Summary of Recent Advances
The adoptive transfer of T cells isolated or engineered to have specificity for diseased cells represents an ideal approach for the targeted therapy of human viral and malignant diseases. The therapeutic potential of adoptive T cell therapy for infections and cancer was demonstrated in rodent models long ago, but the task of translating this approach into an effective clinical therapy has not been easy. Carefully designed clinical trials have evaluated the transfer of antigen-specific T cells in humans, and provided insight into the barriers to efficacy and strategies to improve T cell therapy. The importance of altering the host environment to facilitate persistence and function of transferred T cells and intrinsic properties of T cells that are selected or engineered for therapy in determining their fate in vivo are key issues that have recently emerged and are informing the design of the next generation of clinical trials.
Introduction
An intact and functional T cell compartment is critical for effective immunity to pathogens and there is evidence that T cells can participate in the control and elimination of tumors (1,2). Studies in rodent models of cancer and infectious diseases have demonstrated that the adoptive transfer of T cells of defined antigen specificity can establish or augment immunity and eradicate malignant or infected cells. Adoptive T cell transfer also has therapeutic activity against human viral infections in allogeneic hematopoietic stem cell transplant recipients, a setting in which virus-specific T cells can be readily isolated and expanded from the immunocompetent donor (3-5). It was initially perceived that the major obstacle for applying T cell therapy to human malignancies would be the requirement to isolate and expand tumor-reactive T cells to sufficient numbers to modulate T cell immunity. The identification of tumor associated antigens (TAA) and refined culture techniques have overcome this obstacle for selected tumors such as melanoma. However, in initial studies the infusion of large numbers of T cells or T cell clones specific for TAA failed to completely eradicate tumors in the majority of patients, at least in part due to the short persistence of the transferred T cells in vivo (6-9). This review will summarize insights that have been derived from clinical trials of T cell immunotherapy that have led to progress in developing improved regimens for establishing a durable and functional T cell response using adoptive T cell transfer.
T cell therapy for opportunistic virus infections and virus induced malignancy
A notable success of adoptive T cell transfer is its use to prevent or treat opportunistic virus infections in allogeneic hematopoietic stem cell transplant (HCT) recipients. Regimens for HCT often employ myeloablative doses of chemotherapy and radiation to treat the underlying malignancy and facilitate engraftment of donor stem cells, and either administer immunosuppressive drugs post transplant or deplete T cells from the donor stem cell graft to prevent graft-versus-host disease (GVHD) (10). These treatments result in a prolonged functional and/or numerical deficit of T cells, and render HCT recipients susceptible to life-threatening infection, both from endogenous latent viruses that reactivate after HCT and from acute community acquired viruses.
Cytomegalovirus
Reactivation of latent cytomegalovirus (CMV) in allogeneic HCT recipients remains a significant cause of morbidity and mortality despite antiviral drug therapy (11). The finding that progressive infection with CMV correlated with deficient CMV-specific CD8+ and CD4+ T cell responses suggested that the adoptive transfer of CMV-specific T cells isolated from the immunocompetent donor might be used to restore protective immunity in the recipient. The initial studies of adoptive immunotherapy for CMV infused CD8+ T cell clones or polyclonal T cell lines that were derived from the donor and selected for recognition of CMV-infected cells and lack of cross reactivity with recipient alloantigens (5,12,13). The enrichment and cloning of CMV-specific T cells required prolonged culture but avoided a risk of GVHD, which is observed frequently if unselected donor T cells are administered to HCT recipients. These studies demonstrated that CD8+ and CD4+ CMV-specific T cells could be adoptively transferred to patients early after HCT with minimal toxicity, and that the transferred cells could persist and function in vivo, and control infection (5,12,13).
Despite this encouraging data, the complex culture methods used to isolate CMV-specific T cells limited the broad and timely application of this approach. More recent efforts have been directed at designing methods to select CMV-specific T cells directly from donor blood. Immunomagnetic selection of antigen-specific T cells has been developed based on binding of tetrameric HLA class I molecules folded with CMV peptides, or the capture of T cells that secrete interferon gamma after antigen stimulation (14,15). The infusion of remarkably small numbers of donor-derived CD8+ T cells selected for binding to HLA class I tetramers containing CMV pp65 or IE-1 peptides to allogeneic HCT recipients with CMV reactivation restored T cell responses to these CMV antigens and reduced the need for antiviral drug therapy (3).
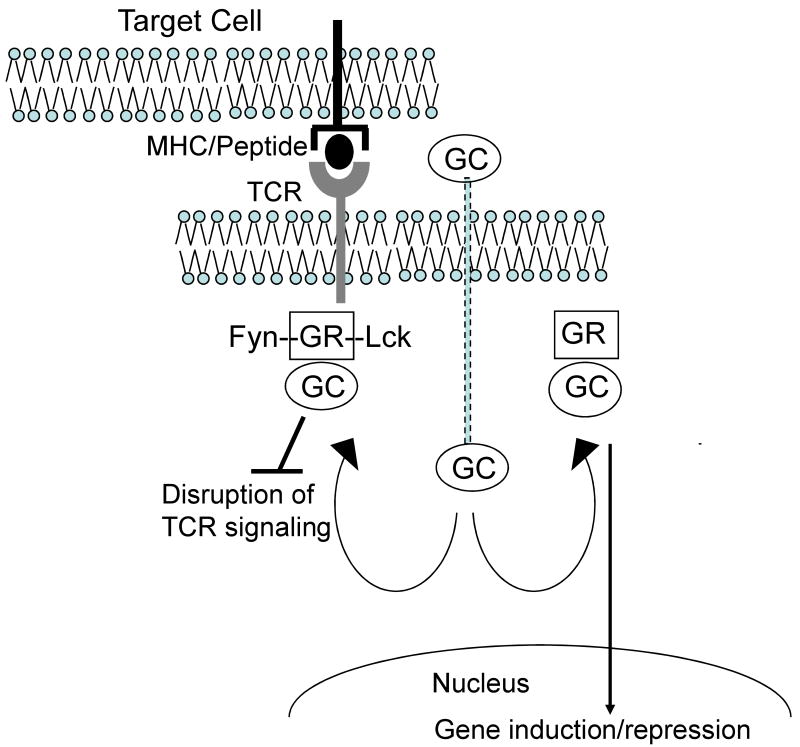
Adoptive T cell therapy for CMV is not uniformly successful in protecting HCT recipients from progressive CMV infection. It is increasingly clear that adoptive transfer of CMV-specific T cells is ineffective in patients that require high doses of glucocorticoids to treat GVHD, which occurs in at least 35% of HLA identical allogeneic HCT recipients that receive a non T cell depleted stem cell graft (16). Glucocorticoids impair T cell antigen receptor signaling and cytokine production, and promote T cell apoptosis, and these effects are mediated through binding to the glucocorticoid receptor (GR), activation of GR-responsive immunosuppressive genes and inhibition of proinflammatory genes (17,18) (Figure 1). Nongenomic effects of the GR in T cells have also been described, including a physical interaction of unligated GR with the TCR complex, which is disrupted by glucocorticoid binding resulting in impaired activation of Lck and Fyn (19) (Figure 1). Thus, patients that receive prolonged treatment with glucocorticoids have frequent reactivations of CMV and are at high risk of toxicity from antiviral drugs and for the development of drug resistant viral variants. In a clinical trial at the Fred Hutchinson Cancer Research Center, the infusion of CD8+ and CD4+ CMV-specific T cell clones failed to restore persistent functional T cell immunity in patients that received prednisone, and these patients remained at risk for CMV viremia. These results suggest that glucocorticoids will be useful for reversing toxicity that might develop after adoptive T cell therapy but pose a problem for reconstituting virus-specific immunity in HCT recipients. T cells can be engineered to be resistant to glucocorticoids by interfering with GR expression by introduction of siRNA or by gene editing with zinc finger nucleases (20). The latter approach is now being evaluated in animal models, and may enable the restoration of a protective glucocorticoid resistant CMV-specific T cell response in this high-risk group of patients.
Figure 1. Glucocorticoids exert negative effects on adoptively transferred T cells through both nongenomic and genomic mechanisms.
The non ligated glucocorticoid receptor (GR) is associated with the T cell receptor signaling complexes containing Fyn and Lck. Immediately upon binding of the GR to glucocorticoid (GC), these multiprotein complexes are disrupted leading to failure of downstream signaling after engagement of the TCR by antigen. Binding of GC to the GR in the cytoplasm also leads to translocation of the bound receptor to the nucleus and regulation of a number of glucocorticoid responsive genes, including repression of proinflammatory genes such as IL-2, and activation of immunosuppressive genes such as MAPK phosphatase-1, and interleukin 10.
Epstein Barr Virus
Severely immunocompromised solid organ and HCT recipients, particularly those that receive a T cell depleted HCT or T cell depleting antibodies to treat GVHD or organ graft rejection, may develop an Epstein Barr virus driven lymphoproliferative disease (EBV-LPD), consisting of EBV-infected B cells that express the highly immunogenic EBNA-3A, 3B, and 3C proteins. Rooney and colleagues have isolated polyclonal EBV-specific T cells containing variable numbers of CD8+ and CD4+ T cells from the blood of HCT donors by repeated in vitro stimulation with EBV transformed B cell lines that express the EBNA proteins, and administered these T cells to the respective recipients with EBV-LPD or at high risk for EBV-LPD. Adoptive T cell therapy targeting EBV was highly effective, both for promoting tumor regression in patients with established EBV-LPD and preventing the development of LPD when used as prophylaxis (4,21)
EBV-LPD can be rapidly progressive and similar to the situation with CMV, the time required to isolate and expand EBV-reactive T cells is a significant obstacle to the routine use of adoptive T cell therapy. Several groups are developing cryopreserved banks of polyclonal EBV-specific T cells from HLA typed volunteer donors so that a cell product could be immediately available to treat severe EBV infections in unrelated transplant recipients. The initial results of infusing partially HLA matched EBV-specific T cell lines to immunocompromised solid organ allograft recipients with EBV-LPD are surprisingly encouraging, both in terms of safety and therapeutic efficacy (22). The degree of immunodeficiency in the recipient is apparently sufficiently severe that the allogeneic T cells are not rejected before mediating antitumor activity.
EBV is also associated with a number of malignancies that occur in immunocompetent individuals. A subset of Hodgkin's disease contains EBV genomes and express a limited number of weakly immunogenic EBV proteins, including LMP-2. Bollard et al have used dendritic cells engineered to express LMP-2 as antigen presenting cells to expand autologous LMP-2-specific T cells from patients with Hodgkin's disease (23). The adoptive transfer of polyclonal T cells containing both CD4+ and CD8+ LMP-2 specific T cells augmented LMP-2 specific T cell immunity and promoted tumor regression in a subset of these patients (23). Studies are also in progress to develop adoptive T cell therapy for the subset of nasopharyngeal carcinomas that are EBV positive (24)
Other Opportunistic Viruses
Effective drug therapy is not available for several other viruses that cause morbidity after allogeneic HCT and might be amenable to adoptive T cell therapy, including adenovirus, community respiratory viruses, and BK virus (25,26). Our knowledge concerning the antigen specificity and protective capacity of T cell responses to these viruses in humans is incomplete, and the frequency of T cells in donor blood is typically much lower than for latent viruses such as CMV and EBV. Nevertheless, efforts are being made to derive T cell products that contain an expanded repertoire of virus specificities and could be used in adoptive therapy. Leen et al. have developed a culture method in which a recombinant adenovirus that encodes the CMVpp65 protein is used to infect EBV-LCL for use in stimulating T cells from HCT donors (27). This results in the simultaneous expansion of T cells specific for adenovirus, CMV and EBV, and the infusion of such T cells into HCT recipients augmented responses to all three viruses and promoted virus clearance. It is likely that additional viral antigens could be incorporated into such culture systems to hasten immune reconstitution to the most prevalent viral pathogens after HCT.
T cell therapy for non-opportunistic persistent viruses
The efficacy of adoptive T cell therapy for viral infections in immunocompromised hosts raises the prospect of using T cell therapy to boost partially effective responses to human immunodeficiency, hepatitis C, and hepatitis B viruses that cause a chronic persistent infection. Early efforts to boost HIV-specific CD8+ T cell responses by adoptive transfer were unsuccessful due to short-term persistence of the transferred T cells in individuals with replicating virus (28). We now know that CD8+ and CD4+ virus-specific T cells in these chronic infections are characterized by a progressive loss of function and viability related to upregulation of inhibitory molecules such as PD-1 and Tim-3 (29-31). Thus, the infusion of autologous T cells engineered to recognize these viruses by introduction of virus-specific TCR genes (32,33), combined with inhibitors of PD-1 or Tim-3 signaling pathways might improve the quantity and function of antiviral T cells. This strategy may carry a risk of immunopathology, particularly if the antigen load is excessive at the time T cell therapy is administered. An approach that is being developed to restore the CD4 deficiency in HIV infection is to engineer autologous CD4 T cells for adoptive transfer that lack the CCR-5 co-receptor for HIV entry using zinc finger nucleases to permanently disrupt the CCR-5 coding sequence (34).
Tumor-specific T cell therapy
Adoptive T cell therapy for human malignancy has proven to be more challenging and less effective than for opportunistic viral infections. This reflects several obstacles, such as the difficulty isolating the rare highly avid T cells that are specific for self-antigens expressed selectively or preferentially by tumor cells from most cancer patients; the requirement that transferred tumor-reactive T cells persist in vivo, traffic to tumor sites and function in an inhospitable immunosuppressive tumor microenvironment; and the potential for antigen or HLA loss tumor cells variants to escape recognition (35-38). As a consequence, even when tumor-reactive T cells have been isolated and expanded from cancer patients, the adoptive transfer of these cells to treat malignancy was usually unsuccessful, and understanding the precise reasons for failure posed a formidable task. A proximal problem that was evident in the initial trials, and one that was distinct from the results of T cell therapy for viral infections after HCT was that the persistence of adoptively transferred tumor-specific T cells in vivo was remarkably short (6,8,9). The basis for the differential persistence of adoptively transferred virus-specific T cells in HCT recipients and tumor-reactive T cells in cancer patients is now being revealed, and reflects both the environment into which the T cells are infused and qualitative attributes of T cells that are isolated and expanded for adoptive transfer.
Depletion of endogenous lymphocytes to improve the efficacy of adoptively transferred tumor-specific T cells
The longest persistence of adoptively transferred T cells in humans was observed when virus-specific T cells were administered to immunodeficient allogeneic HCT recipients early post-transplant, when lymphopenia is typically present (3-5). Although not initially recognized, a lymphopenic environment may contribute significantly to improving the persistence of transferred T cells by reducing competition for cytokines such as IL15 and IL7 that promote lymphocyte proliferation and survival; making “space” available in the lymphoid compartment; and eliminating CD4+ CD25+ regulatory T cells and other cells with suppressor function (39-41). Direct evidence that the induction of lymphopenia improves the persistence of transferred T cells was provided by studies from Rosenberg et al. in which melanoma patients were rendered lymphopenic either by treatment with chemotherapy alone or chemotherapy combined with total body irradiation, prior to the adoptive transfer of 1010-1011 polyclonal melanoma-specific T cells (42-44). In a significant subset of patients including those with advanced metastatic tumors, transferred T cells underwent dramatic in vivo expansion, persisted long term, infiltrated into tumors and promoted tumor regression. As larger numbers of melanoma patients have been treated with T cell therapy following lymphodepletion, it has become clear that the improved antitumor activity that is observed correlates with better persistence of transferred T cells (45). Studies in murine models have confirmed that lymphodepletion can be exploited to improve the antitumor efficacy of transferred effector T cells (TE), and provided evidence that hematopoietic stem cell infusion with lymphodepletion further promotes the antitumor activity of T cell transfer (46).
It is now evident that inducing lymphopenia before adoptive T cell transfer improves the magnitude and duration of cell persistence, but additional studies are needed to determine the mechanisms involved and whether alternative strategies, such as administration of IL7 or IL15 combined with regimens that selectively or preferentially deplete regulatory or suppressor cells, might be equally effective with less toxicity. Additionally, the studies in melanoma have focused on infusing lymphocyte populations that predominantly contain cytolytic CD8+ T cells, and the contribution of tumor-specific CD4+ T cells to antitumor activity has not been extensively explored. A recent case report demonstrated that infusion of a CD4+ T cell clone specific for the NY-ESO TAA in a melanoma patient that did not receive lymphodepleting chemotherapy led to a durable remission, emphasizing that additional study of the CD4 subset is indicated (47).
Isolating T cells for immunotherapy with the intrinsic capacity to persist in vivo
The quality of the T cells that are selected for expansion and adoptive transfer has been identified as an additional critical factor that determines the persistence of transferred effector cells. The T lymphocyte pool from which T cells for adoptive immunotherapy could potentially be isolated contains CD45RA+ CD62L+ naïve (TN), CD45RO+ CD62L+ central memory (TCM), and CD62L- effector memory (TEM) subsets that differ in phenotype, function, and homing (48). After recognition of antigen in vivo, TN cells undergo proliferation and differentiation, resulting in the generation of large numbers of CD62L- effector T cells (TE), most of which die as antigen is cleared leaving a small pool of TCM and TEM cells (49). Memory T cells respond to antigen re-exposure in vivo and in vitro by differentiating again into TE cells. The lifelong maintenance of T cell memory suggests that some cells in the memory pool may be capable of both self-renewal and differentiation, and there is evidence in mice that a subset of memory T cells may be endowed with stem cell like properties (50,51).
Despite the distinct attributes of T cell subsets, the origin of antigen-specific CD8+ or CD4+ T cells isolated for adoptive therapy has not been known with certainty in clinical trials. In the adoptive transfer studies for opportunistic viruses, TE cells were obtained from donors with prior exposure to the pathogen and it is likely that the majority of the cells were derived from either TEM or TCM. In situations where tumor-reactive TE cells are generated from the blood or tumor infiltrates of tumor bearing patients, the cells could be derived from T cells that have been exposed to TAA, but these cells are unlikely to have fully differentiated into memory cells in the presence of persistent antigen. Given the very low frequency of T cells specific for TAA in the blood of cancer patients, it is also possible that tumor reactive TE obtained after in vitro culture with antigen are derived from TN precursors (52).
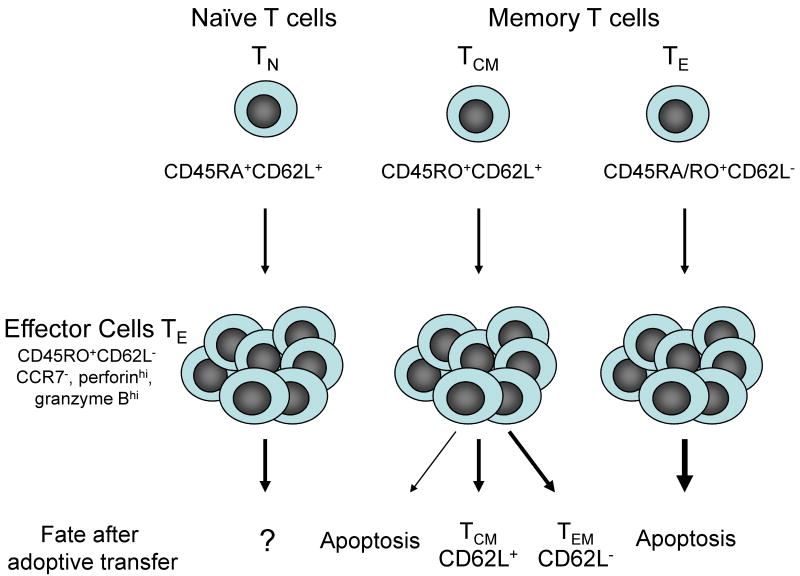
To provide insight into whether the origin of TE cells influenced their ability to persist in vivo after adoptive transfer, we performed studies in nonhuman primates (M. nemestrina) to analyze the fate of CD8+ CMV-specific TE cell clones derived from sort purified TCM and TEM. We selected non-human primates rather than mice because macaque and human T cells are similar in phenotype, function, and regulation, and culture conditions for propagating macaque T cells are identical to those for human T cell therapy. The cells were marked using retroviral vectors that encode with B-lineage molecules to enable tracking the transferred cells in vivo, expanded for at least 30 population doublings before adoptive transfer, and administered intravenously to animals with a full lymphoid compartment and without exogenous cytokines. T cell clones from both subsets had equivalent cytolytic and proliferative capacity, and uniformly expressed a TE cell phenotype (CD62L- CCR7- CD28- CD127-, granzyme Bhi and perforinhi). However, CD8+ CMV-specific TE clones derived from TEM survived in the blood for only a short duration after adoptive transfer, failed to persist in lymph nodes (LN), bone marrow (BM), or peripheral tissues, and did not reacquire phenotypic markers of TCM during their brief life span in vivo. By contrast, TE clones derived from TCM persisted in the blood long-term after adoptive transfer, migrated to memory T cell niches in the LN and BM, reacquired phenotypic properties of TCM and TEM, and responded to antigen challenge (53).
These results reveal profound differences in the survival of TE derived from TCM and TEM after transfer into lymphoreplete hosts. TE cells from TCM have remarkable plasticity in the fates they adopt in vivo. Some of the TE cells derived from TCM reacquire CD62L as well as other markers of TCM and establish reservoirs of long-lived cells in lymph nodes while others adopt a CD62L- TEM phenotype (Figure 2). Although TE derived from TEM have proliferative potential in vitro, these cells uniformly fail to survive in vivo after transfer into lymphoreplete hosts, suggesting that TE derived from TEM are incapable of reverting to the memory pool. It remains possible that lymphodepletion or exogenous cytokines will enable TEM-derived clones to persist, although these cells survive less well in vitro in IL-2, IL-7 and IL-15 compared to TCM-derived T cells (53). Additional studies are in progress to examine the fate of TE derived from TN precursors and to determine if the same results are obtained with CD4+ T cells derived from TCM and TEM subsets. Murine studies using naïve TCR transgenic T cells would suggest that the culture conditions under which the T cells are primed is critical in determining the differentiation program of the cells (54,55).
Figure 2. Fate of adoptively transferred CD8+ effector T cells derived from distinct T cell subsets.
Effector T cells (TE) can be derived from naïve (TN), central memory (TCM), and effector memory (TEM) subsets and express a CD62L-, CCR7-, granzyme Bhi, and perforinhi phenotype. Despite the similar phenotype at the time of adoptive transfer, T cells derived from TEM precursors die rapidly after cell transfer and do not establish persistent T cell memory. T cells derived from TCM precursors survive long term in vivo and revert to both CD62L+ and CD62L- memory cells. A subset of the transferred cells that reexpress CD62L and CCR-7 reside in lymph nodes, downregulate expression of granzyme B and perforin and are capable of responding to antigen challenge. The fate of TE derived from naïve precursors in vitro has not yet been definitively established in a large animal model.
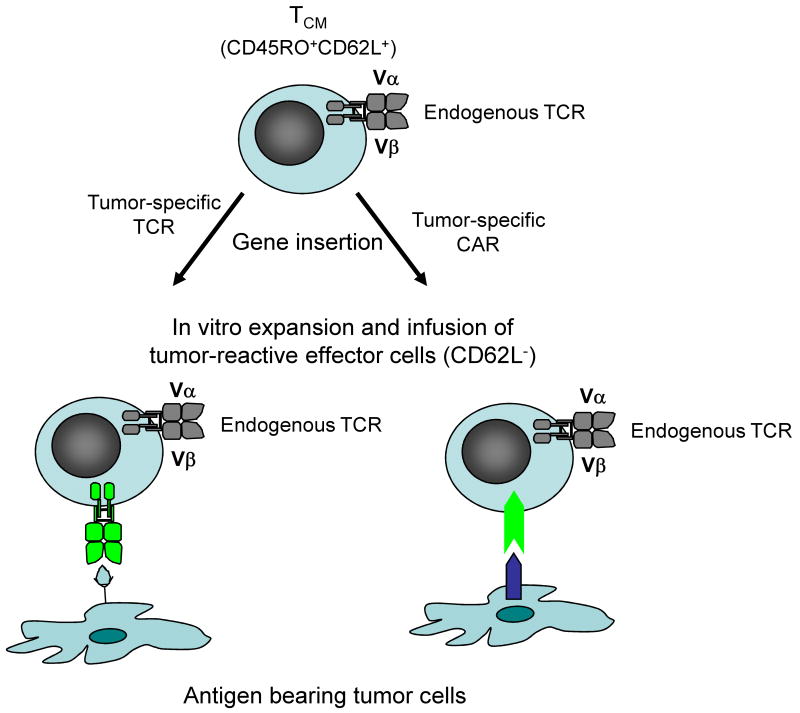
These results have implications for the types of T cells that should be selected for adoptive transfer, and for strategies to derive tumor-reactive T cells for immunotherapy of cancer. It is difficult and often impossible to detect or isolate T cells specific for TAA in blood or tumor infiltrating lymphocytes obtained from patients with solid tumors other than melanoma. However, gene expression profiling of tumors has identified many proteins that are aberrantly expressed or overexpressed in comparison with the normal tissue counterparts and might be targets for T cell therapy. Additionally, sequencing of the genomes of breast and colorectal cancer has identified an average of 90 nonsynonymous mutations, and in silico analysis suggests that many of these encode unique MHC binding epitopes (56,57). The failure of the immune system to respond to a growing tumor in most patients is due to evasion mechanisms including ignorance, local recruitment of regulatory T cells or other suppressor cells, and expression or secretion of inhibitory molecules or cytokines that impair responses locally and/or systemically. However, T cells specific for TAA can be derived by in vitro priming from the naïve repertoire of normal individuals and the T cell receptor (TCR) alpha and beta genes isolated from these T cells and inserted into T cells from the cancer patient to confer a desired specificity for an MHC/peptide complex (58,59). Indeed, many groups are now assembling libraries of TCRs that target human TAA and could be used to engineer tumor-reactive T cells for therapy. In a similar fashion, chimeric antigen receptors (CAR) fashioned by fusing single chain antibody domains to the TCR ζ chain alone or in combination with costimulatory signaling domains, can be introduced into T cells to target surface molecules expressed on tumor cells and overcome the requirement for MHC restriction (60). The demonstration of the superior engraftment properties of TE derived from TCM would suggest that selection or enrichment of TCM prior to insertion to tumor targeting receptors will provide a superior T cell product for adoptive therapy, and may overcome the inconsistent cell persistence observed in initial studies (58,61) (Figure 3). The results of a recent study of T cell therapy for neuroblastoma support this hypothesis. TE cells derived from cultures of EBV-specific TE cells and engineered with a CAR specific for the diasialoganglioside GD2 on neuroblastoma exhibited superior persistence compared with unselected T cells activated with anti CD3 monoclonal antibody and engineered with the identical CAR (62). The results suggest that intrinsic programming of the EBV-specific T cells and/or recognition of antigen through the endogenous TCR were important for in vivo persistence of the tumor-reactive T cells.
Figure 3. Engineering tumor-reactive effector T cells by insertion of genes that encode tumor-specific T cell receptors or chimeric antigen receptors into central memory T cells.
The superior persistence of effector T cells derived from virus-specific TCM suggests that this subset of T cells should be isolated based on their expression of CD45RO and CD62L for insertion of genes that encode TCR or chimeric antigen receptors (CAR) to target molecules on tumor cells. After insertion of the tumor-targeting receptors, the engineered T cells could be expanded in vitro and despite differentiating to cytolytic effector cells will retain the intrinsic capacity to persist in vivo after adoptive transfer and revert to the memory pool.
Conclusions
Substantial progress has been made in understanding the requirements to effectively utilize adoptive T cell therapy for human viral infections and melanoma. The encouraging results now being achieved in small clinical trials have diminished previous pessimism, and the field appears poised to apply adoptive therapy more broadly to human malignancies. However, the techniques required for this endeavor remain complex and involve isolating or engineering by gene transfer T cells that recognize malignant cells, expanding the tumor-reactive cells in vitro, and conditioning the host to promote the survival and function of transferred T cells. Malignancies are formidable adversaries and employ many strategies to overcome immune recognition, which may include defects in the processing or presentation of TAA, recruitment of suppressor cells, and production of factors that disable tumor-reactive T cells. Obtaining T cells with the capacity to persist in vivo and achieving a quantitatively large tumor-reactive T cell response by conditioning the host before adoptive transfer represent key steps in improving the efficacy of adoptive T cell transfer. It will be essential that future clinical trials evaluate the characteristics of tumors that fail to respond to therapy to derive insights into the properties of tumor cells that limit therapeutic efficacy, and lead to the rationale modifications to improve efficacy.
Acknowledgments
The authors acknowledge support from National Institutes of Health grants CA114536, AI053193 and CA18029 and from the Thomsen Family Postdoctoral Fellowship.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Carolina Berger, D3-100, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue NE, Seattle, WA. cberger@fhcrc.org.
Cameron J. Turtle, D3-100, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue NE, Seattle, WA. cturtle@fhcrc.org
Michael C. Jensen, City of Hope National Medical Center, Duarte, CA. mjensen@coh.org
Stanley R. Riddell, D3-100, Fred Hutchinson Cancer Research Center, 1100 Fairview Avenue NE, Seattle, WA. sriddell@fhcrc.org
References
- 1.Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol. 2004;22:329–60. doi: 10.1146/annurev.immunol.22.012703.104803. [DOI] [PubMed] [Google Scholar]
- 2.Galon J, Costes A, Sanchez-Cabo F, Kirilovsky A, Mlecnik B, Lagorce-Pages C, Tosolini M, Camus M, Berger A, Wind P, Zinzindohoue F, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Pages F. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–4. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 3.Cobbold M, Khan N, Pourgheysari B, Tauro S, McDonald D, Osman H, Assenmacher M, Billingham L, Steward C, Crawley C, Olavarria E, Goldman J, Chakraverty R, Mahendra P, Craddock C, Moss PA. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med. 2005;202:379–86. doi: 10.1084/jem.20040613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rooney CM, Smith CA, Ng CY, Loftin SK, Sixbey JW, Gan Y, Srivastava DK, Bowman LC, Krance RA, Brenner MK, Heslop HE. Infusion of cytotoxic T cells for the prevention and treatment of Epstein-Barr virus-induced lymphoma in allogeneic transplant recipients. Blood. 1998;92:1549–55. [PubMed] [Google Scholar]
- 5.Walter EA, Greenberg PD, Gilbert MJ, Finch RJ, Watanabe KS, Thomas ED, Riddell SR. Reconstitution of cellular immunity against cytomegalovirus in recipients of allogeneic bone marrow by transfer of T-cell clones from the donor. N Engl J Med. 1995;333:1038–44. doi: 10.1056/NEJM199510193331603. [DOI] [PubMed] [Google Scholar]
- 6.Dudley ME, Wunderlich J, Nishimura MI, Yu D, Yang JC, Topalian SL, Schwartzentruber DJ, Hwu P, Marincola FM, Sherry R, Leitman SF, Rosenberg SA. Adoptive transfer of cloned melanoma-reactive T lymphocytes for the treatment of patients with metastatic melanoma. J Immunother. 2001;24:363–73. doi: 10.1097/00002371-200107000-00012. 1997. [DOI] [PubMed] [Google Scholar]
- 7.Gattinoni L, Powell DJ, Jr, Rosenberg SA, Restifo NP. Adoptive immunotherapy for cancer: building on success. Nat Rev Immunol. 2006;6:383–93. doi: 10.1038/nri1842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mackensen A, Meidenbauer N, Vogl S, Laumer M, Berger J, Andreesen R. Phase I study of adoptive T-cell therapy using antigen-specific CD8+ T cells for the treatment of patients with metastatic melanoma. J Clin Oncol. 2006;24:5060–9. doi: 10.1200/JCO.2006.07.1100. [DOI] [PubMed] [Google Scholar]
- 9.Yee C, Thompson JA, Byrd D, Riddell SR, Roche P, Celis E, Greenberg PD. Adoptive T cell therapy using antigen-specific CD8+ T cell clones for the treatment of patients with metastatic melanoma: in vivo persistence, migration, and antitumor effect of transferred T cells. Proc Natl Acad Sci U S A. 2002;99:16168–73. doi: 10.1073/pnas.242600099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Appelbaum FR. The current status of hematopoietic cell transplantation. Annu Rev Med. 2003;54:491–512. doi: 10.1146/annurev.med.54.101601.152456. [DOI] [PubMed] [Google Scholar]
- 11.Boeckh M, Leisenring W, Riddell SR, Bowden RA, Huang ML, Myerson D, Stevens-Ayers T, Flowers ME, Cunningham T, Corey L. Late cytomegalovirus disease and mortality in recipients of allogeneic hematopoietic stem cell transplants: importance of viral load and T-cell immunity. Blood. 2003;101:407–14. doi: 10.1182/blood-2002-03-0993. [DOI] [PubMed] [Google Scholar]
- 12.Einsele H, Roosnek E, Rufer N, Sinzger C, Riegler S, Loffler J, Grigoleit U, Moris A, Rammensee HG, Kanz L, Kleihauer A, Frank F, Jahn G, Hebart H. Infusion of cytomegalovirus (CMV)-specific T cells for the treatment of CMV infection not responding to antiviral chemotherapy. Blood. 2002;99:3916–22. doi: 10.1182/blood.v99.11.3916. [DOI] [PubMed] [Google Scholar]
- 13.Peggs KS, Verfuerth S, Pizzey A, Khan N, Guiver M, Moss PA, Mackinnon S. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet. 2003;362:1375–7. doi: 10.1016/S0140-6736(03)14634-X. [DOI] [PubMed] [Google Scholar]
- 14.Becker C, Pohla H, Frankenberger B, Schuler T, Assenmacher M, Schendel DJ, Blankenstein T. Adoptive tumor therapy with T lymphocytes enriched through an IFN-gamma capture assay. Nat Med. 2001;7:1159–62. doi: 10.1038/nm1001-1159. [DOI] [PubMed] [Google Scholar]
- 15.Knabel M, Franz TJ, Schiemann M, Wulf A, Villmow B, Schmidt B, Bernhard H, Wagner H, Busch DH. Reversible MHC multimer staining for functional isolation of T-cell populations and effective adoptive transfer. Nat Med. 2002;8:631–7. doi: 10.1038/nm0602-631. [DOI] [PubMed] [Google Scholar]
- 16.Hahn T, McCarthy PL, Jr, Zhang MJ, Wang D, Arora M, Frangoul H, Gale RP, Hale GA, Horan J, Isola L, Maziarz RT, van Rood JJ, Gupta V, Halter J, Reddy V, Tiberghien P, Litzow M, Anasetti C, Pavletic S, Ringden O. Risk Factors for Acute Graft-Versus-Host Disease After Human Leukocyte Antigen-Identical Sibling Transplants for Adults With Leukemia. J Clin Oncol. 2008 doi: 10.1200/JCO.2008.17.6545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ashwell JD, Lu FW, Vacchio MS. Glucocorticoids in T cell development and function. Annu Rev Immunol. 2000;18:309–45. doi: 10.1146/annurev.immunol.18.1.309. [DOI] [PubMed] [Google Scholar]
- 18.Franchimont D. Overview of the actions of glucocorticoids on the immune response: a good model to characterize new pathways of immunosuppression for new treatment strategies. Ann N Y Acad Sci. 2004;1024:124–37. doi: 10.1196/annals.1321.009. [DOI] [PubMed] [Google Scholar]
- 19.Lowenberg M, Verhaar AP, van den Brink GR, Hommes DW. Glucocorticoid signaling: a nongenomic mechanism for T-cell immunosuppression. Trends Mol Med. 2007;13:158–63. doi: 10.1016/j.molmed.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Durai S, Mani M, Kandavelou K, Wu J, Porteus MH, Chandrasegaran S. Zinc finger nucleases: custom-designed molecular scissors for genome engineering of plant and mammalian cells. Nucleic Acids Res. 2005;33:5978–90. doi: 10.1093/nar/gki912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gottschalk S, Heslop HE, Rooney CM. Adoptive immunotherapy for EBV-associated malignancies. Leuk Lymphoma. 2005;46:1–10. doi: 10.1080/10428190400002202. [DOI] [PubMed] [Google Scholar]
- *22.Haque T, Wilkie GM, Jones MM, Higgins CD, Urquhart G, Wingate P, Burns D, McAulay K, Turner M, Bellamy C, Amlot PL, Kelly D, MacGilchrist A, Gandhi MK, Swerdlow AJ, Crawford DH. Allogeneic cytotoxic T-cell therapy for EBV-positive posttransplantation lymphoproliferative disease: results of a phase 2 multicenter clinical trial. Blood. 2007;110:1123–31. doi: 10.1182/blood-2006-12-063008. [DOI] [PubMed] [Google Scholar]; This study reports the results of infusing allogeneic EBV-specific T cells derived from HLA partially matched unrelated donors to 33 patients with EBV post transplant lymphoproliferative disorder. The T cell infusions were well tolerated and the overall response rate was 64% at 5 weeks and 52% at 6 months, demonstrating that allogeneic T cells can persist and mediate an antitumor effect in these highly immunocompromised patients.
- 23.Bollard CM, Gottschalk S, Leen AM, Weiss H, Straathof KC, Carrum G, Khalil M, Wu MF, Huls MH, Chang CC, Gresik MV, Gee AP, Brenner MK, Rooney CM, Heslop HE. Complete responses of relapsed lymphoma following genetic modification of tumor-antigen presenting cells and T-lymphocyte transfer. Blood. 2007;110:2838–45. doi: 10.1182/blood-2007-05-091280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Louis CU, Straathof K, Bollard CM, Gerken C, Huls MH, Gresik MV, Wu MF, Weiss HL, Gee AP, Brenner MK, Rooney CM, Heslop HE, Gottschalk S. Enhancing the in vivo expansion of adoptively transferred EBV-specific CTL with lymphodepleting CD45 monoclonal antibodies in NPC patients. Blood. 2008 doi: 10.1182/blood-2008-05-157222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Boeckh M. The challenge of respiratory virus infections in hematopoietic cell transplant recipients. Br J Haematol. 2008;143:455–67. doi: 10.1111/j.1365-2141.2008.07295.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wong AS, Cheng VC, Yuen KY, Kwong YL, Leung AY. High frequency of polyoma BK virus shedding in the gastrointestinal tract after hematopoietic stem cell transplantation: a prospective and quantitative analysis. Bone Marrow Transplant. 2008 doi: 10.1038/bmt.2008.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *27.Leen AM, Myers GD, Sili U, Huls MH, Weiss H, Leung KS, Carrum G, Krance RA, Chang CC, Molldrem JJ, Gee AP, Brenner MK, Heslop HE, Rooney CM, Bollard CM. Monoculture-derived T lymphocytes specific for multiple viruses expand and produce clinically relevant effects in immunocompromised individuals. Nat Med. 2006;12:1160–6. doi: 10.1038/nm1475. [DOI] [PubMed] [Google Scholar]; This study describes a cell culture system that can be used to simultaneously elicit T cells specific for cytomegalovirus, Epstein Barr virus, and adenovirus for adoptive immunotherapy of immunodeficient stem cell transplant recipients. The approach is based on the infection of donor EBV transformed B lymphocytes with a replication defective adenovirus that encoded the immunodominant cytomegalovirus pp65 protein and the use of these B cells as antigen presenting cells. The adoptive transfer of T cells elicited by this approach augmented T cell immunity to all three viruses and mediated antiviral activity.
- 28.Brodie SJ, Lewinsohn DA, Patterson BK, Jiyamapa D, Krieger J, Corey L, Greenberg PD, Riddell SR. In vivo migration and function of transferred HIV-1-specific cytotoxic T cells. Nat Med. 1999;5:34–41. doi: 10.1038/4716. [DOI] [PubMed] [Google Scholar]
- 29.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, Mncube Z, Duraiswamy J, Zhu B, Eichbaum Q, Altfeld M, Wherry EJ, Coovadia HM, Goulder PJ, Klenerman P, Ahmed R, Freeman GJ, Walker BD. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 30.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, Gyenes G, Vali B, Hyrcza MD, Yue FY, Kovacs C, Sassi A, Loutfy M, Halpenny R, Persad D, Spotts G, Hecht FM, Chun TW, McCune JM, Kaul R, Rini JM, Nixon DF, Ostrowski MA. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008 doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Joseph A, Zheng JH, Follenzi A, Dilorenzo T, Sango K, Hyman J, Chen K, Piechocka-Trocha A, Brander C, Hooijberg E, Vignali DA, Walker BD, Goldstein H. Lentiviral vectors encoding human immunodeficiency virus type 1 (HIV-1)-specific T-cell receptor genes efficiently convert peripheral blood CD8 T lymphocytes into cytotoxic T lymphocytes with potent in vitro and in vivo HIV-1-specific inhibitory activity. J Virol. 2008;82:3078–89. doi: 10.1128/JVI.01812-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Varela-Rohena A, Molloy PE, Dunn SM, Li Y, Suhoski MM, Carroll RG, Milicic A, Mahon T, Sutton DH, Laugel B, Moysey R, Cameron BJ, Vuidepot A, Purbhoo MA, Cole DK, Phillips RE, June CH, Jakobsen BK, Sewell AK, Riley JL. Control of HIV-1 immune escape by CD8 T cells expressing enhanced T-cell receptor. Nat Med. 2008 doi: 10.1038/nm.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **34.Perez EE, Wang J, Miller JC, Jouvenot Y, Kim KA, Liu O, Wang N, Lee G, Bartsevich VV, Lee YL, Guschin DY, Rupniewski I, Waite AJ, Carpenito C, Carroll RG, Orange JS, Urnov FD, Rebar EJ, Ando D, Gregory PD, Riley JL, Holmes MC, June CH. Establishment of HIV-1 resistance in CD4+ T cells by genome editing using zinc-finger nucleases. Nat Biotechnol. 2008;26:808–16. doi: 10.1038/nbt1410. [DOI] [PMC free article] [PubMed] [Google Scholar]; This paper describes the use of zinc finger nucleases that target the DNA sequence encoding the first transmembrane domain of the CCR-5 co-receptor to permanently disrupt this gene in CD4+ T cells and confer genotypic and phenotypic resistance to HIV infection. This strategy could provide a means to reconstitute or preserve the CD4+ memory T cell pool in HIV infection by adoptive transfer of CCR-5 gene edited T cells.
- 35.Drake CG, Jaffee E, Pardoll DM. Mechanisms of immune evasion by tumors. Adv Immunol. 2006;90:51–81. doi: 10.1016/S0065-2776(06)90002-9. [DOI] [PubMed] [Google Scholar]
- 36.Kaiser BK, Yim D, Chow IT, Gonzalez S, Dai Z, Mann HH, Strong RK, Groh V, Spies T. Disulphide-isomerase-enabled shedding of tumour-associated NKG2D ligands. Nature. 2007;447:482–6. doi: 10.1038/nature05768. [DOI] [PubMed] [Google Scholar]
- 37.Thomas DA, Massague J. TGF-beta directly targets cytotoxic T cell functions during tumor evasion of immune surveillance. Cancer Cell. 2005;8:369–80. doi: 10.1016/j.ccr.2005.10.012. [DOI] [PubMed] [Google Scholar]
- 38.Vence L, Palucka AK, Fay JW, Ito T, Liu YJ, Banchereau J, Ueno H. Circulating tumor antigen-specific regulatory T cells in patients with metastatic melanoma. Proc Natl Acad Sci U S A. 2007;104:20884–9. doi: 10.1073/pnas.0710557105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Colombo MP, Piconese S. Regulatory-T-cell inhibition versus depletion: the right choice in cancer immunotherapy. Nat Rev Cancer. 2007;7:880–7. doi: 10.1038/nrc2250. [DOI] [PubMed] [Google Scholar]
- 40.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–7. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tan JT, Ernst B, Kieper WC, LeRoy E, Sprent J, Surh CD. Interleukin (IL)-15 and IL-7 jointly regulate homeostatic proliferation of memory phenotype CD8+ cells but are not required for memory phenotype CD4+ cells. J Exp Med. 2002;195:1523–32. doi: 10.1084/jem.20020066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dudley ME, Wunderlich JR, Robbins PF, Yang JC, Hwu P, Schwartzentruber DJ, Topalian SL, Sherry R, Restifo NP, Hubicki AM, Robinson MR, Raffeld M, Duray P, Seipp CA, Rogers-Freezer L, Morton KE, Mavroukakis SA, White DE, Rosenberg SA. Cancer regression and autoimmunity in patients after clonal repopulation with antitumor lymphocytes. Science. 2002;298:850–4. doi: 10.1126/science.1076514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dudley ME, Wunderlich JR, Yang JC, Sherry RM, Topalian SL, Restifo NP, Royal RE, Kammula U, White DE, Mavroukakis SA, Rogers LJ, Gracia GJ, Jones SA, Mangiameli DP, Pelletier MM, Gea-Banacloche J, Robinson MR, Berman DM, Filie AC, Abati A, Rosenberg SA. Adoptive cell transfer therapy following non-myeloablative but lymphodepleting chemotherapy for the treatment of patients with refractory metastatic melanoma. J Clin Oncol. 2005;23:2346–57. doi: 10.1200/JCO.2005.00.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- **44.Dudley ME, Yang JC, Sherry R, Hughes MS, Royal R, Kammula U, Robbins PF, Huang J, Citrin DE, Leitman SF, Wunderlich J, Restifo NP, Thomasian A, Downey SG, Smith FO, Klapper J, Morton K, Laurencot C, White DE, Rosenberg SA. Adoptive cell therapy for patients with metastatic melanoma: evaluation of intensive myeloablative chemoradiation preparative regimens. J Clin Oncol. 2008;26:5233–9. doi: 10.1200/JCO.2008.16.5449. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study updates the results of adoptive T cell therapy for patients with advanced melanoma with in vitro expanded autologous tumor infiltrating lymphocytes that were administered after various lymphodepleting regimens that increase the circulating levels of interleukin 7 and interleukin 15. The results in 93 patients show an objective response rate of 56%, which is superior to the historical experience when tumor infiltrating lymphocytes were administered without lymphodepleting chemotherapy.
- 45.Robbins PF, Dudley ME, Wunderlich J, El-Gamil M, Li YF, Zhou J, Huang J, Powell DJ, Jr, Rosenberg SA. Cutting edge: persistence of transferred lymphocyte clonotypes correlates with cancer regression in patients receiving cell transfer therapy. J Immunol. 2004;173:7125–30. doi: 10.4049/jimmunol.173.12.7125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wrzesinski C, Paulos CM, Gattinoni L, Palmer DC, Kaiser A, Yu Z, Rosenberg SA, Restifo NP. Hematopoietic stem cells promote the expansion and function of adoptively transferred antitumor CD8 T cells. J Clin Invest. 2007;117:492–501. doi: 10.1172/JCI30414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *47.Hunder NN, Wallen H, Cao J, Hendricks DW, Reilly JZ, Rodmyre R, Jungbluth A, Gnjatic S, Thompson JA, Yee C. Treatment of metastatic melanoma with autologous CD4+ T cells against NY-ESO-1. N Engl J Med. 2008;358:2698–703. doi: 10.1056/NEJMoa0800251. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is a case report describing the regression of melanoma metastasis after adoptive transfer of an autologous CD4+ T cell clone specific for the NY-ESO-1 antigen. Tumor regression was associated with augmentation of T cell responses to other melanoma antigens consistent with the induction of epitope spreading. This report highlights the potential use of CD4+ T cells in adoptive T cell therapy of human malignancies.
- 48.Sallusto F, Geginat J, Lanzavecchia A. Central memory and effector memory T cell subsets: function, generation, and maintenance. Annu Rev Immunol. 2004;22:745–63. doi: 10.1146/annurev.immunol.22.012703.104702. [DOI] [PubMed] [Google Scholar]
- 49.Wherry EJ, Teichgraber V, Becker TC, Masopust D, Kaech SM, Antia R, von Andrian UH, Ahmed R. Lineage relationship and protective immunity of memory CD8 T cell subsets. Nat Immunol. 2003;4:225–34. doi: 10.1038/ni889. [DOI] [PubMed] [Google Scholar]
- *50.Chang JT, Palanivel VR, Kinjyo I, Schambach F, Intlekofer AM, Banerjee A, Longworth SA, Vinup KE, Mrass P, Oliaro J, Killeen N, Orange JS, Russell SM, Weninger W, Reiner SL. Asymmetric T lymphocyte division in the initiation of adaptive immune responses. Science. 2007;315:1687–91. doi: 10.1126/science.1139393. [DOI] [PubMed] [Google Scholar]; This study provides evidence that asymmetric cell division during the activation of a naïve T cell may in part explain the distinct fates that are adopted by T cells during an immune response. The model presented demonstrates the unequal partitioning of fate determining proteins to daughter cells that determines their differentiation into effector or central memory T cells.
- 51.Zhang Y, Joe G, Hexner E, Zhu J, Emerson SG. Host-reactive CD8+ memory stem cells in graft-versus-host disease. Nat Med. 2005;11:1299–305. doi: 10.1038/nm1326. [DOI] [PubMed] [Google Scholar]
- 52.Ho WY, Nguyen HN, Wolfl M, Kuball J, Greenberg PD. In vitro methods for generating CD8+ T-cell clones for immunotherapy from the naive repertoire. J Immunol Methods. 2006;310:40–52. doi: 10.1016/j.jim.2005.11.023. [DOI] [PubMed] [Google Scholar]
- **53.Berger C, Jensen MC, Lansdorp PM, Gough M, Elliott C, Riddell SR. Adoptive transfer of effector CD8+ T cells derived from central memory cells establishes persistent T cell memory in primates. J Clin Invest. 2008;118:294–305. doi: 10.1172/JCI32103. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study uses gene marking to track the fate of clonally derived effector T cells isolated from either central memory or effector memory precursors in a non human primate model. Remarkably, effector cells derived from central memory but not effector memory precursors retained the capacity to persist long term, reacquire a memory phenotype and replenish both central memory and effector memory pools. These results are consistent with the effector memory pool being replenished from central memory T cells, and suggest that deriving effector cells for adoptive therapy from central memory precursors will provide a cell population with greater intrinsic capacity to persist in vivo.
- 54.Hinrichs CS, Spolski R, Paulos CM, Gattinoni L, Kerstann KW, Palmer DC, Klebanoff CA, Rosenberg SA, Leonard WJ, Restifo NP. IL-2 and IL-21 confer opposing differentiation programs to CD8+ T cells for adoptive immunotherapy. Blood. 2008;111:5326–33. doi: 10.1182/blood-2007-09-113050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Klebanoff CA, Gattinoni L, Torabi-Parizi P, Kerstann K, Cardones AR, Finkelstein SE, Palmer DC, Antony PA, Hwang ST, Rosenberg SA, Waldmann TA, Restifo NP. Central memory self/tumor-reactive CD8+ T cells confer superior antitumor immunity compared with effector memory T cells. Proc Natl Acad Sci U S A. 2005;102:9571–6. doi: 10.1073/pnas.0503726102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Segal NH, Parsons DW, Peggs KS, Velculescu V, Kinzler KW, Vogelstein B, Allison JP. Epitope landscape in breast and colorectal cancer. Cancer Res. 2008;68:889–92. doi: 10.1158/0008-5472.CAN-07-3095. [DOI] [PubMed] [Google Scholar]
- 57.Wood LD, Parsons DW, Jones S, Lin J, Sjoblom T, Leary RJ, Shen D, Boca SM, Barber T, Ptak J, Silliman N, Szabo S, Dezso Z, Ustyanksky V, Nikolskaya T, Nikolsky Y, Karchin R, Wilson PA, Kaminker JS, Zhang Z, Croshaw R, Willis J, Dawson D, Shipitsin M, Willson JK, Sukumar S, Polyak K, Park BH, Pethiyagoda CL, Pant PV, Ballinger DG, Sparks AB, Hartigan J, Smith DR, Suh E, Papadopoulos N, Buckhaults P, Markowitz SD, Parmigiani G, Kinzler KW, Velculescu VE, Vogelstein B. The genomic landscapes of human breast and colorectal cancers. Science. 2007;318:1108–13. doi: 10.1126/science.1145720. [DOI] [PubMed] [Google Scholar]
- **58.Morgan RA, Dudley ME, Wunderlich JR, Hughes MS, Yang JC, Sherry RM, Royal RE, Topalian SL, Kammula US, Restifo NP, Zheng Z, Nahvi A, de Vries CR, Rogers-Freezer LJ, Mavroukakis SA, Rosenberg SA. Cancer regression in patients after transfer of genetically engineered lymphocytes. Science. 2006;314:126–9. doi: 10.1126/science.1129003. [DOI] [PMC free article] [PubMed] [Google Scholar]; This is the first study in which autologous T cells were genetically altered to express a tumor-reactive T cell receptor by retrovirus mediated gene transfer and adoptively transferred to treat melanoma. A small number of patients had tumor regression revealing the potential of this approach. The study also reveals issues that need to be resolved including the design of vectors that provide high levels of persistent gene expression.
- 59.Stanislawski T, Voss RH, Lotz C, Sadovnikova E, Willemsen RA, Kuball J, Ruppert T, Bolhuis RL, Melief CJ, Huber C, Stauss HJ, Theobald M. Circumventing tolerance to a human MDM2-derived tumor antigen by TCR gene transfer. Nat Immunol. 2001;2:962–70. doi: 10.1038/ni1001-962. [DOI] [PubMed] [Google Scholar]
- 60.Kershaw MH, Teng MW, Smyth MJ, Darcy PK. Supernatural T cells: genetic modification of T cells for cancer therapy. Nat Rev Immunol. 2005;5:928–40. doi: 10.1038/nri1729. [DOI] [PubMed] [Google Scholar]
- 61.Till BG, Jensen MC, Wang J, Chen EY, Wood BL, Greisman HA, Qian X, James SE, Raubitschek A, Forman SJ, Gopal AK, Pagel JM, Lindgren CG, Greenberg PD, Riddell SR, Press OW. Adoptive immunotherapy for indolent non-Hodgkin lymphoma and mantle cell lymphoma using genetically modified autologous CD20-specific T cells. Blood. 2008;112:2261–71. doi: 10.1182/blood-2007-12-128843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- *62.Pule MA, Savoldo B, Myers GD, Rossig C, Russell HV, Dotti G, Huls MH, Liu E, Gee AP, Mei Z, Yvon E, Weiss HL, Liu H, Rooney CM, Heslop HE, Brenner MK. Virus-specific T cells engineered to coexpress tumor-specific receptors: persistence and antitumor activity in individuals with neuroblastoma. Nat Med. 2008;14:1264–70. doi: 10.1038/nm.1882. [DOI] [PMC free article] [PubMed] [Google Scholar]; This study demonstrates that EBV-specific T cells modified to express a chimeric antigen receptor that targets neuroblastoma have superior in vivo persistence and antitumor activity compared with non virus-specific T cells modified with the same tumor-specific receptor after anti CD3 activation. These results are consistent with the EBV-specific T cells having the potential to persist as memory cells even when modified with a chimeric antigen receptor, although the frequency of persisting T cells was too low to determine their phenotype in vivo.