase of cerebellar long-term depression requires activation of CaMKIV and CREB. Neuron. 1999;23:559–568. doi: 10.1016/s0896-6273(00)80808-9. [DOI] [PubMed] [Google Scholar]
- 2.Armitage BA, Siegelbaum SA. Presynaptic induction and expression of homosynaptic depression at Aplysia sensorimotor neuron synapses. J Neurosci. 1998;18:8770–9. doi: 10.1523/JNEUROSCI.18-21-08770.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Augustine GJ. Regulation of transmitter release at the squid giant synapse by presynaptic delayed rectifier potassium current. J Physiol. 1990;431:343–364. doi: 10.1113/jphysiol.1990.sp018333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Augustine GJ, Charlton MP. Calcium dependence of presynaptic calcium current and post-synaptic response at the squid giant synapse. J Physiol. 1986;381:619–40. doi: 10.1113/jphysiol.1986.sp016347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bailey CH, Thompson EB, Castellucci VF, Kandel ER. Ultrastructure of the synapses of sensory neurons that mediate the gill-withdrawal reflex in Aplysia. J Neurocytol. 1979;8:415–444. doi: 10.1007/BF01214801. [DOI] [PubMed] [Google Scholar]
- 6.Baxter DA, Byrne JH. Serotonergic modulation of two potassium currents in the pleural sensory neurons of Aplysia. J Neurophysiol. 1989;62:665–679. doi: 10.1152/jn.1989.62.3.665. [DOI] [PubMed] [Google Scholar]
- 7.Beique JC, Chapin-Penick EM, Mladenovic L, Andrade R. Serotonergic facilitation of synaptic activity in the developing rat prefrontal cortex. J Physiol. 2004;556:739–754. doi: 10.1113/jphysiol.2003.051284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Blumenfeld H, Spira ME, Kandel ER, Siegelbaum SA. Facilitatory and inhibitory transmitters modulate calcium influx during action potentials in aplysia sensory neurons. Neuron. 1990;5:487–499. doi: 10.1016/0896-6273(90)90088-w. [DOI] [PubMed] [Google Scholar]
- 9.Borst JG, Sakmann B. Calcium influx and transmitter release in a fast CNS synapse. Nature. 1996;383:431–4. doi: 10.1038/383431a0. [DOI] [PubMed] [Google Scholar]
- 10.Borst JG, Sakmann B. Effect of changes in action potential shape on calcium currents and transmitter release in a calyx-type synapse of the rat auditory brainstem. Philos Trans R Soc Lond B Biol Sci. 1999;354:347–55. doi: 10.1098/rstb.1999.0386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Boyle MB, Klein M, Smith SJ, Kandel ER. Serotonin increases intracellular Ca2+ transients in voltage-clamped sensory neurons of Aplysia californica. Proc Natl Acad Sci U S A. 1984;81:7642–7646. doi: 10.1073/pnas.81.23.7642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Braha O, Dale N, Hochner B, Klein M, Abrams TW, Kandel ER. Second messengers involved in the two processes of presynaptic facilitation that contribute to sensitization and dishabituation in Aplysia sensory neurons. Proc Natl Acad Sci U S A. 1990;87:2040–2044. doi: 10.1073/pnas.87.5.2040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Braha O, Edmonds B, Sacktor T, Kandel ER, Klein M. The contributions of protein kinase A and protein kinase C to the actions of 5-HT on the L-type Ca2+ current of the sensory neurons in Aplysia. J Neurosci. 1993;13:1839–1851. doi: 10.1523/JNEUROSCI.13-05-01839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Brette F, Lacampagne A, Salle L, Findlay I, Le Guennec JY. Intracellular Cs+ activates the PKA pathway, revealing a fast, reversible, Ca2+-dependent inactivation of L-type Ca2+ current. Am J Physiol Cell Physiol. 2003;285:C310–C318. doi: 10.1152/ajpcell.00368.2002. [DOI] [PubMed] [Google Scholar]
- 15.Byrne JH, Kandel ER. Presynaptic facilitation revisited: state and time dependence [Review] J Neurosci. 1996;16:425–435. doi: 10.1523/JNEUROSCI.16-02-00425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, van den Pol AN. Presynaptic GABAB autoreceptor modulation of P/Q-type calcium channels and GABA release in rat suprachiasmatic nucleus neurons. J Neurosci. 1998;18:1913–1922. doi: 10.1523/JNEUROSCI.18-05-01913.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Christie BR, Eliot LS, Ito K, Miyakawa H, Johnston D. Different Ca2+ channels in soma and dendrites of hippocampal pyramidal neurons mediate spike-induced Ca2+ influx. J Neurophysiol. 1995;73:2553–7. doi: 10.1152/jn.1995.73.6.2553. [DOI] [PubMed] [Google Scholar]
- 18.Coulson RL, Klein M. Rapid development of synaptic connections and plasticity between sensory neurons and motor neurons of Aplysia in cell culture: implications for learning and regulation of synaptic strength. J Neurophysiol. 1997;77:2316–2327. doi: 10.1152/jn.1997.77.5.2316. [DOI] [PubMed] [Google Scholar]
- 19.Czesnik D, Nezlin L, Rabba J, Muller B, Schild D. Noradrenergic modulation of calcium currents and synaptic transmission in the olfactory bulb of Xenopus laevis tadpoles. Eur J Neurosci. 2001;13:1093–1100. doi: 10.1046/j.0953-816x.2001.01479.x. [DOI] [PubMed] [Google Scholar]
- 20.Deisseroth K, Bito H, Tsien RW. Signaling from synapse to nucleus: postsynaptic CREB phosphorylation during multiple forms of hippocampal synaptic plasticity. Neuron. 1996;16:89–101. doi: 10.1016/s0896-6273(00)80026-4. [DOI] [PubMed] [Google Scholar]
- 21.Dodge FA, Jr, Rahamimoff R. Co-operative action a calcium ions in transmitter release at the neuromuscular junction. J Physiol. 1967;193:419–432. doi: 10.1113/jphysiol.1967.sp008367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dunlap K, Fischbach GD. Neurotransmitters decrease the calcium component of sensory neurone action potentials. Nature. 1978;276:837–839. doi: 10.1038/276837a0. [DOI] [PubMed] [Google Scholar]
- 23.Edmonds B, Klein M, Dale N, Kandel ER. Contributions of two types of calcium channels to synaptic transmission and plasticity. Science. 1990;250:1142–1147. doi: 10.1126/science.2174573. [DOI] [PubMed] [Google Scholar]
- 24.Eliot LS, Kandel ER, Siegelbaum SA, Blumenfeld H. Imaging terminals of Aplysia sensory neurons demonstrates role of enhanced Ca2+ influx in presynaptic facilitation. Nature. 1993;361:634–637. doi: 10.1038/361634a0. [DOI] [PubMed] [Google Scholar]
- 25.Fedchyshyn MJ, Wang LY. Developmental transformation of the release modality at the calyx of held synapse. J Neurosci. 2005;25:4131–4140. doi: 10.1523/JNEUROSCI.0350-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fernandez-Chacon R, Konigstorfer A, Gerber SH, Garcia J, Matos MF, Stevens CF, Brose N, Rizo J, Rosenmund C, Sudhof TC. Synaptotagmin I functions as a calcium regulator of release probability. Nature. 2001;410:41–9. doi: 10.1038/35065004. [DOI] [PubMed] [Google Scholar]
- 27.Fossier P, Baux G, Tauc L. N- and P-type Ca2+ channels are involved in acetylcholine release at a neuroneuronal synapse: only the N-type channel is the target of neuromodulators. Proc Natl Acad Sci U S A. 1994;91:4771–4775. doi: 10.1073/pnas.91.11.4771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ghirardi M, Braha O, Hochner B, Montarolo PG, Kandel ER, Dale N. Roles of PKA and PKC in facilitation of evoked and spontaneous transmitter release at depressed and nondepressed synapses in Aplysia sensory neurons. Neuron. 1992;9:479–489. doi: 10.1016/0896-6273(92)90185-g. [DOI] [PubMed] [Google Scholar]
- 29.Graef IA, Mermelstein PG, Stankunas K, Neilson JR, Deisseroth K, Tsien RW, Crabtree GR. L-type calcium channels and GSK-3 regulate the activity of NF-ATc4 in hippocampal neurons. Nature. 1999;401:703–708. doi: 10.1038/44378. [DOI] [PubMed] [Google Scholar]
- 30.Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219:400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- 31.Haydon PG, Henderson E, Stanley EF. Localization of individual calcium channels at the release face of a presynaptic nerve terminal. Neuron. 1994;13:1275–1280. doi: 10.1016/0896-6273(94)90414-6. [DOI] [PubMed] [Google Scholar]
- 32.Hochner B, Kandel ER. Modulation of a transient K+ current in the pleural sensory neurons of Aplysia by serotonin and cAMP: implications for spike broadening. Proc Natl Acad Sci U S A. 1992;89:11476–11480. doi: 10.1073/pnas.89.23.11476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Huang CC, Wang SJ, Gean PW. Selective enhancement of P-type calcium currents by isoproterenol in the rat amygdala. J Neurosci. 1998;18:2276–2282. doi: 10.1523/JNEUROSCI.18-06-02276.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Huang YY, Kandel ER. 5-Hydroxytryptamine Induces a Protein Kinase A/Mitogen-Activated Protein Kinase-Mediated and Macromolecular Synthesis-Dependent Late Phase of Long-Term Potentiation in the Amygdala. J Neurosci. 2007;27:3111–3119. doi: 10.1523/JNEUROSCI.3908-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Isaacson JS. GABAB receptor-mediated modulation of presynaptic currents and excitatory transmission at a fast central synapse. J Neurophysiol. 1998;80:1571–1576. doi: 10.1152/jn.1998.80.3.1571. [DOI] [PubMed] [Google Scholar]
- 36.Klein M. Differential cyclic AMP dependence of facilitation at Aplysia sensorimotor synapses as a function of prior stimulation: augmentation versus restoration of transmitter release. J Neurosci. 1993;13:3793–3801. doi: 10.1523/JNEUROSCI.13-09-03793.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Klein M. Synaptic augmentation by 5-HT at rested Aplysia sensorimotor synapses: independence of action potential prolongation. Neuron. 1994;13:159–166. doi: 10.1016/0896-6273(94)90466-9. [DOI] [PubMed] [Google Scholar]
- 38.Klein M, Kandel ER. Mechanism of calcium current modulation underlying presynaptic facilitation and behavioral sensitization in Aplysia. Proc Natl Acad Sci U S A. 1980;77:6912–6916. doi: 10.1073/pnas.77.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Lando L, Zucker RS. Ca2+ cooperativity in neurosecretion measured using photolabile Ca2+ chelators. J Neurophysiol. 1994;72:825–30. doi: 10.1152/jn.1994.72.2.825. [DOI] [PubMed] [Google Scholar]
- 40.Llinas R, Sugimori M, Silver RB. Microdomains of high calcium concentration in a presynaptic terminal. Science. 1992;256:677–679. doi: 10.1126/science.1350109. [DOI] [PubMed] [Google Scholar]
- 41.Marinesco S, Carew TJ. Serotonin release evoked by tail nerve stimulation in the CNS of aplysia: characterization and relationship to heterosynaptic plasticity. J Neurosci. 2002;22:2299–312. doi: 10.1523/JNEUROSCI.22-06-02299.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mckernan MG, Shinnickgallagher P. Fear conditioning induces a lasting potentiation of synaptic currents in vitro. Nature. 1997;390:607–611. doi: 10.1038/37605. [DOI] [PubMed] [Google Scholar]
- 43.Nair SG, Gudelsky GA. Activation of 5-HT2 receptors enhances the release of acetylcholine in the prefrontal cortex and hippocampus of the rat. Synapse. 2004;53:202–207. doi: 10.1002/syn.20054. [DOI] [PubMed] [Google Scholar]
- 44.Pisani A, Bonsi P, Catania MV, Giuffrida R, Morari M, Marti M, Centonze D, Bernardi G, Kingston AE, Calabresi P. Metabotropic glutamate 2 receptors modulate synaptic inputs and calcium signals in striatal cholinergic interneurons. J Neurosci. 2002;22:6176–6185. doi: 10.1523/JNEUROSCI.22-14-06176.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Robitaille R, Adler EM, Charlton MP. Strategic location of calcium channels at transmitter release sites of frog neuromuscular synapses. Neuron. 1990;5:773–779. doi: 10.1016/0896-6273(90)90336-e. [DOI] [PubMed] [Google Scholar]
- 46.Royer S, Coulson RL, Klein M. Switching off and on of synaptic sites at aplysia sensorimotor synapses. J Neurosci. 2000;20:626–38. doi: 10.1523/JNEUROSCI.20-02-00626.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Sakaba T, Neher E. Quantitative relationship between transmitter release and calcium current at the calyx of held synapse. J Neurosci. 2001;21:462–476. doi: 10.1523/JNEUROSCI.21-02-00462.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Schacher S, Montarolo P, Kandel ER. Selective short- and long-term effects of serotonin, small cardioactive peptide, and tetanic stimulation on sensorimotor synapses of Aplysia in culture. J Neurosci. 1990;10:3286–3294. doi: 10.1523/JNEUROSCI.10-10-03286.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Schacher S, Proshansky E. Neurite regeneration by Aplysia neurons in dissociated cell culture: modulation by Aplysia hemolymph and the presence of the initial axonal segment. J Neurosci. 1983;3:2403–2413. doi: 10.1523/JNEUROSCI.03-12-02403.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shahrezaei V, Cao A, Delaney KR. Ca2+ from one or two channels controls fusion of a single vesicle at the frog neuromuscular junction. J Neurosci. 2006;26:13240–13249. doi: 10.1523/JNEUROSCI.1418-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Sherff CM, Carew TJ. Coincident induction of long-term facilitation at sensory-motor synapses in Aplysia: presynaptic and postsynaptic factors. Neurobiol Learn Mem. 2002;78:498–507. doi: 10.1006/nlme.2002.4092. [DOI] [PubMed] [Google Scholar]
- 52.Smith SJ, Buchanan J, Osses LR, Charlton MP, Augustine GJ. The spatial distribution of calcium signals in squid presynaptic terminals. J Physiol. 1993;472:573–593. doi: 10.1113/jphysiol.1993.sp019963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Spafford JD, Munno DW, Van Nierop P, Feng ZP, Jarvis SE, Gallin WJ, Smit AB, Zamponi GW, Syed NI. Calcium channel structural determinants of synaptic transmission between identified invertebrate neurons. J Biol Chem. 2003;278:4258–4267. doi: 10.1074/jbc.M211076200. [DOI] [PubMed] [Google Scholar]
- 54.Stanley EF. Single calcium channels and acetylcholine release at a presynaptic nerve terminal. Neuron. 1993;11:1007–1011. doi: 10.1016/0896-6273(93)90214-c. [DOI] [PubMed] [Google Scholar]
- 55.Sun ZY, Kauderer B, Schacher S. Differential distribution of functional receptors for neuromodulators evoking short-term heterosynaptic plasticity in Aplysia sensory neurons. J Neurosci. 1996;16:7540–7549. doi: 10.1523/JNEUROSCI.16-23-07540.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Vargas G, Yeh TY, Blumenthal DK, Lucero MT. Common components of patch-clamp internal recording solutions can significantly affect protein kinase A activity. Brain Res. 1999;828:169–173. doi: 10.1016/s0006-8993(99)01306-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Wang BW, Liao WN, Chang CT, Wang SJ. Facilitation of glutamate release by nicotine involves the activation of a Ca2+/calmodulin signaling pathway in rat prefrontal cortex nerve terminals. Synapse. 2006;59:491–501. doi: 10.1002/syn.20267. [DOI] [PubMed] [Google Scholar]
- 58.Westenbroek RE, Hoskins L, Catterall WA. Localization of Ca2+ channel subtypes on rat spinal motor neurons, interneurons, and nerve terminals. J Neurosci. 1998;18:6319–6330. doi: 10.1523/JNEUROSCI.18-16-06319.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Williams GV, Rao SG, Goldman-Rakic PS. The physiological role of 5-HT2A receptors in working memory. J Neurosci. 2002;22:2843–2854. doi: 10.1523/JNEUROSCI.22-07-02843.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Wu LG, Saggau P. Presynaptic inhibition of elicited neurotransmitter release. Trends Neurosci. 1997;20:204–212. doi: 10.1016/s0166-2236(96)01015-6. [DOI] [PubMed] [Google Scholar]


