Abstract
Ultrasound contrast agents, in the form of gas-filled microbubbles, are becoming popular in perfusion monitoring; they are employed as molecular imaging agents. Microbubbles are manufactured from biocompatible materials, they can be injected intravenously, and some are approved for clinical use. Microbubbles can be destroyed by ultrasound irradiation. This destruction phenomenon can be applied to targeted drug delivery and enhancement of drug action. The ultrasonic field can be focused at the target tissues and organs; thus, selectivity of the treatment can be improved, reducing undesirable side effects. Microbubbles enhance ultrasound energy deposition in the tissues and serve as cavitation nuclei, increasing intracellular drug delivery. DNA delivery and successful tissue transfection is observed in the areas of the body where ultrasound is applied after intravascular administration of microbubbles and plasmid DNA. Accelerated blood clot dissolution in the areas of insonation by cooperative action of thrombolytic agents and microbubbles is demonstrated in several clinical trials.
Keywords: Ultrasound, Ultrasound contrast agent, Molecular Imaging, Microbubbles, Echo, Acoustic, Triggered Release
1. Introduction
The general goal of drug delivery and targeting is to improve the efficacy of drug action in the region of the disease while reducing undesired side effects, such as toxicity, in the healthy tissues. Lately, studies that combine drugs with an externally applied “trigger” are gaining attention. This approach controls drug action and/or deposition in the targeted region by an external energy field, such as light (photodynamic therapy), neutron beam (boron neutron capture therapy), magnetic field (targeted accumulation of magnetic drug carrier in the tissues close to the magnet), or mechanical energy. In order to improve the delivery of drugs and therapeutic genes, mechanical energy has been applied in the form of ultrasound irradiation. Ultrasound waves have numerous advantages as a “controlling” field for drug delivery. Ultrasound is already widely applied in medicine, and current technology allows easy focusing and penetration deep into the body. Ultrasound has been known to improve drug delivery into tissues and cells [1]. Furthermore, presence of ultrasound energy deposition foci in the tissues (e.g., in the case of inertial or non-inertial cavitation) provides highly efficient ways of perturbing cell membranes and increasing their permeability. Gas-filled microbubbles administered by intravascular route can serve as cavitation nuclei and aid in a wide range of ultrasound-mediated drug delivery applications.
In this review, we will describe a variety of microbubble formulations, their in vitro/in vivo behavior, their interaction with ultrasound, and their use for imaging, targeting, and controlled release. Bioeffects in tissues and cells due to microbubble-ultrasound interactions will be discussed, followed by the potential therapeutic applications of such interactions. We will specifically focus on the developing applications of microbubbles as drug delivery and potentiation vehicles: microbubbles can be either co-administered with drugs or pre-loaded with pharmaceutical agents for triggered release and tissue deposition. As this is a dynamic, rapidly expanding field, it will not be possible to cover all of the existing literature. General development trends will be illustrated with experimental data and discussed.
2. Microbubble design and imaging applications
Ultrasonography is currently the most widely used diagnostic medical imaging modality. It is non-invasive and relatively low in cost, and it uses portable, real-time imaging equipment. It also avoids hazardous ionizing radiation. An ultrasound transducer placed on the skin or inside the body broadcasts ultrasound pressure wave pulses, which are partially reflected or scattered by the interfaces between different tissues or structures in the body. Some of the scattered sound waves return to the transducer. The imaging system converts these signals into electrical pulses and digitizes them. The time intervals between pulse transmission and reception, as well as the speed of sound in the tissue, are known. Therefore, an image that is based on scattered sound signals can be generated. However, blood, a liquid phase material with low compressibility, scatters ultrasound poorly. Ultrasound images of blood can be improved by the use of contrast agents, which increase the scattering and reflection of ultrasonic waves.
The use of microbubble-based contrast agents was first introduced in echocardiography by Gramiak and Shah in 1968 [2]. They demonstrated that intracardiac injection of agitated saline improved aortic root delineation due to scattering of ultrasound by air microbubbles present in the solution. Since then, other agitated contrast agents (hydrogen peroxide, dextrose, indocyanine green dye, iodinated contrast) have been used. However, these formulations were unable to pass through the pulmonary capillaries and could opacify the left-sided cardiac chambers only when administrated by intracoronary or aortic root injection. In 1984, Feinstein et al. introduced the use of sonication to create microbubbles that were stable and small enough to transit through the pulmonary microcirculation from right to left heart [3, 4].
Microbubbles are small (typically 1 – 8 μm in diameter) gas-filled microspheres. Currently, microbubble contrast agents are capable of unobstructed movement through the vasculature, including capillaries, after intravenous administration. The first-generation microbubbles (e.g. Albunex®) were filled with air. Because of the high solubility of air in blood and a thin (10–15 nm) protein shell coat that was not a good barrier against gas diffusion, these microbubbles disappeared from the bloodstream within seconds after administration [5]. Inert, high molecular weight gases like perfluorocarbons or sulfur hexafluoride are used in second- and third-generation contrast agents. Their decreased solubility and low diffusion coefficient prolong the lifespan of microbubbles within the circulation. The gas core is generally surrounded by a protein (albumin), lipid, surfactant, or biocompatible polymer shell (from 2 to 500 nm thick). Encapsulation improves stability against gas loss, dissolution, and microbubble coalescence, and it produces a more standard size distribution. A surfactant layer increases the half-life of the microbubble by decreasing surface tension, in some instances to near-zero values [6]. The composition of the shell determines the stiffness of the bubbles, their resistance to rupture in the ultrasound pressure field, and the ease with which they are recognized and cleared by the reticuloendothelial system.
The acoustic backscatter of microbubbles may be orders of magnitude greater than the backscatter of blood and the majority of other tissues and organs. This is due to the high acoustic impedance mismatch between gases and blood or soft tissue. It makes microbubbles useful as contrast agents for ultrasound imaging, especially in echocardiography. The microbubble acoustic backscatter signal is dependent on the compressibility of the gas, the size of microbubbles, the thickness, viscosity and density of the bubble shell, the properties of the surrounding medium, and the frequency and power of the applied ultrasound [7, 8]. As microbubbles become larger, they become more echogenic (the ultrasound scattering cross-section of a microbubble is directly proportional to the sixth power of its radius [9]). However, a compromise must be made that limits the maximum size of useful bubbles for intravascular use because the largest particle able to transit via normal pulmonary and systemic capillary beds does not normally exceed 6 to 8 μm in diameter. Furthermore, one must take into account that the microbubble size within the local milieu may change because of potential bubble coalescence, gas core expansion or contraction, and inward/outward diffusion of gases, depending on the temperature, vapor pressure, hydrostatic pressure, acoustic pressure, etc. While larger bubbles with a diameter of several micrometers may provide the most efficient acoustic response, submicron-sized bubbles were reported to demonstrate reasonable acoustic backscatter [10, 11]. Those particles sufficiently small enough to leave the bloodstream and enter the interstitial space might open an interesting cache of opportunities in diagnostics and therapy, such as direct access of contrast and drug delivery agents to tumor cells.
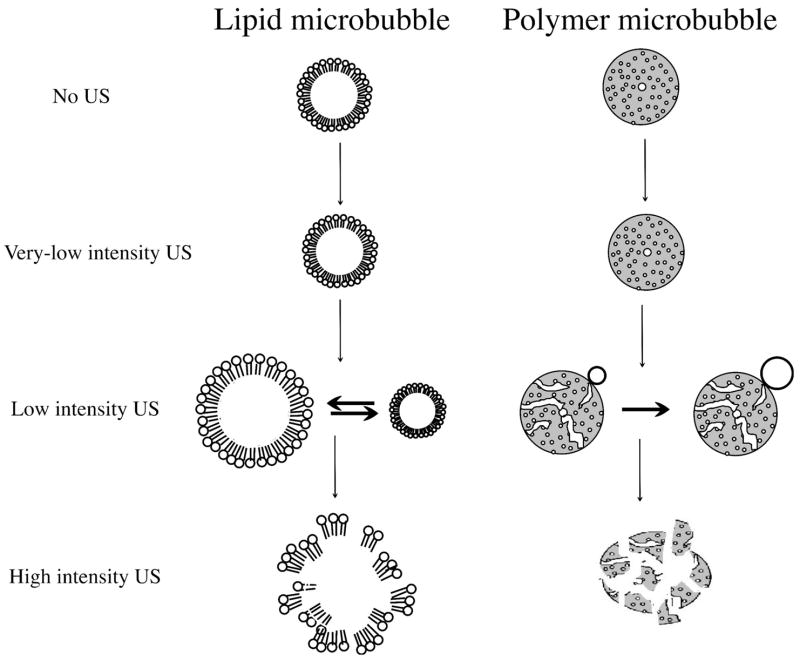
Behavior of lipid microbubbles depends on the amplitude of ultrasound to which they are exposed (Figure 1). At very low acoustic power (mechanical index < 0.05–0.1), the microbubble oscillates in a relatively symmetrical, linear manner. The changes in the bubble volume are thus inversely proportional to pressure variations caused by ultrasound. This bubble vibration produces ultrasound scattering with a frequency equal to the transmitted frequency (linear backscatter).
Figure 1.
Schematic representation of lipid (left column) and polymer (right column) microbubble interaction with ultrasound of increasing intensity (top to bottom).
At a slightly higher mechanical index (MI) of 0.1 to 0.3, often referred to as low-power imaging, the bubble becomes somewhat more resistant to compression than to expansion. This phenomenon is known as stable or non-inertial cavitation and leads to non-linear oscillations and backscatter at a variety of frequencies (harmonics, sub-harmonics and ultra-harmonics). The detection of these harmonic frequency signals has been implemented in ultrasound imaging equipment for some years. Harmonic imaging markedly enhances the bubble-to-tissue backscatter signal ratio since tissue does not produce significant harmonic backscatter at this range of mechanical index. This forms the basis for the ultrasound contrast-specific harmonic imaging techniques (e.g. phase inversion imaging, ultraharmonic imaging, harmonic power Doppler imaging) and plays a role in the improvement of endocardial border delineation and left ventricular opacification (LVO) imaging [12].
At even higher acoustic pressures (MI > 0.3–0.6), the microbubble undergoes forced expansion and compression, which result in its destruction by either outward diffusion of the gas during the compression phase or diffusion via large shell defects, or by complete fragmentation of the microbubble shell and the gas core [13, 14], (Figure 2). At high acoustic power, the peak wall velocity for gas-liquid interface motion during microbubble collapse may reach 700 m/s. These rapid events are accompanied by the exhibition of highly transient, non-linear scattering and inertial cavitation.
Figure 2.
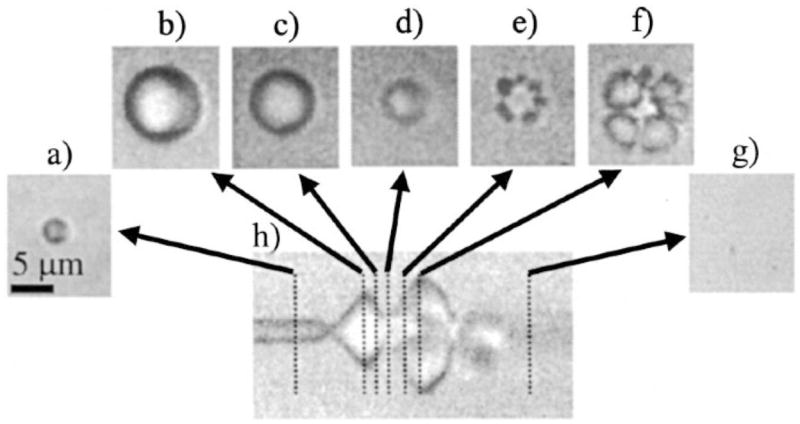
Optical frame images and streak image corresponding to the oscillation and fragmentation of a contrast agent microbubble, where fragmentation occurs during compression. The bubble has an initial diameter of 3 μm, shown in a. The streak image in h shows the diameter of the bubble as a function of time, and dashed lines indicate the times at which the two-dimensional frame images in a-g were acquired relative to the streak image. (Reproduced with permission from ref. [14], Copyright, 2000, American Institute of Physics).
Microbubble destruction is also an important feature for quantifying perfusion with intermittent triggered imaging methods. If microbubbles are administered as a continuous infusion, their destruction and the evaluation of myocardial replenishment rate (assessed with a sequence of variable-delay, high-MI ultrasound pulses synchronized with cardiac contractions) provide an estimate of the mean myocardial microbubble velocity [15, 16]. Since microbubbles possess rheology properties similar to red blood cells, bubble perfusion data reflects red blood cell flow [17, 18]. There exists another variant of destruction-replenishment imaging methods, which is more suitable for motion-free situations: a single high-intensity pulse followed by real-time (e.g., 30 frames per second) low-power nondestructive imaging. Ultrasound contrast is used to measure perfusion in a variety of organs and tissues, such as kidneys [19], brain [20], skin graft, [21] and skeletal muscles [22], as well as to improve tumor diagnosis by detecting tumor vasculature and angiogenesis [23].
Polymeric microbubbles exhibit quite different behavior in response to ultrasound as compared to lipid-shelled microbubbles [24]. At low ultrasound intensity, the stiff polymeric shell will not oscillate actively. As ultrasound pressure is increased above a threshold value, shell defects or cracks will form, through which the encapsulated gas can escape (Figure 1). Experimental evidence obtained with high-speed camera microscopy of insonated bubbles suggests that application of high intensity ultrasound results in complete breakdown of the microbubbles (Figure 3).
Figure 3.
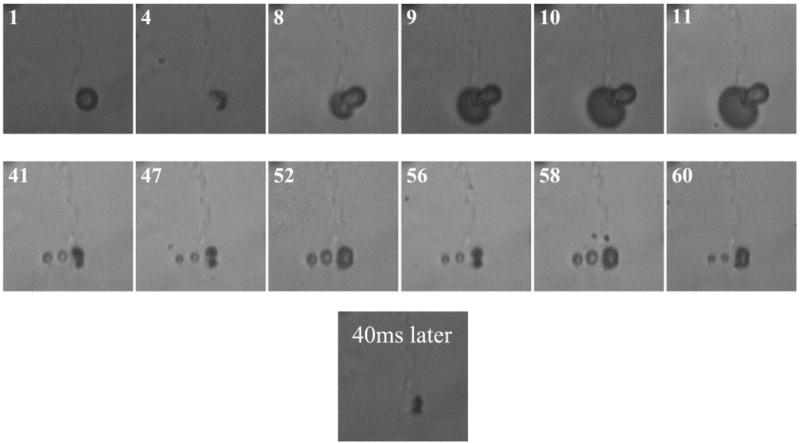
Optical frames showing initial PB127 microsphere (frame 1), shell fissure and gas escape (frames 4,8 to 11) under ultrasound of 1.7MHz, four cycles, MI of 1.4 and formation of new free bubbles. Two new free bubbles (frames 41, 47 to 60) demonstrating oscillations under ultrasound of 1.7 MHz, four cycles and MI of 0.25. Last displayed frame shows an optical recording taken 40 ms later, demonstrating bubble disappearance due to dissolution. (Reproduced with permission from ref. [146], Copyright, 2005, Elsevier)
3. Ligand-mediated targeting of microbubbles and potential use for drug delivery
The development of targeted contrast agents extended ultrasound-based imaging to the depiction of molecular and cellular processes. The principle relies on the accumulation of the contrast agent at the specific molecular sites in the body.
For active targeting, microbubbles are designed to adhere selectively to cellular epitopes and receptors. Since the microbubbles remain within the vasculature because of their micron size, specific marker molecules have to be located in the intravascular space, e.g., on the luminal surface of vascular endothelium. For instance, cell adhesion molecules overexpressed on endothelium during pathological events can be targeted. These molecular markers often play an important role in processes such as wound healing, leukocyte and granulocyte extravasation, lymphocyte homing and so forth. A wide variety of ligands, including antibodies, carbohydrates and peptides may be used to target the bubbles. Monoclonal antibodies (mAb) are able to bind a large range of epitopes with a very high specificity and selectivity. However, because of the murine origin of most monoclonal antibodies in the experimental studies, they will elicit an unacceptable immune response in human patients. Recombinant gene technology has made it possible to create humanized antibodies and antibody fragments, which are less immunogenic. Additionally, it is even possible to tailor their affinity and produce multivalent and multispecific fragments [25]. Peptides have the advantages of being less immunogenic than antibodies due to their small size (typically 5 to 15 amino acids), and they are often inexpensive.
Several groups reported, in vitro as well as in vivo, a significantly enhanced microbubble adhesion to activated endothelium [26], rejecting tissues [27], neovasculature endothelium [28–31], lymph node-related vasculature [32], or activated platelets [33] by targeting ICAM-1, VCAM-1, integrins, P- and E-selectin, or GIIb/IIIa receptors. Weller et al. demonstrated that simultaneous targeting to multiple ligands (ICAM-1 via anti-ICAM-1 mAb and selectins via sialyl Lewis carbohydrates) could synergistically increase microbubble adhesion strength to the activated endothelium in vitro [34].
The coupling of ligands to the microbubble may either be covalent or non-covalent. In the former case, the ligand is attached to the headgroup of phospholipids composing the microbubble shell either directly or via an extended polymer spacer arm [35]. For non-covalent binding strategies, avidin-biotin bridging is a commonly used technique due to the wide array of available biotinylated ligands and excellent affinity of avidin for biotin (KD 10−15 M). However, for in vivo studies, avidin, which is heavily glycosylated and carries a strong overall positive charge, can alter microbubble biodistribution and may result in strong nonspecific adhesion of bubbles. Being a foreign (chicken egg) protein, avidin will cause undesired immune response in mammals. Streptavidin, a biotin-binding protein from Streptomyces avidinii, may be a preferred bridging molecule for microbubble animal trials because it is not glycosylated, and its isoelectric point, which is slightly acidic, is closer to neutral. However, repeated injections of streptavidin-carrying particles in humans will still be difficult to justify because of the risk of an immune response.
A preferred linking scheme of the ligand to the bubbles is a covalent chemical bond, such as a peptide, ester, disulfide or thioether [36]. It may be a significant advantage to place the ligand at the end of an intermediary spacer arm like a poly(ethylene glycol) (PEG) molecular tether, connecting it to the bubble surface indirectly. The length and the flexibility of the polymer tether as well as the targeting ligand density on the bubble surface are important features for the success of microbubble targeting. The tethered ligand should be separated far (tens of nm) from the bubble surface; it should have a high degree of translational freedom to bind to its target efficiently and rapidly. The microbubble shell should not interfere with the ligand-receptor binding [37, 38]. Borden et al. recently described a different, novel surface architecture in which the tethered ligand is buried in a polymeric “overbrush” [39]. The ligand is instantly revealed and made available for targeting only after the application of ultrasound radiation force. This approach may decrease both the undesired non-specific binding to the tissues in the non-insonated areas and undesired uptake of contrast agent particles by the reticuloendothelial system.
The contact between the microbubble and its target can be enhanced by external application of an ultrasonic field. A microbubble, driven by an ultrasound field near its resonance frequency, experiences net primary and secondary ultrasound radiation forces, also known as Bjerknes forces. Ultrasound can displace microbubbles over significant distances (up to millimeters) in the direction of the ultrasound propagation and can cause attraction between microbubbles leading to aggregate formation [40–45]. Thus, the microbubbles can be concentrated on the target located on the vessel wall.
All of these design approaches and considerations can be applied not only in the case of molecular ultrasound imaging, but also for targeted energy deposition and drug delivery. Targeted bubbles will selectively accumulate only at the endothelium of the diseased tissues, but not in the other areas; therefore, focusing of the ultrasound beam may not be necessary to achieve selective treatment of the target tissue while avoiding non-targeted normal regions of the body.
4. Microbubbles in drug delivery: co-administration of microbubbles and drug
4.1 Bioeffects of ultrasound in the presence of microbubbles
As described above, high acoustic pressures can lead to rapid (microsecond) disintegration of microbubbles. This phenomenon is widely used in diagnostic ultrasonography, but it can also induce bioeffects in certain conditions. Both in vitro and in vivo experiments reported the occurrence of hemolysis, microvascular leakages, capillary ruptures, petechial hemorrhages [46–52], mild elevations of troponin-T in blood, cardiomyocyte injury [53–56], inflammatory cell infiltrations and premature ventricular contractions [57–59] in tissues when insonating in the presence of contrast agents. No tissue damage has been noted when ultrasound or microbubbles were not used in combination (e.g., see [60] for a review). The extent of the undesired bioeffects can be accentuated by a combination of several factors, such as high concentration of contrast agent, the delivery method (intra-arterial vs. intravenous), the ultrasound system transducer and settings (low ultrasound frequency and high pressure), low attenuation by the tissues located between the insonation focus and the transducer, the ultrasound imaging mode (intermittent), and the type of targeted tissue.
The medical significance of these observations should be further investigated to assure patient safety. At the same time, consistent induction of these bioeffects, caused by the interaction between ultrasound and microbubbles, opens opportunities for therapeutic applications. For example, injuring the vasculature of a tumor might encourage tumor regression.
4.2 Thrombolysis via ultrasound-assisted microbubble destruction
Three decades ago Trubestein et al. reported the potential for high-frequency ultrasound to dissolve intra-arterial thrombi [61, 62]. This technique, called sonothrombolysis, has proven efficacious when applied in combination with fibrinolytic agents (e.g. urokinase and tissue plasminogen activator). Sonothrombolysis at lower frequencies (kHz instead of MHz) caused less heating and allowed a broader and more uniform acoustic field; one of the possible explanations of this effect could be more efficient cavitation at lower ultrasound frequency.
During the last decade, studies demonstrating that intravenously administered microbubbles were able to enhance the thrombolytic effects of ultrasound, either with or without the presence of these fibrinolytic agents, often resulting in complete vessel recanalization, progressed from animal and in vitro experimentation to clinical trials [63–65]. This phenomenon is explained by the ability of microbubbles to lower the threshold energy needed for cavitation [66]. Cavitation creates high-speed microstreams (or microjets) of sufficient magnitude to promote clot lysis. The induced changes in the fibrin mesh accelerate the transport and penetration of fibrinolytic agents into the clot, provide more efficient use of thrombolytic enzymes, and achieve faster clot dissolution without the release of large amounts of the potentially hazardous clot fragments into the medium. Clinical trials now in progress may bring this technique into widespread practical use in a broad range of clot dissolution scenarios, from dialysis graft [67] to stroke [68] to myocardial infarction [69]. The importance of this technique goes beyond simply lowering the dose of expensive thrombolytic agents; it helps reduce the existing risk of hemorrhaging, while accelerating clot dissolution and limiting ischemic damage to the tissues, and thus improving patient outcome.
4.3 Enhancement of vascular permeability by insonated microbubbles
Intravital microscopy observation of the results of in vivo destruction of microbubbles induced by ultrasound was reported by Skyba et al. [70]. It was shown that immediate rupture of the microvessels occurred at the location of microbubble destruction, with extravasation of red blood cells in the interstitial space as a consequence. This observation opened a variety of possibilities in using microbubbles for therapeutic purposes, besides their use as diagnostic contrast agents. Normally, cells, plasmid DNA, and pharmaceutical drug carrier particles have difficulty crossing the endothelial layer and escaping from the bloodstream. The destruction of microbubbles creates conditions to help overcome the endothelial lining barrier in the targeted tissue. Successful interstitial delivery of engineered red blood cells and nondeformable polymer latex spheres of various sizes, up to 500 nm [71] has been demonstrated. The delivery effect was dependent on the power of ultrasound applied [59, 72].
Because of these microscale injuries induced by ultrasound-mediated microbubble destruction, proinflammatory cells, platelets, and marrow-derived stem cells are recruited into the tissues; they elicit an angiogenic and arteriogenic response [73]. Microvascular remodeling thus occurs in occluded capillaries, but blood flow can also be increased in normal skeletal muscles [70, 74]. The simultaneous injection of cells and/or factors (like bone marrow mononuclear cells [75, 76] or granulocyte colony-stimulating factor [77]) that are known to stimulate arterio- and angiogenesis by binding to injured vascular endothelium and supplying additional angiogenetic factors has been proven to enhance neovessel formation when compared to treatments performed without ultrasound.
The endothelial lining in the brain, the blood-brain-barrier, which is normally impenetrable even for the majority of low molecular weight drugs, can be reversibly “softened” by ultrasound-mediated microbubble destruction (using a transcranial ultrasound transducer), and thus the entry of drugs into the central nervous system can be improved. Compared to other strategies that have known limitations, this technique shows only minimal vascular effects (tiny regions of extravasated red blood cells [78]) and is safer because of its non-invasive character [79–81].
4.4 Enhancement of cell membrane permeability by insonated microbubbles
The ability of microbubbles to act as cavitation nuclei when destroyed can increase cell membrane permeability, if cells are located in close proximity to microbubbles. Although ultrasound had been proven to increase cell membrane permeability on its own [82], the use of microbubbles has a significant additive effect. Several explanations are offered for this phenomenon called ‘sonoporation’. First, the microjets cause shear stress on the cell membrane and create transient, non-lethal holes in the plasma membrane, through which a drug or gene is able to diffuse [83, 84]. These pores were visualized by scanning electron microscopy [85, 86], and the mechanism of action could be revealed using a high frame rate camera [87]. Second, the generation of intracellular reactive oxygen species, following the application of ultrasound, might contribute to permeabilization of the cell membrane [88–90] without affecting the cell viability. The local, transient temperature increase due to the absorption and dissipation of ultrasound energy may also influence phospholipid bilayer fluidity and thus cell permeability [91]. Other scenarios are the involvement of active transport mechanisms, such as endocytosis and phagocytosis in the uptake of microbubbles, and the fusion of lipid-based microbubbles with the phospholipid cell membrane.
Intracellular drug delivery after the exposure of cells to insonated microbubbles has mainly been demonstrated using model fluorescently labeled molecules with different sizes (propidium iodide, calcein, FITC-dextran, TO-PRO®-1) as well as particles, such as polymer beads [92–95], that do not translocate across intact cell membranes. Fluorescent microscopy shows an increased number of label-positive cells for the experiments that combine microbubbles and ultrasound compared to cells exposed to ultrasound or microbubbles separately. Although only a few studies were performed using anti-cancer drugs combined with ultrasound [96–98], they confirmed that lower drug dose was necessary to achieve therapeutic effect.
4.5 Gene delivery by ultrasound and microbubbles: co-administration of bubbles
Several types of vector systems have been developed for therapeutic delivery of genetic material, but the widespread clinical application of gene therapy has not become a reality so far. Investigations into alternative gene delivery strategies are underway. Perturbation of the cell membrane and the vessel wall by insonated microbubbles increased permeability and interstitial delivery as described above. Hydrodynamic gene delivery techniques [99], as well as intramuscular direct injection of plasmid DNA [100], are well known, so the success of ultrasound and microbubbles in aiding transfection can be anticipated. Experiments involving the co-administration of microbubbles and plasmid DNA were mainly carried out with genes coding for marker proteins (e.g., luciferase, green or red fluorescent protein, beta-galactosidase, and similar). Application of ultrasound in combination with bubbles and plasmid results in an improved transfection efficiency compared to the naked plasmid DNA alone in vitro and in vivo [27, 101–108]. Similar results were observed by delivering synthetic oligonucleotides (antisense or siRNA), which modulate gene expression by inactivating the gene of interest, instead of inserting a therapeutic gene [109].
5. Drug-loaded microbubbles: ultrasound-triggered delivery systems
The examples above show the benefits of ultrasound-mediated microbubble destruction with co-administration of a drug or gene, but the effects remain spatially limited to the close proximity of the insonated microbubbles. Time limitations also exist: perturbations in a cell membrane caused by ultrasound-related effects are very short-lived. Therefore, the drug to be delivered should be located in the immediate vicinity of the insonated bubbles. Consequently, the microbubbles themselves have been proposed as carrier vehicles for drugs and genes. A schematic representation of the different drug loading strategies is depicted in Figure 4: microbubbles can have drug molecules incorporated within the thick polymer shell or inside the gas core (or multiple cores) of thick-shelled bubbles. Drug could be attached to the external surface of the thin lipid monolayer bubbles by covalent or noncovalent bonds, or incorporated in liposomes or nanoparticles that are then associated with the bubble surface.
Figure 4.
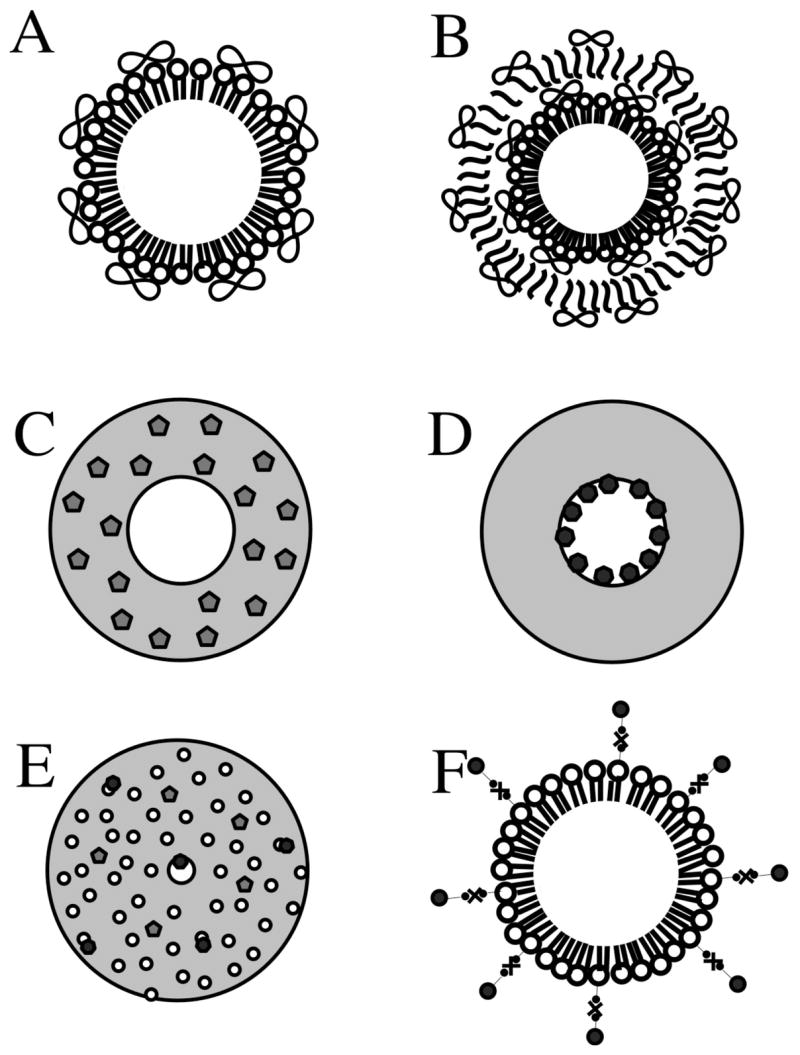
Schematic representation of loading strategies of drugs and genes on microbubbles. (A) Non-covalently binding of DNA to the surface of cationic lipid microbubbles. (B) Multilayered structure based on a lipid microbubble sequentially coated with DNA and poly-L-lysine layers. Polymeric microbubbles with central hollow core surrounded by a thick polymeric shell. (C) Polymeric microbubble loaded with hydrophobic drug loaded in the shell phase. (D) Polymeric microbubble with hydrophilic drug loaded in the internal void. (E) Internal structure of polymeric microbubbles: water-phase is dispersed through the polymer matrix, forming upon lyophilization a plurality of cavities distributed over the particle volume. (F) Attachment of liposomes or nanoparticles to the surface of microbubbles through biotin-avidin-biotin bridging system. The drawing is not to scale.
5.1 Microbubbles as gene delivery vehicles
Lipid microbubbles are currently the most commonly used form of contrast agents in ultrasonography; manufacturing of lipid monolayer-coated bubbles is easy and straightforward. Microbubble shell composition can be easily adjusted by adding a variety of lipids to the emulsion mixture preparation. The positively charged groups of some synthetic lipids make electrostatic interactions possible between the lipids and plasmid DNA, which possesses an overall negative charge. To load DNA on the charged microbubble shell, a plasmid is simply mixed with the lipid microbubbles immediately prior to use. DNA coupled to the surface of the microbubbles remains intact even after insonation. Furthermore, DNA in the electrostatic complex is also protected from enzymatic degradation [110–112]. The adherence of DNA to the microbubbles enhances the transfection efficiency compared to co-administration of bubbles and DNA. A higher local concentration is obtained by releasing the gene from the bubbles by focused ultrasound in the immediate proximity to permeabilized vessels and cells (Figure 5); at the same time, plasmids in non-targeted regions of the body should not be able to penetrate into non-targeted cells and tissues and will be degraded. This results in high specificity with a low transfection in non-insonated regions.
Figure 5.

Intravital microscopy of the cremaster muscle in mice following intravascular injection of cationic microbubbles bearing YOYO-1-labeled plasmid. (A) Cremaster microcirculation 1 min after IV injection of microbubbles illustrating stable conjugation of plasmid to microbubbles during microvascular transit. All microbubbles in the field transited unimpeded. (B) Microvessel rupture and peri-vascular hemorrhage with fluorescent DNA deposition at a site of microbubble destruction. (C,D) Deposition of fluorescent plasmid DNA in the perivascular tissue of cremasteric venules following US-mediated destruction of microbubbles without vessel rupture. Scale bar - 20 μm. (Reproduced with permission from ref. [110], Copyright, 2003, Elsevier).
However, in this configuration the loading capacity of the microbubbles is restricted to their surface area, and therefore, high amounts of microbubbles have to be injected to provide the desired effect. The development of multilayered microbubbles by sequential adsorption of DNA and poly-L-lysine onto the positive microbubble template allows higher DNA content and even compartmentalization by the so-called layer-by-layer (LBL) deposition technique (Figure 4B). An alternative, which also separates the plasmid from the biological fluids until the bubble is insonated and destroyed, was suggested recently: plasmid DNA was incorporated inside the gas core of biodegradable polymer microbubbles [113]. This approach will not only protect the plasmid from host nucleases, but it will also attain high plasmid load per bubble.
A number of studies were performed wherein microbubbles were used to transfect myocardium, vascular endothelium, or skeletal muscles with plasmid DNA or viral transgenes coding for luciferase or antisense oligonucleotides. Although the results show significant increase in the transfection rates, overall efficiencies still remain relatively low when compared to standard adenoviral or lipofection techniques [110, 114–126]. An important advantage of ultrasound-mediated microbubble-assisted transfection is in the ability to achieve DNA delivery and expression only in the insonated areas and not in the non-targeted organs. Even if transfection efficacy is not very high but the encoded protein is produced in the target tissue in an amount sufficient to achieve functional biological response, therapeutic intervention will be successful [125]. One of the methods of improving transfection efficacy is to combine ultrasound-sensitive microbubbles with retroviral particles engineered to be unable to enter cells on their own [126]. This approach was implemented by using the same positively charged liposomes as for plasmid immobilization; a reasonable transfection efficacy was observed with such hybrid particles. Caution should still be recommended because viral particles, even when immobilized on the bubble surface, might cause undesired immune responses after systemic administration.
5.2 Microbubble drug carriers: ultrasound-activated drug release and delivery
Unger et al. demonstrated that it was possible to incorporate hydrophobic drugs in lipid microbubbles by simply adding them to the lipid phase prior to bubble preparation [127]. However, the use of monolayer-based lipid microbubbles is not the most beneficial for drug delivery of most hydrophobic pharmaceutical entities because the amount of drug carried in such a bubble preparation is quite limited. The thin shell (few nanometers) restricts the loading capacity, and it may not be able to prevent leakage of drugs from the bubble during their circulation through non-targeted areas of the body. Therefore, other strategies have to be developed. One of the approaches is to prepare microbubbles with a thick lipid shell containing triglyceride oil phase and a drug dissolved in it [128]; however, this approach is limited to hydrophobic fat-soluble drugs, such as paclitaxel. An alternative that would allow incorporation of water-soluble materials, such as proteins or plasmid DNA, would be to use a solid (polymeric) microbubble shell.
Polymeric microbubbles possess a thick, hard shell, which permits a much higher loading capacity. They are usually manufactured by a double water-in-oil-emulsion technique resulting in a microsphere, the precursor of the microbubble, with an internal water-phase and an external organic phase containing the dissolved polymer (e.g. poly(lactide-co-glycolide) or poly(lactide-co-ethylene glycol)). Polymers of this kind are fully biocompatible, biodegradable, and approved by the FDA for parenteral applications. The organic solvent is removed by evaporation or extraction, while the internal water-phase is eliminated during lyophilization or spray-drying. Ideally, in such a bubble a single hollow core is present, surrounded by a polymeric shell. Most often, though, the thin section samples analyzed with scanning or transmission electron microscopy reveal an internally porous structure with multiple voids. Volatile compounds such as camphor or ammonium bicarbonate can be added to the preparation to help increase the porosity of the particles [129]. These bubbles can be stored for long periods (perhaps for many years) in sealed vials in dry lyophilized state; they are gradually degraded through hydrolysis when resuspended in water. The advantage of polymeric microbubbles is that both hydrophobic and hydrophilic drugs can be incorporated. Depending on its solubility, the drug is added in either the organic or aqueous phase prior to emulsification. Proteins (enzymes, hormones) are encapsulated with high efficiency, and they are still intact after release, with limited loss of activity [130–137]. Encapsulation efficacy is influenced by the process parameters such as the dispersion technique and applied shear, the polymer composition, temperature, the presence and type of surfactants, plasticizer agents and protein stabilizers, the ionic strength etc. The rate of drug release from the polymer microbubbles may also depend on the lipophilicity and water solubility of the drug. After destruction of the microbubble by ultrasound, the weak interactions between a hydrophilic drug and the polymer fragments will be broken easily, and the drug will be liberated rapidly. On the contrary, a hydrophobic drug will be released slowly, and gradually from the polymer fragments of destroyed microbubbles deposited in the tissue.
A novel bubble preparation method described by Böhmer is based on ink-jet printing technology. It enables manufacturing of polymer microbubbles with a narrow size distribution [138].The procedure involves preparation of ink-jetted microparticles with an added slow-evaporating organic solvent core component that is removed by lyophilization. Because there is no aqueous internal core in the particle structure, these microbubbles can only encapsulate hydrophobic drug molecules.
Alternatively, the polymer shell can be filled with a perfluoropentane core, a liquid at room temperature that becomes gaseous at body temperature. Recently, doxorubicin-containing biodegradable block copolymer micelles sonicated in the presence of perfluoropentane were described as a nanobubble formulation that could extravasate and reach tumor interstitial space after intravenous administration. [139]. These particles upon insonation of tumors released the drug from the biodegradable polymer shell, which resulted in tumor regression in an animal model. Nanobubbles in the tumor mass coalesced into larger microbubbles and could be detected by ultrasound imaging. Perfluoropentane liquid nanoparticle emulsion was investigated as Echogen ultrasound contrast agent. Echogen reached extensive clinical trials, but did not gain widespread clinical acceptance and is not currently available. Prior to perfluoropentane Echogen emulsion intravenous administration, hypobaric activation of the formulation was required to form gas microbubbles; otherwise acoustic backscatter efficacy remained low [140]. The production of polymeric microbubbles is considerably simplified using the perfluoropentane phase-shift approach. However, perfluoropentane is not a good solvent even for hydrophobic drug substances. Hence, the drug could not be dissolved in the nanoparticle core. Therefore, this system will limit the load of doxorubicin and similar molecules that could be incorporated in the bubble shell and slowly released from it (Figure 4C).
5.3 Multi-particle assemblies for ultrasound-mediated drug delivery
If one wants to broaden the spectrum of drugs associated with bubbles to include proteins, enzymes, antibodies and hydrophilic substances, want to improve loading capacity, or if the drug can’t survive the harsh conditions of microbubble preparation, one could use multi-particle assemblies. Liposomes or nanoparticles that entrap the drug can be prepared separately and then coupled to the surface of the microbubbles [141–144]. In this case, both lipid- and polymer shelled microbubbles could be used. The simplest way to accomplish this arrangement for preclinical proof of principle studies is via a biotin-avidin-biotin bridging system (Figure 4F), but the covalent attachment of drug carriers to the microbubble surface is also feasible. When the microbubble is destroyed by ultrasound in the target tissue, the energy released in the form of high shear flow, microjets, and microstreaming, will cause rupture of the membrane of microbubble-associated liposomes and subsequently release the encapsulated drug [143–145].
6. The future
The use of microbubbles as a tool for drug delivery enhancement has an enormous clinical potential, especially in oncology and vascular applications. Whereas free drugs often possess harmful side effects, their encapsulation in microbubbles and subsequent local release, deposition, and potentiation in the target tissue by ultrasound triggering will help improve the therapeutic index, lower the incidence of adverse events, and achieve successful therapy. Microbubbles combined with ultrasound offer a possibility to optimize the action of the currently approved drugs and drug delivery systems, such as liposomes, by improving their pharmacokinetics and delivery to the target. Especially promising in this respect is the use of pharmaceutical agents that are harmless, nontoxic, and completely inactive until subjected to ultrasound; for example, the plasmid DNA-microbubble complexes would not transfect the tissues to which ultrasound is not applied. Overall, the set of ideas to be developed in this area is now clear. Future decades will show which of these drug and gene delivery approaches will be most useful in the practical clinical arena.
7. Conclusions
Intravascular microbubbles can improve drug penetration into tissues when combined with focused ultrasound treatment, such as through the blood-brain barrier. Drug substances, including plasmid DNA, can be attached to or incorporated in the microbubble particles for ultrasound-triggered release in the insonated organs and tissues. Co-administration of ultrasound, microbubbles and thrombolytic enzymes results in rapid and minimally invasive clot lysis, target vessel recanalization and positive patient outcome, such as for treatment of stroke.
Overall, ultrasound-assisted drug delivery combined with microbubble contrast agents will aid in the treatment of debilitating diseases.
Acknowledgments
Support from University of Virginia Cardiovascular Division, Cardiovascular Research Center and Cardiovascular Imaging center is appreciated. A. Klibanov’s research is supported in part via NIH 5R01EB002185 and 1R21CA102880. S. Hernot visiting fellowship at University of Virginia is supported by Vrije Universiteit Brussel Laboratory of In Vivo Cellular and Molecular Imaging. Collaboration with Philips Research (C.T. Chin, W. Shi, C. Hall and M. Bohmer) is gratefully acknowledged. Generous donation of laboratory equipment by Mallinckrodt Inc (Hazelwood MO) to A. Klibanov’s laboratory at University of Virginia is appreciated.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Kassan DG, Lynch AM, Stiller MJ. Physical enhancement of dermatologic drug delivery: Iontophoresis and phonophoresis. J Am Acad Dermatol. 1996;34:657–66. doi: 10.1016/s0190-9622(96)80069-7. [DOI] [PubMed] [Google Scholar]
- 2.Gramiak R, Shah PM. Echocardiography of the aortic root. Invest Radiol. 1968;3:356–66. doi: 10.1097/00004424-196809000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Feinstein SB, Ten Cate FJ, Zwehl W, Ong K, Maurer G, Tei C, Shah PM, Meerbaum S, Corday E. Two-dimensional contrast echocardiography. I. In vitro development and quantitative analysis of echo contrast agents. J Am Coll Cardiol. 1984;3:14–20. doi: 10.1016/s0735-1097(84)80424-6. [DOI] [PubMed] [Google Scholar]
- 4.Ten Cate FJ, Feinstein S, Zwehl W, Meerbaum S, Fishbein M, Shah PM, Corday E. Two-dimensional contrast echocardiography. Ii. Transpulmonary studies. J Am Coll Cardiol. 1984;3:21–7. doi: 10.1016/s0735-1097(84)80425-8. [DOI] [PubMed] [Google Scholar]
- 5.Kabalnov A, Klein D, Pelura T, Schutt E, Weers J. Dissolution of multicomponent microbubbles in the bloodstream: 1. Theory. Ultrasound Med Biol. 1998;24:739–49. doi: 10.1016/s0301-5629(98)00034-9. [DOI] [PubMed] [Google Scholar]
- 6.Lee S, Kim DH, Needham D. Equilibrium and dynamic interfacial tension measurements at microscopic interfaces using a micropipet technique. 2. Dynamics of phospholipid monolayer formation and equilibrium tensions at the water-air interface. Langmuir. 2001;17:5544–5550. [Google Scholar]
- 7.Lindner JR, Wei K. Contrast echocardiography. Curr Probl Cardiol. 2002;27:454–519. doi: 10.1067/mcd.2002.129364. [DOI] [PubMed] [Google Scholar]
- 8.de Jong N, Hoff L, Skotland T, Bom N. Absorption and scatter of encapsulated gas filled microspheres: Theoretical considerations and some measurements. Ultrasonics. 1992;30:95–103. doi: 10.1016/0041-624x(92)90041-j. [DOI] [PubMed] [Google Scholar]
- 9.de Jong N, Bouakaz A, Frinking P. Basic acoustic properties of microbubbles. Echocardiography. 2002;19:229–40. doi: 10.1046/j.1540-8175.2002.00229.x. [DOI] [PubMed] [Google Scholar]
- 10.Oeffinger BE, Wheatley MA. Development and characterization of a nano-scale contrast agent. Ultrasonics. 2004;42:343–7. doi: 10.1016/j.ultras.2003.11.011. [DOI] [PubMed] [Google Scholar]
- 11.Patel D, Dayton P, Gut J, Wisner E, Ferrara KW. Optical and acoustical interrogation of submicron contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 2002;49:1641–51. doi: 10.1109/tuffc.2002.1159844. [DOI] [PubMed] [Google Scholar]
- 12.Cohen JL, Cheirif J, Segar DS, Gillam LD, Gottdiener JS, Hausnerova E, Bruns DE. Improved left ventricular endocardial border delineation and opacification with optison (fs069), a new echocardiographic contrast agent. Results of a phase iii multicenter trial. J Am Coll Cardiol. 1998;32:746–52. doi: 10.1016/s0735-1097(98)00311-8. [DOI] [PubMed] [Google Scholar]
- 13.Chomas JE, Dayton P, Allen J, Morgan K, Ferrara KW. Mechanisms of contrast agent destruction. IEEE Trans Ultrason Ferroelectr Freq Control. 2001;48:232–48. doi: 10.1109/58.896136. [DOI] [PubMed] [Google Scholar]
- 14.Chomas JE, Dayton PA, May D, Allen J, Klibanov A, Ferrara K. Optical observation of contrast agent destruction. Applied Physics Letters. 2000;77:1056–1058. [Google Scholar]
- 15.Wei K, Skyba DM, Firschke C, Jayaweera AR, Lindner JR, Kaul S. Interactions between microbubbles and ultrasound: In vitro and in vivo observations. J Am Coll Cardiol. 1997;29:1081–8. doi: 10.1016/s0735-1097(97)00029-6. [DOI] [PubMed] [Google Scholar]
- 16.Jayaweera AR, Kaul S. Quantifying myocardial blood flow with contrast echocardiography. Am J Card Imaging. 1993;7:317–35. [PubMed] [Google Scholar]
- 17.Jayaweera AR, Edwards N, Glasheen WP, Villanueva FS, Abbott RD, Kaul S. In vivo myocardial kinetics of air-filled albumin microbubbles during myocardial contrast echocardiography. Comparison with radiolabeled red blood cells. Circ Res. 1994;74:1157–65. doi: 10.1161/01.res.74.6.1157. [DOI] [PubMed] [Google Scholar]
- 18.Keller MW, Segal SS, Kaul S, Duling B. The behavior of sonicated albumin microbubbles within the microcirculation: A basis for their use during myocardial contrast echocardiography. Circ Res. 1989;65:458–67. doi: 10.1161/01.res.65.2.458. [DOI] [PubMed] [Google Scholar]
- 19.Wei K, Le E, Bin JP, Coggins M, Thorpe J, Kaul S. Quantification of renal blood flow with contrast-enhanced ultrasound. J Am Coll Cardiol. 2001;37:1135–40. doi: 10.1016/s0735-1097(00)01210-9. [DOI] [PubMed] [Google Scholar]
- 20.Rim SJ, Leong-Poi H, Lindner JR, Couture D, Ellegala D, Mason H, Durieux M, Kassel NF, Kaul S. Quantification of cerebral perfusion with “Real-time” Contrast-enhanced ultrasound. Circulation. 2001;104:2582–7. doi: 10.1161/hc4601.099400. [DOI] [PubMed] [Google Scholar]
- 21.Christiansen JP, Leong-Poi H, Amiss LR, Drake DB, Kaul S, Lindner JR. Skin perfusion assessed by contrast ultrasound predicts tissue survival in a free flap model. Ultrasound Med Biol. 2002;28:315–20. doi: 10.1016/s0301-5629(01)00523-3. [DOI] [PubMed] [Google Scholar]
- 22.Dawson D, Vincent MA, Barrett EJ, Kaul S, Clark A, Leong-Poi H, Lindner JR. Vascular recruitment in skeletal muscle during exercise and hyperinsulinemia assessed by contrast ultrasound. Am J Physiol Endocrinol Metab. 2002;282:E714–20. doi: 10.1152/ajpendo.00373.2001. [DOI] [PubMed] [Google Scholar]
- 23.Ferrara KW, Merritt CR, Burns PN, Foster FS, Mattrey RF, Wickline SA. Evaluation of tumor angiogenesis with us: Imaging, doppler, and contrast agents. Acad Radiol. 2000;7:824–39. doi: 10.1016/s1076-6332(00)80631-5. [DOI] [PubMed] [Google Scholar]
- 24.Bloch SH, Dayton PA, Wan M, Ferrara KW. Optical observation of lipid- and polymer-shelled ultrasound microbubble contrast agents. Appl Phys Lett. 2004;84:631–633. [Google Scholar]
- 25.Van de Wiele C, Revets H, Mertens N. Radioimmunoimaging. Advances and prospects. Q J Nucl Med Mol Imaging. 2004;48:317–25. [PubMed] [Google Scholar]
- 26.Villanueva FS, Jankowski RJ, Klibanov S, Pina ML, Alber SM, Watkins SC, Brandenburger GH, Wagner WR. Microbubbles targeted to intercellular adhesion molecule-1 bind to activated coronary artery endothelial cells. Circulation. 1998;98:1–5. doi: 10.1161/01.cir.98.1.1. [DOI] [PubMed] [Google Scholar]
- 27.Weller GE, Lu E, Csikari MM, Klibanov AL, Fischer D, Wagner WR, Villanueva FS. Ultrasound imaging of acute cardiac transplant rejection with microbubbles targeted to intercellular adhesion molecule-1. Circulation. 2003;108:218–24. doi: 10.1161/01.CIR.0000080287.74762.60. [DOI] [PubMed] [Google Scholar]
- 28.Ellegala DB, Leong-Poi H, Carpenter JE, Klibanov AL, Kaul S, Shaffrey ME, Sklenar J, Lindner JR. Imaging tumor angiogenesis with contrast ultrasound and microbubbles targeted to alpha(v)beta3. Circulation. 2003;108:336–41. doi: 10.1161/01.CIR.0000080326.15367.0C. [DOI] [PubMed] [Google Scholar]
- 29.Leong-Poi H, Christiansen J, Heppner P, Lewis CW, Klibanov AL, Kaul S, Lindner JR. Assessment of endogenous and therapeutic arteriogenesis by contrast ultrasound molecular imaging of integrin expression. Circulation. 2005;111:3248–54. doi: 10.1161/CIRCULATIONAHA.104.481515. [DOI] [PubMed] [Google Scholar]
- 30.Weller GE, Wong MK, Modzelewski RA, Lu E, Klibanov AL, Wagner WR, Villanueva FS. Ultrasonic imaging of tumor angiogenesis using contrast microbubbles targeted via the tumor-binding peptide arginine-arginine-leucine. Cancer Res. 2005;65:533–9. [PubMed] [Google Scholar]
- 31.Wheatley MA, Lathia JD, Oum KL. Polymeric ultrasound contrast agents targeted to integrins: Importance of process methods and surface density of ligands. Biomacromolecules. 2007;8:516–22. doi: 10.1021/bm060659i. [DOI] [PubMed] [Google Scholar]
- 32.Hauff P, Reinhardt M, Briel A, Debus N, Schirner M. Molecular targeting of lymph nodes with l-selectin ligand-specific us contrast agent: A feasibility study in mice and dogs. Radiology. 2004;231:667–73. doi: 10.1148/radiol.2313030425. [DOI] [PubMed] [Google Scholar]
- 33.Schumann PA, Christiansen JP, Quigley RM, McCreery TP, Sweitzer RH, Unger EC, Lindner JR, Matsunaga TO. Targeted-microbubble binding selectively to gpiib iiia receptors of platelet thrombi. Invest Radiol. 2002;37:587–93. doi: 10.1097/00004424-200211000-00001. [DOI] [PubMed] [Google Scholar]
- 34.Weller GE, Villanueva FS, Tom EM, Wagner WR. Targeted ultrasound contrast agents: In vitro assessment of endothelial dysfunction and multi-targeting to icam-1 and sialyl lewisx. Biotechnol Bioeng. 2005;92:780–8. doi: 10.1002/bit.20625. [DOI] [PubMed] [Google Scholar]
- 35.Kim DH, Klibanov AL, Needham D. The influence of tiered layers of surface-grafted poly(ethylene glycol) on receptor-ligand-mediated adhesion between phospholipid monolayer-stabilized microbubbles and coated glass beads. Langmuir. 2000;16:2808–2817. [Google Scholar]
- 36.Bloemen PG, Henricks PA, van Bloois L, van den Tweel MC, Bloem AC, Nijkamp FP, Crommelin DJ, Storm G. Adhesion molecules: A new target for immunoliposome-mediated drug delivery. FEBS Lett. 1995;357:140–4. doi: 10.1016/0014-5793(94)01350-a. [DOI] [PubMed] [Google Scholar]
- 37.Jeppesen C, Wong JY, Kuhl TL, Israelachvili JN, Mullah N, Zalipsky S, Marques CM. Impact of polymer tether length on multiple ligand-receptor bond formation. Science. 2001;293:465–8. doi: 10.1126/science.293.5529.465. [DOI] [PubMed] [Google Scholar]
- 38.Weller GE, Villanueva FS, Klibanov AL, Wagner WR. Modulating targeted adhesion of an ultrasound contrast agent to dysfunctional endothelium. Ann Biomed Eng. 2002;30:1012–9. doi: 10.1114/1.1513565. [DOI] [PubMed] [Google Scholar]
- 39.Borden MA, Sarantos MR, Stieger SM, Simon SI, Ferrara KW, Dayton PA. Ultrasound radiation force modulates ligand availability on targeted contrast agents. Mol Imaging. 2006;5:139–47. [PMC free article] [PubMed] [Google Scholar]
- 40.Rychak JJ, Klibanov AL, Hossack JA. Acoustic radiation force enhances tareted delivery of ultrasound contrast microbubbles : In vitro verification. IEEE Trans Ultrason Ferroelectr Freq Control. 2005;52:421–433. doi: 10.1109/tuffc.2005.1417264. [DOI] [PubMed] [Google Scholar]
- 41.Dayton PA, Allen JS, Ferrara KW. The magnitude of radiation force on ultrasound contrast agents. J Acoust Soc Am. 2002;112:2183–92. doi: 10.1121/1.1509428. [DOI] [PubMed] [Google Scholar]
- 42.Dayton PA, Morgan KE, Klibanov ALS, Brandenburger G, Nightingale KR, Ferrara KW. A preliminary evaluation of the effects of primary and secondary radiation forces on acoustic contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 1997;44:1264–1277. [Google Scholar]
- 43.Zhao S, Borden M, Bloch SH, Kruse D, Ferrara KW, Dayton PA. Radiation-force assisted targeting facilitates ultrasonic molecular imaging. Mol Imaging. 2004;3:135–48. doi: 10.1162/1535350042380317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dayton P, Klibanov A, Brandenburger G, Ferrara K. Acoustic radiation force in vivo: A mechanism to assist targeting of microbubbles. Ultrasound Med Biol. 1999;25:1195–201. doi: 10.1016/s0301-5629(99)00062-9. [DOI] [PubMed] [Google Scholar]
- 45.Dayton PA, Zhao S, Bloch SH, Schumann P, Penrose K, Matsunaga TO, Zutshi R, Doinikov A, Ferrara KW. Application of ultrasound to selectively localize nanodroplets for targeted imaging and therapy. Mol Imaging. 2006;5:160–74. [PMC free article] [PubMed] [Google Scholar]
- 46.Li P, Cao LQ, Dou CY, Armstrong WF, Miller D. Impact of myocardial contrast echocardiography on vascular permeability: An in vivo dose response study of delivery mode, pressure amplitude and contrast dose. Ultrasound Med Biol. 2003;29:1341–9. doi: 10.1016/s0301-5629(03)00988-8. [DOI] [PubMed] [Google Scholar]
- 47.Skyba DM, Price RJ, Linka AZ, Skalak TC, Kaul S. Direct in vivo visualization of intravascular destruction of microbubbles by ultrasound and its local effects on tissue. Circulation. 1998;98:290–3. doi: 10.1161/01.cir.98.4.290. [DOI] [PubMed] [Google Scholar]
- 48.Miller DL, Quddus J. Diagnostic ultrasound activation of contrast agent gas bodies induces capillary rupture in mice. Proc Natl Acad Sci U S A. 2000;97:10179–84. doi: 10.1073/pnas.180294397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ay T, Havaux X, Van Camp G, Campanelli B, Gisellu G, Pasquet A, Denef JF, Melin JA, Vanoverschelde JL. Destruction of contrast microbubbles by ultrasound: Effects on myocardial function, coronary perfusion pressure, and microvascular integrity. Circulation. 2001;104:461–6. doi: 10.1161/hc3001.092038. [DOI] [PubMed] [Google Scholar]
- 50.Miller DL, Gies RA. Gas-body-based contrast agent enhances vascular bioeffects of 1.09 mhz ultrasound on mouse intestine. Ultrasound Med Biol. 1998;24:1201–8. doi: 10.1016/s0301-5629(98)00063-5. [DOI] [PubMed] [Google Scholar]
- 51.Li P, Armstrong WF, Miller DL. Impact of myocardial contrast echocardiography on vascular permeability: Comparison of three different contrast agents. Ultrasound Med Biol. 2004;30:83–91. doi: 10.1016/j.ultrasmedbio.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Wible JH, Jr, Galen KP, Wojdyla JK, Hughes MS, Klibanov AL, Brandenburger GH. Microbubbles induce renal hemorrhage when exposed to diagnostic ultrasound in anesthetized rats. Ultrasound Med Biol. 2002;28:1535–46. doi: 10.1016/s0301-5629(02)00651-8. [DOI] [PubMed] [Google Scholar]
- 53.Miller DL, Li P, Gordon D, Armstrong WF. Histological characterization of microlesions induced by myocardial contrast echocardiography. Echocardiography. 2005;22:25–34. doi: 10.1111/j.0742-2822.2005.03184.x. [DOI] [PubMed] [Google Scholar]
- 54.Miller DL, Li P, Dou C, Gordon D, Edwards CA, Armstrong WF. Influence of contrast agent dose and ultrasound exposure on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Radiology. 2005;237:137–43. doi: 10.1148/radiol.2371041467. [DOI] [PubMed] [Google Scholar]
- 55.Miller DL, Dou C, Armstrong WF. The influence of agent delivery mode on cardiomyocyte injury induced by myocardial contrast echocardiography in rats. Ultrasound Med Biol. 2005;31:1257–63. doi: 10.1016/j.ultrasmedbio.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 56.Miller DL, Driscoll EM, Dou C, Armstrong WF, Lucchesi BR. Microvascular permeabilization and cardiomyocyte injury provoked by myocardial contrast echocardiography in a canine model. J Am Coll Cardiol. 2006;47:1464–8. doi: 10.1016/j.jacc.2005.09.078. [DOI] [PubMed] [Google Scholar]
- 57.van Der Wouw PA, Brauns AC, Bailey SE, Powers JE, Wilde AA. Premature ventricular contractions during triggered imaging with ultrasound contrast. J Am Soc Echocardiogr. 2000;13:288–94. doi: 10.1067/mje.2000.103865. [DOI] [PubMed] [Google Scholar]
- 58.Zachary JF, Hartleben SA, Frizzell LA, O’Brien WD., Jr Arrhythmias in rat hearts exposed to pulsed ultrasound after intravenous injection of a contrast agent. J Ultrasound Med. 2002;21:1347–56. doi: 10.7863/jum.2002.21.12.1347. discussion 1343–45. [DOI] [PubMed] [Google Scholar]
- 59.Vancraeynest D, Havaux X, Pouleur AC, Pasquet A, Gerber B, Beauloye C, Rafter P, Bertrand L, Vanoverschelde JL. Myocardial delivery of colloid nanoparticles using ultrasound-targeted microbubble destruction. Eur Heart J. 2006;27:237–45. doi: 10.1093/eurheartj/ehi479. [DOI] [PubMed] [Google Scholar]
- 60.Miller DL. Overview of experimental studies of biological effects of medical ultrasound caused by gas body activation and inertial cavitation. Prog Biophys Mol Biol. 2007;93:314–30. doi: 10.1016/j.pbiomolbio.2006.07.027. [DOI] [PubMed] [Google Scholar]
- 61.Trubestein G, Engel C, Etzel F, Sobbe A, Cremer H, Stumpff U. Thrombolysis by ultrasound. Clin Sci Mol Med Suppl. 1976;3:697s–698s. doi: 10.1042/cs051697s. [DOI] [PubMed] [Google Scholar]
- 62.Trubestein G. [removal of intravascular thrombi by means of ultrasonics. Experimental study with a new method and its clinical use] Fortschr Med. 1978;96:755–60. [PubMed] [Google Scholar]
- 63.Tachibana K, Tachibana S. Albumin microbubble echo-contrast material as an enhancer for ultrasound accelerated thrombolysis. Circulation. 1995;92:1148–50. doi: 10.1161/01.cir.92.5.1148. [DOI] [PubMed] [Google Scholar]
- 64.Porter TR, LeVeen RF, Fox R, Kricsfeld A, Xie F. Thrombolytic enhancement with perfluorocarbon-exposed sonicated dextrose albumin microbubbles. Am Heart J. 1996;132:964–8. doi: 10.1016/s0002-8703(96)90006-x. [DOI] [PubMed] [Google Scholar]
- 65.Molina CA, Ribo M, Rubiera M, Montaner J, Santamarina E, Delgado-Mederos R, Arenillas JF, Huertas R, Purroy F, Delgado P, Alvarez-Sabin J. Microbubble administration accelerates clot lysis during continuous 2-mhz ultrasound monitoring in stroke patients treated with intravenous tissue plasminogen activator. Stroke. 2006;37:425–9. doi: 10.1161/01.STR.0000199064.94588.39. [DOI] [PubMed] [Google Scholar]
- 66.Birnbaum Y, Iakobishvili Z, Porter A, Hasdai D, Atar S, Siegel RJ, Battler A. Microparticle-containing oncotic solutions augment in-vitro clot disruption by ultrasound. Thromb Res. 2000;98:549–57. doi: 10.1016/s0049-3848(00)00214-0. [DOI] [PubMed] [Google Scholar]
- 67.Culp WC, Porter TR, McCowan TC, Roberson PK, James CA, Matchett WJ, Moursi M. Microbubble-augmented ultrasound declotting of thrombosed arteriovenous dialysis grafts in dogs. J Vasc Interv Radiol. 2003;14:343–347. doi: 10.1097/01.rvi.0000058409.01661.b4. [DOI] [PubMed] [Google Scholar]
- 68.Pagola J, Ribo M, Alvarez-Sabin J, Lange M, Rubiera M, Molina CA. Timing of recanalization after microbubble-enhanced intravenous thrombolysis in basilar artery occlusion. Stroke. 2007;38:2931–4. doi: 10.1161/STROKEAHA.107.487454. [DOI] [PubMed] [Google Scholar]
- 69.Nesser HJ, Karia DH, Tkalec W, Pandian NG. Therapeutic ultrasound in cardiology. Herz. 2002;27:269–78. doi: 10.1007/s00059-002-2362-y. [DOI] [PubMed] [Google Scholar]
- 70.Song J, Cottler PS, Klibanov AL, Kaul S, Price RJ. Microvascular remodeling and accelerated hyperemia blood flow restoration in arterially occluded skeletal muscle exposed to ultrasonic microbubble destruction. Am J Physiol Heart Circ Physiol. 2004;287:H2754–61. doi: 10.1152/ajpheart.00144.2004. [DOI] [PubMed] [Google Scholar]
- 71.Price RJ, Skyba DM, Kaul S, Skalak TC. Delivery of colloidal particles and red blood cells to tissue through microvessel ruptures created by targeted microbubble destruction with ultrasound. Circulation. 1998;98:1264–7. doi: 10.1161/01.cir.98.13.1264. [DOI] [PubMed] [Google Scholar]
- 72.Song J, Chappell JC, Qi M, VanGieson EJ, Kaul S, Price RJ. Influence of injection site, microvascular pressure and ultrasound variables on microbubble-mediated delivery of microspheres to muscle. J Am Coll Cardiol. 2002;39:726–31. doi: 10.1016/s0735-1097(01)01793-4. [DOI] [PubMed] [Google Scholar]
- 73.Yoshida J, Ohmori K, Takeuchi H, Shinomiya K, Namba T, Kondo I, Kiyomoto H, Kohno M. Treatment of ischemic limbs based on local recruitment of vascular endothelial growth factor-producing inflammatory cells with ultrasonic microbubble destruction. J Am Coll Cardiol. 2005;46:899–905. doi: 10.1016/j.jacc.2005.05.052. [DOI] [PubMed] [Google Scholar]
- 74.Song J, Qi M, Kaul S, Price RJ. Stimulation of arteriogenesis in skeletal muscle by microbubble destruction with ultrasound. Circulation. 2002;106:1550–5. doi: 10.1161/01.cir.0000028810.33423.95. [DOI] [PubMed] [Google Scholar]
- 75.Imada T, Tatsumi T, Mori Y, Nishiue T, Yoshida M, Masaki H, Okigaki M, Kojima H, Nozawa Y, Nishiwaki Y, Nitta N, Iwasaka T, Matsubara H. Targeted delivery of bone marrow mononuclear cells by ultrasound destruction of microbubbles induces both angiogenesis and arteriogenesis response. Arterioscler Thromb Vasc Biol. 2005;25:2128–34. doi: 10.1161/01.ATV.0000179768.06206.cb. [DOI] [PubMed] [Google Scholar]
- 76.Zen K, Okigaki M, Hosokawa Y, Adachi Y, Nozawa Y, Takamiya M, Tatsumi T, Urao N, Tateishi K, Takahashi T, Matsubara H. Myocardium-targeted delivery of endothelial progenitor cells by ultrasound-mediated microbubble destruction improves cardiac function via an angiogenic response. J Mol Cell Cardiol. 2006;40:799–809. doi: 10.1016/j.yjmcc.2006.03.012. [DOI] [PubMed] [Google Scholar]
- 77.Miyake Y, Ohmori K, Yoshida J, Ishizawa M, Mizukawa M, Yukiiri K, Kohno M. Granulocyte colony-stimulating factor facilitates the angiogenesis induced by ultrasonic microbubble destruction. Ultrasound Med Biol. 2007 doi: 10.1016/j.ultrasmedbio.2007.05.017. [DOI] [PubMed] [Google Scholar]
- 78.McDannold N, Vykhodtseva N, Raymond S, Jolesz FA, Hynynen K. Mri-guided targeted blood-brain barrier disruption with focused ultrasound: Histological findings in rabbits. Ultrasound Med Biol. 2005;31:1527–37. doi: 10.1016/j.ultrasmedbio.2005.07.010. [DOI] [PubMed] [Google Scholar]
- 79.Treat LH, McDannold N, Vykhodtseva N, Zhang Y, Tam K, Hynynen K. Targeted delivery of doxorubicin to the rat brain at therapeutic levels using mri-guided focused ultrasound. Int J Cancer. 2007;121:901–7. doi: 10.1002/ijc.22732. [DOI] [PubMed] [Google Scholar]
- 80.Hynynen K. Focused ultrasound for blood-brain disruption and delivery of therapeutic molecules into the brain. Expert Opin Drug Deliv. 2007;4:27–35. doi: 10.1517/17425247.4.1.27. [DOI] [PubMed] [Google Scholar]
- 81.Schlachetzki F, Holscher T, Koch HJ, Draganski B, May A, Schuierer G, Bogdahn U. Observation on the integrity of the blood-brain barrier after microbubble destruction by diagnostic transcranial color-coded sonography. J Ultrasound Med. 2002;21:419–29. doi: 10.7863/jum.2002.21.4.419. [DOI] [PubMed] [Google Scholar]
- 82.Lawrie A, Brisken AF, Francis SE, Tayler DI, Chamberlain J, Crossman DC, Cumberland DC, Newman CM. Ultrasound enhances reporter gene expression after transfection of vascular cells in vitro. Circulation. 1999;99:2617–20. doi: 10.1161/01.cir.99.20.2617. [DOI] [PubMed] [Google Scholar]
- 83.Ward M, Wu J, Chiu JF. Ultrasound-induced cell lysis and sonoporation enhanced by contrast agents. J Acoust Soc Am. 1999;105:2951–7. doi: 10.1121/1.426908. [DOI] [PubMed] [Google Scholar]
- 84.Wu J, Ross JP, Chiu JF. Reparable sonoporation generated by microstreaming. J Acoust Soc Am. 2002;111:1460–4. doi: 10.1121/1.1420389. [DOI] [PubMed] [Google Scholar]
- 85.Tachibana K, Uchida T, Ogawa K, Yamashita N, Tamura K. Induction of cell-membrane porosity by ultrasound. Lancet. 1999;353:1409. doi: 10.1016/S0140-6736(99)01244-1. [DOI] [PubMed] [Google Scholar]
- 86.Ogawa K, Tachibana K, Uchida T, Tai T, Yamashita N, Tsujita N, Miyauchi R. High-resolution scanning electron microscopic evaluation of cell-membrane porosity by ultrasound. Med Electron Microsc. 2001;34:249–53. doi: 10.1007/s007950100022. [DOI] [PubMed] [Google Scholar]
- 87.van Wamel A, Bouakaz A, Versluis M, de Jong N. Micromanipulation of endothelial cells: Ultrasound-microbubble-cell interaction. Ultrasound Med Biol. 2004;30:1255–8. doi: 10.1016/j.ultrasmedbio.2004.07.015. [DOI] [PubMed] [Google Scholar]
- 88.Basta G, Venneri L, Lazzerini G, Pasanisi E, Pianelli M, Vesentini N, Del Turco S, Kusmic C, Picano E. In vitro modulation of intracellular oxidative stress of endothelial cells by diagnostic cardiac ultrasound. Cardiovasc Res. 2003;58:156–61. doi: 10.1016/s0008-6363(02)00665-x. [DOI] [PubMed] [Google Scholar]
- 89.Juffermans LJ, Dijkmans PA, Musters RJ, Visser CA, Kamp O. Transient permeabilization of cell membranes by ultrasound-exposed microbubbles is related to formation of hydrogen peroxide. Am J Physiol Heart Circ Physiol. 2006;291:H1595–601. doi: 10.1152/ajpheart.01120.2005. [DOI] [PubMed] [Google Scholar]
- 90.Kondo T, Misik V, Riesz P. Effect of gas-containing microspheres and echo contrast agents on free radical formation by ultrasound. Free Radic Biol Med. 1998;25:605–12. doi: 10.1016/s0891-5849(98)00106-3. [DOI] [PubMed] [Google Scholar]
- 91.Miller DL, Gies RA. The interaction of ultrasonic heating and cavitation in vascular bioeffects on mouse intestine. Ultrasound Med Biol. 1998;24:123–8. doi: 10.1016/s0301-5629(97)00209-3. [DOI] [PubMed] [Google Scholar]
- 92.van Wamel A, Kooiman K, Emmer M, ten Cate FJ, Versluis M, de Jong N. Ultrasound microbubble induced endothelial cell permeability. J Control Release. 2006;116:e100–2. doi: 10.1016/j.jconrel.2006.09.071. [DOI] [PubMed] [Google Scholar]
- 93.Keyhani K, Guzman HR, Parsons A, Lewis TN, Prausnitz MR. Intracellular drug delivery using low-frequency ultrasound: Quantification of molecular uptake and cell viability. Pharm Res. 2001;18:1514–20. doi: 10.1023/a:1013066027759. [DOI] [PubMed] [Google Scholar]
- 94.Nie F, Xu HX, Tang Q, Lu MD. Microbubble-enhanced ultrasound exposure improves gene transfer in vascular endothelial cells. World J Gastroenterol. 2006;12:7508–13. doi: 10.3748/wjg.v12.i46.7508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Hallow DM, Mahajan AD, Prausnitz MR. Ultrasonically targeted delivery into endothelial and smooth muscle cells in ex vivo arteries. J Control Release. 2007;118:285–93. doi: 10.1016/j.jconrel.2006.12.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sonoda S, Tachibana K, Uchino E, Yamashita T, Sakoda K, Sonoda KH, Hisatomi T, Izumi Y, Sakamoto T. Inhibition of melanoma by ultrasound-microbubble-aided drug delivery suggests membrane permeabilization. Cancer Biol Ther. 2007;6 doi: 10.4161/cbt.6.8.4485. [DOI] [PubMed] [Google Scholar]
- 97.Kipshidze NN, Porter TR, Dangas G, Yazdi H, Tio F, Xie F, Hellinga D, Wolfram R, Seabron R, Waksman R, Abizaid A, Roubin G, Iyer S, Colombo A, Leon MB, Moses JW, Iversen P. Novel site-specific systemic delivery of rapamycin with perfluorobutane gas microbubble carrier reduced neointimal formation in a porcine coronary restenosis model. Catheter Cardiovasc Interv. 2005;64:389–94. doi: 10.1002/ccd.20285. [DOI] [PubMed] [Google Scholar]
- 98.van Wamel A, Bouakaz A, Bernard B, ten Cate F, de Jong N. Radionuclide tumour therapy with ultrasound contrast microbubbles. Ultrasonics. 2004;42:903–6. doi: 10.1016/j.ultras.2003.11.013. [DOI] [PubMed] [Google Scholar]
- 99.Suda T, Liu D. Hydrodynamic gene delivery: Its principles and applications. Mol Ther. 2007 doi: 10.1038/sj.mt.6300314. [DOI] [PubMed] [Google Scholar]
- 100.Tsurumi Y, Takeshita S, Chen D, Kearney M, Rossow ST, Passeri J, Horowitz JR, Symes JF, Isner JM. Direct intramuscular gene transfer of naked DNA encoding vascular endothelial growth factor augments collateral development and tissue perfusion. Circulation. 1996;94:3281–90. doi: 10.1161/01.cir.94.12.3281. [DOI] [PubMed] [Google Scholar]
- 101.Endoh M, Koibuchi N, Sato M, Morishita R, Kanzaki T, Murata Y, Kaneda Y. Fetal gene transfer by intrauterine injection with microbubble-enhanced ultrasound. Mol Ther. 2002;5:501–8. doi: 10.1006/mthe.2002.0577. [DOI] [PubMed] [Google Scholar]
- 102.Taniyama Y, Tachibana K, Hiraoka K, Aoki M, Yamamoto S, Matsumoto K, Nakamura T, Ogihara T, Kaneda Y, Morishita R. Development of safe and efficient novel nonviral gene transfer using ultrasound: Enhancement of transfection efficiency of naked plasmid DNA in skeletal muscle. Gene Ther. 2002;9:372–80. doi: 10.1038/sj.gt.3301678. [DOI] [PubMed] [Google Scholar]
- 103.Lawrie A, Brisken AF, Francis SE, Cumberland DC, Crossman DC, Newman CM. Microbubble-enhanced ultrasound for vascular gene delivery. Gene Ther. 2000;7:2023–7. doi: 10.1038/sj.gt.3301339. [DOI] [PubMed] [Google Scholar]
- 104.Ohta S, Suzuki K, Tachibana K, Yamada G. Microbubble-enhanced sonoporation: Efficient gene transduction technique for chick embryos. Genesis. 2003;37:91–101. doi: 10.1002/gene.10232. [DOI] [PubMed] [Google Scholar]
- 105.Taniyama Y, Tachibana K, Hiraoka K, Namba T, Yamasaki K, Hashiya N, Aoki M, Ogihara T, Yasufumi K, Morishita R. Local delivery of plasmid DNA into rat carotid artery using ultrasound. Circulation. 2002;105:1233–9. doi: 10.1161/hc1002.105228. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi M, Kido K, Aoi A, Furukawa H, Ono M, Kodama T. Spinal gene transfer using ultrasound and microbubbles. J Control Release. 2006 doi: 10.1016/j.jconrel.2006.10.026. [DOI] [PubMed] [Google Scholar]
- 107.Nishida K, Doita M, Takada T, Kakutani K, Miyamoto H, Shimomura T, Maeno K, Kurosaka M. Sustained transgene expression in intervertebral disc cells in vivo mediated by microbubble-enhanced ultrasound gene therapy. Spine. 2006;31:1415–9. doi: 10.1097/01.brs.0000219945.70675.dd. [DOI] [PubMed] [Google Scholar]
- 108.Guo DP, Li XY, Sun P, Tang YB, Chen XY, Chen Q, Fan LM, Zang B, Shao LZ, Li XR. Ultrasound-targeted microbubble destruction improves the low density lipoprotein receptor gene expression in hepg2 cells. Biochem Biophys Res Commun. 2006;343:470–4. doi: 10.1016/j.bbrc.2006.02.179. [DOI] [PubMed] [Google Scholar]
- 109.Kodama T, Tan PH, Offiah I, Partridge T, Cook T, George AJ, Blomley MJ. Delivery of oligodeoxynucleotides into human saphenous veins and the adjunct effect of ultrasound and microbubbles. Ultrasound Med Biol. 2005;31:1683–91. doi: 10.1016/j.ultrasmedbio.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 110.Christiansen JP, French BA, Klibanov AL, Kaul S, Lindner JR. Targeted tissue transfection with ultrasound destruction of plasmid-bearing cationic microbubbles. Ultrasound Med Biol. 2003;29:1759–67. doi: 10.1016/s0301-5629(03)00976-1. [DOI] [PubMed] [Google Scholar]
- 111.Lentacker I, De Geest BG, Vandenbroucke RE, Peeters L, Demeester J, De Smedt SC, Sanders NN. Ultrasound-responsive polymer-coated microbubbles that bind and protect DNA. Langmuir. 2006;22:7273–8. doi: 10.1021/la0603828. [DOI] [PubMed] [Google Scholar]
- 112.Lentacker I, De Smedt SC, Demeester J, Sanders NN. Microbubbles which bind and protect DNA against nucleases. J Control Release. 2006;116:e73–5. doi: 10.1016/j.jconrel.2006.09.057. [DOI] [PubMed] [Google Scholar]
- 113.Hauff P, Seemann S, Reszka R, Schultze-Mosgau M, Reinhardt M, Buzasi T, Plath T, Rosewicz S, Schirner M. Evaluation of gas-filled microparticles and sonoporation as gene delivery system: Feasibility study in rodent tumor models. Radiology. 2005;236:572–8. doi: 10.1148/radiol.2362040870. [DOI] [PubMed] [Google Scholar]
- 114.Teupe C, Richter S, Fisslthaler B, Randriamboavonjy V, Ihling C, Fleming I, Busse R, Zeiher AM, Dimmeler S. Vascular gene transfer of phosphomimetic endothelial nitric oxide synthase (s1177d) using ultrasound-enhanced destruction of plasmid-loaded microbubbles improves vasoreactivity. Circulation. 2002;105:1104–9. doi: 10.1161/hc0902.104720. [DOI] [PubMed] [Google Scholar]
- 115.Bekeredjian R, Chen S, Frenkel PA, Grayburn PA, Shohet RV. Ultrasound-targeted microbubble destruction can repeatedly direct highly specific plasmid expression to the heart. Circulation. 2003;108:1022–6. doi: 10.1161/01.CIR.0000084535.35435.AE. [DOI] [PubMed] [Google Scholar]
- 116.Shohet RV, Chen S, Zhou YT, Wang Z, Meidell RS, Unger RH, Grayburn PA. Echocardiographic destruction of albumin microbubbles directs gene delivery to the myocardium. Circulation. 2000;101:2554–6. doi: 10.1161/01.cir.101.22.2554. [DOI] [PubMed] [Google Scholar]
- 117.Vannan M, McCreery T, Li P, Han Z, Unger E, Kuersten B, Nabel E, Rajagopalan S. Ultrasound-mediated transfection of canine myocardium by intravenous administration of cationic microbubble-linked plasmid DNA. J Am Soc Echocardiogr. 2002;15:214–8. doi: 10.1067/mje.2002.119913. [DOI] [PubMed] [Google Scholar]
- 118.Frenkel PA, Chen S, Thai T, Shohet RV, Grayburn PA. DNA-loaded albumin microbubbles enhance ultrasound-mediated transfection in vitro. Ultrasound Med Biol. 2002;28:817–22. doi: 10.1016/s0301-5629(02)00518-5. [DOI] [PubMed] [Google Scholar]
- 119.Chen S, Ding JH, Bekeredjian R, Yang BZ, Shohet RV, Johnston SA, Hohmeier HE, Newgard CB, Grayburn PA. Efficient gene delivery to pancreatic islets with ultrasonic microbubble destruction technology. Proc Natl Acad Sci U S A. 2006;103:8469–74. doi: 10.1073/pnas.0602921103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Chen S, Shohet RV, Bekeredjian R, Frenkel P, Grayburn PA. Optimization of ultrasound parameters for cardiac gene delivery of adenoviral or plasmid deoxyribonucleic acid by ultrasound-targeted microbubble destruction. J Am Coll Cardiol. 2003;42:301–8. doi: 10.1016/s0735-1097(03)00627-2. [DOI] [PubMed] [Google Scholar]
- 121.Bekeredjian R, Chen S, Grayburn PA, Shohet RV. Augmentation of cardiac protein delivery using ultrasound targeted microbubble destruction. Ultrasound Med Biol. 2005;31:687–91. doi: 10.1016/j.ultrasmedbio.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 122.Erikson JM, Freeman GL, Chandrasekar B. Ultrasound-targeted antisense oligonucleotide attenuates ischemia/reperfusion-induced myocardial tumor necrosis factor-alpha. J Mol Cell Cardiol. 2003;35:119–30. doi: 10.1016/s0022-2828(02)00289-4. [DOI] [PubMed] [Google Scholar]
- 123.Kipshidze NN, Porter TR, Dangas G, Yazdi H, Tio F, Xie F, Hellinga D, Fournadjiev J, Wolfram R, Seabron R, Waksman R, Abizaid A, Roubin G, Iyer S, Leon MB, Moses JW, Iversen P. Systemic targeted delivery of antisense with perflourobutane gas microbubble carrier reduced neointimal formation in the porcine coronary restenosis model. Cardiovasc Radiat Med. 2003;4:152–9. doi: 10.1016/S1522-1865(03)00184-7. [DOI] [PubMed] [Google Scholar]
- 124.Hashiya N, Aoki M, Tachibana K, Taniyama Y, Yamasaki K, Hiraoka K, Makino H, Yasufumi K, Ogihara T, Morishita R. Local delivery of e2f decoy oligodeoxynucleotides using ultrasound with microbubble agent (optison) inhibits intimal hyperplasia after balloon injury in rat carotid artery model. Biochem Biophys Res Commun. 2004;317:508–14. doi: 10.1016/j.bbrc.2004.03.070. [DOI] [PubMed] [Google Scholar]
- 125.Leong-Poi H, Kuliszewski MA, Lekas M, Sibbald M, Teichert-Kuliszewska K, Klibanov AL, Stewart DJ, Lindner JR. Therapeutic arteriogenesis by ultrasound-mediated vegf165 plasmid gene delivery to chronically ischemic skeletal muscle. Circ Res. 2007;101:295–303. doi: 10.1161/CIRCRESAHA.107.148676. [DOI] [PubMed] [Google Scholar]
- 126.Taylor SL, Rahim AA, Bush NL, Bamber JC, Porter CD. Targeted retroviral gene delivery using ultrasound. J Gene Med. 2007;9:77–87. doi: 10.1002/jgm.1003. [DOI] [PubMed] [Google Scholar]
- 127.Unger EC, McCreery TP, Sweitzer RH. Mrx-501: A novel ultrasound contrast agent with therapeutic properties. Acad Radiol. 1998;5(suppl 1):S247–S249. doi: 10.1016/s1076-6332(98)80119-0. [DOI] [PubMed] [Google Scholar]
- 128.Unger EC, McCreery TP, Sweitzer RH, Caldwell VE, Wu Y. Acoustically active lipospheres containing paclitaxel: A new therapeutic ultrasound contrast agent. Invest Radiol. 1998;33:886–92. doi: 10.1097/00004424-199812000-00007. [DOI] [PubMed] [Google Scholar]
- 129.El-Sherif DM, Lathia JD, Le NT, Wheatley MA. Ultrasound degradation of novel polymer contrast agents. J Biomed Mater Res A. 2004;68:71–8. doi: 10.1002/jbm.a.20032. [DOI] [PubMed] [Google Scholar]
- 130.Blanco D, Alonso MJ. Protein encapsulation and release from poly(lactide-co-glycolide) microspheres: Effect of the protein and polymer properties and of the co-encapsulation of surfactants. Eur J Pharm Biopharm. 1998;45:285–94. doi: 10.1016/s0939-6411(98)00011-3. [DOI] [PubMed] [Google Scholar]
- 131.Rosa GD, Iommelli R, La Rotonda MI, Miro A, Quaglia F. Influence of the co-encapsulation of different non-ionic surfactants on the properties of plga insulin-loaded microspheres. J Control Release. 2000;69:283–95. doi: 10.1016/s0168-3659(00)00315-1. [DOI] [PubMed] [Google Scholar]
- 132.Deng XM, Li XH, Yuan ML, Xiong CD, Huang ZT, Jia WX, Zhang YH. Optimization of preparative conditions for poly-dl-lactide- polyethylene glycol microspheres with entrapped vibrio cholera antigens. J Control Release. 1999;58:123–31. doi: 10.1016/s0168-3659(98)00147-3. [DOI] [PubMed] [Google Scholar]
- 133.Li X, Zhang Y, Yan R, Jia W, Yuan M, Deng X, Huang Z. Influence of process parameters on the protein stability encapsulated in poly-dl-lactide-poly(ethylene glycol) microspheres. J Control Release. 2000;68:41–52. doi: 10.1016/s0168-3659(00)00235-2. [DOI] [PubMed] [Google Scholar]
- 134.Li X, Deng X, Huang Z. In vitro protein release and degradation of poly-dl-lactide-poly(ethylene glycol) microspheres with entrapped human serum albumin: Quantitative evaluation of the factors involved in protein release phases. Pharm Res. 2001;18:117–24. doi: 10.1023/a:1011043230573. [DOI] [PubMed] [Google Scholar]
- 135.Pistel KF, Kissel T. Effects of salt addition on the microencapsulation of proteins using w/o/w double emulsion technique. J Microencapsul. 2000;17:467–83. doi: 10.1080/026520400405723. [DOI] [PubMed] [Google Scholar]
- 136.Frauke Pistel K, Breitenbach A, Zange-Volland R, Kissel T. Brush-like branched biodegradable polyesters, part iii. Protein release from microspheres of poly(vinyl alcohol)-graft-poly(d,l-lactic-co-glycolic acid) J Control Release. 2001;73:7–20. doi: 10.1016/s0168-3659(01)00231-0. [DOI] [PubMed] [Google Scholar]
- 137.Yang YY, Chia HH, Chung TS. Effect of preparation temperature on the characteristics and release profiles of plga microspheres containing protein fabricated by double-emulsion solvent extraction/evaporation method. J Control Release. 2000;69:81–96. doi: 10.1016/s0168-3659(00)00291-1. [DOI] [PubMed] [Google Scholar]
- 138.Bohmer MR, Schroeders R, Steenbakkers JA, de Winter SH, Duineveld PA, Lub J, Nijssen WP, Pikkemaat JA, Stapert HR. Preparation of monodisperse polymer particles and capsules by ink-jet printing. Colloids and surfaces A : Physicochem Eng Aspects. 2006;289:96–104. [Google Scholar]
- 139.Rapoport N, Gao Z, Kennedy A. Multifunctional nanoparticles for combining ultrasonic tumor imaging and targeted chemotherapy. J Natl Cancer Inst. 2007;99:1095–106. doi: 10.1093/jnci/djm043. [DOI] [PubMed] [Google Scholar]
- 140.Forsberg F, Roy R, Merton DA, Rawool NM, Liu JB, Huang M, Kessler D, Goldberg BB. Conventional and hypobaric activation of an ultrasound contrast agent. Ultrasound Med Biol. 1998;24:1143–50. doi: 10.1016/s0301-5629(98)00062-3. [DOI] [PubMed] [Google Scholar]
- 141.Lum AF, Borden MA, Dayton PA, Kruse DE, Simon SI, Ferrara KW. Ultrasound radiation force enables targeted deposition of model drug carriers loaded on microbubbles. J Control Release. 2006;111:128–34. doi: 10.1016/j.jconrel.2005.11.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Kheirolomoom A, Dayton PA, Lum AF, Little E, Paoli EE, Zheng H, Ferrara KW. Acoustically-active microbubbles conjugated to liposomes: Characterization of a proposed drug delivery vehicle. J Control Release. 2007;118:275–84. doi: 10.1016/j.jconrel.2006.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Klibanov AL, Rinkevich D, McConaughy JE, Lewis C, Lindner JR, Kaul S. Ultrasound-triggered release of microbubble-associated material: Site-targeted drug release and deposition. 32nd Annual Symposium on Controlled Release of Bioactive Materials Honolulu : Controlled Release Society. 2004:735. [Google Scholar]
- 144.Lewis CW, McConaughy J, Rinkevich D, Klibanov AL, Lindner JR. Development of a novel microbubble agent for ultrasound-mediated targeted drug delivery. Circulation. 2004;110(suppl 5):511. [Google Scholar]
- 145.Marmottant P, Hilgenfeldt S. Controlled vesicle deformation and lysis by single oscillating bubbles. Nature. 2003;423:153–6. doi: 10.1038/nature01613. [DOI] [PubMed] [Google Scholar]
- 146.Bouakaz A, Versluis M, de Jong N. High-speed optical observations of contrast agent destruction. Ultrasound Med Biol. 2005;31:391–9. doi: 10.1016/j.ultrasmedbio.2004.12.004. [DOI] [PubMed] [Google Scholar]