Abstract
Inorganic phosphate (Pi) is an important polyanion needed for ATP synthesis and bone formation. Since it is found at millimolar levels in plasma it is usually incorporated as a constituent of artificial cerebrospinal fluid (ACSF) formulations for maintaining brain slices. In this paper we show that Pi limits the extracellular zinc concentration by inducing metal precipitation. We present data suggesting that amino acids like histidine may counteract the Pi-induced zinc precipitation by the formation of soluble zinc complexes. We propose that the interplay between Pi and amino acids in the extracellular space may influence the availability of metals for cellular uptake.
Introduction
An essential step in the process of unraveling the mechanisms of action potential generation, synaptic transmission and muscular contraction was identifying the nature of the ionic species necessary for the operation of excitable cells (Burton 1975). This began with Ringer’s demonstration (Ringer 1883) that extracellular calcium was necessary for cardiac contraction and set in motion the process of formulating media used to sustain cells in vitro. An important step was Krebs and Henseleit’s (1932) realization that the constituents of normal plasma would be a good starting point for formulating physiological salines. Building on these foundations McIlwain developed the in-vivo brain slice preparation, one that has been enormously influential in the progress of neuroscience (Li & Mc 1957).
Inorganic phosphate (Pi, orthophosphate) is an essential ion in living organisms playing indispensable roles in ATP synthesis and bone mineralization, among other processes. Pi exists in two predominant forms at physiological pH; HPO42− and H2PO4− at a ~4:1 ratio. Intracellular Pi is sustained at a concentration of about 2mM in most mammals and is a key determinant of the free energy available from ATP hydrolysis (Erecinska & Silver 1989). Plasma Pi levels vary considerably in different vertebrates (Furman et al. 1997). In human plasma, the normal Pi level is ~ 1.1 mM but fluctuates more widely than calcium, and exhibits circadian variations. The concentration of Pi in the CSF of mammals is ~0.4 mM however little is known about the concentration in the interstitial space.
Little information is available on the mechanism of Pi uptake into cells of the CNS. A protein initially identified as a Pi transporter (Ni et al. 1994) was subsequently shown to carry glutamate into synaptic vesicles (Bellocchio et al. 2000, Takamori et al. 2000) and its Pi transporting capabilities are uncertain. Pi transport has been well characterized in the kidneys where it is transported by members of the Slc34 (Murer et al. 2004) and Slc20 (Collins et al. 2004) families for monovalent and divalent species respectively, in sodium-dependent processes.
There are abundant opportunities for solid minerals to form from the complex mixtures of ions in and around cells, particularly between metals and polyanions like Pi. In a solution with known concentrations of ions it is possible to predict the formation of precipitates from the solubility products (Ksp) for the various ion combinations. As a thermodynamic parameter, the Ksps however gives no indication of how fast the precipitate takes to form. One common form hydroxyapatite (Ca10(PO4)6(OH)2) taks a long time, others like hopeite (Zn3(PO4)2:4H2O) form within a few milliseconds.
Zinc is found at a high concentration in certain glutamatergic vesicles within the mammalian forebrain and it has been proposed to be released and act as a neuromodulator (Smart et al. 2004, Paoletti et al. 2008). There is a potential chemical impediment to the free release of zinc ions, namely, that zinc-phosphate has a very low solubility product (9.1 × 10− 36 M5), which limits the concentration of free zinc ions in a solution with a high concentration of Pi. In this communication we show that in brain slices the extracellular free zinc concentration is indeed limited by precipitation. Moreover, we demonstrate that this limitation can be overcome by the provision of amino acids like histidine that increases the solubility of the metal.
Materials and methods
Brain Slices
All animal procedures were in accordance with the NIH Guide for the Care and use of Laboratory Animals and approved by the Institutional Animal Care and Use committee of the University of Iowa. Sprague-Dawley rats (21–60 day old, male) were decapitated, the brain removed and placed in ice-cold normal saline containing (in mM); 125 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgSO4, 25 NaHCO3, 1.25 NaH2PO4, and 10 glucose, bubbled with 95% O2-5% CO2. The composition of PFS was (in mM): 125 NaCl, 2.5 KCl, 2 CaCl2, 1.3 MgSO4, 26 NaHCO3 and 10 glucose bubbled with carbogen. To avoid zinc contamination high purity reagents were used, while avoiding metals, glass and plastics that can leach zinc into solutions (Kay 2004). Slices were cut at a thickness of 500 μm on a McIlwain tissue chopper, and held in an interface chamber at room temperature for at least one hour prior to loading with the zinc indicator. FluoZin-3 AM (5μM) was applied to slices together with 0.04 % w/v pluronic for an hour.
Imaging brain slices
The slices were stabilized with a U-shaped stainless steel piece cross strung with nylon fibers in a temperature controlled chamber (RC-27L; Warner Instruments). Solutions were provided by a gravity feed system controlled by a VC-6 six channel valve controller (Warner Instruments). Images were acquired on an Olympus Optical BX50WI upright microscope. Illumination was provided by a monochromator set at 480 nm (T.I.L.L. Photonics), passed through a dichroic (Q495lp; Chroma Technology) and then through a filter (HQ530/60; Chroma Technology)onto the faceplate of a Princeton Instruments cooled CCD camera. Data were acquired by the MetaFluor program (Universal Imaging Corporation) and images were analyzed using ImageJ (NIH). No black-level adjustment was applied to images. The fluorescence intensity was expressed as % ΔF/Fo = % (F−Fo)/Fo, where F is the fluorescence intensity and Fo the fluorescence intensity at time zero. The dark current of the CCD was subtracted from all intensity measurements. All data are expressed as mean ± SEM.
Synaptic responses
Bipolar tungsten stimulating electrodes (A-M Systems Inc. ~4 MΩ), coupled to a stimulus isolation unit (AMPI) were placed in the upper half of the stratum radiatum and the field potentials were recorded with glass electrodes (2–5 MΩ) coupled to an Axoclamp-2B (Axon Instruments), connected to a low-noise amplifier (SR 560, Stanford Research Systems) to a Digidata 1322A A/D (Axon Instruments) using Clampex 9.2 software. fEPSPs were stimulated with a pulse of 60 μs duration and the current was adjusted to give a response approximately 60% of the maximal amplitude. Slices that did not produce a maximum fEPSP amplitude >1 mV were discarded.
Light scattering
The 90° light scattering (excitation 480 nm, emission 484 nm) was determined in a Hitachi F-4500 spectrofluorimeter in a rapidly stirred methacrylate cuvette whose temperature was controlled by a circulating water bath (32 °C). The solution was continuously bubbled with 95% O2-5% CO2.
ICP-OES
To measure the amount of zinc precipitated, solutions were prepared with different concentrations of zinc, centrifuged at 15,500 g for 30 minutes at 32 °C and the zinc concentration of the supernatant was then determined on Varian 720 ICP Optical Emission Spectrometer. Yttrium was used as an internal standard. Standard curves were constructed using zinc solutions of 10,30 and 50 μM. The following lines were used to establish calibration curves and calculate the zinc concentration: 202.548, 206.200, 213.857 nm; internal standard: Y line 371.021 nm.
Particle size distributions were measured on a Malvern instruments Zetasizer, Nano series; Following parameters are applied to our experiments: reflective index for Zn3(PO4)2.H2O4 is 1.594 and absorption is 0.1.
Elemental Analysis of precipitates
The EDS analysis was performed on a Hitachi S-3400N variable pressure SEM equipped with a Bruker AXS Quantax x-ray microanalysis system. Quantitation was determined with standard-less analysis using the peak to background ZAF method.
Calculation of precipitate formation
Precipitate formation was calculated using the program MINTEQA2 (Allison 2003). The NIST database of formation constants was used and all calculations were performed under 5% CO2 at 32 °C.
Reagents
FluoZin-3 AM (Molecular Probes, Invitrogen), TPEN (Fluka) and other reagents were from Sigma.
Results
Zinc precipitation is induced by Pi in normal ACSF
The provision of zinc to brain slices above a few micromolar poses something of a problem in most artificial CSF formulations (ACSF), because they typically include inorganic phosphate that will precipitate zinc and thus limit the availability of free zinc ions. Slices can be sustained in phosphate free solutions and appear to exhibit normal synaptic responses and cellular activity, however, the effects of withholding phosphate are as yet unknown. To increase the solubility of zinc while preserving Pi we added to normal saline histidine, which forms both mono and bis-histidine complexes with zinc (Martell & Hancock 1996)but does not act as a neurotransmitter (Godfraind et al. 1973).
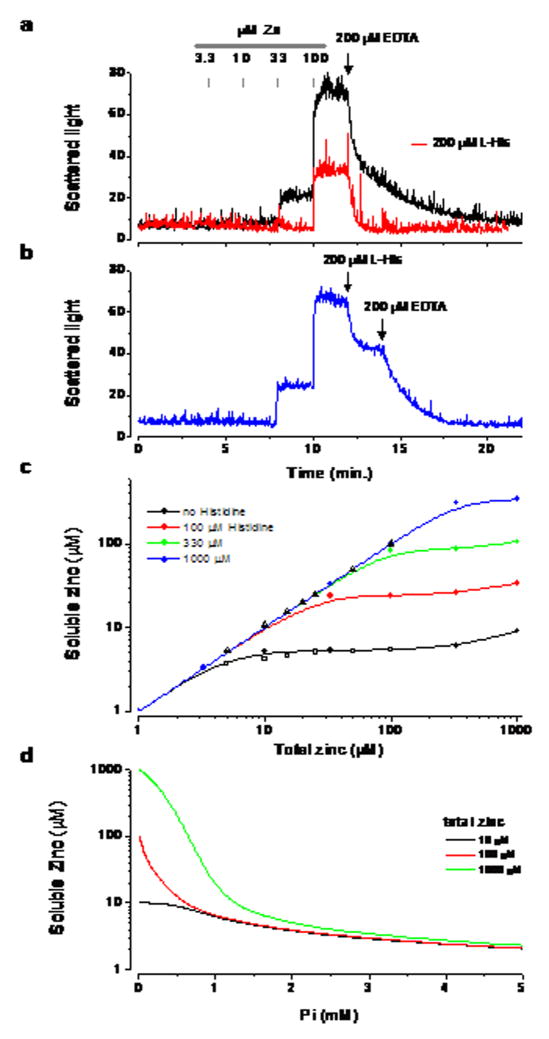
To detect the formation of precipitate in physiological solutions we used 90° light scattering in a cuvette within a spectrofluorimeter. In PFS (phosphate free saline) the addition of zinc up to a concentration of 330 μM led to no discernable precipitate formation, which is consistent with the high solubility of the zinc-bicarbonate complex. In normal saline, however, additions of zinc above about ~10μM led to the formation of a precipitate (Fig. 1a&b). Adding histidine increased the solubility of zinc in normal saline, prevented the formation of precipitate and led to the dissolution of existing precipitate (Fig. 1a&b).
Fig. 1.
Precipitation of zinc by phosphate (a) Formation of precipitates measured by light scattering in a spectrofluorimeter. Black line in the absence of histidine, red in its presence (200 μM). (b) Addition of histidine to ACSF after the addition of zinc. (c) The concentration of soluble zinc species in ACSF as function of zinc added calculated by MINTEQA2 (solid colored circles) at different concentrations of histidine. Values measured with ICP-OES are shown by the open symbols; squares in ACSF triangles in PFS. (d) Influence of Pi concentration on zinc solubility calculated with MINTEQA2.
The geochemical modeling program MINTEQA2 was used to calculate the concentration of soluble zinc species in different saline formulations (Allison 2003). The program takes into account all possible species that might form in a mixture and uses empirical thermodynamic parameters to calculate the equilibrium concentration of species, including any that might precipitate out of solution. The soluble zinc species considered are Zn2+, ZnOH+, Zn(OH)2, Zn(OH)3−, Zn(OH)4−2, ZnCl+, ZnCl2, ZnCl3−, ZnCl4−2, ZnOHCl, ZnSO4, Zn(SO4)2−2, ZnCO3, ZnHCO3+. Fifty insoluble species were considered in the calculation and the only one that formed was Zn3(PO4)2:4H2O. Fig. 1c shows estimates of the soluble zinc concentration in normal saline as a function of the added zinc concentration. In histidine-free saline without the soluble zinc concentration could not exceed ~ 4 μM. The addition of progressively higher concentrations of histidine led to an increase in the concentration of soluble zinc species. For example with 100 μM zinc and 200 μM histidine, there was 24.4 μM of Zn.His, 17.8 μM of Zn.His2 and 2.84 μM of all the other soluble zinc species. Since there is some uncertainty about the extracellular Pi concentration we calculated the solubility of zinc at different Pi concentrations and this is plotted in Fig. 1d.
To test the predications of the modeling program we assessed the solubility of zinc in ACSF using ICP-OES (inductively coupled plasma optical emission spectroscopy) with different zinc concentrations. The solutions were centrifuged to remove any precipitate and the supernatant was analyzed by ICP-OES. The results are shown in Fig. 1c and there is an excellent correspondence between the solubility measured by ICP-OES (open symbols) and that estimated by MINTEQA2 (closed symbols).
In human CSF the total amino acid concentration is ~ 700 μM (~500μM glutamine) and the histidine concentration ~12 μM (Davson et al. 1993, Wishart et al. 2008). Glutamine leads to little if any increase in the solubility of zinc compared to that in normal saline alone. In normal saline with 100 μM zinc and 500 μM glutamine, the free zinc concentration as determined by ICP-OES was 4.4 ± 0.2 (n=5), (5.9 μM from MINTEQA2) and was uninfluenced by the addition of more zinc. Citrate can chelate zinc and is found at a concentration of 200–400 μM in CSF (Wishart et al. 2008). With 400 μM citrate in normal saline the addition of 100 μM zinc only leads to a free zinc concentration of 13.9 μM (MINTEQA2 calculation).
The composition of the material that precipitated out after the addition of zinc to ACSF was determined by EDS (Energy dispersive X-ray spectroscopy, Fig. 2b). Atomic percentages are 60.9±4 O, 15.9±0.7 P, 4.6± 0.3 Ca and 18.7±0.5 Zn (n=4, from two different samples), with trace amounts of K and Mg2. The analysis of the precipitate is consistent with an amorphous compound composed largely of Zn3(PO4)2·4H2O (Hopeite) and CaZn2(PO4)2·2(H2O) (Scholzite).
Fig. 2.
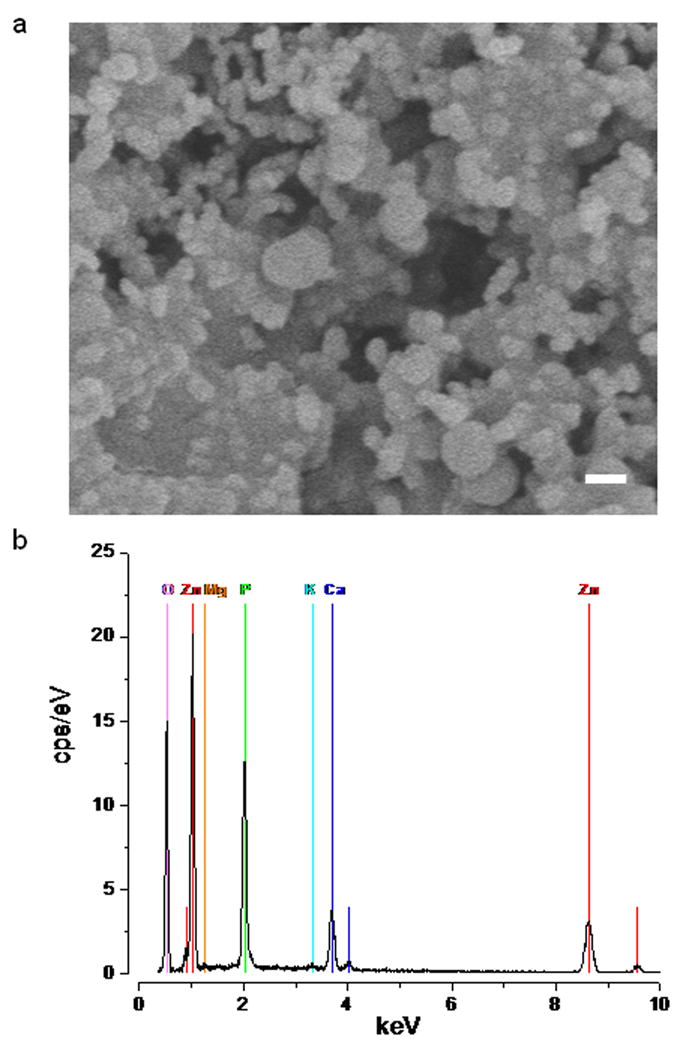
Analysis of the precipitates formed on the addition of zinc to normal saline. (a) Scanning EM of particles precipitated by the addition of zinc to ACSF (scale bar 100 nm). (b) EDS of the precipitate.
The size distribution of the precipitate in normal saline was assessed by light scattering and found to be polydisperse. The mean particle size of the precipitate formed one minute after zinc addition was 688nm and the minimum particle size ~200nm. This is consistent with scanning EM images of the precipitate (Fig. 2a).
Pi reduces zinc influx into brain slices
To assess the availability of zinc in neuronal tissue we used zinc transport into rat brain slices loaded with the acetoxymethyl (AM) ester form of the zinc-sensitive fluorescent indicator FluoZin-3 (Gee et al. 2002). In such slices the fluorescence was elevated slightly above the tissue autofluorescence, suggesting that there is some chelatable zinc within cells. Application of the membrane impermeant chelator Ca-EDTA did not lead to a substantial reduction in the signal, whereas the membrane permeable chelators diethyldithiocarbamate (DEDTC) or N,N,N′,N′-Tetrakis-(2-pyridylmethyl) -Ethylenediamine (TPEN) induced a decrease in fluorescence, consistent with the intracellular location of the zinc-indicator complex (data not shown). FluoZin-3 exhibits little response to calcium or magnesium, even in the millimolar range, and any elevations of fluorescence intensity are only likely to arise from the formation of a zinc-FluoZin-3 complex(Zhao et al. 2008b).
Numerous scattered puncta were visible when viewing the parenchyma of FluoZin-3 loaded neocortical slices with little evidence of perikaryal or nuclear staining. Staining was most evident on the outer most aspect of the slices extending ~ 20 μm into the slice. From these images it seems that FluoZin-3 enters synaptic vesicles rendering the endogenous zinc visible, which is consistent with the known ability of AM derivatives to load intracellular membranes (Thomas et al. 2000). To test this hippocampal slices were labeled with FluoZin-3 AM, which led to a faint but clear demarcation of areas that are highlighted by the Timm’s stain, namely the hilus and stratum lucidum, showing that FluoZin-3 enters zinc-rich synaptic vesicles (Fig. 3).
Fig. 3.

The hilus of a hippocampal slice loaded with FluoZin-3 AM. str. gr. – stratum granulosum (scale bar 10 μm).
To measure the zinc influx we imaged neocortical slices around layer 4, which includes all cellular elements, viz. dendrites, somata, synaptic boutons, glia and axons. In hippocampal slice experiments no differences were found in recording from the pyramidal cell layer as opposed to recordings from areas outside of this. Although we cannot be certain about the compartment that zinc translocates into, in this communication we have simply employed it as an assay to monitor zinc passage into cells.
In normal saline the application of 100 μM zinc led to a small elevation of fluorescence in FluoZin-3 AM loaded neocortical slices, consistent with the precipitation of zinc by phosphate (Fig. 4a). The co-application of histidine (200 μM) with 100 μM zinc boosted the metal influx. Histidine by itself in normal saline did not increase the fluorescence intensity (data not shown), which suggests that it does not appropriate enough zinc in the saline or slice to induce any metal influx. In contrast, the addition of 100 μM zinc in PFS induced a substantial metal influx that was not augmented by histidine (Fig. 4b).
Fig. 4.
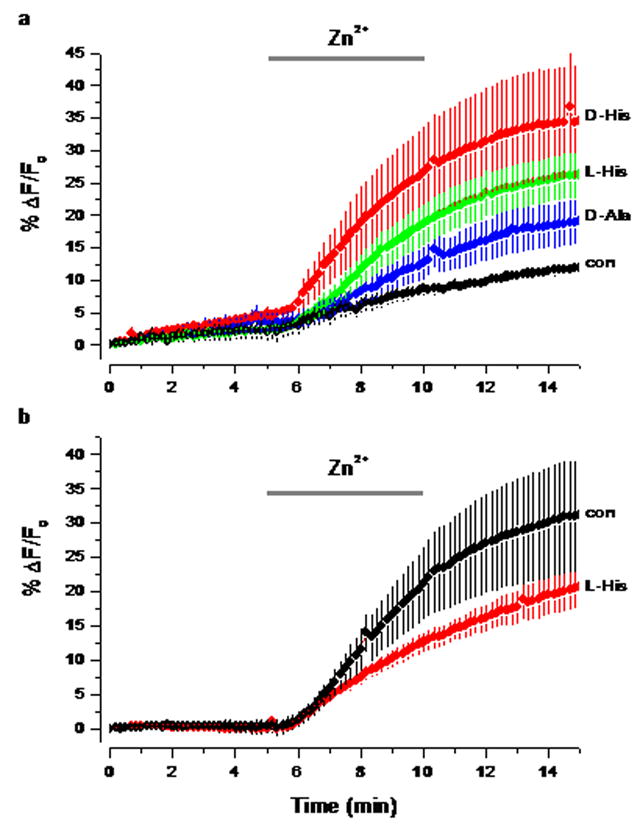
The influence of Pi and histidine on the influx of zinc into neocortical slices. In all experiments the fluorescence was measured in slices loaded with FluoZin-3 AM in an area of interest roughly midway through the neocortex. (a) Influences of amino acids (200 μM) on zinc uptake in ACSF. The period of zinc (100 μM) and amino acid application is indicated by a horizontal bar. Control (con) no amino acid was added (n=4), D-Ala (n=4), L-His (n=41) and D-His (n=6)(b) Zinc uptake in PFS. Control (con, n=5) and L-His (n=5).
There is some evidence from experiments on intestine that zinc and histidine are cotransported into cells. To test this hypothesis we co-applied either L or D-histidine with zinc. There was little difference between the augmentation of zinc transport by L or D histidine (Fig. 4a). This suggests that the amino acid is probably not interacting with a stereospecific transporter. Both L and D alanine, which form weaker complexes with zinc than histidine also augmented the zinc transport (Fig. 4a). In addition, transport was not inhibited by the presence of gylcyl-sarcosine which acts as a substrate for the dipeptide/tripeptide transporters, which have been suggested to carry metal-amino acid complexes (Conrad & Ahearn 2005). All these findings suggest that histidine is simply increasing the solubility of zinc in solution, rather than acting as a specific carrier.
To determine if the outcome of our experiments resulted from an edge artifact, because the probe only labeled the outer most aspect of the slice, we performed experiments where FluoZin-3 AM was injected into the depths of the slice. Under these conditions similar results were obtained to those shown in Fig. 4a (data not shown).
Although we did not systematically study the temperature dependence of zinc transport, no transport was evident at 24°C, while at 32°C it was. This suggests that the process has a high Q10, consistent with an active transport mechanism.
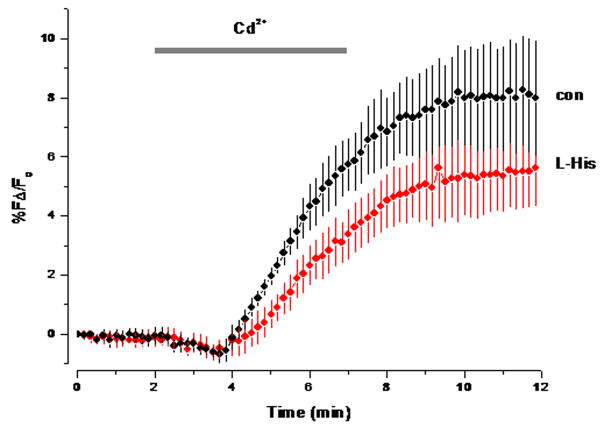
We also investigated the solubility and transport of cadmium because it has similar properties to zinc and appears to be able to translocate into some cells as a ‘rogue hitchhiker’ on zinc transporters (He et al. 2006). Cadmium also increases the fluorescence of FluoZin-3, though only to ~30% of the level of zinc (Zhao et al. 2008a). Unlike zinc, cadmium is not precipitated by Pi in normal saline; we found this both empirically and in theoretical calculations. Application of cadmium to slices first led to a decline and then to an increase in fluorescence. Histidine, however, did not increase the rate of cadmium transport, consistent with the solubility of cadmium in normal saline. The latency of the fluorescence increase induced by cadmium application was longer than that in zinc applications (~2 vs ~1min) (Fig. 5). This suggests that either the rate of cadmium transport is considerably slower than zinc or that cadmium displaces zinc from endogenous chelators like metallothionein and so induces an increase in fluorescence.
Fig. 5.
Cadmium uptake in neocortical slices as measured by FluoZin-3. The horizontal gray bar represent the time period when Cd (100μM) and L-His (200 μM) were applied (n=3 in both cases).
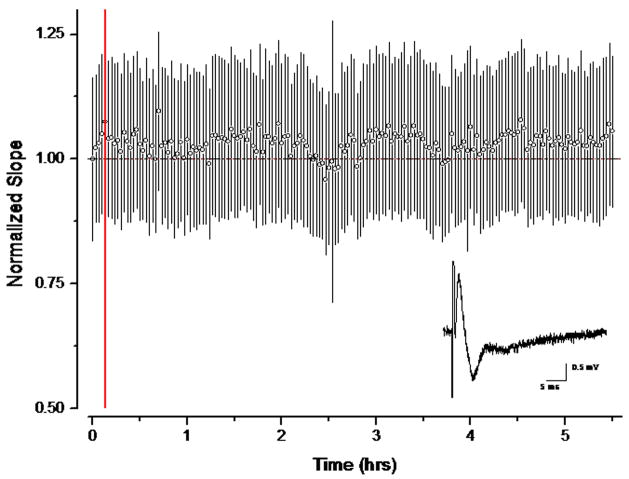
To determine the effect of removing Pi from ACSF on synaptic transmission we measured the field potentials evoked by stimulation of the Schaffer collaterals in the stratum radiatum of region CA1 of rat hippocampal slices. Over a period of 5.5 hours there was no decrease in the slope of the field EPSP (Fig. 6).
Fig. 6.
Effect of Pi removal on fESPS evoked by stimulation of the Schaffer collaterals. The pathway was stimulated every 5s with a 60 μs pulse. The graph plots the slope of the fESPS averaged over one minute, normalized to the initial value, for 3 slices. Error propagation was carried through all the calculations. The redline indicates the time PFS flow commenced.
Discussion
We have shown here that in brain slices, the presence of phosphate in physiological saline limits the concentration that soluble zinc can reach after metal application by the formation of zinc phosphate precipitates. In addition, we have shown that amino acids like histidine can increase zinc solubility in the presence of Pi, but they do not play a direct role in the transport of zinc. These results have clear implications for experiments where exogenous zinc is applied to brain slices. For example, it accounts for Molnar and Nadler’s (2001) observation that the presence of phosphate inhibited the action of exogenous zinc on GABA receptors. Our results also have perhaps less obvious implications for zinc release and uptake in vivo that we will discuss below. Moreover, our experiments have cast a spotlight on a rather underappreciated yet ubiquitious anion, Pi.
It is widely believed that synaptic zinc acts as a neuromodulator, being released during exocytosis and then diffusing into the synaptic cleft (Frederickson et al. 2005). In contrast our laboratory has provided evidence which conflicts with this idea and instead we have suggested that rather than being released, zinc is presented to the extracellular space while bound to exocytosed vesicular proteins (Kay 2003, Kay & Toth 2006). We have termed this scenario ‘externalization’ in contrast to simple release (Kay & Toth 2008). The presence of extracellular Pi poses a problem for the zinc release hypothesis, as precipitates could form if large amounts of zinc are released. Whether they do form will depend on the concentration of zinc chelators, small ligands, macromolecules, Pi, zinc and pH. We cannot, however, exclude the existence of zinc chelators more powerful than those so far identified.
The concentration of glutamate within vesicles is estimated to be ~ 300 mM, but after exocytosis it declines very rapidly as the molecule diffuses within the synaptic cleft (Barbour & Hausser 1997). Glutamate has a low affinity for zinc, nevertheless at high concentrations it can solubilize zinc. For example, at a zinc concentration of 100 μM in normal saline if the glutamate concentration is above ~ 30 mM no precipitate forms, however below this concentration progressive amounts of precipitate form with half the zinc being precipitated with ~14 mM glutamate (MINTEQA2 calculations).
Histidine has been proposed to augment the transport of zinc by forming a 2:1 complex (Sivarama Sastry et al. 1960) that is either transported as a unit or hands zinc off to a transporter. Histidine has been found to facilitate zinc transport in erythrocytes (Aiken et al. 1992) and in the intestines of rats (Wapnir et al. 1983), trout (Glover & Hogstrand 2002) and lobsters (Conrad & Ahearn 2007). However, our results suggest that in brain slices histidine simply increases the solubility of zinc and does not serve to facilitate the transport of zinc.
Our finding that histidine does not augment zinc transport in PFS suggests that under these conditions there is little phosphate in the extracellular space of brain slices. Similarly, because histidine increases zinc transport in normal saline, this indicates that the amino acid concentration is rather low in slices. In human CSF the total amino acid concentration is ~ 700 μM (~500μM glutamine) and the histidine concentration ~12 μM (Davson et al. 1993, Wishart et al. 2008). This concentration of histidine is too low to increase the solubility of zinc in the presence of Pi.
However the fact that in normal saline histidine augments zinc transport does not imply that there are no amino acids in the extracellular space. It could be that the zinc-phosphate particles do not penetrate through the extracellular space, and too little amino acid is likely to leach out to solubilize it. It is likely that the amino acid levels in the extracellular space of brain slices are diminished as they diffuse into the bathing solution. However, there is evidence that extracellular glutamate and perhaps glycine levels remain elevated in slices (Sah et al. 1989).
It does not seem to have been widely appreciated that Pi is an essential component of the extracellular medium that goes beyond its role in pH regulation. If Pi is removed from saline bathing neocortical cells the intracellular ATP and Pi levels remain stable for 30 min but both decline to ~60% of control levels after an hour (Glinn et al. 1997). Furthermore, synaptosomes derived from chronically phosphate deprived rats show an increase in cytosolic calcium and a decrease in ATP (Massry et al. 1991).
Controlled precipitation plays an important role in skeleton formation and other biomineralization processes (Dorozhkin & Epple 2002). On the other hand the uncontrolled formation of insoluble aggregates plays a prominent role in the pathogenesis of atherosclerosis (Giachelli et al. 2001) and may do so in a number of neuropathologies, including Alzheimer’s disease, Huntington’s disease and ALS. We would like to suggest that the formation of inorganic precipitates could serve as a nucleus for the accretion of molecules and ions. However, if one is to implicate precipitation in a neuropathology it is important to exclude aggregates that arise during the analysis. In this regard it is instructive to consider the case of entities termed ‘nanobacteria’ that on closer examination turned out to be calcium carbonate particles (Martel & Young 2008).
Aggregates of material with a predominantly inorganic basis have been described in a number of neuropathologies that have for the most part been ascribed to the precipitation of calcium. The development of calcifications within the brain has been noted in the case of ischemia and exitotoxicity (Mahy et al. 1999); no zinc was found in this case (N. Mahy personal communication). Calcifications have also been described in the case of Fahr’s syndrome in the basal ganglia (Bouras et al. 1996), spasmodic dysphonia (Simonyan et al. 2008) and in Urbach-Wiethe disease in the amygdala (Thornton et al. 2008); some zinc is found in the case of Fahr’s syndrome. It is also worth noting that excess intracellular Pi may lead to the precipitation of calcium as it does in the case of skeletal muscle sarcoplasmic reticulum, limiting calcium mobilization (Dutka et al. 2005).
Though Pi is usually incorporated in solutions used for sustaining brain slices, there are cases where its omission is seemingly without effect (Miles 1990), but this has not been studied systematically. In cultured neurons, many investigators leave out phosphate from the minimal solutions used to perform physiological experiments but preserve it in the media used to culture the cells.
The saline formulations used currently for sustaining brain slices are based on the concentrations of ions in plasma. The plasma concentration of Pi is around 1.1 mM in humans and tends to be higher in other animals. In rats the plasma Pi concentration is 3.2±0.1 mM and in CSF 0.47±0.01 mM (Mulroney et al. 2004). The latter is close to the concentration in humans and it is likely but not certain that the extracellular concentration is similar. Most mammalian slice ACSF formulations have Pi at a concentration of around 1 mM; to mimic CSF more closely it may be worthwhile shifting to a concentration of ~0.5 mM.
It is worth considering whether Pi might play roles other than that of the substrate for ATP synthesis. For example might it act as an allosteric regulator of ion channels and transporters? Moreover, the outward directed Pi gradient could be employed in carrier mediated mechanisms to transport ions, although none have thus far been identified.
There is a pressing need for experiments to determine the range of concentrations of Pi in the extracellular space and to determine the effect of changes in Pi on neuronal and synaptic activity. NMR measurements in the intact brain can distinguish between extra and intracellular Pi and may provide a means for assessing the extracellular Pi concentration (Gilboe et al. 1998).
To the best of our knowledge there have been no systematic studies of the effect of phosphate free ACSF on synaptic transmission. We found that phosphate removal for up to 5.5 hours had no effect on transmission at the Schaffer collateral CA1 synapse. This suggests that intact neuronal tissue either has considerable reserves of Pi or that it is endowed with an exceptional capacity to reclaim Pi that passes into the extracellular space. Until it becomes feasible to measure extracellular Pi, it is not possible to say whether or not Pi levels are sustained in slices held in PFS.
It is clear that the role of Pi extends beyond that of a pH buffering agent. There is little information on the mechanisms to control the intra and extracellular Pi levels, which could have a profound impact on the precipitation of metals. Moreover, the levels of amino acids, like histidine could play an important role in formation of metal-phosphate precipitates.
Perhaps because phosphate has been identified as part of the pH buffer system of physiological salines, it has not received much attention as a necessary component of media. Inorganic phosphate seems to have become part of the scenery when in fact it is a significant supporting player in cellular biology.
Acknowledgments
We would like to thank Dr. Sarah Larsen for the use of her ICP-OES and particle size analyzer, Daphne Lison, Drs. Richard Miles and Bill Shuttleworth and for helpful comments on earlier versions of this manuscript. Drs. Wei Chen, Pierre Paoletti and Kristina Simonyan for useful suggestions. This work was supported by grants from NINDS (NS47508 to A.R.K.) and NIEHS through the University of Iowa Environmental Health Sciences Research Center, NIEHS/NIH P30 ES0560. I.N. was supported in part by a fellowship from the Center for Biocatalysis & Bioprocessing. We thank Jean Ross and Dr. Jonas Baltrusaitis of the Central Microscopy Research Facility (funded by NSF grant CHE 0320387) for performing the elemental analysis on precipitates.
References
- Aiken SP, Horn NM, Saunders NR. Effects of amino acids on zinc transport in rat erythrocytes. J Physiol (Lond) 1992;445:69–80. doi: 10.1113/jphysiol.1992.sp018912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison JD. MINTEQA2 for Windows. Allison Geoscience Consultants, Inc; 2003. [Google Scholar]
- Barbour B, Hausser M. Intersynaptic diffusion of neurotransmitter. Trends Neurosci. 1997;20:377–384. doi: 10.1016/s0166-2236(96)20050-5. [DOI] [PubMed] [Google Scholar]
- Bellocchio EE, Reimer RJ, Fremeau RT, Jr, Edwards RH. Uptake of glutamate into synaptic vesicles by an inorganic phosphate transporter. Science. 2000;289:957–960. doi: 10.1126/science.289.5481.957. [DOI] [PubMed] [Google Scholar]
- Bouras C, Giannakopoulos P, Good PF, Hsu A, Hof PR, Perl DP. A laser microprobe mass analysis of trace elements in brain mineralizations and capillaries in Fahr’s disease. Acta neuropathologica. 1996;92:351–357. doi: 10.1007/s004010050529. [DOI] [PubMed] [Google Scholar]
- Burton RF. Ringer Solutions and Physiological Salines. John Wright & Sons; 1975. [Google Scholar]
- Collins JF, Bai L, Ghishan FK. The SLC20 family of proteins: dual functions as sodium-phosphate cotransporters and viral receptors. Pflugers Arch. 2004;447:647–652. doi: 10.1007/s00424-003-1088-x. [DOI] [PubMed] [Google Scholar]
- Conrad EM, Ahearn GA. 3H-L-histidine and 65Zn(2+) are cotransported by a dipeptide transport system in intestine of lobster Homarus americanus. The Journal of experimental biology. 2005;208:287–296. doi: 10.1242/jeb.01401. [DOI] [PubMed] [Google Scholar]
- Conrad EM, Ahearn GA. Transepithelial transport of zinc and L: -histidine across perfused intestine of American lobster, Homarus americanus. Journal of comparative physiology. 2007;177:297–307. doi: 10.1007/s00360-006-0129-0. [DOI] [PubMed] [Google Scholar]
- Davson H, Zlokovic B, Rakic L, Segal MB. An introduction to the blood-brain barrier. CRC Press, Inc; Boca Raton, FL: 1993. [Google Scholar]
- Dorozhkin SV, Epple M. Biological and medical significance of calcium phosphates. Angew Chem Int Ed Engl. 2002;41:3130–3146. doi: 10.1002/1521-3773(20020902)41:17<3130::AID-ANIE3130>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Dutka TL, Cole L, Lamb GD. Calcium phosphate precipitation in the sarcoplasmic reticulum reduces action potential-mediated Ca2+ release in mammalian skeletal muscle. Am J Physiol Cell Physiol. 2005;289:C1502–1512. doi: 10.1152/ajpcell.00273.2005. [DOI] [PubMed] [Google Scholar]
- Erecinska M, Silver IA. ATP and brain function. J Cereb Blood Flow Metab. 1989;9:2–19. doi: 10.1038/jcbfm.1989.2. [DOI] [PubMed] [Google Scholar]
- Frederickson CJ, Koh JY, Bush AI. The neurobiology of zinc in health and disease. Nature reviews. 2005;6:449–462. doi: 10.1038/nrn1671. [DOI] [PubMed] [Google Scholar]
- Furman S, Lichtstein D, Ilani A. Sodium-dependent transport of phosphate in neuronal and related cells. Biochim Biophys Acta. 1997;1325:34–40. doi: 10.1016/s0005-2736(96)00238-6. [DOI] [PubMed] [Google Scholar]
- Gee KR, Zhou ZL, Qian WJ, Kennedy R. Detection and imaging of zinc secretion from pancreatic beta-cells using a new fluorescent zinc indicator. J Am Chem Soc. 2002;124:776–778. doi: 10.1021/ja011774y. [DOI] [PubMed] [Google Scholar]
- Giachelli CM, Jono S, Shioi A, Nishizawa Y, Mori K, Morii H. Vascular calcification and inorganic phosphate. Am J Kidney Dis. 2001;38:S34–37. doi: 10.1053/ajkd.2001.27394. [DOI] [PubMed] [Google Scholar]
- Gilboe DD, Kintner DB, Anderson ME, Fitzpatrick JH., Jr NMR-based identification of intra-and extracellular compartments of the brain Pi peak. Journal of neurochemistry. 1998;71:2542–2548. doi: 10.1046/j.1471-4159.1998.71062542.x. [DOI] [PubMed] [Google Scholar]
- Glinn M, Ni BH, Paul SM. Inorganic phosphate enhances phosphonucleotide concentrations in cultured fetal rat cortical neurons. Brain research. 1997;757:85–92. doi: 10.1016/s0006-8993(97)00162-5. [DOI] [PubMed] [Google Scholar]
- Glover CN, Hogstrand C. Amino acid modulation of in vivo intestinal zinc absorption in freshwater rainbow trout. The Journal of experimental biology. 2002;205:151–158. doi: 10.1242/jeb.205.1.151. [DOI] [PubMed] [Google Scholar]
- Godfraind JM, Krnjevic K, Maretic H, Pumain R. Inhibition of cortical neurones by imidazole and some derivatives. Canadian journal of physiology and pharmacology. 1973;51:790–797. doi: 10.1139/y73-122. [DOI] [PubMed] [Google Scholar]
- He L, Girijashanker K, Dalton TP, Reed J, Li H, Soleimani M, Nebert DW. ZIP8, member of the solute-carrier-39 (SLC39) metal-transporter family: characterization of transporter properties. Molecular pharmacology. 2006;70:171–180. doi: 10.1124/mol.106.024521. [DOI] [PubMed] [Google Scholar]
- Kay AR. Evidence for chelatable zinc in the extracellular space of the hippocampus, but little evidence for synaptic release of Zn. J Neuroscience. 2003;23:6847–6855. doi: 10.1523/JNEUROSCI.23-17-06847.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR. Detecting and minimizing zinc contamination in physiological solutions. BMC Physiol. 2004;4:4. doi: 10.1186/1472-6793-4-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Toth K. Is zinc a neuromodulator? Science signaling. 2008;1:re3. doi: 10.1126/stke.119re3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AR, Tóth K. The influence of location of a fluorescent zinc-probe in brain slices on its response to synaptic activation. J Neurophysiol. 2006;95:1949–1956. doi: 10.1152/jn.00959.2005. [DOI] [PubMed] [Google Scholar]
- Krebs HA, Henseleit K. Untersuchungen über die Harnsoffbildung im Tierkörper. Hoppe-Seyler’s Zeitschrift fur physiologische Chemie. 1932;210:33–46. [Google Scholar]
- Li CL, Mc IH. Maintenance of resting membrane potentials in slices of mammalian cerebral cortex and other tissues in vitro. J Physiol. 1957;139:178–190. doi: 10.1113/jphysiol.1957.sp005885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahy N, Prats A, Riveros A, Andres N, Bernal F. Basal ganglia calcification induced by excitotoxicity: an experimental model characterised by electron microscopy and X-ray microanalysis. Acta neuropathologica. 1999;98:217–225. doi: 10.1007/s004010051072. [DOI] [PubMed] [Google Scholar]
- Martel J, Young JD. Purported nanobacteria in human blood as calcium carbonate nanoparticles. Proc Natl Acad Sci U S A. 2008;105:5549–5554. doi: 10.1073/pnas.0711744105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martell AE, Hancock RD. Metal Complexes in Aqueous Solution: Modern Inorganic Chemistry. Plenum Press; New York, NY: 1996. [Google Scholar]
- Massry SG, Hajjar SM, Koureta P, Fadda GZ, Smogorzewski M. Phosphate depletion increases cytosolic calcium of brain synaptosomes. Am J Physiol. 1991;260:F12–18. doi: 10.1152/ajprenal.1991.260.1.F12. [DOI] [PubMed] [Google Scholar]
- Miles R. Synaptic excitation of inhibitory cells by single CA3 hippocampal pyramidal cells of the guinea-pig in vitro. J Physiol. 1990;428:61–77. doi: 10.1113/jphysiol.1990.sp018200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnar P, Nadler JV. Lack of effect of mossy fiber-released zinc on granule cell GABA(A) receptors in the pilocarpine model of epilepsy. J Neurophysiol. 2001;85:1932–1940. doi: 10.1152/jn.2001.85.5.1932. [DOI] [PubMed] [Google Scholar]
- Mulroney SE, Woda CB, Halaihel N, Louie B, McDonnell K, Schulkin J, Haramati A, Levi M. Central control of renal sodium-phosphate (NaPi-2) transporters. Am J Physiol Renal Physiol. 2004;286:F647–652. doi: 10.1152/ajprenal.00354.2002. [DOI] [PubMed] [Google Scholar]
- Murer H, Forster I, Biber J. The sodium phosphate cotransporter family SLC34. Pflugers Arch. 2004;447:763–767. doi: 10.1007/s00424-003-1072-5. [DOI] [PubMed] [Google Scholar]
- Ni B, Rosteck PR, Jr, Nadi NS, Paul SM. Cloning and expression of a cDNA encoding a brain-specific Na(+)-dependent inorganic phosphate cotransporter. Proc Natl Acad Sci U S A. 1994;91:5607–5611. doi: 10.1073/pnas.91.12.5607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paoletti P, Vergnano AM, Barbour B, Casado M. Zinc at glutamatergic synapses. Neuroscience. 2008 doi: 10.1016/j.neuroscience.2008.01.061. [DOI] [PubMed] [Google Scholar]
- Ringer S. A further contribution regarding the influence of the different constituents of blood on the contraction of the heart. J Physiol. 1883;4:29–42. doi: 10.1113/jphysiol.1883.sp000120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sah P, Hestrin S, Nicoll RA. Tonic activation of NMDA receptors by ambient glutamate enhances excitability of neurons. Science. 1989;246:815–818. doi: 10.1126/science.2573153. [DOI] [PubMed] [Google Scholar]
- Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined diffusion tensor imaging and neuropathological study. Brain. 2008;131:447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sivarama Sastry K, Viswanathan L, Ramaiah A, Sarma PS. Studies on the binding of 65Zn by equine erythrocytes in vitro. Biochem J. 1960;74:561–567. doi: 10.1042/bj0740561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smart TG, Hosie AM, Miller PS. Zn2+ ions: modulators of excitatory and inhibitory synaptic activity. Neuroscientist. 2004;10:432–442. doi: 10.1177/1073858404263463. [DOI] [PubMed] [Google Scholar]
- Takamori S, Rhee JS, Rosenmund C, Jahn R. Identification of a vesicular glutamate transporter that defines a glutamatergic phenotype in neurons. Nature. 2000;407:189–194. doi: 10.1038/35025070. [DOI] [PubMed] [Google Scholar]
- Thomas D, Tovey SC, Collins TJ, Bootman MD, Berridge MJ, Lipp P. A comparison of fluorescent Ca2+ indicator properties and their use in measuring elementary and global Ca2+ signals. Cell Calcium. 2000;28:213–223. doi: 10.1054/ceca.2000.0152. [DOI] [PubMed] [Google Scholar]
- Thornton HB, Nel D, Thornton D, van Honk J, Baker GA, Stein DJ. The neuropsychiatry and neuropsychology of lipoid proteinosis. The Journal of neuropsychiatry and clinical neurosciences. 2008;20:86–92. doi: 10.1176/jnp.2008.20.1.86. [DOI] [PubMed] [Google Scholar]
- Wapnir RA, Khani DE, Bayne MA, Lifshitz F. Absorption of zinc by the rat ileum: effects of histidine and other low-molecular-weight ligands. The Journal of nutrition. 1983;113:1346–1354. doi: 10.1093/jn/113.7.1346. [DOI] [PubMed] [Google Scholar]
- Wishart DS, Lewis MJ, Morrissey JA, et al. The human cerebrospinal fluid metabolome. Journal of chromatography. 2008 doi: 10.1016/j.jchromb.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Zhao J, Bertoglio BA, Devinney MJ, 2nd, Dineley KE, Kay AR. The interaction of biological and noxious metals with the zinc probes FluoZin-3 and Newport Green. Analytical biochemistry. 2008a doi: 10.1016/j.ab.2008.09.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Bertoglio BA, Gee KR, Kay AR. The zinc indicator FluoZin-3 is not perturbed significantly by physiological levels of calcium or magnesium. Cell Calcium. 2008b doi: 10.1016/j.ceca.2008.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]