Aerobic organisms use molecular oxygen (dioxygen; O2) to generate chemical energy in the form of adenine triphosphate (ATP). Energy transforms cellular structure to function, a defining property of life. Due to its favorable thermodynamic properties, O2 appears to have been selected during biological evolution to serve as the terminal electron acceptor in the reduction of carbon-based fuels to generate ATP by oxidative phosphorylation. That O2 is essential to sustain human life is perhaps best illustrated during an acute cardiopulmonary arrest—commonly referred to as “code blue” on the medical wards. Indeed, the “A-B-Cs” of basic life support is to ensure gas-exchange via the lungs and O2 delivery to internal organs: “A” for airway, “B” for breathing, and “C” for circulation. A less well appreciated role of O2 is in the evolution of organismal size, multicellularity, and biological complexity. An understanding of the key role of O2 in the evolution of complex life and mammalian physiology may provide novel insights of O2, and its metabolites (reactive oxygen species), in the pathophysiology of diseases that affect the lung.
O2 AND METAZOAN EVOLUTION
Multiple lines of evidence from evolutionary biology (1, 2), geochemistry (3), and systems biology (4) build a compelling case for a central role of O2 in the evolution of complex multicellular life on earth (5, 6). The oldest life forms are estimated to have been present over 3.7 billion years (Gyr) ago (7). In the relative absence of O2 and under the strong reducing conditions that existed on the primordial earth's surface, early unicellular life forms relied on metabolic pathways that used electron acceptors such as CO2 and SO4. Between about 2.5 and 2.2 Gyr ago, however, the earth's atmospheric concentration of O2 rose significantly (3). This rise in atmospheric O2 is thought to have been due to biological and geological factors that led to an increase in O2 production relative to its consumption. Biological processes, most notably the emergence of cyanobacteria capable of oxygenic photosynthesis (8), were an important source of O2 production during this early period. Geological events such as the burial of organic carbon (9) and a shift from submarine to subaerial volcanism (10) accounted for decreased O2 consumption.
The availability of O2 was a major coup for early life forms, since it allowed for the use of energy-efficient metabolic pathways. O2 is well suited to serve as an electron acceptor in the oxidation of carbon-based fuels for several reasons. First, the reduction of O2 provides the largest free energy release per electron transfer, with the exception of fluorine. Second, unlike fluorine, ground state triplet O2 is a diradical with its outer electrons in parallel spin, allowing for its greater stability and consequent accumulation in the earth's atmosphere. Third, aerobic metabolism yields at least 4-fold more energy per molecule of glucose oxidized than the most efficient anaerobic pathways. Fourth, ability of O2 to diffuse across biological membranes and to bind heme moieties in proteins (e.g., hemoglobin and cytochromes) facilitates O2 delivery to systemic organs and mitochondrial electron transfer functions. Finally, the biochemical symmetry of oxygenic photosynthesis and aerobic respiration (H2O → O2 → H2O cycle) maintains homeostasis within our planetary biosphere.
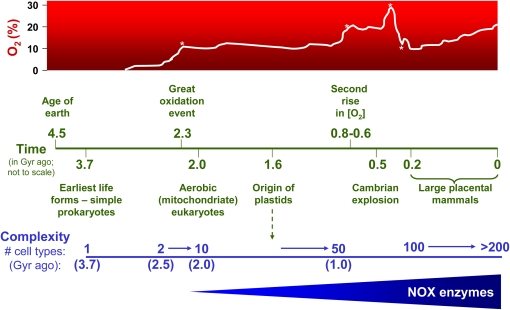
Increasing complexity in metazoan evolution has been linked to rising atmospheric O2 levels and the ability to use O2 to generate energy more efficiently (11). The evolution of the lung and circulatory system would have been critical for the transport of O2 to internal tissues/organs in larger, more complex metazoans, otherwise limited by the diffusibility of O2 across multiple cell layers. Complexity in organismal structure and function has been linked with the number of differentiated cell types and overall size of adult organisms (12). Estimates of the maximum number of cell types of common ancestors, combined with divergence times, showed an increase from 2 to approximately 10 cell types between 2.5 and 2.0 Gyr ago, and from 10 to 50 cell types between 1.5 and 1.0 Gyr ago (11) (Figure 1). The most prolific increase in speciation, however, occurred during the “Cambrian explosion,” approximately 540 million years ago. A second major rise in O2 concentration is thought to have been a critical factor (13), among other events such as mass extinctions (14), that created the “perfect storm” for this evolutionary diversification. A larger increase in O2 concentration occurred just before ∼ 300 million years ago, and this period was associated with the emergence of reptile and insect gigantism; the subsequent drop in O2 concentration during the Permian-Triassic period (∼ 260–245 million years ago) appears to have led to their extinction (15). The rise of O2 levels from approximately 10% (205 million years ago) to the current approximately 21% corresponds with vertebrate evolution and emergence of its key features: endothermy, placentation, and body size (humans are estimated to have > 200 different “cell types”) (1) (Figure 1).
Figure 1.
Temporal relationships between estimated atmospheric O2 concentrations, evolutionary diversification, and biological complexity. The first period of rapid O2 accumulation occurred approximately 2.3 billion years (Gyr) ago (the “great oxidation event,” denoted by the first asterisk on the O2 time line), followed by the first emergence of aerobic eukaryotes and multicellularity. The evolution of plastids approximately 1.6 Gyr ago provided eukaryotes the ability to generate their own O2, which appears to have triggered a second phase in the expansion of multicellularity (10–50 cell types between 1.5 and 1.0 Gyr ago) (11). The second major increase in atmospheric O2 between 0.8 and 0.6 Gyr ago (denoted by the second asterisk on the O2 time line), is thought to have been an important factor in the Cambrian explosion (∼ 0.54 Gyr) associated with a period of the most rapid and prolific speciation (13). A third rise in atmospheric O2 to levels greater than 30% approximately 0.3 Gyr ago (denoted by the third asterisk on the O2 time line) has been linked to the emergence of “gigantism” in several arthropod groups and reptile-like animals; the rapid drop in O2 concentration that followed (∼ 260–240 million years ago; fourth asterisk on the O2 time line) preceded mass extinctions of these species (15). The rise of O2 from about 10% to 21% over the past 205 million years is thought to have been a key factor in evolution of large placental mammals (1). Specialized O2-reducing enzymes dedicated to reactive oxygen species (ROS) generation, the NADPH oxidase (NOX) family, are primarily expressed in eukaryotes with an increasing number of NOX homologs that emerged in more complex metazoans (38, 39).
O2 IN CELLULAR PHYSIOLOGY: MORE THAN AEROBIC METABOLISM
The physiologic role of O2 in metazoan species is not limited to mitochondrial respiration. Recent biochemical networks analysis demonstrate that O2 is among the most-used compounds in a myriad of metabolic and biosynthetic pathways, superseding even adenosine triphosphate (ATP) and nicotinamide adenine dinucleotide (NAD+) (4). In this analysis, O2 was predicted to be involved in more that 103 metabolic reactions not found in anaerobes (4, 5). O2 is involved in a large number of biosynthetic pathways. Aerobes appear to have adopted O2-dependent mechanisms for synthesis of monounsaturated fatty acids, tyrosine, and nicotinic acid, biomolecules that are also found in more archaic anaerobes; and in a number of macromolecules that carry out more specialized cell functions found exclusively in aerobic metazoans (16). The O2-dependent synthesis of sterols and polyunsaturated fatty acids (16), key components of cell membranes, may have allowed for the biogenesis of intracellular organelles essential for cellular compartmentalization. O2 is also required for a number of biosynthetic reactions for specialized cell functions, such as formation of the connective tissue proteins, hydroxyproline and hydroxylysine (17, 18), synthesis of the visual pigment, retinal from β-carotene (19), and histone demethylation reactions that regulate chromatin remodeling (20).
ROS GENERATION FROM O2 METABOLISM: A DOUBLE-EDGED SWORD
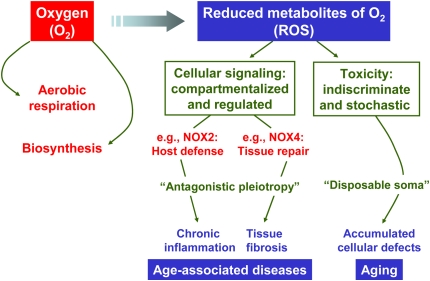
Although O2-dependent biosynthetic reactions and aerobic respiration have significant advantages, the use of O2 in these biological processes represents a double-edged sword. The generation of ROS, either as by-products of O2 metabolism (unintended) or by specialized enzymes (intended), has the potential to cause damage to cellular macromolecules such as proteins, lipid, and DNA. ROS that are capable of such damage include, but are not limited to, superoxide anion (O2·−), hydrogen peroxide (H2O2), and hydroxyl radical (OH·). To counteract toxicity from ROS, all aerobic species possess conserved cellular defense strategies such as that afforded by antioxidant enzymes (e.g., superoxide dismutase, catalase, peroxiredoxins, and glutathione peroxidase) (21). A relative increase in ROS production over antioxidant capacity is often referred to as a state of “oxidative stress” and is implicated in aging and age-associated diseases (22, 23). A causal role of ROS in these processes is not incontrovertible; nevertheless, the oxidative stress theory of aging remains as viable as any other to explain this complex phenomenon (24). ROS may contribute to age-associated pathologies by their cumulative injurious effects on cells/tissues in an indiscriminate and stochastic manner (Figure 2).
Figure 2.
The “oxygen paradox” of fitness/survival during early life of complex metazoans followed by aging and age-associated diseases in later life. The unique biochemical properties of O2 and ROS were exploited and selected during the evolution of complex multicellular life, based on their utility for aerobic respiration, biosynthesis of macromolecules, signal transduction, innate immunity/host defense, and tissue repair. However, the generation of O2/ROS may also exert “undesired” consequences in the aging adult organism by at least two different mechanisms. First, by the selection of genes that, while serving useful purposes in early reproductive life, exert pathobiologic effects in the elderly—a concept akin to the “theory of antagonisitic pleiotropy.” Second, ROS generation by mitochondrial and/or other enzymatic cellular sources mediate nonspecific, irreversible damage to tissues, including cellular and matrix components, that result in progressive organ dysfunction in a stochastic but nevertheless cumulative manner—a concept analogous to the “disposable soma theory.” While the former is perhaps a better explanation for specific age-associated disorders, the latter is a plausible theory for the more generalized aging process.
ROS IN CELLULAR PHYSIOLOGY: MORE THAN MEDIATORS OF CELLULAR INJURY
In addition to its recognized toxic effects, the concept that ROS serve “useful purposes” in normal physiologic processes has gained significant attention in recent years. An example of such a role is the ROS-generating NADPH oxidase (NOX) enzyme, gp91phox (NOX2), which serves host defense against invading microbes (25). In addition, a role for ROS in the transmission of biochemical signals from cell surface receptor–ligand interactions has been increasingly recognized (26, 27). The precise mechanisms of signal transduction are not known; however, one plausible and well-studied mechanism involves the reversible oxidization of cysteine residues on protein tyrosine phosphatases (28, 29). In addition to cellular signaling, there are a number of diverse biological contexts in which extracellular H2O2, via peroxidase-catalyzed reactions, mediates tyrosine crosslinking of extracellular matrix (ECM) proteins; examples include the pathogen-evoked hypersensitivity response to restrict spread of pathogens in plants (30), protection of the freshly fertilized egg from polyspermy in sea urchins (31, 32), stabilization of cuticular extracellular matrix (ECM) in Caenorhabditis elegans (33), and the conferring of “resilient” mechanical properties to resilin found in joints and tendons of insects (34). In mammals, the biosynthesis of thyroxine involves the activity of an H2O2-generating enzyme, a member of the NOX/DUOX gene family (35). The ability of the pro-fibrotic cytokine, transforming growth factor-β1 (TGF-β1), to induce crosslinking of (myo)fibroblast-derived extracellular matrix proteins is dependent on H2O2 in the presence of an extracellular heme peroxidase (36); the specific mammalian peroxidase(s) that mediate such reactions in physiologic/pathophysiologic contexts in vivo requires further study.
EVOLUTION OF THE ROS-GENERATING NOX GENE FAMILY
Just as the unique chemical/thermodynamic properties of O2 were harnessed for aerobic respiration and biosynthesis during biological evolution, it appears that the chemistry and reactivity of ROS may have also been adopted for purposes of cell signaling/regulation (Figure 2). This is best exemplified by the NOX enzymes, whose primary function is the regulated and compartmentalized generation of ROS (37). Intriguingly, the molecular evolution of NOX family enzymes appears to have increased in number and diversity with increasing complexity during metazoan evolution (38, 39). NOX enzymes are expressed in all multicellular eukaryotes, including fungi, plants, and animals, but not in prokaryotes (39). Most mammalian species, in particular humans, express seven NOX homologs (NOX1-5 and DUOX1-2), while C. elegans and Drosophila melanogaster each express two members of this gene family (39, 40). The diversification of NOX gene family with metazoan evolution suggests adaptive roles for these ROS-generating enzymes in more specialized organismal functions. For example, the newest member of this family, NOX3, is found in reptiles, birds, and mammals (39); it is highly expressed in the inner ear, and is required for vestibular sensory function and for perception of gravitational motion and balance (41). Such specialized functions may have been essential (or, at least beneficial) to the adaptation of these species to land. NOX4 appears to be present in all chordates, but not in other metazoans (39). NOX4 is required for the differentiation and activation of myofibroblasts (42, 43), key cellular mediators of wound repair responses and fibrosis. This raises the possibility that NOX4 may have emerged as an early adaptation of chordates to tissue injury and host repair responses (Figure 2).
NOX ENZYMES AND ANTAGONISTIC PLEIOTROPY
The use of O2 and ROS in normal physiologic processes presents an apparent paradox for the fitness and survival of mammalian species (44). While the use of O2 and generation of ROS have “useful,” physiologic purposes, they may also play a role in aging and age-associated degenerative diseases. One explanation for this paradox is the concept of antagonistic pleiotropy, which posits that genes selected because of their beneficial effects during early/reproductive life may also mediate deleterious effects in later life, a theory that was originally proposed to explain cellular senescence (45, 46). NOX2, an enzyme that was present in early multicellular eukaryotes as a critical component of the innate immune response and conserved in mammals, may also play a role in chronic inflammatory conditions in mature adults. The more recently identified NOX4 homolog and its function in myofibroblast differentiation/activation supports the possibility that this gene may also function in an “antagonistically pleiotropic” manner. While NOX4 and myofibroblasts may regulate wound repair responses in early life, this regulatory pathway may prove to be detrimental by promoting tissue fibrosis and organ dysfunction in later life (Figure 2). Myofibroblasts are key mediators of fibrogenesis in a number of human fibrotic disorders, including idiopathic pulmonary fibrosis, a disease typically associated with aging. One wonders if aging and age-associated diseases are the “price we pay” for the evolutionary conservation and diversification of O2/ROS-using metabolic pathways to produce more energy-efficient, larger, and more complex species. On the other hand, life on our planet would look very different without having had the availability of O2 in our biosphere. An understanding of the evolutionary selection and the (difficult) choices made during evolution of complex metazoans has the potential to provide novel insights into disease pathogenesis and innovative strategies for treatment of age-associated human maladies.
Acknowledgments
The author thanks members of his laboratory, and colleagues at the University of Michigan, and many others outside this institution for stimulating discussions on topics related to the content of this article.
This work was supported in part by National Institutes of Health grants R01 HL67967 and P50 HL74024 to V.J.T.
Originally Published in Press as DOI: 10.1165/rcmb.2008-0360PS on October 31, 2008
Conflict of Interest Statement: The author does not have a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.Falkowski PG, Katz ME, Milligan AJ, Fennel K, Cramer BS, Aubry MP, Berner RA, Novacek MJ, Zapol WM. The rise of oxygen over the past 205 million years and the evolution of large placental mammals. Science 2005;309:2202–2204. [DOI] [PubMed] [Google Scholar]
- 2.Acquisti C, Kleffe J, Collins S. Oxygen content of transmembrane proteins over macroevolutionary time scales. Nature 2007;445:47–52. [DOI] [PubMed] [Google Scholar]
- 3.Bekker A, Holland HD, Wang PL, Rumble D III, Stein HJ, Hannah JL, Coetzee LL, Beukes NJ. Dating the rise of atmospheric oxygen. Nature 2004;427:117–120. [DOI] [PubMed] [Google Scholar]
- 4.Raymond J, Segre D. The effect of oxygen on biochemical networks and the evolution of complex life. Science 2006;311:1764–1767. [DOI] [PubMed] [Google Scholar]
- 5.Falkowski PG. Evolution: tracing oxygen's imprint on earth's metabolic evolution. Science 2006;311:1724–1725. [DOI] [PubMed] [Google Scholar]
- 6.Catling DC, Glein CR, Zahnle KJ, McKay CP. Why O2 is required by complex life on habitable planets and the concept of planetary “oxygenation time”. Astrobiology 2005;5:415–438. [DOI] [PubMed] [Google Scholar]
- 7.Rosing MT. 13c-depleted carbon microparticles in >3700-ma sea-floor sedimentary rocks from west Greenland. Science 1999;283:674–676. [DOI] [PubMed] [Google Scholar]
- 8.Brocks JJ, Logan GA, Buick R, Summons RE. Archean molecular fossils and the early rise of eukaryotes. Science 1999;285:1033–1036. [DOI] [PubMed] [Google Scholar]
- 9.Hayes JM, Waldbauer JR. The carbon cycle and associated redox processes through time. Philos Trans R Soc Lond B Biol Sci 2006;361:931–950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kump LR, Barley ME. Increased subaerial volcanism and the rise of atmospheric oxygen 2.5 billion years ago. Nature 2007;448:1033–1036. [DOI] [PubMed] [Google Scholar]
- 11.Hedges SB, Blair JE, Venturi ML, Shoe JL. A molecular timescale of eukaryote evolution and the rise of complex multicellular life. BMC Evol Biol 2004;4:2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bonner JT. On the origin of differentiation. J Biosci 2003;28:523–528. [DOI] [PubMed] [Google Scholar]
- 13.Kennedy M, Droser M, Mayer LM, Pevear D, Mrofka D. Late precambrian oxygenation: inception of the clay mineral factory. Science 2006;311:1446–1449. [DOI] [PubMed] [Google Scholar]
- 14.Benton MJ. Diversification and extinction in the history of life. Science 1995;268:52–58. [DOI] [PubMed] [Google Scholar]
- 15.Berner RA, Vandenbrooks JM, Ward PD. Evolution: oxygen and evolution. Science 2007;316:557–558. [DOI] [PubMed] [Google Scholar]
- 16.Goldfine H. The evolution of oxygen as a biosynthetic reagent. J Gen Physiol 1965;49:253–274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fujimoto D, Tamiya N. Incorporation of O from air into hydroxyproline by chick embryo. Biochem J 1962;84:333–335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Towe KM. Oxygen-collagen priority and the early metazoan fossil record. Proc Natl Acad Sci USA 1970;65:781–788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sumper M, Reitmeier H, Oesterhelt D. Biosynthesis of the purple membrane of halobacteria. Angew Chem Int Ed Engl 1976;15:187–194. [DOI] [PubMed] [Google Scholar]
- 20.Forneris F, Binda C, Battaglioli E, Mattevi A. Lsd1: oxidative chemistry for multifaceted functions in chromatin regulation. Trends Biochem Sci 2008;33:181–189. [DOI] [PubMed] [Google Scholar]
- 21.McCord JM, Fridovich I. Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J Biol Chem 1969;244:6049–6055. [PubMed] [Google Scholar]
- 22.Balaban RS, Nemoto S, Finkel T. Mitochondria, oxidants, and aging. Cell 2005;120:483–495. [DOI] [PubMed] [Google Scholar]
- 23.Finkel T. Radical medicine: treating ageing to cure disease. Nat Rev Mol Cell Biol 2005;6:971–976. [DOI] [PubMed] [Google Scholar]
- 24.Kirkwood TB. Understanding the odd science of aging. Cell 2005;120:437–447. [DOI] [PubMed] [Google Scholar]
- 25.Nauseef WM. NOX enzymes in immune cells. Semin Immunopathol 2008;30:195–208. [DOI] [PubMed] [Google Scholar]
- 26.Thannickal VJ, Fanburg BL. Reactive oxygen species in cell signaling. Am J Physiol Lung Cell Mol Physiol 2000;279:L1005–L1028. [DOI] [PubMed] [Google Scholar]
- 27.Lambeth JD. NOX enzymes and the biology of reactive oxygen. Nat Rev Immunol 2004;4:181–189. [DOI] [PubMed] [Google Scholar]
- 28.Rhee SG, Bae YS, Lee SR, Kwon J. Hydrogen peroxide: a key messenger that modulates protein phosphorylation through cysteine oxidation. Sci STKE 2000;2000:PE1. [DOI] [PubMed]
- 29.Xu D, Rovira II, Finkel T. Oxidants painting the cysteine chapel: redox regulation of PTPS. Dev Cell 2002;2:251–252. [DOI] [PubMed] [Google Scholar]
- 30.Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell 1994;79:583–593. [DOI] [PubMed] [Google Scholar]
- 31.Shapiro BM. The control of oxidant stress at fertilization. Science 1991;252:533–536. [DOI] [PubMed] [Google Scholar]
- 32.Wong JL, Wessel GM. Free-radical crosslinking of specific proteins alters the function of the egg extracellular matrix at fertilization. Development 2008;135:431–440. [DOI] [PubMed] [Google Scholar]
- 33.Edens WA, Sharling L, Cheng G, Shapira R, Kinkade JM, Lee T, Edens HA, Tang X, Sullards C, Flaherty DB, et al. Tyrosine cross-linking of extracellular matrix is catalyzed by duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J Cell Biol 2001;154:879–891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Elvin CM, Carr AG, Huson MG, Maxwell JM, Pearson RD, Vuocolo T, Liyou NE, Wong DC, Merritt DJ, Dixon NE. Synthesis and properties of crosslinked recombinant pro-resilin. Nature 2005;437:999–1002. [DOI] [PubMed] [Google Scholar]
- 35.Milenkovic M, De Deken X, Jin L, De Felice M, Di Lauro R, Dumont JE, Corvilain B, Miot F. Duox expression and related H2O2 measurement in mouse thyroid: Onset in embryonic development and regulation by tsh in adult. J Endocrinol 2007;192:615–626. [DOI] [PubMed] [Google Scholar]
- 36.Larios JM, Budhiraja R, Fanburg BL, Thannickal VJ. Oxidative protein cross-linking reactions involving l-tyrosine in transforming growth factor-beta1-stimulated fibroblasts. J Biol Chem 2001;276:17437–17441. [DOI] [PubMed] [Google Scholar]
- 37.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev 2007;87:245–313. [DOI] [PubMed] [Google Scholar]
- 38.Kawahara T, Quinn MT, Lambeth JD. Molecular evolution of the reactive oxygen-generating NADPH oxidase (NOX/DUOX) family of enzymes. BMC Evol Biol 2007;7:109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sumimoto H. Structure, regulation and evolution of NOX-family NADPH oxidases that produce reactive oxygen species. FEBS J 2008;275:3249–3277. [DOI] [PubMed] [Google Scholar]
- 40.Bedard K, Lardy B, Krause KH. NOX family NADPH oxidases: not just in mammals. Biochimie 2007;89:1107–1112. [DOI] [PubMed] [Google Scholar]
- 41.Paffenholz R, Bergstrom RA, Pasutto F, Wabnitz P, Munroe RJ, Jagla W, Heinzmann U, Marquardt A, Bareiss A, Laufs J, et al. Vestibular defects in head-tilt mice result from mutations in Nox3, encoding an NADPH oxidase. Genes Dev 2004;18:486–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Thannickal VJ, Fanburg BL. Activation of an H2O2-generating NADH oxidase in human lung fibroblasts by transforming growth factor beta 1. J Biol Chem 1995;270:30334–30338. [DOI] [PubMed] [Google Scholar]
- 43.Cucoranu I, Clempus R, Dikalova A, Phelan PJ, Ariyan S, Dikalov S, Sorescu D. NAD(P)H oxidase 4 mediates transforming growth factor-beta1-induced differentiation of cardiac fibroblasts into myofibroblasts. Circ Res 2005;97:900–907. [DOI] [PubMed] [Google Scholar]
- 44.Lambeth JD. Nox enzymes, ROS, and chronic disease: an example of antagonistic pleiotropy. Free Radic Biol Med 2007;43:332–347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Williams GC. Pleiotropy, natural selection, and the evolution of senescence. Evolution Int J Org Evolution 1957;11:398–411. [Google Scholar]
- 46.Kirkwood TB, Rose MR. Evolution of senescence: late survival sacrificed for reproduction. Philos Trans R Soc Lond B Biol Sci 1991;332:15–24. [DOI] [PubMed] [Google Scholar]