Abstract
Rationale: Pulmonary nontuberculous mycobacterial (PNTM) disease is increasing, but predisposing features have been elusive.
Objectives: To prospectively determine the morphotype, immunophenotype, and cystic fibrosis transmembrane conductance regulator genotype in a large cohort with PNTM.
Methods: We prospectively enrolled 63 patients with PNTM infection, each of whom had computerized tomography, echocardiogram, pulmonary function, and flow cytometry of peripheral blood. In vitro cytokine production in response to mitogen, LPS, and cytokines was performed. Anthropometric measurements were compared with National Health and Nutrition Examination Survey (NHANES) age- and ethnicity-matched female control subjects extracted from the NHANES 2001–2002 dataset.
Measurements and Main Results: Patients were 59.9 (±9.8 yr [SD]) old, and 5.4 (±7.9 yr) from diagnosis to enrollment. Patients were 95% female, 91% white, and 68% lifetime nonsmokers. A total of 46 were infected with Mycobacterium avium complex, M. xenopi, or M. kansasii; 17 were infected with rapidly growing mycobacteria. Female patients were significantly taller (164.7 vs. 161.0 cm; P < 0.001) and thinner (body mass index, 21.1 vs. 28.2; P < 0.001) than matched NHANES control subjects, and thinner (body mass index, 21.1 vs. 26.8; P = 0.002) than patients with disseminated nontuberculous mycobacterial infection. A total of 51% of patients had scoliosis, 11% pectus excavatum, and 9% mitral valve prolapse, all significantly more than reference populations. Stimulated cytokine production was similar to that of healthy control subjects, including the IFN-γ/IL-12 pathway. CD4+, CD8+, B, and natural killer cell numbers were normal. A total of 36% of patients had mutations in the cystic fibrosis transmembrane conductance regulator gene.
Conclusions: Patients with PNTM infection are taller and leaner than control subjects, with high rates of scoliosis, pectus excavatum, mitral valve prolapse, and cystic fibrosis transmembrane conductance regulator mutations, but without recognized immune defects.
Keywords: immunodeficiency, IFN-γ/IL-12, bronchiectasis, leanness, cystic fibrosis
AT A GLANCE COMMENTARY
Scientific Knowledge on the Subject
Pulmonary nontuberculous mycobacterial (PNTM) infections may target a preexisting morphologic syndrome, but this has never been prospectively studied. A role for cystic fibrosis transmembrane conductance regulator (CFTR) is likely, but has only been seen in one prospective study. The role for immune defects is unknown.
What This Study Adds to the Field
Patients with PNTM infection are taller and leaner than control subjects, with high rates of scoliosis, pectus excavatum, mitral valve prolapse, and cystic fibrosis transmembrane conductance regulator mutations, but without recognized immune defects.
Nontuberculous mycobacteria are ubiquitous environmental organisms that are an increasingly common cause of pulmonary disease in certain populations (1, 2). Pulmonary NTM (PNTM) infection in patients who are non–HIV infected was previously seen in the setting of underlying chronic lung disease, such as chronic obstructive pulmonary disease or cystic fibrosis (CF) (3, 4). Prince and colleagues recognized PNTM infection in elderly white women without preexisting conditions (5). Multiple small pulmonary nodules, bronchiectasis, and a predilection for right middle lobe and lingula involvement were identified (6).
Because patients with disseminated mycobacterial infection in the absence of HIV frequently have discrete mutations in the IFN-γ and IL-12 production and response pathways (7), it has long been suspected that pulmonary nontuberculous mycobacteria in elderly white women may be due to an immune defect. However, despite studies of antigen-driven cytokine production in peripheral blood and bronchoalveolar lavage cells (8, 9), no consistent immune phenotype in PNTM infection has been established.
Morphologic features reported in this population include scoliosis, pectus excavatum, mitral valve prolapse, and thin body habitus (10). Some of these features are reminiscent of complex multisystem disorders, such as hyper-IgE syndrome (due to mutations in STAT3) and Marfan syndrome (due to mutations in fibrillin 1) (11, 12). Voluntary suppression of cough was hypothesized to predispose to pulmonary Mycobacterium avium complex (MAC) infection, termed the “Lady Windermere syndrome” (13, 14).
To clarify the predisposition to PNTM infection, we undertook a prospective study of morphologic, immunologic, and genetic aspects of PNTM infection (this work was presented in part at the annual meeting of the American Thoracic Society in Seattle, Washington, May, 2003).
METHODS
Subjects
From November 2001 to December 2005, we recruited 63 patients with microbiologic and radiographic evidence of active PNTM infection. Patients were accepted for study if they carried a verified diagnosis of PNTM infection without regard to sex, ethnicity, or insurance. Recruitment was accomplished through listing in the National Institutes of Health announcements of protocols, online at http://www.clinicaltrials.gov/, and by self- or physician referral. Patients were categorized according to the organism recovered at the time of diagnosis. A total of 60 patients fulfilled the 2007 American Thoracic Society criteria, whereas three patients had one positive NTM culture from a single sputum sample (15). Patients were prospectively enrolled in a National Institute of Allergy and Infectious Diseases institutional review board–approved observational protocol. A total of 57 patients tested negative for antibodies to HIV; the remaining six patients were not tested, but all had normal numbers of CD4+ cells and no HIV risk factors. To determine if CF transmembrane conductance regulator (CFTR) mutations were important in this population, we excluded from this study patients previously diagnosed with CF. CFTR testing before enrollment varied from none to extensive. All patients were seen at the Warren Grant Magnuson Clinical Center, National Institutes of Health, Bethesda, Maryland, and provided informed consent. A prospective age-, sex-, and race-matched control group (n = 32) was recruited for CFTR mutation analysis.
Data Collection
For all subjects, we took a complete history, reviewed medical records, administered a standardized questionnaire, and performed physical examinations with anthropometric measurements. Anthropometric measurements were performed by a team of three study nurses according to the detailed guidelines set forth in a National Health and Nutrition Examination Survey (NHANES) III video presentation (16). Body measurements and demographic data were obtained from the NHANES (National Bureau of Weights and Standards, Hyattsville, MD) 2001–2002 survey. Of the 11,039 survey participants, 826 were female, 42–85 years of age, either “non-Hispanic white” or “non-black/Mexican” ethnicity, and had no missing values. Their anthropomorphic values were used as controls.
Eight female patients with disseminated NTM infection had the same anthropometric measurements made by the same team of nurses, to serve as severe disease– and sex-matched control subjects.
Clinical Evaluation
Venous blood was obtained for routine laboratory tests, selected antibody titers, and immunologic studies. Computed tomography (CT) scans of the lungs, without intravenous contrast, were reviewed retrospectively by an experienced radiologist. Scoliosis was determined from the posterior–anterior chest radiograph. Pectus excavatum was determined from CT scans of the chest using the pectus severity index (17). Standard two-dimensional echocardiography was analyzed for mitral valve prolapse and mitral regurgitation by previously established criteria (18). Pulmonary function testing was performed on all patients according to American Thoracic Society guidelines (19, 20).
Laboratory Studies
Peripheral blood mononuclear cells (PBMCs) were isolated by centrifugation from heparinized whole blood using lymphocyte-separation gradient (BioWhittaker, Inc., Walkersville, MD) and washed with Hank's balanced salt solution. PBMCs (106/ml) were plated in 1 ml of complete media (RPMI 1640, 2 mM glutamine, 20 mM Hepes, 0.01 mg/ml gentamicin) with 10% fetal calf serum into 24-well plates. Selected wells were stimulated with 1% phytohemagglutinin (PHA) (Life Technologies, Gaithersburg, MD), PHA plus 1 ng/ml IL-12 heterodimer (R&D Systems, Minneapolis, MN), 200 ng/ml Escherichia coli–derived LPS (Sigma Chemical Co., St. Louis, MO), or LPS plus 1,000 U/ml IFN-γ (Genentech, Inc., South San Francisco, CA) for 48 hours at 37°C in 5% CO2. Supernatants were frozen at −80°C for subsequent cytokine determination. Thawed samples were examined for IL-6, IL-10, IFN-γ, tumor necrosis factor (TNF)-α, IL-1b, and IL-12 in duplicate using bioluminescent beads (Bio-Rad Laboratories, Hercules, CA). Control samples from healthy blood bank volunteers were stimulated and analyzed concurrently with experimental samples.
Flow cytometry for surface display of CD2, CD3, CD4, CD8, CD28, CD57, HLA-DR, CD25, CD20, CD16, and CD56 was done using a FACScan flow cytometer (Becton, Dickinson and Co., San Jose, CA) equipped with Cell Quest software (Becton, Dickinson and Co.).
To determine whether clonal proliferation of B or T cells occurred in this syndrome, consensus primers directed to conserved sequences in the variable and junctional regions of both the immunoglobulin heavy-chain gene locus and T-cell receptor γ-chain locus were used to amplify genomic DNA as previously described (21, 22). We defined cases as clonal if they possessed one or two rearrangements that are significantly increased in intensity over the background polyclonal pattern, and restricted patterns as the presence of one or more (usually multiple) distinct rearrangements appearing within the polyclonal background, but which are not of sufficient intensity to be considered significant clonal rearrangements.
CFTR Sequencing
CFTR sequencing from peripheral blood or transformed B cell lines was performed using both standard commercial genetic mutation screening (Genzyme Genetics, Framingham, MA; Ambry Genetics, Aliso Viejo, CA) and full coding region sequencing. For full exon sequencing, reagents supplied with the VariantSEQr Resequencing System (Applied Biosystems, Foster City, CA) CFTR assay were used. Sequencing of CFTR included coverage of exons 1–27, together with 5′ and 3′ untranslated regions (132 and 1,554 base pairs), 15 intronic bases flanking each exon, and 1,000 base pairs 5′ of exon 1. In addition, we used primers described by Zielenski and colleagues for exons 3, 5, 6, 10, 14, and 15 (23). All polymerase chain reaction (PCR) primers were tagged 5′ with M13 forward (F) or reverse (R) primer sequence, respectively. PCR reactions were performed using 10 ng of genomic DNA and following the manufacturer's protocol, with the exception of removing 50% glycerol from the master mix for exon 13, decreasing the annealing temperature from 60°C to 57°C for exons 3, 6, 10, and 52.8°C for exons 5, 14, and 15. Sequencing reactions were prepared using 2 μl of ExoSAP-IT (USB Corp., Cleveland, OH)-digested PCR product, M13-21F and M13R primers, and followed the manufacturer's master mix and thermal cycling conditions. Cycling reactions were performed on either Bio-Rad Tetrad 2 (Bio-Rad Laboratories) or ABI 9700 (Applied Biosystems) thermal cyclers. DNA capillary electrophoresis sequencing was performed on an ABI 3730xl (Applied Biosystems) instrument. Raw data were imported into SeqScape software (Applied Biosystems) in which sequencing alignments and analyses were performed. Sequence data were aligned against the CFTR reference sequence NM_000492 transcript. For the majority of DNAs, the mixed-base threshold was set at 66%. Subsequent DNA sequencing data were analyzed with a 50% mixed-base threshold. Reports were generated listing all base calls deviating from the reference sequence. Reports noted single nucleotide polymorphisms with the resulting amino acid effect. Homozygous/heterozygous insertion–deletion mutations were also reported. The polymorphisms listed in the mutation report were manually verified from chromatograms.
Statistical Analysis
Statistical analyses were performed with SAS software, version 9 (SAS Institute, Inc., Cary, NC). Continuous variables were compared using the Student's t test. Dichotomous variables were compared using the binomial test for one-sample comparisons.
The skewed distribution of our cytokine values were evaluated using the Kruskal-Wallis test. An adjustment for multiple comparisons was not made for the cytokine comparisons, as this was an exploratory analysis of patterns of difference. Cytokine data were displayed with box and whisker plots generated using SPSS software, version 12 (SPSS, Inc., Chicago, IL).
Using SAS, we extracted anthropomorphic measurements for 826 age-/ethnicity-matched female control subjects from the NHANES 2001–2002 database. All analyses were two sided, with significance set to P < 0.05.
RESULTS
We enrolled 63 patients (mean age, 59.9 ± 9.8 [SD] yr). The mean age at onset of symptoms was 50.6 ± 13.1 years, and the mean age at diagnosis was 55.8 ± 10.3 years, with a mean of 5.4 ± 7.9 years from diagnosis to enrollment. Patients were 95% female, 91% white, and 68% lifetime nonsmokers (Table 1). Our cohort was widely geographically distributed at the time of PNTM infection diagnosis, but 59% were in the South Atlantic region, consistent with previous epidemiologic surveys (1). Symptoms at presentation were fatigue (83%), chronic productive cough (78%), often lasting for months, and shortness of breath (65%) (Table 2). Sputum tended to be thick and green. Night sweats (54%), fevers (44%), and hemoptysis (29%) were less common. The mean weight loss ascribed to PNTM infection (or its treatment) was 3.7 ± 5.2 kg.
TABLE 1.
PATIENT DEMOGRAPHICS
| Characteristics | Patients (n = 63) |
|---|---|
| Mean age at enrollment ± SD, yr | 59.9 ± 9.8 |
| Median age, yr | 59 |
| Mean age at onset of symptoms ± SD, yr | 50.6 ± 13.1 |
| Mean age at diagnosis ± SD, yr | 55.8 ± 10.3 |
| Female sex, n (%) | 60 (95.2) |
| Ethnicity | |
| White, n (%) | 57 (90.5) |
| Asian, n (%) | 5 (7.9) |
| Hispanic, n (%) | 1 (1.6) |
| Residence at time of diagnosis, n (%)* | |
| Northeast | 5 (8) |
| South Atlantic | 37 (59) |
| South Central | 4 (6) |
| Midwest | 6 (10) |
| West | 10 (16) |
| Foreign | 1 (2) |
| Nonsmokers† | 43 (68) |
Northeast (PA, NJ, NY, CT, RI, MA,VT, NH, ME); South Atlantic (DE, MD, VA, DC, WV, NC, SC, GA, FL); South Central (KY, TN, MS, AL, OK, AR, LA, TX); Midwest (OH, IN, IL MI, WI, MN, IA, MO, ND, SD, NE, KS); West (MT, ID, WY, NV, UT, CO, AZ, NM, WA, OR, CA, AK, HI).
Defined as lifetime nonsmoker or less than 5-pack-year history of smoking.
TABLE 2.
SYMPTOMS AT TIME OF PRESENTATION
| Symptom | Patients (n = 63) |
|---|---|
| Cough, n (%) | 49 (78) |
| Phlegm | 42 (67) |
| Description of phlegm, n (n = 42) | |
| Thick | 37 |
| Green | 23 |
| Yellow | 11 |
| Clear | 4 |
| Hemoptysis, n (%) | 18 (29) |
| Fever, n (%) | 28 (44) |
| Fatigue, n (%) | 52 (83) |
| Shortness of breath, n (%) | 41 (65) |
| Night sweats, n (%) | 34 (54) |
| Mean weight loss attributed to PNTM infection or chemotherapy at time of enrollment ± SD, kg | 3.7 ± 5.2 |
Definition of abbreviation: PNTM = pulmonary nontuberculous mycobacterial.
A total of 44 patients were infected with at least one subspecies of the MAC; 17 patients had rapid growing mycobacteria, 16 due to M. abscessus, and one due to M. chelonae. One case each of M. kansasii and M. xenopi was included (Table 3).
TABLE 3.
MICROBIOLOGY
| Organism | Patients (n = 63) |
|---|---|
| MAC, n (%) | 44 (71) |
| M. avium, n | 8 |
| M. intracellularae, n | 18 |
| M. avium and M. intracellularae, n* | 3 |
| X-cluster, n | 2 |
| MAC (unspeciated), n | 13 |
| Rapid growing mycobacteria, n (%) | 17 (24) |
| M. abscessus, n | 16 |
| M. chelonae, n | 1 |
| Other mycobacteria, n | |
| M. kansasii | 1 |
| M. xenopi | 1 |
Definition of abbreviation: MAC = Mycobacterium avium complex.
Both species recovered simultaneously on multiple occasions from sputum.
Because nontuberculous mycobacteria are ubiquitous in water and soil, but not transmitted from human to human, we looked for behavioral links through a comprehensive questionnaire of activities, exposures, and habits. We found no distinct patterns of water consumption, bathing preferences, use of hot tubs, pets, or gardening (Table 4).
TABLE 4.
SURVEY QUESTIONS
| Condition | Patients (n = 63) |
|---|---|
| Exposures, n (%) | |
| Drink city water | 52 (83) |
| Drink well water | 18 (29) |
| Drink bottled water | 19 (30) |
| Shower | 34 (54) |
| Bath | 11 (17) |
| Shower and bath | 18 (29) |
| Swim in lake/ocean | 27 (43) |
| Swim in pool | 45 (71) |
| Swim weekly in pool for parts of the year | 28 (44) |
| Use of hot tub or Jacuzzi more than once a year | 11 (17) |
| Gardening | 36 (57) |
| Pets | 28 (44) |
| Menstrual history | |
| Mean age at menarche ± SD, yr | 12.8 ± 1.5 |
| Reached menopause, n | 55 |
| Mean age at menopause ± SD, yr | 47.2 ± 6.2 |
| Surgical oopherectomy, n (%) | 17/55 (31) |
| Hormone replacement therapy, n (%) | 22/55 (40) |
| Prior respiratory history, n (%) | |
| No respiratory issues prior to PNTM infection | 45 (71) |
| Recurrent pneumonias or bronchitis as a child* | 14 (22) |
| Do you agree with the statement: “It is socially unacceptable to cough”? | 20 (32) |
Definition of abbreviation: PNTM = pulmonary nontuberculous mycobacterial.
Defined as more than three pneumonias during childhood, or more than three episodes of bronchitis per year as a child.
PNTM infection onset is typically postmenopausal. Because estrogen has extensive effects on bone and tissue (24), we collected menstrual histories (Table 4). The mean age at menarche was 12.8 ± 1.5 years, and the mean age at menopause was 47.2 ± 6.2 years. Of those who reached menopause, 28.3% underwent a hysterectomy and 40% reported ever using hormone replacement therapy. The age at menarche did not differ significantly from the NHANES population (12.8 vs. 12.77 yr). Patients with PNTM reported a slightly later menopause than the NHANES control subjects (47.2 vs. 45.2 yr; P = 0.04). Hysterectomies were more common in the NHANES control group than in the patients with PNTM infection (45.9 vs. 28.3%; P = 0.01), but the percentage of those who reported ever being on hormone replacement therapy was not statistically different (40 vs. 50%; P = 0.20).
The majority of patients (71%) reported no respiratory issues before PNTM infection, whereas 22% reported recurrent pneumonias or bronchitis in childhood (Table 4). However, contrary to the behavioral hypothesis dubbed the “Lady Windermere syndrome,” 68% of patients reported no aversion to coughing in public, as determined by an eight-question survey (13).
We screened all patients for CF and α1-antitrypsin deficiency. A total of 23 of 63 patients (36.5%) had at least one mutation in the CFTR gene; 7 of them (11.1% overall) had two mutations. The most common mutation was delF508, seen in 9 of the 23 (39.1%) patients with identified CFTR mutations (Table 5). Other previously reported mutations included R117H, V754M, D1152H, R75Q, S1235R, G576A, R668C, R31C, and R1162L (25, 26). The prevalence of CFTR mutations is higher than both the general population and a group of 32 age-/sex-matched normal subjects. In the age-/sex-matched group, there were five individuals with single CFTR mutations (15.6%). Two individuals had delF508 (6.2%), two had S912L, and one had I507V.
TABLE 5.
UNDERLYING FACTORS
| Feature | Patients (n = 63) |
|---|---|
| CFTR mutation, n (%) | 23 (36.5) |
| delF508, n | 9 |
| R117H, n | 2 |
| V754M, n | 2 |
| R75Q, n | 2 |
| D1152H, n | 1 |
| S1235R, n | 1 |
| R1162L, n | 1 |
| G576A, n | 1 |
| R668C, n | 1 |
| R31C, n | 1 |
| E681V, n | 1 |
| 406-6T>C, n | 1 |
| 5′ UTR-680 T>G, n | 1 |
| −741T>G, n | 1 |
| R170H, n | 1 |
| 4375-36delT, n | 1 |
| α1-Antitrypsin, n | |
| <100 mg/dl | 2 |
| Not completed | 16 |
Definition of abbreviations: CFTR = cystic fibrosis transmembrane conductance regulator; UTR = untranslated region.
Sweat chloride testing was performed on 55 patients, and 10 (18%) had borderline-positive or positive tests (Table 6). Interestingly, only 3 of the 10 patients with PNTM infection who had an elevated sweat chloride test carried CFTR mutations. Two patients had low serum α1-antitrypsin levels out of 47 patients tested (Table 5).
TABLE 6.
MUTATION STATUS BY SWEAT CHLORIDE LEVELS
| Sweat Chloride (mmol/L)
|
|||
|---|---|---|---|
| No. of CFTR Mutations | <40 | 40 to ≤60 | >60 |
| 0 | 23 | 6 | 1 |
| 1 | 16 | 2 | 0 |
| >1 | 6 | 0 | 1 |
Definition of abbreviation: CFTR = cystic fibrosis transmembrane conductance regulator.
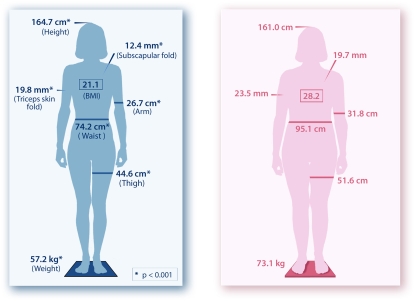
Patients with PNTM infection had a distinct body morphotype (Figure 1). They were taller (164.7 vs. 161.0 cm) and leaner (body mass index [BMI] [weight in kg/(height in m)2], 21.1 vs. 28.2) than NHANES control subjects, and all skinfold and circumference measurements were significantly leaner as well (P < 0.001 for all). The difference in BMI between patients with PNTM and control subjects remained significant even when using the patient's self-reported weight before the onset of PNTM infection (BMI, 22.3 vs. 28.2; P < 0.001). To try to address concerns about disease severity and NTM-specific weight loss, nine women with disseminated NTM infection without identified molecular defects followed during the course of the study were also measured. Patients with PNTM were significantly leaner (BMI, 21.1 vs. 26.4; P = 0.002) than disseminated NTM infection.
Figure 1.
(Left panel) Schematic depiction of the anthropometrics of the women with pulmonary nontuberculous mycobacterial (PNTM) (n = 60) compared with (right panel) National Health and Nutrition Examination Survey age-, sex-, and race-matched control subjects. *P < 0.001.
A total of 51% of patients with PNTM infection had scoliosis compared with 1.9% reported in the general population (P < 0.0001) (Table 7) (27). Pectus excavatum occurred in 11% of patients with PNTM infection compared with the estimated incidence of less than 1% in the general population (P < 0.001) (28). In a retrospective review, Iseman and colleagues reported scoliosis and pectus excavatum in 52 and 27% of patients with PNTM infection respectively (10). Transthoracic echocardiography was completed on 56 patients. We found that 9% had mitral valve prolapse, including one case of nonclassic mitral valve prolapse, which is statistically different from the 2.4% incidence in the Framingham cohort (P = 0.004) (18). Importantly, other mild cardiac anomalies, including trace mitral regurgitation (39%) and mild mitral regurgitation (22%), were similar to the incidences found in the Framingham cohort (29).
TABLE 7.
MORPHOLOGIC FEATURES
| Measurement | No. (%) with PNTM Infection (n = 63) | Population Values, % (Ref No.) | P Values (χ2) |
|---|---|---|---|
| Scoliosis | 32 (51) | 1.9 (26) | <0.001 |
| Pectus excavatum | 7 (11) | 1 (27) | <0.001 |
| Mitral valve prolapse | 5/56 (9) | 2.4 (18) | 0.004 |
Definition of abbreviation: PNTM = pulmonary nontuberculous mycobacterial.
Because Marfan syndrome is also associated with leanness, increased height, scoliosis, and mitral valve prolapse, we examined body segment ratios and other physical findings that are highly correlated with Marfan syndrome. The frequency of high-arched palate in PNTM was 79%, which is similar to the reported rate of 60–75% in Marfan syndrome (30). However, none of our 63 patients met major criteria for skeletal involvement under the revised (Ghent) diagnostic criteria for Marfan syndrome (31). The mean upper:lower body segment ratio in PNTM infection was 0.99 (vs. <0.86 in Marfan), and the mean armspan:height ratio was 1.00 (vs. >1.05 in Marfan). Only 38% had positive wrist and thumb signs, and only 24% had the benign joint hypermobility syndrome, compared with 85% of patients with Marfan syndrome (32). Therefore, this is a morphologic syndrome distinct from Marfan syndrome.
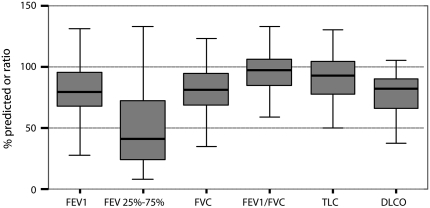
Bronchiectasis was most prevalent in the middle lobe (90%) and lingula (73%) (Table 8), higher than distribution proportions (51 and 60%, respectively) in a retrospective review by Lynch and colleagues (6). Nodules and cavities also followed a similar distribution, but the overall prevalence of bronchiectasis was higher in our prospectively identified cohort than in prior retrospective surveys of CT scans of NTM culture–positive patients (6, 33, 34). The prevalence of cavitary disease was lower in our cohort than in prior surveys (35). For most patients, the predominant form of bronchiectasis was cylindrical (61%), but saccular- (43%) and cystic (9%)-predominant presentations were also seen. Pulmonary function studies (Figure 2) showed mild obstruction, with decreased forced expiratory flow 25–75% (45.4% of predicted), suggesting small airways involvement similar to previously reported series (36).
TABLE 8.
RADIOGRAPHIC FEATURES
| Feature | Location | Number (%) (n = 63) |
|---|---|---|
| Bronchiectasis | Right upper lobe | 25 (40) |
| Right middle lobe | 57 (90) | |
| Right lower lobe | 37 (59) | |
| Left upper lobe | 15 (24) | |
| Lingula* | 46 (73) | |
| Left lower lobe | 36 (57) | |
| Predominant type of bronchiectasis | Cylindrical | 34 (61) |
| Saccular | 24 (43) | |
| Cystic | 5 (9) | |
| Nodules | Right upper lobe | 35 (56) |
| Right middle lobe | 45 (71) | |
| Right lower lobe | 46 (73) | |
| Left upper lobe | 28 (44) | |
| Lingula | 37 (59) | |
| Left lower lobe | 42 (67) | |
| Cavities | Right upper lobe | 11 (17) |
| Right middle lobe | 6 (10) | |
| Right lower lobe | 3 (5) | |
| Left upper lobe | 3 (5) | |
| Lingula | 1 (2) | |
| Left lower lobe | 2 (3) |
For this analysis, the lingula was considered separately from upper division of the left upper lobe.
Figure 2.
Pulmonary function tests are shown for patients with PNTM infection. Data are given as percent predicted values. FEF25–75 = forced expiratory flow between 25 and 75% of FVC; DlCO = diffusing capacity for carbon monoxide.
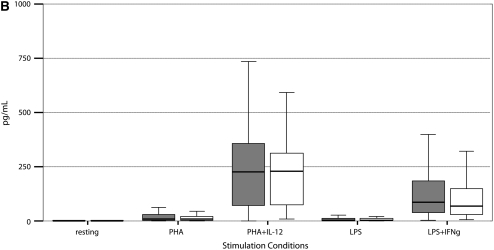
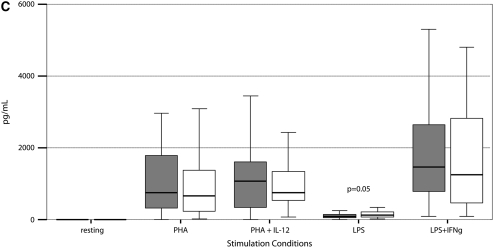
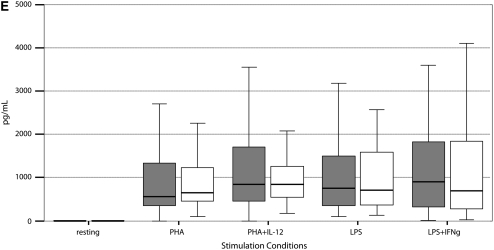
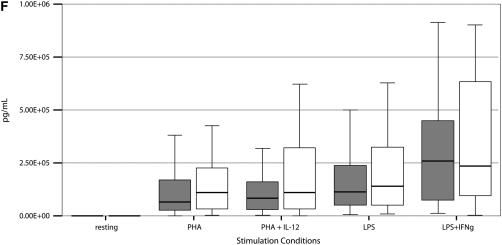
Stimulated cytokine production and response from PBMCs of 55 PNTM-infected patients studied with bioluminescent beads showed no consistent immune phenotype (Figure 3). In particular, there were no defects in IFN-γ/IL-12 production or response of the magnitude seen in disseminated NTM disease (37). There was a modest decrease in IFN-γ production in response to PHA plus IL-12 (PNTM-infected patients, 7,504 pg/ml vs. normal subjects, 10,384 pg/ml; P = 0.03), and a modest decrease in IL-1β response to LPS (PNTM-infected patients, 1,113 pg/ml vs. normal subjects, 1,184 pg/ml; P = 0.03).
Figure 3.
(A–F) Cytokine stimulation and response for peripheral blood mononuclear cells in 55 patients. Dark boxes, patients; open boxes, controls. In each box plot, the median value is indicated by the thick horizontal line, and the 25th and 75th percentiles are indicated by the upper and lower margins of the box, respectively. The whiskers denote the last value that is inside 1.5 times the interquartile range. Extreme values, if any, have been hidden to preserve the scale of each chart. Only P values that are below 0.05 are shown. Stimulation of (A) IFN-γ, (B) IL-12; (C) TNF-α, (D) IL-1b, (E) IL-10, and (F) IL-6.
Patients with PNTM had slightly fewer lymphocytes than healthy normal control subjects, but still fell well within the normal ranges for CD4+, CD8+, B, and natural killer cells (Table 9). A total of 56 of the 63 patients underwent B and T-cell clonality studies. One patient had a clonal immunoglobulin gene heavy-chain gene rearrangement. Abnormal T-cell receptor γ gene rearrangement patterns were seen in 20 of 56 (36%) cases, with eight (14%) showing distinct clonal rearrangements, and another 12 (22%) showing restricted rearrangement patterns. However, no patient had clinical lymphoproliferative disease.
TABLE 9.
FLOW CYTOMETRY
| PNTM-infected Patients, Mean Values (n = 61) | Percentile, Normal Values (n = 40)
|
||
|---|---|---|---|
| Immune Cell Type | 5th | 95th | |
| CD3 | 1,061 | 650 | 2,108 |
| CD2 | 1,264 | 992 | 2,079 |
| CD3/α-β | 1,022 | 659 | 1,812 |
| CD3/γ-δ | 36 | 9 | 163 |
| CD4 | 694 | 362 | 1,275 |
| CD8 | 427 | 344 | 911 |
| CD4/CD3 | 690 | 358 | 1,259 |
| CD8/CD3 | 322 | 194 | 836 |
| CD3+/CD4−/CD8− | 25 | 12 | 102 |
| CD28 | 991 | 771 | 1,891 |
| CD8/CD57 | 228 | 0 | 478 |
| CD3+/CD8/CD57 | 157 | 0 | 239 |
| CD3/HLA-DR | 197 | 0 | 291 |
| CD3/CD25 | 462 | 193 | 1,248 |
| CD20 | 166 | 49 | 424 |
| CD16+orCD56+/CD3− | 203 | 87 | 505 |
| CD16+orCD56+/CD3+ | 94 | 24 | 516 |
Definition of abbreviation: PNTM = pulmonary nontuberculous mycobacterial.
DISCUSSION
We studied patients with PNTM in a comprehensive prospective manner for morphologic and immune phenotypes. Our skewed referral population had severe, often refractory pulmonary infection, and consequently provides the best chance of capturing a distinct clinical phenotype associated with this infection.
Patients with PNTM infection were significantly leaner than age-/sex-/ethnicity-matched control subjects in both central (waist circumference) and distal (arm circumference) measurements. Although patients attributed an average of 3.7 kg of weight loss to their disease, they were on average 3.7 cm taller than the NHANES control subjects (P < 0.001), a difference unlikely to be caused by infection acquired late in life. Ziedalski and colleagues (26) recently reported low BMI values in their patients with PNTM infection, but without height values it was unclear whether these patients had acquired weight loss or intrinsic leanness. We tried to control for infection-specific BMI in our PNTM cohort by comparison to patients with disseminated NTM infection. The mean BMI of the women in our PNTM cohort was significantly lower than the mean BMI of the eight patients with disseminated NTM infection (21.1 vs. 26.8, respectively; P = 0.002), as well as lower than the mean BMI of the NHANES control population. Therefore, our predominantly female cohort is significantly taller and leaner than either NHANES control subjects or women with disseminated MAC infection. This morphotype is a cardinal feature of PNTM disease that is not induced by illness or mycobacterial infection per se. The additional aspects of scoliosis, pectus excavatum, and mitral valve prolapse are distinct from other connective tissue syndromes, such as Marfan syndrome (38) or hyper-IgE recurrent infection (Job's) syndrome (12).
Nontuberculous mycobacteria are widely recovered from municipal water sources, and often survive chlorination, leading to concern about modern and avoidable exposures (39, 40). We identified no common causal water exposure. Tanaka and colleagues (41) found no distinct association of PNTM with specific water exposures in a Japanese population. Although these findings are not controlled for exposures in a large, uninfected population, they strongly suggest that exposures to showers, hot tubs, or swimming pools are not, in and of themselves, etiologic.
The increasing female predominance of PNTM is striking, and suggests some role for sex hormones, as does the postmenopausal onset of disease. However, there was no correlation between age at menarche or menopause and either age at diagnosis or onset of symptoms (data not shown), suggesting that the total amount of lifetime estrogen exposure is not a marker for susceptibility to NTM infection. Tsuyuguchi and colleagues (42) found that exogenous estrogen enhanced the anti-MAC activity of murine macrophages, and that oophorectomy of mice increased the number of MAC colonies recovered from the lung after intratracheal infection. Recently, polymorphisms in the estrogen receptor gene have been found to be overrepresented in women with adolescent idiopathic scoliosis (43), suggesting a role for estrogen in scoliosis. However, the increased height, scoliosis, and mitral valve prolapse we describe predate the onset of PNTM infection. Therefore, we believe that this morphotype is a marker of an underlying immune, epithelial, or mucociliary impairment. However, leanness, mitral valve prolapse, and scoliosis have long been associated in women (44).
Although the IFN-γ/IL-12 axis is central to susceptibility to disseminated NTM disease, there have not been persuasive reports of its role in lung disease. PNTM infections are common in CF, but infection in CF does not occur outside the lung, and immunity in CF is generally normal. Interestingly, PNTM infections increase with age in CF, and are more common in those with milder disease (45). To test the integrity of the lymphocyte and monocyte limbs of immunity, we stimulated cells from patients with PNTM and normal subjects. We specifically avoided the use of mycobacterial antigens, because of the suppression of antigen-induced responses in the setting of active disease, as repeatedly shown in leishmaniasis, leprosy, and tuberculosis. T-cell responses to the mitogen PHA were entirely normal, as were IFN-γ responses. There was a slight decrease in IFN-γ production in response to PHA plus IL-12, as well as a slight decrease in IL-1β production in response to LPS. The underlying causes of these mild abnormalities is important to determine, but these abnormalities are different from those associated with disseminated disease, in which profound defects in IFN-γ production, IL-12 production, or response to those cytokines are found. Vankayalapati and colleagues (8) found that peripheral blood monocytes from patients with active pulmonary MAC produced more IL-10 than M. avium sensitin-responsive control subjects, which they hypothesized led to diminished IFN-γ, IL-12, and TNF-α; mitogen responses were not reported. Safdar and colleagues (46) reported marked decrease in IFN-γ secretion in response to stimulation with PHA and phorbol myristate, despite normal levels of intracellular IFN-γ levels as measured by flow cytometry. Kwon and colleagues (47) used conditions and stimuli similar to ours, and found markedly diminished production of IFN-γ, IL-12p40, and TNF-α. We did not confirm either of these reports in our larger cohort.
Clonality of lymphocytes often raises concern for monoclonal proliferation or malignancy. However, it is increasingly appreciated that nonneoplastic mono- or oligoclonality may occur among reactive lymphocytes and increases with age (48). Because none of the current patients have developed cytopenias or overt hematologic malignancy, it is interesting to speculate that the T-cell receptor gene abnormalities in these patients may reflect the emergence of specific T-cell populations in response to chronic infection.
PNTM in previously healthy, postmenopausal women, chronic obstructive lung disease in nonsmokers (44), and adult bronchiectasis in nonsmokers (49) are emerging entities. Of the 22 patients with chronic obstructive lung disease identified by Birring and colleagues (44), 83% were female, the mean age was 70 years, and a third had autoimmune organ disease, suggesting a possible autoimmune association. Autoimmune disease was not prominent in our cohort. Similarly, of the 103 patients identified by King and colleagues (49), 63% were female, and the mean age was 56 years. The predominance of elderly women in these pulmonary syndromes suggests interrelated manifestations of pulmonary disease in postmenopausal women, of which PNTM is only one. It is also possible that PNTM is a cumulative result of multiple factors, including morphotype and other causes of bronchiectasis.
Ziedalski and colleagues (26) reported a 50% prevalence of CFTR mutations in their NTM cohort, whereas we found 36.5%, frequencies substantially higher than the general population. Most of our patients were heterozygotes or compound heterozygotes, with normal sweat chloride levels. Although delF508 was our most common mutation, as it is in the general population, we found no delF508 homozygotes. Despite our use of commercial tests and efforts at full gene sequencing, uncommon, novel, or difficult-to-detect CFTR mutations, allelic deletions, or duplications may have been missed. Therefore, our value of 36.5% of the PNTM population affected should be seen as a minimum estimate, whereas the real number may be higher. Normal sweat chloride levels in the majority of cases suggest that, even if there are undetected mutations, they do not lead to the same impairment of CFTR as seen in classic CF. Because at least half of the patients reported here and by Ziedalski and colleagues (26) did not have mutations identified in CFTR, and almost all cases were lacking other features of classic CF, the distinct clinical entity of PNTM infection cannot be ascribed to mutations in CFTR alone.
Patients with PNTM infection did not confirm voluntary factors, such as cough suppression, or specific environmental exposures, such as showers, as etiologies of their disease. Cytokine and immune phenotyping were not consistent with previous reports or defects that predispose to disseminated NTM disease. We found that patients with PNTM were taller and leaner than NHANES control subjects, had high rates of scoliosis, pectus excavatum, and mitral valve prolapse, as well as high rates of mutation in the CFTR. These findings identify PNTM as a disease largely affecting women with a complex preexisting morphotype, making an underlying genetic defect likely.
Originally Published in Press as DOI: 10.1164/rccm.200805-686OC on August 14, 2008
Conflict of Interest Statement: None of the authors has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
References
- 1.O'Brien RJ, Geiter LJ, Snider DE Jr. The epidemiology of nontuberculous mycobacterial diseases in the United States: results from a national survey. Am Rev Respir Dis 1987;135:1007–1014. [DOI] [PubMed] [Google Scholar]
- 2.Marras TK, Daley CL. Epidemiology of human pulmonary infection with nontuberculous mycobacteria. Clin Chest Med 2002;23:553–567. [DOI] [PubMed] [Google Scholar]
- 3.Olivier KN, Weber DJ, Wallace RJ Jr, Faiz AR, Lee JH, Zhang Y, Brown-Elliot BA, Handler A, Wilson RW, Schechter MS, et al. Nontuberculous mycobacteria. I: Multicenter prevalence study in cystic fibrosis. Am J Respir Crit Care Med 2003;167:828–834. [DOI] [PubMed] [Google Scholar]
- 4.Aksamit TR. Mycobacterium avium complex pulmonary disease in patients with pre-existing lung disease. Clin Chest Med 2002;23:643–653. [DOI] [PubMed] [Google Scholar]
- 5.Prince DS, Peterson DD, Steiner RM, Gottlieb JE, Scott R, Israel HL, Figueroa WG, Fish JE. Infection with mycobacterium avium complex in patients without predisposing conditions. N Engl J Med 1989;321:863–868. [DOI] [PubMed] [Google Scholar]
- 6.Lynch DA, Simone PM, Fox MA, Bucher BL, Heinig MJ. CT features of pulmonary mycobacterium avium complex infection. J Comput Assist Tomogr 1995;19:353–360. [DOI] [PubMed] [Google Scholar]
- 7.Dorman SE, Holland SM. Interferon-gamma and interleukin-12 pathway defects and human disease. Cytokine Growth Factor Rev 2000;11:321–333. [DOI] [PubMed] [Google Scholar]
- 8.Vankayalapati R, Wizel B, Samten B, Griffith DE, Shams H, Galland MR, Von Reyn CF, Girard WM, Wallace RJ Jr, Barnes PF. Cytokine profiles in immunocompetent persons infected with mycobacterium avium complex. J Infect Dis 2001;183:478–484. [DOI] [PubMed] [Google Scholar]
- 9.Yamazaki Y, Kubo K, Sekiguchi M, Honda T. Analysis of BAL fluid in M. avium–intracellulare infection in individuals without predisposing lung disease. Eur Respir J 1998;11:1227–1231. [DOI] [PubMed] [Google Scholar]
- 10.Iseman MD, Buschman DL, Ackerson LM. Pectus excavatum and scoliosis: thoracic anomalies associated with pulmonary disease caused by Mycobacterium avium complex. Am Rev Respir Dis 1991;144:914–916. [DOI] [PubMed] [Google Scholar]
- 11.Guide SV, Holland SM. Host susceptibility factors in mycobacterial infection: genetics and body morphotype. Infect Dis Clin North Am 2002;16:163–186. [DOI] [PubMed] [Google Scholar]
- 12.Holland SM, DeLeo FR, Elloumi HZ, Hsu AP, Uzel G, Brodsky N, Freeman AF, Demidowich A, Davis J, Turner ML, et al. STAT3 mutations in the hyper-IgE syndrome. N Engl J Med 2007;357:1608–1619. [DOI] [PubMed] [Google Scholar]
- 13.Reich JM, Johnson RE. Mycobacterium avium complex pulmonary disease presenting as an isolated lingular or middle lobe pattern: the Lady Windermere syndrome. Chest 1992;101:1605–1609. [DOI] [PubMed] [Google Scholar]
- 14.Dhillon SS, Watanakunakorn C. Lady Windermere syndrome: middle lobe bronchiectasis and mycobacterium avium complex infection due to voluntary cough suppression. Clin Infect Dis 2000;30:572–575. [DOI] [PubMed] [Google Scholar]
- 15.Griffith DE, Aksamit T, Brown-Elliott BA, Catanzaro A, Daley C, Gordin F, Holland SM, Horsburgh R, Huitt G, Iademarco MF, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med 2007;175:367–416. [DOI] [PubMed] [Google Scholar]
- 16.National Health and Nutrition Examination Survey (NHANES). Anthropometry procedures manual [Internet]. (Revised 2000 Dec; accessed 2008 Sep 25). Atlanta: Centers for Disease Control and Prevention. Available from: http://www.cdc.gov/nchs/data/nhanes/bm.pdf
- 17.Haller JA Jr, Kramer SS, Lietman SA. Use of CT scans in selection of patients for pectus excavatum surgery: a preliminary report. J Pediatr Surg 1987;22:904–906. [DOI] [PubMed] [Google Scholar]
- 18.Freed LA, Levy D, Levine RA, Larson MG, Evans JC, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical outcome of mitral-valve prolapse. N Engl J Med 1999;341:1–7. [DOI] [PubMed] [Google Scholar]
- 19.Lung function testing: selection of reference values and interpretative strategies. American Thoracic Society. Am Rev Respir Dis 1991;144:1202–1218. [DOI] [PubMed] [Google Scholar]
- 20.American Thoracic Society. Standardization of spirometry, 1994 update. Am J Respir Crit Care Med 1995;152:1107–1136. [DOI] [PubMed] [Google Scholar]
- 21.McCarthy KP, Sloane JP, Kabarowski JH, Matutes E, Wiedemann LM. A simplified method of detection of clonal rearrangements of the T-cell receptor-gamma chain gene. Diagn Mol Pathol 1992;1:173–179. [PubMed] [Google Scholar]
- 22.Ramasamy I, Brisco M, Morley A. Improved PCR method for detecting monoclonal immunoglobulin heavy chain rearrangement in B cell neoplasms. J Clin Pathol 1992;45:770–775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zielenski J, Rozmahel R, Bozon D, Kerem B, Grzelczak Z, Riordan JR, Rommens J, Tsui LC. Genomic DNA sequence of the cystic fibrosis transmembrane conductance regulator (CFTR) gene. Genomics 1991;10:214–228. [DOI] [PubMed] [Google Scholar]
- 24.Compston JE. Sex steroids and bone. Physiol Rev 2001;81:419–447. [DOI] [PubMed] [Google Scholar]
- 25.Pignatti PF, Bombieri C, Marigo C, Benetazzo M, Luisetti M. Increased incidence of cystic fibrosis gene mutations in adults with disseminated bronchiectasis. Hum Mol Genet 1995;4:635–639. [DOI] [PubMed] [Google Scholar]
- 26.Ziedalski TM, Kao PN, Henig NR, Jacobs SS, Ruoss SJ. Prospective analysis of cystic fibrosis transmembrane regulator mutations in adults with bronchiectasis or pulmonary nontuberculous mycobacterial infection. Chest 2006;130:995–1002. [DOI] [PubMed] [Google Scholar]
- 27.Shands AR Jr, Eisberg HB. The incidence of scoliosis in the state of Delaware; a study of 50,000 minifilms of the chest made during a survey for tuberculosis. J Bone Joint Surg Am 1955;37-A:1243–1249. [PubMed] [Google Scholar]
- 28.Clark JB, Grenville-Mathers R. Pectus excavatum. Br J Dis Chest 1962;56:202–205. [DOI] [PubMed] [Google Scholar]
- 29.Singh JP, Evans JC, Levy D, Larson MG, Freed LA, Fuller DL, Lehman B, Benjamin EJ. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am J Cardiol 1999;83:897–902. [DOI] [PubMed] [Google Scholar]
- 30.Poole AE. Craniofacial aspects of the Marfan syndrome. Birth Defects Orig Artic Ser 1989;25:73–81. [PubMed] [Google Scholar]
- 31.De Paepe A, Devereux RB, Dietz HC, Hennekam RC, Pyeritz RE. Revised diagnostic criteria for the Marfan syndrome. Am J Med Genet 1996;62:417–426. [DOI] [PubMed] [Google Scholar]
- 32.Grahme R, Pyeritz RE. The Marfan syndrome: joint and skin manifestation are prevalent and correlated. Br J Rheumatol 1995;34:126–131. [DOI] [PubMed] [Google Scholar]
- 33.Hollings NP, Wells AU, Wilson R, Hansell DM. Comparative appearances of non-tuberculous mycobacteria species: a CT study. Eur Radiol 2002;12:2211–2217. [DOI] [PubMed] [Google Scholar]
- 34.Moore EH. Atypical mycobacterial infection in the lung: CT appearance. Radiology 1993;187:777–782. [DOI] [PubMed] [Google Scholar]
- 35.Wittram C, Weisbrod GL. Mycobacterium avium complex lung disease in immunocompetent patients: radiography–CT correlation. Br J Radiol 2002;75:340–344. [DOI] [PubMed] [Google Scholar]
- 36.Kubo K, Yamazaki Y, Masubuchi T, Takamizawa A, Yamamoto H, Koizumi T, Fujimoto K, Matsuzawa Y, Honda T, Hasegawa M, et al. Pulmonary infection with Mycobacterium avium–intracellulare leads to air trapping distal to the small airways. Am J Respir Crit Care Med 1998;158:979–984. [DOI] [PubMed] [Google Scholar]
- 37.Dorman SE, Holland SM. Mutation in the signal-transducing chain of the interferon-gamma receptor and susceptibility to mycobacterial infection. J Clin Invest 1998;101:2364–2369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Glesby MJ, Pyeritz RE. Association of mitral valve prolapse and systemic abnormalities of connective tissue: a phenotypic continuum. JAMA 1989;262:523–528. [PubMed] [Google Scholar]
- 39.Wallace RJ Jr. Nontuberculous mycobacteria and water: a love affair with increasing clinical importance. Infect Dis Clin North Am 1987;1:677–686. [PubMed] [Google Scholar]
- 40.Wallace RJ Jr, Brown BA, Griffith DE. Nosocomial outbreaks/pseudo-outbreaks caused by nontuberculous mycobacteria. Annu Rev Microbiol 1998;52:453–490. [DOI] [PubMed] [Google Scholar]
- 41.Tanaka G, Shojima J, Matsushita I, Nagai H, Kurashima A, Nakata K, Toyota E, Kobayashi N, Kudo K, Keicho N. Pulmonary Mycobacterium avium complex infection: association with nramp1 polymorphisms. Eur Respir J 2007;30:90–96. [DOI] [PubMed] [Google Scholar]
- 42.Tsuyuguchi K, Suzuki K, Matsumoto H, Tanaka E, Amitani R, Kuze F. Effect of oestrogen on Mycobacterium avium complex pulmonary infection in mice. Clin Exp Immunol 2001;123:428–434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu J, Qiu Y, Zhang L, Sun Q, Qiu X, He Y. Association of estrogen receptor gene polymorphisms with susceptibility to adolescent idiopathic scoliosis. Spine 2006;31:1131–1136. [DOI] [PubMed] [Google Scholar]
- 44.Birring SS, Brightling CE, Bradding P, Entwisle JJ, Vara DD, Grigg J, Wardlaw AJ, Pavord ID. Clinical, radiologic, and induced sputum features of chronic obstructive pulmonary disease in nonsmokers: a descriptive study. Am J Respir Crit Care Med 2002;166:1078–1083. [DOI] [PubMed] [Google Scholar]
- 45.Olivier KN. The natural history of nontuberculous mycobacteria in patients with cystic fibrosis. Paediatr Respir Rev 2004;5(Suppl A):S213–S216. [DOI] [PubMed] [Google Scholar]
- 46.Safdar A, White DA, Stover D, Armstrong D, Murray HW. Profound interferon gamma deficiency in patients with chronic pulmonary nontuberculous mycobacteriosis. Am J Med 2002;113:756–759. [DOI] [PubMed] [Google Scholar]
- 47.Kwon YS, Kim EJ, Lee SH, Suh GY, Chung MP, Kim H, Kwon OJ, Koh WJ. Decreased cytokine production in patients with nontuberculous mycobacterial lung disease. Lung 2007;185:337–341. [DOI] [PubMed] [Google Scholar]
- 48.Schwab R, Szabo P, Manavalan JS, Weksler ME, Posnett DN, Pannetier C, Kourilsky P, Even J. Expanded CD4+ and CD8+ T cell clones in elderly humans. J Immunol 1997;158:4493–4499. [PubMed] [Google Scholar]
- 49.King PT, Holdsworth SR, Freezer NJ, Villanueva E, Holmes PW. Characterisation of the onset and presenting clinical features of adult bronchiectasis. Respir Med 2006;100:2183–2189. [DOI] [PubMed] [Google Scholar]