Summary
T cell responses are regulated by integrating positive and negative signals from costimulatory and inhibitory receptors. While the function of specific T cell costimulatory molecules during infections has been appreciated for some time, recent observations have now revealed a critical role for inhibitory receptors in regulating T cell responses to pathogens, especially during chronic infections. A key emerging principle is that there is considerable diversity in the number and type of inhibitory receptors that can be expressed by T cells during both acute and chronic infections. These distinct inhibitory pathways appear to cooperate in regulating T cell function, could have distinct mechanisms of action, and are likely to provide novel therapeutic targets during persisting infections and other diseases.
Introduction
Upon infection, naïve antigen-specific T cells become activated, differentiate and develop into functional effector and memory T cells or, during chronic infections, exhausted T cells. T cells integrate multiple signals during differentiation, but the precise pathways that control optimal T cell memory versus exhaustion remain incompletely understood. This review focuses on our current understanding of inhibitory receptors during T cell responses following acute versus chronic viral infections. In discussing these inhibitory receptors and their roles in regulating functional and dysfunctional T cell responses during infection it is informative to also examine the role of costimulatory pathways.
Initial activation of a naïve T cell occurs upon interaction of the TCR with specific peptide presented by MHC molecules. This is the major signal for T cell activation and differentiation (signal 1), but additional signals from costimulation (signal 2) and inflammation (signal 3) are required for an effective T cell response. In the absence of coordinated stimulation from signals 1, 2, and 3, proper T cell activation and differentiation does not occur [1-3]. For example, TCR signaling in the absence of signal 2 from costimulation induces T cell anergy in vitro [2]. In vivo, a poor costimulatory environment or a preferential engagement of coinhibitory pathways during T cell priming can lead to tolerance [4],[5]. Several years ago the model for T cell activation versus anergy or tolerization was a simple one. Our developing understanding of signal 3 from inflammation and the increased number and diversity of costimulatory and inhibitory receptors is revealing the decision between full activation and tolerance/dysfunction to be more complex. We are only just beginning to understand the interplay between different costimulatory and inhibitory receptor signals and how they regulate initial T cell activation and division, clonal deletion, the generation and maintenance of memory T cells and T cell exhaustion.
Diversity of costimulatory and inhibitory receptors on T cells
Recent work has highlighted the diversity of costimulatory and inhibitory molecules on T cells (Table 1). Many of these receptors are part of the Ig superfamily and specifically the CD28:B7 subfamily [1]. CD28 family members include the costimulatory receptors CD28 and ICOS and the inhibitory receptors CTLA-4, PD-1 and BTLA. The TNF:TNFR family also contains several costimulatory molecules such as CD27, CD40L, 4-1BB, OX40 and LIGHT that bind the ligands CD70, CD40, 4-1BBL, OX40L and HVEM respectively [6,7]. Interestingly, the CD28 family member BTLA and another Ig superfamily molecule CD160 bind the TNF family member HVEM in unusual examples of cross-family interactions [8,9]. Inhibitory receptors can bind the same ligand as a costimulatory molecule, as is the case for CD28 and CTLA-4 binding B7-1 and B7-2 or LIGHT, BTLA and CD160 binding HVEM or unique ligands not shared with a costimulatory receptor (e.g. PD-1 and PD-L1 and PD-L2) [1]. These interactions can be complex and PD-L1, for example, has recently been demonstrated to bind to B7-1 and deliver a bidirectional inhibitory signal [10].
Table 1. Costimulatory and inhibitory receptors.
| Receptor or Ligand on T cell | Superfamily | Cellular Expression pattern | T cell expression | Binds | Costimulatory or Inhibitory | References |
|---|---|---|---|---|---|---|
| CD28 | Ig (CD28 family) | T cells | Constitutive on Naïve | B7.1, B7.2 | Costimulatory | [1] |
| ICOS | Ig (CD28 family) | T cells, NKT cells, NK cells | Induced upon activation | ICOSL (B7RP1) | Costimulatory | [1] |
| CD40L | TNF/TNFR | T cells | Induced upon activation | CD40 | Costimulatory | [6] |
| OX40 | TNF/TNFR | T cells | Induced upon activation | OX40L | Costimulatory | [6] |
| 4-1BB | TNF/TNFR | T cells, DC subset, FDCs, eosinophils, NK cells | Induced upon activation | 4ABBL | Costimulatory | [6] |
| CD27 | TNF/TNFR | T cells, NK cells, B cells | Constitutive on Naïve | CD70 | Costimulatory | [6] |
| LIGHT | TNF/TNFR | T cells, monocytes, NK cells, immature DC | Induced upon activation | HVEM | Costimulatory | [6] |
| CTLA4 | Ig (CD28 family) | T cells | Induced upon activation | B7.1/B7.2 | Inhibitory | [1] |
| PD-1 | Ig (CD28 family) | T cells, NKT cells, B cells monocytes | Induced upon activation | PD-L1/PD-L2 | Inhibitory | [1] |
| PD-L1 | Ig (CD28 family) | T cells, NKT cells, B cells monocytes, DC, endothelial cells, hepatocytes, etc. | Constitutive and upregulated upon activation | B7-1 | Inhibitory | [1,10] |
| BTLA | Ig (CD28 family) | T cells, B cells, DC (macs + NK in B6 mice only) | Constitutive on Naïve | HVEM | Inhibitory | [61] |
| CD160 | Ig | HVEM and MHCI (low affinity) | Induced upon activation | HVEM | Inhibitory | [8] |
| TIM3 | Ig | T cells, macs, DCs | Induced upon activation | GALECTIN 9, phosphatidyl serine | Inhibitory | [16] |
| 2B4 | Ig (CD2 family) | T cells, NK cells, dendritic epidermal T cells, monocytes | Induced upon activation | CD48 | Costimulatoryan d Inhibitory | [12] |
| LAG3 | Ig | T cell, NK cells, B cells | Induced upon activation | MHC-II | Inhibitory | [11,58] |
| CD200R | Ig | T cells, macrophages, DCs, neutrophils, basophils, mast cells | Constitutive on Naïve | CD200 | Inhibitory? | [17] |
| PIR-B | ? | T cell, B cells, DCs | ? | MHC-I | Inhibitory | [57] |
| NKG2A | C-lectin | T cells, NK cells | Induced upon activation | Qa-1B, non-classical MHC-I, stress induced molecules (e.g. Rae-1, MICA, MICB) | Inhibitory | [57] |
| Ly49 family (mice) | C-lectin | T cells, NK cells, NK T cells monocytes/macrophages | ? | MHC-I | Costimulatory and Inhibitory | [14] |
| KIRs (human) | Ig | T cells | Induced upon activation | HLA-1 | Inhibitory | [14] |
| GP49B | Ig | T cells, macrophages, neutrophils, and NK cells, mast cells | ? | αvβ3 | Inhibitory | [57] |
The receptors and ligands described above are major players in T cell activation and inhibition. However, the number of cell surface molecules that can regulate T cell responses is ever increasing. Recently, molecules including LAG-3 [11], 2B4/CD244 [12,13], CD30-CD30L [6], NK receptors [14], the Tim molecules (Tim1-4) [15,16], CD200R [17], Siglecs [18] and others have been implicated in regulating T cell responses. It is currently unclear to what degree there is functional overlap or specificity in these pathways and whether there are common themes and principles about costimulation and inhibition by these different inhibitory receptors.
Costimulatory and inhibitory receptors during infections
Costimulatory and inhibitory receptors are thought to help the immune system balance productive immune responses to foreign antigens versus tolerance to self-antigens. Indeed, a number of costimulatory pathways can augment T cell responses to acute infections. During some viral infections, the absence of costimulatory pathways can lead to a substantial quantitative reduction in the primary antiviral T cell response [1,19] though the level of costimulation required can depend on the type of infection. Primary T cell responses to influenza virus are substantially diminished in mice lacking various costimulatory molecules while T cell responses to acute LCMV infection are only modestly reduced in the same KO mice [1,6]. Similar examples for other infections also exist [20,21].
While there is considerable evidence that inhibitory receptors can influence T cell responses to self antigens [5], the role of inhibitory receptors in T cell priming and memory T cell differentiation during acute infection is less clear. CTLA-4 KO CD8 T cells respond normally to acute LCMV infection [22] and CTLA-4 expressing T cells appear to have normal proliferative responses and effector function during acute LCMV or vaccinia virus infection [23,24]. However, inhibitory receptor pathways can negatively regulate immunity to rabies virus, adenovirus and Sendai virus [11,25,26]. During acute infection with rabies virus, the absence of PD-L1 led to better control of virus and a greater accumulation of CD8 T cells in the CNS [25]. When PD-1 KO mice were infected with adenovirus, T cells expanded more in peripheral tissues leading to rapid clearance of the virus [26]. LAG-3 can also regulate acute T cell responses; LAG-3 KO mice infected with Sendai virus had increased numbers of virus-specific CD4 and CD8 T cells 30 days post infection [11]. While much of the data examining the role of inhibitory receptors during acute infection is consistent with a negative role for these pathways, two recent studies with Listeria monocytogenes (LM) infection suggest a possible alternative role for the PD-1 pathway in this setting [27,28]. In these studies, PD-L1 blockade delayed the kinetics of CD8+ T cell priming, reduced the magnitude of T cell expansion and diminished secondary T cell responses [27,28]. It is unclear whether this role for the PD-1 pathway during LM infection reflects increased T cell death when PD-1:PD-L1 is blocked or a true positive signal through this pathway under some circumstances. Additional studies should help clarify the precise role of the PD-1 pathway in different infections. However, the majority of studies are consistent with a negative role for most inhibitory pathways during infection.
Costimulatory and inhibitory receptors during chronic infection
Many of the costimulatory receptor KO mice discussed above have profound defects in T cell responses and viral control during chronic infections [29-34] suggesting a critical role for costimulation when pathogens persist. Indeed, enhancing costimulation can in some circumstances improve T cell response during chronic infections. For example, 4-1BB has a key role during Friend leukemia virus infection [34] and stimulation of the 4-1BB pathway led to expansion of HIV-specific CD8+ T cells in vitro most likely by regulating CD8 T cell survival [35]. It will be interesting to define how augmenting other costimulatory signals influences T cell responses during chronic viral infections.
There is now accumulating evidence that inhibitory receptors have a prominent role in regulating T cell responses during chronic viral infections. In contrast to acute infections where T cells expand, contract and form functional, long-lived memory cells, during chronic infection T cells develop functional deficiencies that prevent optimal control of infection [36]. This loss of function or exhaustion is hierarchical, with IL-2, cytotoxicity and robust proliferation lost early, TNFα persisting for longer and IFN-γ lost only at more extreme stages of exhaustion [36]. T cell exhaustion been described during a number of chronic infections in mice as well as human infections with HIV, HBV and HCV [37-39]. These functional defects are likely a key reason for failure of immunological control of these persisting pathogens and recent insights indicate a central role for inhibitory receptors in regulating T cell exhaustion.
A functional role for inhibitory receptors during chronic infection was initially demonstrated during chronic LCMV infection where blockade of the PD-1:PD-L1 pathway rejuvenated CD8 T cell function and enhanced viral control [40]. The PD-1 pathway also appears to regulate T cell responses during other mouse models of chronic infection [41], primate infection with SIV [42,43] and in humans infected with HIV [38,44-48], HBV [37,49,50], HCV [39,51,52]; where disrupting this pathway in vitro improves T cell responses. In vivo blockade of the PD-1 pathway during SIV infection of macaques not only improves T cell (and B cell) responses, but also lowers viral load and improves survival of the primates receiving treatment [53]. Thus, there is clearly a role for the PD-1:PD-L1 pathway during chronic infections and manipulation of this pathway has therapeutic potential.
There is reason to be cautiously optimistic about the clinical promise of PD-1 pathway blockade. This intervention alone, however, does not completely restore optimal T cell function and the level of functional recovery and even the precise impact of PD-1 blockade on different effector functions and/or apoptosis differs depending on the study [42,46-48]. Some insights into the nature of partial reversal of exhaustion and the range of responses to PD-1 pathway blockade might come from recent studies demonstrating that populations of exhausted CD8 T cells are heterogeneous [54]. For example, exhausted LCMV-specific CD8 T cells with intermediate PD-1 expression were potently re-invigorated by PD-1:PD-L1 blockade, but there was little if any benefit for the PD-1Hi subset of exhausted CD8 T cells [54]. A similar situation might also exist during chronic HCV infection in humans where PD-1Lo HCV-specific CD8 T cells from the blood responded to PD-1 pathway blockade while PD-1Hi HCV-specific CD8 T cells from the livers of the same individuals did not [55].
Gene expression profiling studies suggested the presence of a number of other known or potential inhibitory receptors on exhausted CD8 T cells [56]. In addition to PD-1, LCMV-specific exhausted CD8 T cells also expressed 2B4, LAG-3, CTLA-4, PirB, GP49 and CD160 [57]. Many of these inhibitory receptors were co-expressed by the same exhausted CD8 T cell and as the severity of the infection increased, both the amount of each receptor and the number of different inhibitory receptors expressed per cell increased [57]. Thus, the PD-1Hi subset of exhausted CD8 T cells also co-expressed multiple other inhibitory receptors. Blockade of the PD-1 pathway together with a second inhibitory receptor LAG-3 demonstrated cooperation between these inhibitory pathways and led to a synergistic restoration of CD8 T cell function and viral control [57]. Several recent studies have demonstrated that HIV-specific T cells can also express more than one inhibitory receptor. Kaufmann et al showed that PD-1 and CTLA-4 are coexpressed by HIV-specific CD4+ T cells [38]. Both PD-1 and CTLA-4 negatively regulated the function of HIV-specific CD4 T cells and co-expression patterns and combined blockade of these inhibitory receptors in vitro suggested cooperative inhibition could occur. One interesting observation was that while CTLA-4 and PD-1 co-regulated HIV-specific CD4 T cells, HIV-specific CD8 T cells expressed substantial amounts of PD-1, but not CTLA-4 suggesting that CD4 and CD8 T cell responses could be regulated by distinct sets of inhibitory receptors. A second study recently demonstrated the expression and inhibitory role of Tim-3 on HIV-specific T cells [15]. Interestingly, Tim-3 and PD-1 were expressed by sometimes distinct and sometimes partially overlapping populations of CD8+ T cells depending on the subject [15]. One concept that emerges from these studies is that there is diversity in the cell surface inhibitory pathways available to negatively regulate T cell responses to chronic infections. Specific inhibitory receptors can be expressed by distinct T cell subsets resulting in differential regulation, while other inhibitory receptors are co-expressed and can operate synergistically. Thus, as with costimulatory molecules, the precise inhibitory receptors of therapeutic interest might depend on the type and severity of infection, whether CD4 or CD8 T cells are of interest as well as other factors such as the activation state of the T cell.
Mechanisms of costimulatory and inhibitory receptor pathways
A mechanistic understanding of how inhibitory receptors restrain T cell responses would not only aid in our understanding of memory T cell differentiation following chronic viral infections, but could also provide novel targets for therapeutics. Unfortunately, for many inhibitory receptors there is little information in this area. Again, examining some aspects of costimulation may provide a guide. For the best-studied costimulatory pathways such as CD28, engagement of the costimulatory receptor compliments TCR signaling both quantitatively and qualitatively. For example, CD28 signaling lowers the threshold for activation, enhances IL2 production and CD25 expression, improves survival by upregulating Bcl-XL, and promotes progression through the cell cycle [1]. In addition, the coupling of CD28 costimulation to glucose metabolism via AKT [58] is likely a key factor in ensuring robust and sustained T cell responses upon initial activation. While costimulatory molecules of the TNF family can also enhance T cell proliferation and cytokine production and regulate Bcl-2 and Bcl-XL expression, they do so through different signaling pathways. For example, 4-1BB signals via TRAF1, TRAF2 and ERK [6]. Thus, while the end result of costimulation by different pathways can be similar the mechanistic intermediates can be quite distinct.
In contrast to many costimulatory pathways, the coinhibitory molecules CTLA-4 [59], PD-1 [60], BTLA [61] and CD160 [8] all inhibit IL-2 production and these four receptors as well as LAG-3 [11] can negatively regulate cell cycle progression. CTLA-4 inhibits CD28 augmented gene transcription, but does not prevent gene regulation by TCR alone [62]. In contrast, PD-1 appears to block both TCR signals [63,64] and CD28 signals [65]. Interestingly, though PD-1 and CTLA-4 both negatively regulate CD28 signaling and AKT activation, they do so by different mechanisms [65]. While the signaling mechanism for some inhibitory pathways is beginning to be defined, there is far less known about the intracellular mechanisms of action of inhibitory receptors compared to costimulatory receptors. Given the similarities in downstream targets, it is tempting to speculate that molecules such as CTLA-4 and PD-1 have evolved to specifically antagonize CD28 (and perhaps ICOS) mediated positive costimulation. It will be interesting to examine if other costimulatory pathways such as TNF receptor family members are resistant to or can overcome CTLA-4 or PD-1 mediated inhibition.
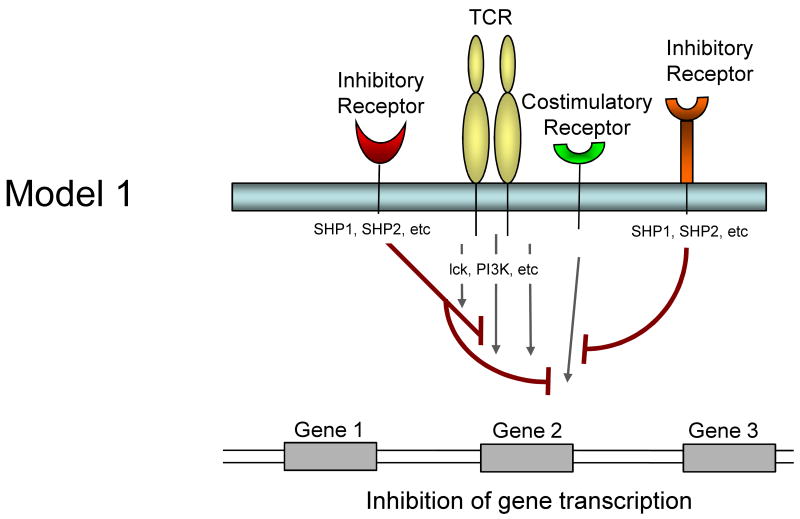
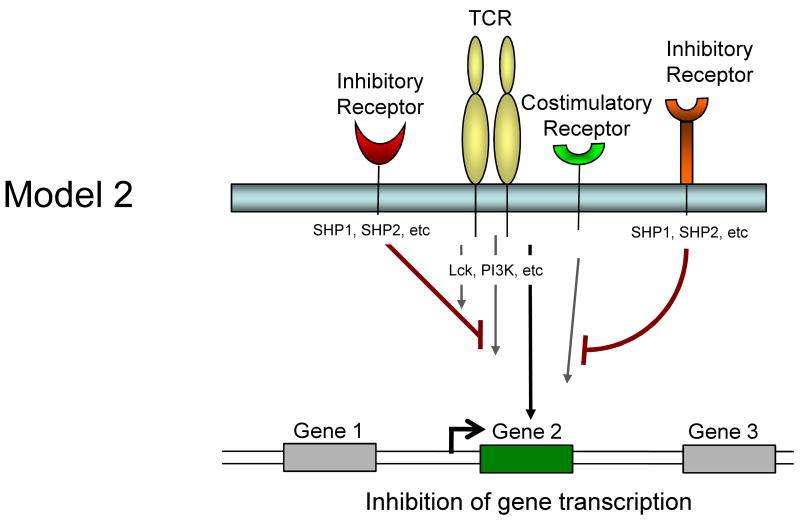
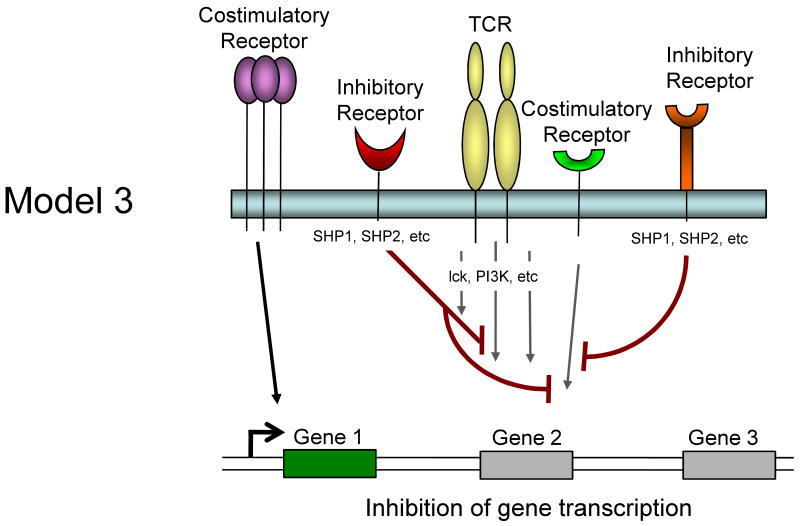
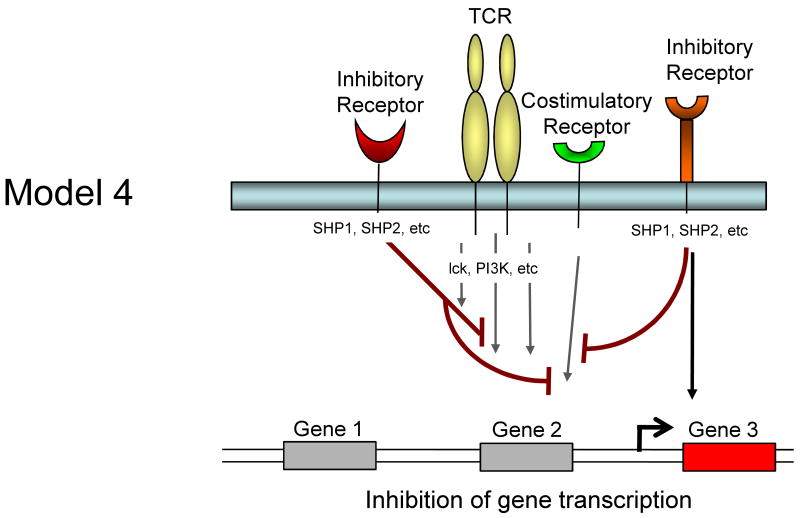
It is currently unclear whether inhibitory receptors merely block the induction of gene expression by TCR/costimulation or whether inhibitory receptor signaling could actually induce alternative gene transcription. Several models for how inhibitory receptor signals might alter gene expression in T cells are possible and are described in Figure 1. First, inhibitory receptor signaling could simply block all signals from the TCR and costimulation leading to an attenuation or elimination of TCR induced gene expression. The effect of this inhibition would be a broad quantitative reduction in gene expression. A temporal delay in expression of inhibitory versus costimulatory receptors would likely be more consistent with an abbreviated activation signal rather than a complete block if this model were intact. Second, inhibitory receptors might selectively block some, but not all signals from TCR and costimulation. For example, while CTLA-4 and/or PD-1 could block CD28-mediated AKT activation and could recruit Shp-1, Shp-2 (and/or SHIP) to dephosphorylate LCK and other substrates, some partial TCR signaling could still occur. The effect might be the induction of only a subset of genes normally associated with T cell activation and perhaps a qualitatively altered response. A third possibility is that ligation of one inhibitory receptor (e.g. PD-1) might lead to inhibition of a specific costimulatory pathway (e.g. CD28), but leave other costimulatory pathways intact. In this situation, qualitative differences in costimulation could alter the course of T cell activation and differentiation. A fourth possibility is that inhibitory receptors actively induce or regulate gene expression independent of their inhibition of TCR or costimulatory signaling. For example, inhibitory molecules might recruit and/or activate selective signaling pathways that lead to new gene transcription (or active repression). This latter model would likely occur with some partial TCR signaling since there is little evidence that inhibitory receptor signaling has in impact without TCR engagement. These models are not mutually exclusive and these and other potential mechanisms could occur for different inhibitory receptor pathways.
Figure 1.
Model showing possible mechanisms of T cell regulation by inhibitory receptors. Model 1: Both TCR-mediated signals and costimulation are inhibited by inhibitory receptors resulting in a termination or quantitative reduction in gene transcription. Model 2: Only some of the TCR and costimulatory-induced signals are inhibited resulting in qualitatively altered gene transcription. Model 3: Inhibitory receptors may regulate some costimulatory pathways while allowing others to signal normally resulting in qualitatively different costimulatory signals. Model 4: Inhibitory receptor signaling could block costimulation-induced signaling and partially block TCR signaling but also induce the transcription of novel genes.
Conclusion and future directions
T cells can express a diverse array of both costimulatory and inhibitory receptors and these molecules can positively or negatively regulate T cell function. In some cases pathogens have exploited negative regulatory pathways to facilitate persistence and inhibitory receptors appear to play a prominent role in T cell dysfunction during persisting infections. In vitro and in vivo blockade studies have revealed the therapeutic potential of modulating costimulatory and inhibitory receptors on T cells. A better understanding of the complexity of co-expression of inhibitory and costimulatory receptors on T cells in different settings is likely to reveal T cell functions and pathways regulated by specific receptors. It is likely there will be opportunities to therapeutically “tune” T cell responses by modulating specific receptor:ligand pathways. Since these same costimulatory and inhibitory pathways are used not only during T cell responses to pathogens, but also in anti-tumor immunity and also autoimmunity, a better mechanistic understanding of the regulation of T cell responses by diverse and co-expressed positive and negative regulatory molecules could have broad therapeutic implications.
Acknowledgments
This work was supported by grants from the National Institutes of Health ((HHSN26620050030C, AI071309 and AI077098 to E.J.W.) and the Foundation for NIH through the Grand Challenges in Global Health Initiative (to E.J.W.)) and the American Heart Association (A.C.).
Footnotes
-
Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al.: Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med 2008, 205:2763-2779.In this paper, the authors show that HIV-specific T cells from the peripheral blood of HIV-infected individuals expressed Tim-3 and that blocking Tim-3 signaling enhanced the function of the HIV-specific T cells. Given the recent studies examining PD-1 and CTLA-4 on HIV-specific T cells, this study suggests a third inhibitory molecule that may be important in developing therapies to HIV.
-
Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ: CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol 2008, 9:176-185.This paper demonstrates that CD160 is a receptor for HVEM and that, similar to BTLA, binding of HVEM to CD160 results in negative signaling to the T cell resulting in inhibition of proliferation and cytokine production.
-
Rowe JH, Johanns TM, Ertelt JM, Way SS: PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol 2008, 180:7553-7557.Blockade of PD-1 was examined to determine its role in regulating T cell responses to acute infection with Listeria monocytogenes. Surprisingly, blockade of this pathway resulted in reduced primary and secondary T cell responses. This paper is significant important because it not only demonstrated that inhibitory receptors can regulate T cell responses to acute infection but that the role of the PD-1 pathway may not be negative in all cases.
-
Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R: Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity 2007, 27:670-684.Gene expression profiles of LCMV-specific T cells from acute and chronic LCMV-infected mice were examined and compared with naïve T cells. A total of 490 genes were upregulated or downregulated by at least 2-fold in the exhausted CD8+ T cells identifying a number of inhibitory receptors, transcription factors and metabolic pathways that may be novel targets for immunotherapy.
-
Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ: Coregulation of CD8(+) T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol 2008.This paper demonstrates that exhausted CD8 T cells from chronically infected LCMV mice upregulate a wide range of inhibitory receptors. Furthermore, in vivo blockade of two of the molecules (PD-L1 and LAG3) show that whilst aLAG3 alone had no effect on the frequency of LCMV-specific CD8 T cells, dual blockade had a greater effect than aPD-L1 alone. Moreover, dual blockade also had a greater effect on rescuing of CD8 T cell function than aPD-L1 alone leading to significantly lower viral titers. This study shows inhibitory receptors can work synergistically and suggests that combination therapies have considerable potential.
-
Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al.: Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol 2007, 8:1246-1254.Kaufmann et al demonstrated that CD4+ T cells from the blood of HIV-infected individuals co-express PD-1 and CTLA-4. CTLA-4 appears to be upregulated specifically by HIV-specific CD4+ T cells but not CD8+ T cells suggesting that inhibitory receptors can differentially regulate CD4 versus CD8 T cell responses. Both PD-1 and CTLA-4 negatively regulated the function of HIV-specific CD4 T cells.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References and recommended reading
- 1.Greenwald RJ, Freeman GJ, Sharpe AH. The B7 family revisited. Annu Rev Immunol. 2005;23:515–548. doi: 10.1146/annurev.immunol.23.021704.115611. [DOI] [PubMed] [Google Scholar]
- 2.Schwartz RH. T cell anergy. Annu Rev Immunol. 2003;21:305–334. doi: 10.1146/annurev.immunol.21.120601.141110. [DOI] [PubMed] [Google Scholar]
- 3.Mescher MF, Curtsinger JM, Agarwal P, Casey KA, Gerner M, Hammerbeck CD, Popescu F, Xiao Z. Signals required for programming effector and memory development by CD8+ T cells. Immunol Rev. 2006;211:81–92. doi: 10.1111/j.0105-2896.2006.00382.x. [DOI] [PubMed] [Google Scholar]
- 4.Goldberg MV, Maris CH, Hipkiss EL, Flies AS, Zhen L, Tuder RM, Grosso JF, Harris TJ, Getnet D, Whartenby KA, et al. Role of PD-1 and its ligand, B7-H1, in early fate decisions of CD8 T cells. Blood. 2007;110:186–192. doi: 10.1182/blood-2006-12-062422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Redmond WL, Sherman LA. Peripheral tolerance of CD8 T lymphocytes. Immunity. 2005;22:275–284. doi: 10.1016/j.immuni.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 6.Watts TH. TNF/TNFR family members in costimulation of T cell responses. Annu Rev Immunol. 2005;23:23–68. doi: 10.1146/annurev.immunol.23.021704.115839. [DOI] [PubMed] [Google Scholar]
- 7.Croft M. Costimulation of T cells by OX40, 4-1BB, and CD27. Cytokine Growth Factor Rev. 2003;14:265–273. doi: 10.1016/s1359-6101(03)00025-x. [DOI] [PubMed] [Google Scholar]
- 8.Cai G, Anumanthan A, Brown JA, Greenfield EA, Zhu B, Freeman GJ. CD160 inhibits activation of human CD4+ T cells through interaction with herpesvirus entry mediator. Nat Immunol. 2008;9:176–185. doi: 10.1038/ni1554. [DOI] [PubMed] [Google Scholar]
- 9.Gonzalez LC, Loyet KM, Calemine-Fenaux J, Chauhan V, Wranik B, Ouyang W, Eaton DL. A coreceptor interaction between the CD28 and TNF receptor family members B and T lymphocyte attenuator and herpesvirus entry mediator. Proc Natl Acad Sci U S A. 2005;102:1116–1121. doi: 10.1073/pnas.0409071102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27:111–122. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Workman CJ, Cauley LS, Kim IJ, Blackman MA, Woodland DL, Vignali DA. Lymphocyte activation gene-3 (CD223) regulates the size of the expanding T cell population following antigen activation in vivo. J Immunol. 2004;172:5450–5455. doi: 10.4049/jimmunol.172.9.5450. [DOI] [PubMed] [Google Scholar]
- 12.Lee KM, McNerney ME, Stepp SE, Mathew PA, Schatzle JD, Bennett M, Kumar V. 2B4 acts as a non-major histocompatibility complex binding inhibitory receptor on mouse natural killer cells. J Exp Med. 2004;199:1245–1254. doi: 10.1084/jem.20031989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Laouar A, Manocha M, Wan M, Yagita H, van Lier RA, Manjunath N. Cutting Edge: Distinct NK receptor profiles are imprinted on CD8 T cells in the mucosa and periphery during the same antigen challenge: role of tissue-specific factors. J Immunol. 2007;178:652–656. doi: 10.4049/jimmunol.178.2.652. [DOI] [PubMed] [Google Scholar]
- 14.Lanier LL. Evolutionary struggles between NK cells and viruses. Nat Rev Immunol. 2008;8:259–268. doi: 10.1038/nri2276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jones RB, Ndhlovu LC, Barbour JD, Sheth PM, Jha AR, Long BR, Wong JC, Satkunarajah M, Schweneker M, Chapman JM, et al. Tim-3 expression defines a novel population of dysfunctional T cells with highly elevated frequencies in progressive HIV-1 infection. J Exp Med. 2008;205:2763–2779. doi: 10.1084/jem.20081398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kuchroo VK, Umetsu DT, DeKruyff RH, Freeman GJ. The TIM gene family: emerging roles in immunity and disease. Nat Rev Immunol. 2003;3:454–462. doi: 10.1038/nri1111. [DOI] [PubMed] [Google Scholar]
- 17.Snelgrove RJ, Goulding J, Didierlaurent AM, Lyonga D, Vekaria S, Edwards L, Gwyer E, Sedgwick JD, Barclay AN, Hussell T. A critical function for CD200 in lung immune homeostasis and the severity of influenza infection. Nat Immunol. 2008;9:1074–1083. doi: 10.1038/ni.1637. [DOI] [PubMed] [Google Scholar]
- 18.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat Rev Immunol. 2007;7:255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 19.Bertram EM, Dawicki W, Watts TH. Role of T cell costimulation in anti-viral immunity. Semin Immunol. 2004;16:185–196. doi: 10.1016/j.smim.2004.02.006. [DOI] [PubMed] [Google Scholar]
- 20.Fang M, Sigal LJ. Direct CD28 costimulation is required for CD8+ T cell-mediated resistance to an acute viral disease in a natural host. J Immunol. 2006;177:8027–8036. doi: 10.4049/jimmunol.177.11.8027. [DOI] [PubMed] [Google Scholar]
- 21.Shahinian A, Pfeffer K, Lee KP, Kundig TM, Kishihara K, Wakeham A, Kawai K, Ohashi PS, Thompson CB, Mak TW. Differential T cell costimulatory requirements in CD28-deficient mice. Science. 1993;261:609–612. doi: 10.1126/science.7688139. [DOI] [PubMed] [Google Scholar]
- 22.Bachmann MF, Waterhouse P, Speiser DE, McKall-Faienza K, Mak TW, Ohashi PS. Normal responsiveness of CTLA-4-deficient anti-viral cytotoxic T cells. J Immunol. 1998;160:95–100. [PubMed] [Google Scholar]
- 23.Raue HP, Slifka MK. Pivotal advance: CTLA-4+ T cells exhibit normal antiviral functions during acute viral infection. J Leukoc Biol. 2007;81:1165–1175. doi: 10.1189/jlb.0806535. [DOI] [PubMed] [Google Scholar]
- 24.Homann D, Dummer W, Wolfe T, Rodrigo E, Theofilopoulos AN, Oldstone MB, von Herrath MG. Lack of intrinsic CTLA-4 expression has minimal effect on regulation of antiviral T-cell immunity. J Virol. 2006;80:270–280. doi: 10.1128/JVI.80.1.270-280.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lafon M, Megret F, Meuth SG, Simon O, Velandia Romero ML, Lafage M, Chen L, Alexopoulou L, Flavell RA, Prehaud C, et al. Detrimental contribution of the immuno-inhibitor B7-H1 to rabies virus encephalitis. J Immunol. 2008;180:7506–7515. doi: 10.4049/jimmunol.180.11.7506. [DOI] [PubMed] [Google Scholar]
- 26.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198:39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Seo SK, Jeong HY, Park SG, Lee SW, Choi IW, Chen L, Choi I. Blockade of endogenous B7-H1 suppresses antibacterial protection after primary Listeria monocytogenes infection. Immunology. 2008;123:90–99. doi: 10.1111/j.1365-2567.2007.02708.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rowe JH, Johanns TM, Ertelt JM, Way SS. PDL-1 blockade impedes T cell expansion and protective immunity primed by attenuated Listeria monocytogenes. J Immunol. 2008;180:7553–7557. doi: 10.4049/jimmunol.180.11.7553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Suresh M, Whitmire JK, Harrington LE, Larsen CP, Pearson TC, Altman JD, Ahmed R. Role of CD28-B7 interactions in generation and maintenance of CD8 T cell memory. J Immunol. 2001;167:5565–5573. doi: 10.4049/jimmunol.167.10.5565. [DOI] [PubMed] [Google Scholar]
- 30.Whitmire JK, Flavell RA, Grewal IS, Larsen CP, Pearson TC, Ahmed R. CD40-CD40 ligand costimulation is required for generating antiviral CD4 T cell responses but is dispensable for CD8 T cell responses. J Immunol. 1999;163:3194–3201. [PubMed] [Google Scholar]
- 31.Fuse S, Bellfy S, Yagita H, Usherwood EJ. CD8+ T cell dysfunction and increase in murine gammaherpesvirus latent viral burden in the absence of 4-1BB ligand. J Immunol. 2007;178:5227–5236. doi: 10.4049/jimmunol.178.8.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fuse S, Zhang W, Usherwood EJ. Control of memory CD8+ T cell differentiation by CD80/CD86-CD28 costimulation and restoration by IL-2 during the recall response. J Immunol. 2008;180:1148–1157. doi: 10.4049/jimmunol.180.2.1148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kemball CC, Lee ED, Szomolanyi-Tsuda E, Pearson TC, Larsen CP, Lukacher AE. Costimulation requirements for antiviral CD8+ T cells differ for acute and persistent phases of polyoma virus infection. J Immunol. 2006;176:1814–1824. doi: 10.4049/jimmunol.176.3.1814. [DOI] [PubMed] [Google Scholar]
- 34.Robertson SJ, Messer RJ, Carmody AB, Mittler RS, Burlak C, Hasenkrug KJ. CD137 costimulation of CD8+ T cells confers resistance to suppression by virus-induced regulatory T cells. J Immunol. 2008;180:5267–5274. doi: 10.4049/jimmunol.180.8.5267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang C, Wen T, Routy JP, Bernard NF, Sekaly RP, Watts TH. 4-1BBL induces TNF receptor-associated factor 1-dependent Bim modulation in human T cells and is a critical component in the costimulation-dependent rescue of functionally impaired HIV-specific CD8 T cells. J Immunol. 2007;179:8252–8263. doi: 10.4049/jimmunol.179.12.8252. [DOI] [PubMed] [Google Scholar]
- 36.Shin H, Wherry EJ. CD8 T cell dysfunction during chronic viral infection. Curr Opin Immunol. 2007;19:408–415. doi: 10.1016/j.coi.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 37.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, Laccabue D, Zerbini A, Cavalli A, Missale G, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81:4215–4225. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kaufmann DE, Kavanagh DG, Pereyra F, Zaunders JJ, Mackey EW, Miura T, Palmer S, Brockman M, Rathod A, Piechocka-Trocha A, et al. Upregulation of CTLA-4 by HIV-specific CD4+ T cells correlates with disease progression and defines a reversible immune dysfunction. Nat Immunol. 2007;8:1246–1254. doi: 10.1038/ni1515. [DOI] [PubMed] [Google Scholar]
- 39.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, Ferrari C. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80:11398–11403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Barber DL, Wherry EJ, Masopust D, Zhu B, Allison JP, Sharpe AH, Freeman GJ, Ahmed R. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 41.Isogawa M, Furuichi Y, Chisari FV. Oscillating CD8(+) T cell effector functions after antigen recognition in the liver. Immunity. 2005;23:53–63. doi: 10.1016/j.immuni.2005.05.005. [DOI] [PubMed] [Google Scholar]
- 42.Petrovas C, Price DA, Mattapallil J, Ambrozak DR, Geldmacher C, Cecchinato V, Vaccari M, Tryniszewska E, Gostick E, Roederer M, et al. SIV-specific CD8+ T cells express high levels of PD1 and cytokines but have impaired proliferative capacity in acute and chronic SIVmac251 infection. Blood. 2007;110:928–936. doi: 10.1182/blood-2007-01-069112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, Ahmed R, Amara RR. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81:5819–5828. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.D'Souza M, Fontenot AP, Mack DG, Lozupone C, Dillon S, Meditz A, Wilson CC, Connick E, Palmer BE. Programmed death 1 expression on HIV-specific CD4+ T cells is driven by viral replication and associated with T cell dysfunction. J Immunol. 2007;179:1979–1987. doi: 10.4049/jimmunol.179.3.1979. [DOI] [PubMed] [Google Scholar]
- 45.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, Mackey EW, Miller JD, Leslie AJ, DePierres C, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443:350–354. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 46.Petrovas C, Casazza JP, Brenchley JM, Price DA, Gostick E, Adams WC, Precopio ML, Schacker T, Roederer M, Douek DC, et al. PD-1 is a regulator of virus-specific CD8+ T cell survival in HIV infection. J Exp Med. 2006;203:2281–2292. doi: 10.1084/jem.20061496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Trautmann L, Janbazian L, Chomont N, Said EA, Gimmig S, Bessette B, Boulassel MR, Delwart E, Sepulveda H, Balderas RS, et al. Upregulation of PD-1 expression on HIV-specific CD8+ T cells leads to reversible immune dysfunction. Nat Med. 2006;12:1198–1202. doi: 10.1038/nm1482. [DOI] [PubMed] [Google Scholar]
- 48.Zhang JY, Zhang Z, Wang X, Fu JL, Yao J, Jiao Y, Chen L, Zhang H, Wei J, Jin L, et al. PD-1 up-regulation is correlated with HIV-specific memory CD8+ T-cell exhaustion in typical progressors but not in long-term nonprogressors. Blood. 2007;109:4671–4678. doi: 10.1182/blood-2006-09-044826. [DOI] [PubMed] [Google Scholar]
- 49.Boettler T, Panther E, Bengsch B, Nazarova N, Spangenberg HC, Blum HE, Thimme R. Expression of the interleukin-7 receptor alpha chain (CD127) on virus-specific CD8+ T cells identifies functionally and phenotypically defined memory T cells during acute resolving hepatitis B virus infection. J Virol. 2006;80:3532–3540. doi: 10.1128/JVI.80.7.3532-3540.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Zhang Z, Zhang JY, Wherry EJ, Jin B, Xu B, Zou ZS, Zhang SY, Li BS, Wang HF, Wu H, et al. Dynamic programmed death 1 expression by virus-specific CD8 T cells correlates with the outcome of acute hepatitis B. Gastroenterology. 2008;134:1938–1949. 1949, e1931–1933. doi: 10.1053/j.gastro.2008.03.037. [DOI] [PubMed] [Google Scholar]
- 51.Radziewicz H, Ibegbu CC, Fernandez ML, Workowski KA, Obideen K, Wehbi M, Hanson HL, Steinberg JP, Masopust D, Wherry EJ, et al. Liver-infiltrating lymphocytes in chronic human hepatitis C virus infection display an exhausted phenotype with high levels of PD-1 and low levels of CD127 expression. J Virol. 2007;81:2545–2553. doi: 10.1128/JVI.02021-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Golden-Mason L, Palmer B, Klarquist J, Mengshol JA, Castelblanco N, Rosen HR. Upregulation of PD-1 expression on circulating and intrahepatic hepatitis C virus-specific CD8+ T cells associated with reversible immune dysfunction. J Virol. 2007;81:9249–9258. doi: 10.1128/JVI.00409-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Velu V, Titanji K, Zhu B, Husain S, Pladevega A, Lai L, Vanderford TH, Chennareddi L, Silvestri G, Freeman GJ, et al. Enhancing SIV-specific immunity in vivo by PD-1 blockade. Nature. 2008 doi: 10.1038/nature07662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Blackburn SD, Shin H, Freeman GJ, Wherry EJ. Selective expansion of a subset of exhausted CD8 T cells by alphaPD-L1 blockade. Proc Natl Acad Sci U S A. 2008;105:15016–15021. doi: 10.1073/pnas.0801497105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Nakamoto N, Kaplan DE, Coleclough J, Li Y, Valiga ME, Kaminski M, Shaked A, Olthoff K, Gostick E, Price DA, et al. Functional restoration of HCV-specific CD8 T cells by PD-1 blockade is defined by PD-1 expression and compartmentalization. Gastroenterology. 2008;134:1927–1937. 1937, e1921–1922. doi: 10.1053/j.gastro.2008.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wherry EJ, Ha SJ, Kaech SM, Haining WN, Sarkar S, Kalia V, Subramaniam S, Blattman JN, Barber DL, Ahmed R. Molecular signature of CD8+ T cell exhaustion during chronic viral infection. Immunity. 2007;27:670–684. doi: 10.1016/j.immuni.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 57.Blackburn SD, Shin H, Haining WN, Zou T, Workman CJ, Polley A, Betts MR, Freeman GJ, Vignali DA, Wherry EJ. Coregulation of CD8(+) T cell exhaustion by multiple inhibitory receptors during chronic viral infection. Nat Immunol. 2008 doi: 10.1038/ni.1679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Frauwirth KA, Riley JL, Harris MH, Parry RV, Rathmell JC, Plas DR, Elstrom RL, June CH, Thompson CB. The CD28 signaling pathway regulates glucose metabolism. Immunity. 2002;16:769–777. doi: 10.1016/s1074-7613(02)00323-0. [DOI] [PubMed] [Google Scholar]
- 59.Brunner MC, Chambers CA, Chan FK, Hanke J, Winoto A, Allison JP. CTLA-4-Mediated inhibition of early events of T cell proliferation. J Immunol. 1999;162:5813–5820. [PubMed] [Google Scholar]
- 60.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, Collins M, Honjo T, Freeman GJ, Carreno BM. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32:634–643. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 61.Krieg C, Han P, Stone R, Goularte OD, Kaye J. Functional analysis of B and T lymphocyte attenuator engagement on CD4+ and CD8+ T cells. J Immunol. 2005;175:6420–6427. doi: 10.4049/jimmunol.175.10.6420. [DOI] [PubMed] [Google Scholar]
- 62.Riley JL, Mao M, Kobayashi S, Biery M, Burchard J, Cavet G, Gregson BP, June CH, Linsley PS. Modulation of TCR-induced transcriptional profiles by ligation of CD28, ICOS, and CTLA-4 receptors. Proc Natl Acad Sci U S A. 2002;99:11790–11795. doi: 10.1073/pnas.162359999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Keir ME, Latchman YE, Freeman GJ, Sharpe AH. Programmed death-1 (PD-1):PD-ligand 1 interactions inhibit TCR-mediated positive selection of thymocytes. J Immunol. 2005;175:7372–7379. doi: 10.4049/jimmunol.175.11.7372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Sheppard KA, Fitz LJ, Lee JM, Benander C, George JA, Wooters J, Qiu Y, Jussif JM, Carter LL, Wood CR, et al. PD-1 inhibits T-cell receptor induced phosphorylation of the ZAP70/CD3zeta signalosome and downstream signaling to PKCtheta. FEBS Lett. 2004;574:37–41. doi: 10.1016/j.febslet.2004.07.083. [DOI] [PubMed] [Google Scholar]
- 65.Parry RV, Chemnitz JM, Frauwirth KA, Lanfranco AR, Braunstein I, Kobayashi SV, Linsley PS, Thompson CB, Riley JL. CTLA-4 and PD-1 receptors inhibit T-cell activation by distinct mechanisms. Mol Cell Biol. 2005;25:9543–9553. doi: 10.1128/MCB.25.21.9543-9553.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]