In the article entitled “Lack of MK2 Inhibits Myofibroblast Formation and Exacerbates Pulmonary Fibrosis” in this issue of the AJRCMB (pp. 507–517), the authors Liu and coworkers extend their previous in vitro findings regarding the role of the kinase MK2 in myofibroblast differentiation (1). The authors present evidence that MK2−/− mice develop more severe pulmonary fibrosis in response to bleomycin, despite the fact that the mice appear to be deficient in the differentiation of myofibroblasts. The bleomycin-treated MK2−/− mice have many vimentin-positive cells in the lung, but few α–smooth muscle actin (α-SMA)–positive cells in fibroproliferative lesions. Further, MK2 loss in vivo does not result in complete loss of α-SMA expression, as α-SMA expression persists in vascular smooth muscle cells in vivo (see Liu and colleagues, Figure 4). Lungs from MK2-null mice also have more soluble collagen after bleomycin compared with wild-type mice, and their embryonic fibroblasts express increased collagen, and exhibit impaired migration in an in vitro wound assay. These are novel and important findings, particularly if the implication that lung fibrosis is pathogenically distinct from myofibroblast differentiation were found to be true in future work.
Myofibroblasts were originally identified more than 30 years ago and later characterized as cells of high contractile activity, rich in the α-isoform of smooth muscle actin in stress fibers, and of high synthetic activity (matrix proteins, cytokines and chemokines, proteases/inhibitors) (2). Based on these features, and their localization at sites of active wound healing, these cells have historically been considered to be the major regulators and effectors of the matrix remodeling required for normal wound healing in many organs. Furthermore, localization of collagen-expressing myofibroblasts to areas of fibrosis in experimental pulmonary fibrosis supports the notion that myofibroblast persistence results in pathologic tissue scarring (3). In vivo cell lineage tracing and adoptive transfer approaches have shed new light on the origin and role of the myofibroblast in fibrosis in the lung and other organs.
ORIGIN OF THE SPECIES
Emerging evidence suggests that the cell of origin for myofibroblasts is partly a function of the organ to be repaired. In the case of vascular remodeling, pericytes and resident smooth muscle cells appear to be the sources of myofibroblasts, whereas stellate cells and epithelial cells predominate in hepatic and renal injury, respectively. In the lung, local resident tissue fibroblasts, bronchoalveolar stem cells, bone marrow–derived and circulating mesenchymal progenitor cells (fibrocytes), and endothelial and epithelial cells (through endothelial/epithelial–mesenchymal transition) have all been proposed to participate in the injury-repair process.
In the specific case of experimental pulmonary fibrosis, circulating fibrocytes (CD45+, Collagen I+, and CD34+ or CD13+) can be recruited to sites of experimental pulmonary fibrosis, and/or cultured from injured lung minces, and contribute to the fibrotic process (4–6). These cells will express α-SMA after long-term in vitro culture (7). In contrast, chimeric mice with green fluorescent protein–labeled bone marrow–derived cells develop ample accumulation of these cells to sites of injury/fibrosis, but little differentiation of bone marrow–derived cells into intrapulmonary myofibroblasts (7). Lineage tracking approaches have also demonstrated epithelial to mesenchymal transition and its regulation as a function of the extracellular matrix (8). Thus, better definition of the cell of origin of myofibroblasts in specific situations is needed, as is further clarification of the actual fibrosis effector cell in vivo.
α-SMA: JUST ONE OF THE GANG, OR MASTER REGULATOR OF MYOFIBROBLAST PHENOTYPE?
In mammals, there are at least six different isoforms of actin that are 95% homologous, but encoded for by different genes. These include the skeletal and cardiac α-actins, the vascular and visceral α and γ smooth muscle actins, and the cytoplasmic β and γ actins. The cytoplasmic actins are enriched in nonmuscle cells and function in cell motility and endocytosis. The skeletal and cardiac α-actins form contractile sarcomeres, while smooth muscle myofibrils are formed by α and γ smooth muscle actins. As α-SMA is the most abundant protein in vascular smooth muscle cells, it is not surprising that mice deficient in α-SMA have deranged vascular contractility and dysregulated blood flow, despite a compensatory up-regulation of skeletal α-actin (9).
Gain- and loss-of-function studies of α-SMA using knockout mice and adenoviral-mediated gene expression have surprisingly identified α-SMA as an antifibrotic molecule in experimental renal fibrosis (10). This finding parallels that noted by Liu and colleagues in this issue of the Journal. Although direct reading of the pathogenic role of α-SMA cannot be gleaned from these results, there are clear consequences of modulating α-SMA expression on fibrosis in vivo. Furthermore, although α-SMA–null renal mesangial cells had fewer actin stress fibers than did their wild-type counterparts, they had compensatory enhancement of expression of other smooth muscle genes, including SMγ-actin, skeletal muscle α-actin, and the SM myosin chain isoform (10). Together these results suggest the existence of a feedback compensatory mechanism designed to maintain cellular actin content and smooth muscle phenotype.
As implied by the mesangial cell findings, levels of expression of α-SMA alone may be insufficient to indicate the extent of myofibroblastic differentiation. This is exemplified by Thy-1(−) and Thy-1(+) rat lung fibroblasts, where both cell types express α-SMA, but they differ significantly in contractility, in expression of myosin, desmin, and in myogenic regulatory factors such as myogenin and MyoD (11). As perhaps all myofibroblasts are not created equal, it would be very useful to evaluate the role of myofibroblasts in a gain/loss of function manner in vivo in several different organs using complementary fibrosis models. Once collated, such a series of results would better define the phenotypic differences among myofibroblasts from different organs and their respective roles in the fibrobroliferative process.
MUSCLING IN ON PULMONARY FIBROSIS
α-SMA–expressing cells are present in fibroproliferative lesions in bleomycin-injured lungs and in fibroblastic foci in idiopathic pulmonary fibrosis (12). The most obvious mechanism whereby myofibroblasts could alter lung fibrosis is through their contractile properties, hence the older term “muscular cirrhosis of the lung” (13). The relative contributions of myofibroblast-generated pulmonary “wound” contraction, relative to that of excess and disorganized collagenous matrix, to the deranged lung mechanics remains unclear. However, myofibroblasts also produce collagen and other matrix proteins, proteases, protease inhibitors, growth factors, cytokines and chemokines, and affect alveolar epithelial cell fate (2). Thus they are capable and poised to regulate and/or directly affect the fibroproliferative process. Precisely which phenotypic features of the myofibroblast are responsible for its fibrosis-modulating effects is an area of active investigation.
LIVE STRONG: MYOFIBROBLAST DIFFERENTIATION AND SURVIVAL
Basic local tissue requirements for the generation of myofibroblasts are the presence of active TGF-β, the presence of specialized matrix-derived biochemical signals, and a high extracellular mechanical stress (14). A rigid extracellular matrix supports the development of large mature focal cell–matrix adhesions, and facilitates α-SMA recruitment to cytoskeletal fibers, thereby enhancing contractile activity. The molecular mechanism of TGF-β induction of α-SMA gene expression involves complex interactions among multiple transcrptional regulators including blockade of Smad-3 binding to the Smad-binding element by gut-enriched Kruppel-like factor (GKLF), as well as binding of Kruppel-like factors (i.e., Sp-1) to the TGF-β control element of the α-SMA promoter (15). Epigenetic regulation of α-SMA expression is also demonstrable upon modulation of telomerase activity (16, 17).
Current evidence suggests that myofibroblasts are terminally differentiated cells, with minimal proliferative and migratory potential, and that they are removed by apoptosis. The triggering of the myofibroblast apoptoic response in vivo may be through changes in the mechanical properties of the matrix, nitric oxide–induced apoptosis, loss of growth factor–induced Akt survival signaling, and or sensitization by TNF-α (2, 18). Interestingly, modulation of focal adhesion kinase (FAK), and/or prosurvival signaling, alters fibrotic tissue formation in mice (19).
MK2: KEY PLAYER IN MYOFIBROBLAST DIFFERENTIATION SIGNALING?
MK2 is in the family of mitogen-activated protein kinase (MAPK)-activated protein kinases (MAP kinase kinases, or MKKs). This study introduces MK2 as a new important signaling intermediate in fibrogenesis, although important roles for its upstream signaling partner, p38 MAPK, have been described. Having demonstrated in a previous study that stress fiber formation and α-SMA expression are inhibited in MK2−/− murine embryonic fibroblasts (MEFs), in part through MK2 effects on α-SMA mRNA stability (20), the authors in this study now address the in vivo significance of disrupted MK2 signaling with regard to fibrosis. Given the vast literature regarding the role of myofibroblasts in fibrosis in multiple organs, one would have predicted a reduction in fibrosis in the bleomycin-challenged MK2-null mice, compared with their wild-type counterparts. Remarkably, the MK2-null mice exhibit an increase in fibrosis after bleomycin challenge. To explain the apparent discrepancy between increased biochemical and histopathologic evidence of fibrosis despite decreased fibroblast α-SMA in vitro and in vivo, the authors performed additional in vitro studies demonstrating increased proliferation, increased collagen, and decreased migration in MK2-null MEFs. Although it would have been preferable to use mature lung fibroblasts rather than embryonic fibroblasts; the findings do provide ample food for thought regarding myofibroblast phenotype regulation.
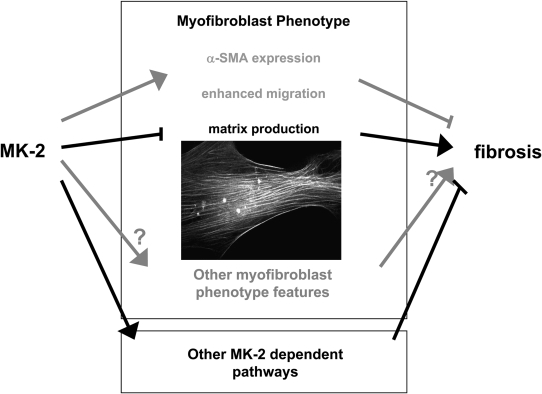
It is useful to consider what these studies tell us about the signaling that mediates fibroblast phenotype regulation. MK2 has multiple kinase substrates, including the heat-shock proteins HSP25 and HSP27, cytoskeleton-associated proteins, mRNA binding proteins, transcription factors, and cell cycle– and apoptosis-related molecules (21). Although p38, which is known to regulate α-SMA via the serum response element (SRE), is upstream of MK2, previous studies by this group demonstrated that TGF-β stimulation of SRE via p38 is intact in MK2−/− fibroblasts (20), suggesting divergent effects on signaling. Functionally, MK2 is known to affect inflammatory signaling, cell cycle control, actin remodeling, cell migration, gene promoter repression, and stability of multiple mRNAs, some of which (e.g., uPA, cyclooxygenase-2 and IL-6) are important in fibrosis (21). The effects of MK2 on cell migration in the present study are striking, with arrested migration in MK2−/− MEFs. Based on their previous studies in endothelial cells, the authors transfected MK2 MEFs with “phosphomimicking” HSP27, but were unable to rescue the migratory defect in fibroblasts. They conclude that the migration defect is due to deficient α-SMA expression. This is an interesting but not fully supported consideration. Usually, fully differentiated myofibroblasts are considered less migratory than nonmyofibroblasts (22). However, this is not always the case: myofibroblasts lacking Thy-1 express higher levels of muscle proteins and are more contractile, yet are also more migratory than their Thy-1(+) counterparts (11, 23). The nonmigratory phenotype of MK2−/− cells is also surprising in light of the bleomycin studies, as the majority of the literature supports an increased migratory phenotype in fibrosis (24). Overall, the absence of MK2 enhances some profibrotic features (proliferation, collagen expression) and inhibits others (α-SMA, migration), but the balance in vivo is profibrotic (see Figure 1). It is possible that the effects of MK2 on gene expression via promoter repression and mRNA stability trump its other functions; global analysis of gene expression in these cells might provide further insights.
Figure 1.
Role of MK2 in myofibroblast differentiation and pulmonary fibrosis. MK2-dependent signaling exacerbates pulmonary fibrosis but abrogates some “pro-fibrotic” myofibroblastic phenotypic features. Alternatively, MK2's effects on fibrosis may be independent of its effects on myofibroblast differentiation. Inset depicts α-SMA–containing stress fibers in TGF-β1–stimulated human lung fibroblasts.
NOTABLE FOR THEIR ABSENCE
Finally, these findings reinforce the concept that in fibrosis, some molecules are notable for their absence. MK2 joins caveolin-1, E- and P-selectins, uteroglobin, surfactant protein C, Nrf2, and Thy-1 among molecules whose absence exacerbates pulmonary fibrosis (12, 25–30). These and others may act as “fibrosis suppressor genes,” which may be as instructive in fibrosis as tumor suppressors have been in cancer biology. Thus, in microarray studies, the genes turned off may be just as important as the genes turned on. These types of molecules may also offer novel therapeutic options—for example, via gene therapy, molecular replacement, or molecular mimicry. They affect different cell types, and different portions of the complex biological cascades leading to fibrosis; and, as the study of Liu and coworkers reinforces, their effects in vitro may not always predict their roles in vivo. We are left once again with the lesson that through a combination of careful in vitro, in vivo, and translational studies, and by thinking outside the box, we can better understand the complex network of tissue repair and fibrogenic signaling. α-SMA has taught us a lot about myofibroblasts and their roles in wound healing and fibrosis. Much remains to be known, including the cell(s) of origin of myofibroblasts in specific injury/repair situations, what phenotypic features of the myofibroblast mediate its fibrosis-modulating effects, and what signaling molecules/pathways determine the myofibroblast phenotype and fate. Once known, we can achieve the ultimate goal of modulating its activity to treat fibrosis. Building on decades of excellent and ongoing research, the best may be yet to come. . . .
Acknowledgments
The authors thank Cassie Woodley and Janice Buffett for their secretarial assistance.
This work was supported by grants from the Children's Center for Research and Innovation (to J.S.H.), National Institutes of Health (HL-58655 to M.A.O.), and the Veterans Administration MERIT Review Board (to M.A.O.).
Conflict of Interest Statement: Neither author has a financial relationship with a commercial entity that has an interest in the subject of this manuscript.
Both authors contributed equally to this work.
References
- 1.Liu T, Warburton RR, Guevara OE, Hill NS, Fanburg BL, Gaestel M, Kayyali US. Lack of MK2 inhibits myofibroblast formation and exacerbates pulmonary fibrosis. Am J Respir Cell Mol Biol 2007;37:507–517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hinz B, Phan SH, Thannickal VJ, Galli A, Bochaton-Piallat ML, Gabbiani G. The myofibroblast: one function, multiple origins. Am J Pathol 2007;170:1807–1816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang K, Rekhter MD, Gordon D, Phan SH. Myofibroblasts and their role in lung collagen gene expression during pulmonary fibrosis: a combined immunohistochemical and in situ hybridization study. Am J Pathol 1994;145:114–125. [PMC free article] [PubMed] [Google Scholar]
- 4.Moore BB, Kolodsick JE, Thannickal VJ, Cooke K, Moore TA, Hogaboam C, Wilke CA, Toews GB. CCR2-mediated recruitment of fibrocytes to the alveolar space after fibrotic injury. Am J Pathol 2005;166:675–684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Phillips RJ, Burdick MD, Hong K, Lutz MA, Murray LA, Xue YY, Belperio JA, Keane MP, Strieter RM. Circulating fibrocytes traffic to the lungs in response to CXCL12 and mediate fibrosis. J Clin Invest 2004;114:438–446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Moore BB, Murray L, Das A, Wilke CA, Herrygers AB, Toews GB. The role of CCL12 in the recruitment of fibrocytes and lung fibrosis. Am J Respir Cell Mol Biol 2006;35:175–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hashimoto N, Jin H, Liu T, Chensue SW, Phan SH. Bone marrow-derived progenitor cells in pulmonary fibrosis. J Clin Invest 2004;113:243–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Willis BC, Liebler JM, Luby-Phelps K, Nicholson AG, Crandall ED, du Bois RM, Borok Z. Induction of epithelial-mesenchymal transition in alveolar epithelial cells by transforming growth factor-beta1: potential role in idiopathic pulmonary fibrosis. Am J Pathol 2005;166:1321–1332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Schildmeyer LA, Braun R, Taffet G, Debiasi M, Burns AE, Bradley A, Schwartz RJ. Impaired vascular contractility and blood pressure homeostasis in the smooth muscle alpha-actin null mouse. FASEB J 2000;14:2213–2220. [DOI] [PubMed] [Google Scholar]
- 10.Takeji M, Moriyama T, Oseto S, Kawada N, Hori M, Imai E, Miwa T. Smooth muscle alpha-actin deficiency in myofibroblasts leads to enhanced renal tissue fibrosis. J Biol Chem 2006;281:40193–40200. [DOI] [PubMed] [Google Scholar]
- 11.Sanders YY, Kumbla P, Hagood JS. Enhanced myofibroblastic differentiation and survival in Thy-1(-) lung fibroblasts. Am J Respir Cell Mol Biol 2007;36:226–235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hagood JS, Prabhakaran P, Kumbla P, Salazar L, MacEwen MW, Barker TH, Ortiz LA, Schoeb T, Siegal GP, Alexander CB, et al. Loss of fibroblast Thy-1 expression correlates with lung fibrogenesis. Am J Pathol 2005;167:365–379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Davies D, MacFarlane A, Darke CS, Dodge OG. Muscular hyperplasia (“cirrhosis”) of the lung and bronchial dilatations as features of chronic diffuse fibrosing alveolitis. Thorax 1966;21:272–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hinz B. Masters and servants of the force: the role of matrix adhesions in myofibroblast force perception and transmission. Eur J Cell Biol 2006;85:175–181. [DOI] [PubMed] [Google Scholar]
- 15.Hu B, Wu Z, Liu T, Ullenbruch MR, Jin H, Phan SH. Gut-enriched Kruppel-like factor interaction with Smad3 inhibits myofibroblast differentiation. Am J Respir Cell Mol Biol 2007;36:78–84. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schissel SL, Layne MD. Telomerase, myofibroblasts, and pulmonary fibrosis. Am J Respir Cell Mol Biol 2006;34:520–522. [DOI] [PubMed] [Google Scholar]
- 17.Liu T, Hu B, Chung MJ, Ullenbruch M, Jin H, Phan SH. Telomerase regulation of myofibroblast differentiation. Am J Respir Cell Mol Biol 2006;34:625–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frankel SK, Cosgrove GP, Cha SI, Cool CD, Wynes MW, Edelman BL, Brown KK, Riches DW. TNF-alpha sensitizes normal and fibrotic human lung fibroblasts to Fas-induced apoptosis. Am J Respir Cell Mol Biol 2006;34:293–304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vittal R, Horowitz JC, Moore BB, Zhang H, Martinez FJ, Toews GB, Standiford TJ, Thannickal VJ. Modulation of prosurvival signaling in fibroblasts by a protein kinase inhibitor protects against fibrotic tissue injury. Am J Pathol 2005;166:367–375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sousa AM, Liu T, Guevara O, Stevens J, Fanburg BL, Gaestel M, Toksoz D, Kayyali US. Smooth muscle alpha-actin expression and myofibroblast differentiation by TGF-β are dependent upon MK2. J Cell Biochem 2007;100:1581–1592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gaestel M. MAPKAP kinases—MKs—two's company, three's a crowd. Nat Rev Mol Cell Biol 2006;7:120–130. [DOI] [PubMed] [Google Scholar]
- 22.Hinz B, Dugina V, Ballestrem C, Wehrle-Haller B, Chaponnier C. Alpha-smooth muscle actin is crucial for focal adhesion maturation in myofibroblasts. Mol Biol Cell 2003;14:2508–2519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olman MA, White KE, Ware L, Simmons WL, Pugin J, Benveniste EN, Matthay MA. Acute lung injury edema fluid mediates fibroblast proliferation and gene expression through interleukin-1. J Immunol 2004;172:2668–2677. [DOI] [PubMed] [Google Scholar]
- 24.Barker TH, Grenett HE, MacEwen MW, Tilden SG, Fuller GM, Settleman J, Woods A, Murphy-Ullrich J, Hagood JS. Thy-1 regulates fibroblast focal adhesions, cytoskeletal organization and migration through modulation of p190 RhoGAP and Rho GTPase activity. Exp Cell Res 2004;295:488–496. [DOI] [PubMed] [Google Scholar]
- 25.White ES, Thannickal VJ, Carskadon SL, Dickie EG, Livant DL, Markwart S, Toews GB, Arenberg DA. Integrin alpha4beta1 regulates migration across basement membranes by lung fibroblasts: a role for phosphatase and tensin homologue deleted on chromosome 10. Am J Respir Crit Care Med 2003;168:436–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Cho HY, Reddy SP, Yamamoto M, Kleeberger SR. The transcription factor NRF2 protects against pulmonary fibrosis. FASEB J 2004;18:1258–1260. [DOI] [PubMed] [Google Scholar]
- 27.Horikawa M, Fujimoto M, Hasegawa M, Matsushita T, Hamaguchi Y, Kawasuji A, Matsushita Y, Fujita T, Ogawa F, Takehara K, et al. E- and P-selectins synergistically inhibit bleomycin-induced pulmonary fibrosis. Am J Pathol 2006;169:740–749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lawson WE, Polosukhin VV, Stathopoulos GT, Zoia O, Han W, Lane KB, Li B, Donnelly EF, Holburn GE, Lewis KG, et al. Increased and prolonged pulmonary fibrosis in surfactant protein C-deficient mice following intratracheal bleomycin. Am J Pathol 2005;167:1267–1277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Lee YC, Zhang Z, Mukherjee AB. Mice lacking uteroglobin are highly susceptible to developing pulmonary fibrosis. FEBS Lett 2006;580:4515–4520. [DOI] [PubMed] [Google Scholar]
- 30.Wang XM, Zhang Y, Kim HP, Zhou Z, Feghali-Bostwick CA, Liu F, Ifedigbo E, Xu X, Oury TD, Kaminski N, et al. Caveolin-1: a critical regulator of lung fibrosis in idiopathic pulmonary fibrosis. J Exp Med 2006;203:2895–2906. [DOI] [PMC free article] [PubMed] [Google Scholar]