Abstract
Background:
To assess the efficacy and safety of S-1 and cisplatin with concurrent thoracic radiation for unresectable stage III non-small-cell lung cancer (NSCLC).
Methods:
Eligible patients were 20–74 years old and had histologically or cytologically confirmed NSCLC, a performance status of 0–1, and no prior chemotherapy. Patients were treated with cisplatin (60 mg m−2 on day 1) and S-1 (orally at 40 mg m−2 per dose, b.i.d., on days 1–14), with the treatment repeated every 4 weeks for four cycles. Beginning on day 2, a 60-Gy thoracic radiation dose was delivered in 30 fractions.
Results:
Of 50 patients, 48 were eligible. Partial response was observed in 42 patients (87.5%; 95% CI: 79.1–96.9%). This regimen was well tolerated. Common toxicities included grade 3/4 neutropenia (32%), grade 3/4 leukopenia (32%), grade 3/4 thrombocytopenia (4%), grade 3 febrile neutropenia (6%), grade 3 oesophagitis (10%), and grade 3 pneumonitis (5%). Median progression-free survival was 12.0 months and median overall survival was 33.1 months. The 1- and 2-year survival rates were 89.5 and 56%, respectively.
Conclusion:
This chemotherapy regimen with concomitant radiotherapy is a promising treatment for locally advanced NSCLC because of its high response rates, good survival rates, and mild toxicities.
Keywords: S-1, non-small-cell lung cancer, chemotherapy, radiotherapy, cisplatin
Lung cancer remains the leading cause of cancer-related deaths worldwide (Jemal et al, 2007). Approximately 30% of non-small-cell lung cancer (NSCLC) patients present with locally advanced lung cancer (van Meerbeeck, 2001). A number of randomised clinical trials support the conclusion that combined-modality approaches improve survival compared with radiotherapy alone for locally advanced lung cancer (Le Chevalier et al, 1991; Non-Small Cell Lung Cancer Collaborative Group, 1995). Depending on the strategy used, chemotherapy may have a cytotoxic role by eradicating distant micrometastases, may have a radiosensitising role by improving local control, or it perhaps may have a role in both effects. Recently, two randomised trials using cisplatin plus older agents from the 1980s compared sequential with concurrent chemoradiotherapy and showed superior survival with the concurrent approach (Furuse et al, 1999; Curran et al, 2003). However, distant metastases remain the major site of failure and it is also likely that more effective chemotherapy will be required for further improvement in results.
During the last decade, several new agents, such as paclitaxel, gemcitabine, vinorelbine, and docetaxel, have been proven to be more effective in metastatic NSCLC than the old regimens (Bunn and Kelly, 1998; Johnson, 1999; Hoffman et al, 2000).
Because of dose-limiting toxicities (Choy et al, 1998; Vokes et al, 2002; Gandara et al, 2003; Zatloukal et al, 2004; Fournel et al, 2005; Bedano et al, 2006; Kiura et al, 2008), these new agents have been tested using a reduced dose or split dose within the newer concurrent chemoradiation regimens. As yet, however, these new strategies have not been proven to be more effective than the older regimens.
S-1 is a new oral fluoropyrimidine agent designed to enhance anticancer activity and to reduce gastrointestinal toxicity through the combined use of an oral fluoropyrimidine agent (tegafur), a dihydropyrimidine dehydrogenase inhibitor (5-chloro-2,4-dihydroxypyridine), and an orotate phosphoribosyl transferase inhibitor (potassium oxonate; Shirasaka et al, 2000). S-1 has been shown to have one of the highest levels of response as a single agent for metastatic NSCLC (Kawahara et al, 2001). Moreover, two phase II trials of S-1 plus cisplatin for advanced NSCLC (stage IIIB without any indications for radiotherapy or stage IV) showed a response rate of 32.7–47% and a median survival time of 11–16 months. In addition, it shows very few severe gastrointestinal or haematological toxicities (Ichinose et al, 2004; Endo et al, 2006).
If the same doses of S-1 plus cisplatin used for advanced NSCLC could be concurrently used with thoracic radiotherapy (TRT), this regimen would be expected to have several advantages over previous regimens. First, the radiation-sensitising effect of 5-FU is well known clinically and S-1 also has been shown to act as a radiosensitiser in preclinical models (Fukushima et al, 1998; Harada et al, 2004). Second, in order to achieve radiosensitisation, prolonged exposure is desirable, and as such, oral administration is the most appealing route of administration. In addition, phase I studies have shown that the half-life of 5-FU after oral S-1 administration has been found to be markedly prolonged compared with that of 5-FU after intravenous administration (van Groeningen et al, 2000; Yamada et al, 2003). Therefore, we conducted a phase II trial using S-1 and cisplatin chemotherapy with concurrent TRT for locally advanced NSCLC.
Patients and methods
Patient selection
Patient eligibility requirements for enrollment in this study included cytologically or histologically documented NSCLC and measurable disease at a locally advanced stage IIIA or IIIB. Patients with T1–T3 and N2 disease if medically inoperable, T4 with any node size and extent, and those with N3 disease with any tumour involvement were eligible. Additional eligibility criteria included patient's age from 20 to 74 years, no prior treatment, Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1, projected life expectancy of at least 3 months, a leukocyte count of 4000–12 000 μl−1, platelet count of ⩾100 000 μl−1, haemoglobin level of ⩾9 g per 100 ml, serum bilirubin level of ⩽1.5 mg per 100 ml, serum aspartate aminotransferase and alanine aminotransferase levels of ⩽100 IU l−1, alkaline phosphatase level of twice the upper limit of normal or less, normal creatinine level, and partial pressure of arterial oxygen ⩾65 torr in room air.
For staging, all patients underwent a computed tomography (CT) scan of the thorax, including the upper abdomen, and either a brain CT scan or brain magnetic resonance imaging. A radioisotopic bone scan or a positron emission tomography (PET) scan was also performed in all of the patients. Mediastinoscopies were not performed and lymph node metastases were clinically diagnosed based on the results of a CT scan and/or PET scan.
Patients with significant pleural effusions or pericardial effusion seen on a CT scan were not eligible. Patients who were pregnant or who had concomitant serious diseases, a concomitant malignancy, or symptomatic cerebral involvement were excluded from the study. The institutional ethics committee of each of the participating institutions approved the protocol and all patients provided written informed consent before starting the study.
Treatment plan
Before therapy was begun, a CT scan of the tumour in the chest was performed in order to determine tumour volume. Treatment in eligible patients began with the administration of two cycles of concurrent chemoradiotherapy that consisted of an oral administration of S-1 (40 mg m−2) twice daily for days 1–14 along with a 60-min intravenous infusion of cisplatin (60 mg m−2) on day 1 and then at 4-week intervals. For the radiation treatment, a 60-Gy dose was given in 30 fractions over a 6-week period. At 2 weeks after the last radiation treatment, patients received two more cycles of consolidation chemotherapy that consisted of the same doses and schedule as for the concurrent chemoradiotherapy. The oral doses of S-1 for each patient were assigned based on the body surface area. The three doses administered were 40 mg (body surface area <1.25 m2), 50 mg (1.25< body surface area <1.50 m2), and 60 mg (body surface area ⩾1.50 m2). Supportive care, which included adequate hydration and antiemetics, was provided. The use of granulocyte colony-stimulating factor during radiotherapy was not permitted.
If changes in the laboratory variables after the start of treatment occurred so that a leukocyte count of >3000 μl−1 or a neutrophil count of >1500 μl−1or any of the other entry eligibility criteria for the study were not met, subsequent courses of treatment were withheld until the noted abnormality had resolved. If there was no resolution of the abnormality within 6 weeks, the patient stopped the protocol treatment but was included in the study analysis.
The doses of S-1 were reduced in the event of any of the following toxicities during the previous treatment cycle: grade 4 haematological toxicity, or grade 3 or more non-haematological toxicity. For the subsequent courses, S-1 was reduced from 60, 50, or 40 mg twice daily to 50, 40, and 25 mg twice daily, respectively.
Radiation therapy
Chest irradiation began concurrently on day 2 in all patients. Two different radiation target volumes were planned: the initial large-field target volume, which consisted of primary tumour and mediastinal lymph nodes, and the boost target volume, which consisted of the primary and involved nodes. Radiation therapy was delivered to the large-field volume (40 Gy) followed by a boost volume (20 Gy) with a dose of 2 Gy daily for 5 days each week for 6 weeks by a linear accelerator generating at least 4 MeV photons. The target volume of the primary tumour included the complete extent of the radiographically defined visible primary tumour and lymph nodes with a minimum 1.0- to 2.0-cm margin around the mass. Contralateral hilar lymph nodes were not included. The objectives were to restrict the relative volume of the normal lung treated with a dose of >20 Gy (V20) to <30%, and the maximum spinal cord dose was restricted to <50 Gy.
Evaluation of response and toxicity
All eligible patients who received any portion of the treatment were considered assessable for response and toxicity. Chest X-rays, complete blood counts, and blood chemistry measurements were done weekly. The response was assessed based on the CT scan findings that initially had been used to define the extent of the tumour. The response was evaluated in accordance with the Response Evaluation Criteria in Solid Tumors (Therasse et al, 2000). The response was confirmed for at least 4 weeks (for a complete or partial response) or 6 weeks (for stable disease) after it was first documented.
Progression-free survival (PFS) was defined as the time from registration until objective tumour progression or death. Patients whose disease had not progressed at the time of study treatment discontinuation continued to be assessed until progression was documented. Overall survival (OS) was defined as the time from registration to death from any cause. Progression-free survival and OS were estimated by the Kaplan–Meier method. Adverse events were graded according to the National Cancer Institute Common Terminology Criteria for Adverse Events, Version 3.0.
Follow-up studies included a post-treatment CT scan at 4–6 weeks from the completion of all chemotherapy. Subsequently, follow-up was every 3 months for 3 years, then every 6 months until disease progression.
Statistical analysis
The primary end point of the study was the response rate, which determined the sample size. For the first stage, Simon's minimax two-stage phase II design (Simon, 1989) was used to allow early termination if the preliminary results indicated minimal efficacy. We chose a 90% response rate as the experimental target level and a 70% response rate as the minimal target level with α- and β-errors of 0.1, resulting in a target of 25 patients. If 19 or more responses were observed among the 25 total assessable patients, the treatment was considered worthy of further consideration. For the second stage, an additional 25 patients were enrolled in order to achieve an overall maximum response rate of 90% with a confidence interval (CI) of 15%
Results
Patient characteristics
A total of 50 patients (43 men and 7 women) were enrolled in the trial between August 2005 and April 2007.
A radioisotopic bone scan was performed in 12 patients, a PET scan in 28 patients, and both were performed in 10 patients. In one patient, the PET scan did not show a bone metastasis, but a radioisotopic bone scan showed a vertebral bone metastasis before treatment. In another patient, a PET scan did not show an adrenal metastasis, but adrenal swelling was detected by an abdominal CT scan before treatment. Thus, the two patients were not eligible for the study.
Patient characteristics are summarised in Table 1. The median age was 63 years. The ECOG performance status was 0 for 72% and 1 for 28% of patients. Histological examinations indicated the presence of the following cancer cell types: adenocarcinoma, 60%; squamous, 28%; and other types, 12%. In total, 22 (44%) patients had stage IIIA disease; 26 (52%) patients had stage IIIB; and 2 (4%) patients had stage IV.
Table 1. Patient characteristics.
| Characteristics | No. of patients |
|---|---|
| Total no. of patients | 50 |
| Age, years | |
| Median | 63 |
| Range | 35–74 |
| Sex | |
| Men | 43 |
| Women | 7 |
| Performance status | |
| 0 | 36 |
| 1 | 14 |
| Histology | |
| Adenocarcinoma | 30 |
| Squamous | 14 |
| Other | 6 |
| Stage | |
| IIIA | 22 |
| T1-3N2M0 | 22 |
| IIIB | 26 |
| T1-3N3M0 | 9 |
| T4N0-1M0 | 6 |
| T4N2M0 | 9 |
| T4N3M0 | 2 |
| IV | 2 |
Treatment delivery
There were 48 patients eligible and assessable for toxicity and response. All but one of these patients (98%) completed the concurrent treatment protocol that consisted of two cycles of chemotherapy and radiotherapy with a dose of 60 Gy. Because of grade 3 oesophagitis, one patient was unable to complete the scheduled concurrent chemoradiotherapy. In total, 43 (90%) patients received at least one cycle of consolidation chemotherapy. Consolidation chemotherapy was started on day 57 to day 81 (median, day 63), and 39 (81%) patients completed two cycles of consolidation chemotherapy.
A total of four patients were unable to complete the two cycles of consolidation chemotherapy because of toxicities (grade 3 pneumonitis, grade 5 cerebral haemorrhage, grade 3 fatigue, and delayed recovery of bone marrow function). In accordance with the study protocol, dose reductions were necessary in 8 out of 48 patients during concurrent treatment (17%) and in 14 out of 43 patients during consolidation chemotherapy (32%).
The projected dose intensities of cisplatin and S-1 were 15 and 280 mg m−2 per week, respectively. The actual dose intensity of cisplatin was 11.2–16 mg m−2 per week (mean: 13.8; 92% of the projected dose intensity), and the actual dose intensity of S-1 was 135–296 mg m−2 per week (mean, 243; 87% of the projected dose intensity).
The radiation dose intensity was 6.5–10 Gy per week (mean, 9.77; 97.7% of the projected dose intensity).
Response to treatment
We observed that 24 out of 25 patients had a partial response in the first stage, and therefore an additional 25 patients were enrolled.
Of 48 patients, none had a complete response, whereas 42 patients had a partial response and 6 patients had stable disease. No patients had progressive disease. The overall response rate was 87.5% (95% CI: 79.1–96.9).
Toxicity of treatment
Major grade 3 and 4 toxicities for the concurrent chemoradiotherapy phase are summarised in Table 2. Concurrent chemoradiotherapy was generally well tolerated. Although 5 out of the 48 (10%) assessable patients had grade 3 oesophagitis, none showed grade 4 radiation-associated oesophagitis. The most common grade 3/4 haematological toxicities were neutropenia (which included two patients with grade 3 febrile neutropenia) and leukopenia (25%). Grade 3/4 anaemia and thrombocytopenia were uncommon (two patients each, 4%) and no patients were observed to have grade 3/4 pneumonitis. Other grade 3/4 toxicities were anorexia (3 patients, 6%), fatigue (2 patients, 4%), diarrhoea (2 patients, 4%) and increases in serum alanine aminotransaminase or aspartate aminotransferase levels (one patient, 2%).
Table 2. Major toxicities.
|
Concurrent chemoradiotherapy (N=48)
|
Consolidation chemotherapy (N=43)
|
|||||||
|---|---|---|---|---|---|---|---|---|
|
Grade 3
|
Grade 4
|
Grade 3
|
Grade 4
|
|||||
| Toxicity | No. | % | No. | % | No. | % | No. | % |
| Haematologic | ||||||||
| Leukopenia | 11 | 23 | 1 | 2 | 3 | 7 | 0 | |
| Neutropenia | 11 | 23 | 0 | 3 | 7 | 1 | 2 | |
| Febrile neutropenia | 2 | 4 | 0 | 1 | 2 | 0 | ||
| Thrombocytopenia | 1 | 2 | 1 | 2 | 0 | 0 | ||
| Anaemia | 2 | 4 | 0 | 2 | 5 | 1 | 2 | |
| Non-haematologic | ||||||||
| Nausea | 1 | 2 | 0 | 0 | 0 | |||
| Anorexia | 3 | 6 | 0 | 2 | 5 | 0 | ||
| Fatigue | 2 | 4 | 0 | 1 | 2 | 0 | ||
| ALT, AST | 1 | 2 | 0 | 0 | 0 | |||
| Pneumonitis | 0 | 0 | 0 | 2 | 5 | 0 | ||
| Oesophagitis | 5 | 10 | 0 | 0 | 0 | |||
| Constipation | 2 | 4 | 0 | 0 | 0 | |||
| Diarrhoea | 2 | 4 | 0 | 0 | 0 | 0 | ||
| Cerebral haemorrhage | 1 (Grade5) | 2 | ||||||
ALT=alanine aminotransaminase; AST=aspartate aminotransferase; Concurrent=S-1 plus cisplatin, and TRT (N=48); Consolidation=S-1 plus cisplatin (N=43).
Table 2 also summarises the major grade 3 and 4 toxicities for the 43 patients who received consolidation chemotherapy. The most common grade 3/4 toxicity was neutropenia (which occurred in 4 (10%) patients, which included one patient with grade 3 febrile neutropenia). Grade 3 pneumonitis was observed in 2 (5%) patients. After steroid therapy, both patients responded and showed improvement.
One patient developed hemiparesis on day 34 of the first consolidation chemotherapy treatment. A brain CT scan showed multiple brain metastases with cerebral haemorrhage, and the patient died on the following day. Although no thrombocytopenia and/or neutropenia were observed, we could not exclude the possibility of treatment-related death.
Survival
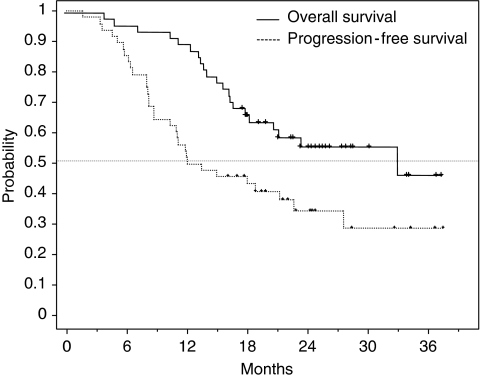
For the current analysis, the median follow-up time was 25.4 months (range, 12–37.6). Of 48 patients, 30 (62.5%) had recurrence of the disease, and to date, 21 of these patients have died. Figure 1 shows OS and PFS for the patients enrolled in the study. The median PFS was 12.0 months and the median OS was 33.1 months. The 1- and 2-year survival rates were 89.5 and 56%, respectively. No correlation was apparent between PFS or OS and sex, age, histology, disease stage, or smoking status.
Figure 1.
Kaplan–Meier overall survival (OS) and progression-free survival (PFS) curves in all patients. The median PFS was 12.0 months and the median OS was 33.1 months.
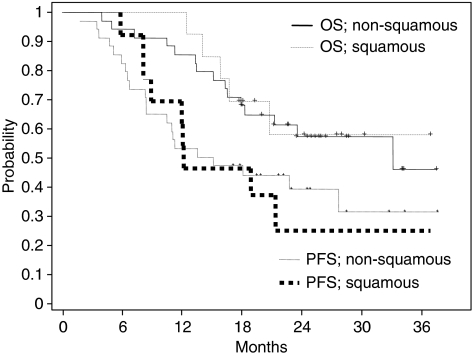
Figure 2 shows OS and PFS curves for the patients with squamous histology and those with non-squamous histology (adenocarcinoma plus other types). There were no differences noted in the survival curves between the squamous and non-squamous histologies.
Figure 2.
Kaplan–Meier overall survival (OS) and progression-free survival (PFS) curves for patients with non-squamous histology (adenocarcinoma plus large cell), and patients with squamous histology.
Among 30 reported sites of first failure, 15 (50%) were distant, 12 (40%) were loco-regional, and 3 (10%) were both loco-regional and distant (Table 3). The brain and lung were the most common sites for distant metastases (17% each).
Table 3. Sites of initial failure.
|
Patients
|
||
|---|---|---|
| First site of failure | No. | % |
| Local | 12 | 40 |
| Local+distant | 3 | 10 |
| Distant | 15 | 50 |
| Brain | 5 | 17 |
| Lung | 5 | 17 |
| Liver | 2 | 7 |
| Bone | 2 | 7 |
| Adrenal | 1 | 3 |
Discussion
This is the first phase II study to investigate the use of the new oral fluoropyrimidine agent, S-1, in the curative-intent therapy setting of stage III NSCLC. Our data indicate positive results with only mild toxicities after treatment consisting of concomitant radiotherapy in addition to S-1 plus cisplatin administered at the same doses as those used for advanced metastatic NSCLC. The median OS of 33.1 months, and the 1- and 2-year survival rates of 89.5 and 56% are very encouraging.
The advantages of using concomitant chemoradiotherapy have been shown by both the West Japan Lung Cancer Group study trial, which evaluated mitomycin, vinblastine, and cisplatin regimens, and the similarly designed Radiation Therapy Oncology Group trial, which evaluated cisplatin and vinblastine (Furuse et al, 1999; Curran et al, 2003). The concurrent chemoradiotherapy arm in the Japanese study resulted in a median OS of 16.5 months with survival rates at 1 and 2 years of 64.1 and 34.6%, respectively (Furuse et al, 1999).
Recently, new agents, such as paclitaxel, vinorelbine, docetaxel, and gemcitabine, have been evaluated in several studies that administered the agents in combination with platinum when using an induction and/or concurrent strategy. However, for many of these newer agents, the dose-limiting toxicities require that lower doses be given during the concurrent phase (Choy et al, 1998; Vokes et al, 2002; Kiura et al, 2003; Belani et al, 2005; Fournel et al, 2005).
The median PFS and the median OS for these new concurrent regimens were 8.4–16.3 and 14.8–23.4 months, respectively. Overall survival rates at 1 and 2 years were 56.3–76 and 25–54%, respectively. These results are not satisfactory. Compared with these studies, the results of our study are exceptionally good.
The increased response rate and prolonged survival seen in this study could explain the radiation-sensitising effect resulting from prolonged exposure to S-1, because it was administered orally on a daily basis. The European Organization for Research and Treatment of Cancer reported a randomised trial that evaluated radiation alone, radiation plus cisplatin at 30 mg m−2, weekly, or radiation plus cisplatin at 6 mg m−2, daily (Schaake-Koning et al, 1992). Results showed an improvement in the OS for radiation plus daily cisplatin as against that for radiation alone. However, the analysis of the patterns of failure indicated there was improvement in regional control only. Therefore, prolonged exposure to S-1 may enhance the radiation effect and contribute to improved local control.
Alternatively, it could be argued that prolonged survival was due to substantial second-line treatment, especially epidermal growth factor receptor (EGFR) tyrosine kinase inhibitors (TKIs), because the median PFS in this study was 12.0 months, which is not exceptionally good compared with previous reports. There were 16 out of 30 relapsed patients receiving substantial second-line treatment. Although we did not analyse EGFR mutational status in this study, 7 out of 30 relapsed patients received EGFR-TKIs. Only one patient responded to an EGFR-TKI, hence the use of EGFR-TKIs cannot exclusively explain the prolonged survival, and it is unclear why we observed so much difference between the median PFS and OS.
Another advantage of using this regimen is that there are only mild toxicities and very few treatment interruptions. Overall, 98% of our study patients completed the concurrent chemoradiotherapy, and 90% of the patients received at least one cycle of consolidation chemotherapy, resulting in a total of 81% of patients completing the planned treatment.
Oesophagitis is the principal toxicity, but only 5 (10%) patients developed grade 3/4 oesophagitis. A possible reason for mild oesophagitis is that potassium oxonate may inhibit the formation of 5-FU nucleotides in the oesophagus, thereby reducing the radiation-sensitising effect in oesophagus rather than in the tumour.
In addition, we observed grade 2 and 3 pulmonary toxicity in only three and two patients, respectively, and there was no grade 5 pulmonary toxicity seen. The reason for the low incidence of severe radiation pneumonitis is unclear, and one possible explanation may be that it was due to the administration of TRT using three-dimensional treatment planning.
Recently, two reports on phase I studies of split-dose S-1 and cisplatin combined with concurrent radiotherapy were published (Kaira et al, 2008; Chikamori et al, 2009). However, this split-dose regimen using cisplatin and S-1 has not been investigated for advanced NSCLC, and we do not know the potential efficacy of this chemotherapy. In addition, we cannot consider our chemoradiotherapy regimen as full-dose chemoradiotherapy, because the actual dose intensities of cisplatin and S-1 in this study were less than the dose intensities of a previous phase II study (70 and 65% for cisplatin and S-1, respectively, compared with the doses in the study reported by Endo et al, 2006).
Differences in the toxicity profile of S-1 as a single agent were observed between Japanese and Caucasians in previous studies (van Groeningen et al, 2000; Hoff et al, 2003). Although haematological toxicities predominated in Japan, diarrhoea was the dose-limiting toxicity in Western countries. In our study, only Japanese patients were enrolled; the toxicity profile of this chemoradiotherapy regimen may be different in Caucasians.
There are several other new agents of interest for stage III NSCLC. In particular, pemetrexed, which is a potent inhibitor of thymidylate synthase, seems promising (Taylor et al, 1992; Schultz et al, 1999). A recent phase III study showed that pemetrexed plus cisplatin yielded efficacy results in advanced NSCLC comparable to those for gemcitabine plus cisplatin (Scagliotti et al, 2008). In addition, pemetrexed plus cisplatin has an excellent safety profile; a recent phase I study showed that a full dose of pemetrexed plus cisplatin in conjunction with full-dose concurrent TRT is well tolerated (Brade et al, 2008). In patients with squamous carcinoma, however, cisplatin plus pemetrexed resulted in significantly worse survival than cisplatin/gemcitabine (Scagliotti et al, 2008). Using pemetrexed in the stage III setting is disadvantageous because stage III NSCLC may include squamous carcinoma more frequently than is seen in stage IV NSCLC. Although S-1 is also a potent inhibitor of thymidylate synthase, we did not observe any differences in either the response rate or survival between the squamous and non-squamous groups. However, because of the small number of patients in this study, we are unable to draw any solid conclusions, and it will be necessary to conduct a larger study to compare the efficacies for the different histology types.
Although we observed a high response rate for this regimen, analysis of the initial sites of failure indicated that local failure still occurred in half of the patients with recurrences, and thus our results highlight the need for further improvement in local control. With regard to local control, currently an additional treatment of interest for stage III NSCLC is dose escalation of TRT that uses three-dimensional treatment planning. In a recent randomised phase II study by the Cancer and Leukemia Group B, this new strategy resulted in a median survival of 24 months and a 1-year survival rate of 66.7% (Socinski et al, 2008). Therefore, we are currently in the process of testing full-dose S-1 and cisplatin chemotherapy in conjunction with concurrent high-dose TRT that uses three-dimensional conformal radiation therapy.
In conclusion, the response rates and survival of the patients enrolled in this trial are most encouraging and compare favourably with the results from similar multimodality studies. A further randomised trial will be necessary to fully evaluate the usefulness of these current findings.
References
- Bedano PM, Neubauer M, Ansari R, Govindan R, Einhorn LH, Bruetman D, White A, Breen T, Juliar B, Hanna N (2006) Phase III study of cisplatin (P) plus etoposide (E) with concurrent chest radiation (XRT) followed by docetaxel (D) vs observation in patients (pts) with stage III non-small cell lung cancer (NSCLC): An interim toxicity analysis of consolidation therapy. J Clin Oncol 2006 ASCO Annu Meet Proc 24 (20 June Suppl): Part I; abstract no. 7043 [Google Scholar]
- Belani CP, Choy H, Bonomi P, Scott C, Travis P, Haluschak J, Curran Jr WJ (2005) Combined chemoradiotherapy regimens of paclitaxel and carboplatin for locally advanced non-small-cell lung cancer: a randomized phase II locally advanced multi-modality protocol. J Clin Oncol 23: 5883–5891 [DOI] [PubMed] [Google Scholar]
- Brade AM, Bezjak A, MacRae R, Laurie SA, Pond GR, Wang L, Dick C, Afinec A, Iscoe N, Shepherd FA (2008) A phase I study of concurrent pemetrexed (P)/cisplatin (C)/radiation (RT) for unresectable stage IIIA/B non-small cell lung cancer (NSCLC). J Clin Oncol 26 (20 May Suppl): abstract no. 7550 [Google Scholar]
- Bunn Jr PA, Kelly K (1998) New chemotherapeutic agents prolong survival and improve quality of life in non-small cell lung cancer: a review of the literature and future directions. Clin Cancer Res 4: 1087–1100 [PubMed] [Google Scholar]
- Chikamori K, Kishino D, Takigawa N, Hotta K, Nogami N, Kamei H, Kuyama S, Gemba K, Takemoto M, Kanazawa S, Ueoka H, Segawa Y, Takata S, Tabata M, Kiura K, Tanimoto M (2009) A phase I study of combination S-1 plus cisplatin chemotherapy with concurrent thoracic radiation for locally advanced non-small cell lung cancer. Lung Cancer 65: 74–79 [DOI] [PubMed] [Google Scholar]
- Choy H, Akerley W, Safran H, Graziano S, Chung C, Williams T, Cole B, Kennedy T (1998) Multiinstitutional phase II trial of paclitaxel, carboplatin, and concurrent radiation therapy for locally advanced non-small-cell lung cancer. J Clin Oncol 16: 3316–3322 [DOI] [PubMed] [Google Scholar]
- Curran WJ, Scott CB, Langer CJ, Komaki R, Lee JS, Hauser S, Movsas B, Wasserman T, Sause W, Cox JD (2003) Long-term benefit is observed in a phase III comparison of sequential vs concurrent chemo-radiation for patients with unresected stage III NSCLC: RTOG 9410. Proc Am Soc Clin Oncol 22; abstract no. 2499a [Google Scholar]
- Endo M, Yamamoto N, Sakai H, Kubota K, Kunitoh H, Nakagawa K, Takeda K, Ichinose Y (2006) Phase II study of 3-weekly S-1 plus cisplatin in patients with advanced non-small cell lung cancer (NSCLC). Proc ESMO; abstract 752P [DOI] [PubMed]
- Fournel P, Robinet G, Thomas P, Souquet PJ, Lena H, Vergnenegre A, Delhoume JY, Le Treut J, Silvani JA, Dansin E, Bozonnat MC, Daures JP, Mornex F, Perol M (2005) Randomized phase III trial of sequential chemoradiotherapy compared with concurrent chemoradiotherapy in locally advanced non-small-cell lung cancer: Groupe Lyon-Saint-Etienne d’Oncologie Thoracique-Groupe Francais de Pneumo-Cancerologie NPC 95-01 Study. J Clin Oncol 23: 5910–5917 [DOI] [PubMed] [Google Scholar]
- Fukushima M, Satake H, Uchida J, Shimamoto Y, Kato T, Takechi T, Okabe H, Fujioka A, Nakano K, Ohshimo H, Takeda S, Shirasaka T (1998) Preclinical antitumor efficacy of S-1: a new oral formulation of 5-fluorouracil on human tumor xenografts. Int J Oncol 13: 693–698 [DOI] [PubMed] [Google Scholar]
- Furuse K, Fukuoka M, Kawahara M, Nishikawa H, Takada Y, Kudoh S, Katagami N, Ariyoshi Y (1999) Phase III study of concurrent vs sequential thoracic radiotherapy in combination with mitomycin, vindesine, and cisplatin in unresectable stage III non-small-cell lung cancer. J Clin Oncol 17: 2692–2699 [DOI] [PubMed] [Google Scholar]
- Gandara DR, Chansky K, Albain KS, Leigh BR, Gaspar LE, Lara Jr PN, Burris H, Gumerlock P, Kuebler JP, Bearden III JD, Crowley J, Livingston R (2003) Consolidation docetaxel after concurrent chemoradiotherapy in stage IIIB non-small-cell lung cancer: phase II Southwest Oncology Group Study S9504. J Clin Oncol 21: 2004–2010 [DOI] [PubMed] [Google Scholar]
- Harada K, Kawaguchi S, Supriatno, Onoue T, Yoshida H, Sato M (2004) Combined effects of the oral fluoropyrimidine anticancer agent, S-1 and radiation on human oral cancer cells. Oral Oncol 40: 713–719 [DOI] [PubMed] [Google Scholar]
- Hoff PM, Saad ED, Ajani JA, Lassere Y, Wenske C, Medgyesy D, Dwivedy S, Russo M, Pazdur R (2003) Phase I study with pharmacokinetics of S-1 on an oral daily schedule for 28 days in patients with solid tumors. Clin Cancer Res 9: 134–142 [PubMed] [Google Scholar]
- Hoffman PC, Mauer AM, Vokes EE (2000) Lung cancer. Lancet 355: 479–485 [DOI] [PubMed] [Google Scholar]
- Ichinose Y, Yoshimori K, Sakai H, Nakai Y, Sugiura T, Kawahara M, Niitani H (2004) S-1 plus cisplatin combination chemotherapy in patients with advanced non-small cell lung cancer: a multi-institutional phase II trial. Clin Cancer Res 10: 7860–7864 [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun MJ (2007) Cancer statistics 2007. CA Cancer J Clin 57: 43–66 [DOI] [PubMed] [Google Scholar]
- Johnson DH (1999) Treatment strategies for metastatic non small-cell lung cancer. Clin Lung Cancer 1: 34–41 [DOI] [PubMed] [Google Scholar]
- Kaira K, Sunaga N, Yanagitani N, Kawata T, Utsugi M, Shimizu K, Ebara T, Kawamura H, Nonaka T, Ishikawa H, Sakurai H, Suga T, Hara K, Hisada T, Ishizuka T, Nakano T, Mori M (2008) Phase I study of oral S-1 plus cisplatin with concurrent radiotherapy for locally advanced non-small-cell lung cancer. Int J Radiat Oncol Biol Phys (in press) [DOI] [PubMed]
- Kawahara M, Furuse K, Segawa Y, Yoshimori K, Matsui K, Kudoh S, Hasegawa K, Niitani H (2001) Phase II study of S-1, a novel oral fluorouracil, in advanced non-small-cell lung cancer. Br J Cancer 85: 939–943 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiura K, Takigawa N, Segawa Y, Kamei H, Takemoto M, Tabata M, Ueoka H, Hiraki S, Matsuo K, Tanimoto M (OLCSG) (2008) Randomized phase III trial of docetaxel and cisplatin combination chemotherapy vs mitomycin, vindesine, and cisplatin combination chemotherapy with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer: OLCSG 0007. J Clin Oncol 26 (20 May Suppl): abstract no. 7515 [Google Scholar]
- Kiura K, Ueoka H, Segawa Y, Tabata M, Kamei H, Takigawa N, Hiraki S, Watanabe Y, Bessho A, Eguchi K, Okimoto N, Harita S, Takemoto M, Hiraki Y, Harada M, Tanimoto M (2003) Phase I/II study of docetaxel and cisplatin with concurrent thoracic radiation therapy for locally advanced non-small-cell lung cancer. Br J Cancer 89: 795–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Chevalier T, Arriagada R, Quoix E, Ruffie P, Martin M, Tarayre M, Lacombe-Terrier MJ, Douillard JY, Laplanche A (1991) Radiotherapy alone vs combined chemotherapy and radiotherapy in nonresectable non-small-cell lung cancer: first analysis of a randomized trial in 353 patients. J Natl Cancer Inst 83: 417–423 [DOI] [PubMed] [Google Scholar]
- Non-Small Cell Lung Cancer Collaborative-Group (1995) Chemotherapy in non-small cell lung cancer: a meta-analysis using updated data on individual patients from 52 randomised clinical trials. Non-small Cell Lung Cancer Collaborative Group. BMJ 311: 899–909 [PMC free article] [PubMed] [Google Scholar]
- Scagliotti GV, Parikh P, von Pawel J, Biesma B, Vansteenkiste J, Manegold C, Serwatowski P, Gatzemeier U, Digumarti R, Zukin M, Lee JS, Mellemgaard A, Park K, Patil S, Rolski J, Goksel T, de Marinis F, Simms L, Sugarman KP, Gandara D (2008) Phase III study comparing cisplatin plus gemcitabine with cisplatin plus pemetrexed in chemotherapy-naive patients with advanced-stage non-small-cell lung cancer. J Clin Oncol 26: 3543–3551 [DOI] [PubMed] [Google Scholar]
- Schaake-Koning C, van den Bogaert W, Dalesio O, Festen J, Hoogenhout J, van Houtte P, Kirkpatrick A, Koolen M, Maat B, Nijs A (1992) Effects of concomitant cisplatin and radiotherapy on inoperable non-small-cell lung cancer. N Engl J Med 326: 524–530 [DOI] [PubMed] [Google Scholar]
- Schultz RM, Patel VF, Worzalla JF, Shih C (1999) Role of thymidylate synthase in the antitumor activity of the multitargeted antifolate, LY231514. Anticancer Res 19: 437–443 [PubMed] [Google Scholar]
- Shirasaka T, Yamamitsu S, Tsuji A, Taguchi T (2000) Conceptual changes in cancer chemotherapy: from an oral fluoropyrimidine prodrug, UFT, to a novel oral fluoropyrimidine prodrug, S-1, and low-dose FP therapy in Japan. Invest New Drugs 18: 315–329 [DOI] [PubMed] [Google Scholar]
- Simon R (1989) Optimal two-stage designs for phase II clinical trials. Control Clin Trials 10: 1–10 [DOI] [PubMed] [Google Scholar]
- Socinski MA, Blackstock AW, Bogart JA, Wang X, Munley M, Rosenman J, Gu L, Masters GA, Ungaro P, Sleeper A, Green M, Miller AA, Vokes EE (2008) Randomized phase II trial of induction chemotherapy followed by concurrent chemotherapy and dose-escalated thoracic conformal radiotherapy (74 Gy) in stage III non-small-cell lung cancer: CALGB 30105. J Clin Oncol 26: 2457–2463 [DOI] [PubMed] [Google Scholar]
- Taylor EC, Kuhnt D, Shih C, Rinzel SM, Grindey GB, Barredo J, Jannatipour M, Moran RG (1992) A dideazatetrahydrofolate analogue lacking a chiral center at C-6, N-[4-[2-(2-amino-3,4-dihydro-4-oxo-7H-pyrrolo[2,3-d]pyrimidin-5-yl)ethyl]benzoyl]-L-glutamic acid, is an inhibitor of thymidylate synthase. J Med Chem 35: 4450–4454 [DOI] [PubMed] [Google Scholar]
- Therasse P, Arbuck SG, Eisenhauer EA, Wanders J, Kaplan RS, Rubinstein L, Verweij J, Van Glabbeke M, van Oosterom AT, Christian MC, Gwyther SG (2000) New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst 92: 205–216 [DOI] [PubMed] [Google Scholar]
- van Groeningen CJ, Peters GJ, Schornagel JH, Gall H, Noordhuis P, de Vries MJ, Turner SL, Swart MS, Pinedo HM, Hanauske AR, Giaccone G (2000) Phase I clinical and pharmacokinetic study of oral S-1 in patients with advanced solid tumors. J Clin Oncol 18: 2772–2779 [DOI] [PubMed] [Google Scholar]
- van Meerbeeck JP (2001) Staging of non-small cell lung cancer: consensus, controversies and challenges. Lung Cancer 34(Suppl 2): S95–S107 [DOI] [PubMed] [Google Scholar]
- Vokes EE, Herndon II JE, Crawford J, Leopold KA, Perry MC, Miller AA, Green MR (2002) Randomized phase II study of cisplatin with gemcitabine or paclitaxel or vinorelbine as induction chemotherapy followed by concomitant chemoradiotherapy for stage IIIB non-small-cell lung cancer: cancer and leukemia group B study 9431. J Clin Oncol 20: 4191–4198 [DOI] [PubMed] [Google Scholar]
- Yamada Y, Hamaguchi T, Goto M, Muro K, Matsumura Y, Shimada Y, Shirao K, Nagayama S (2003) Plasma concentrations of 5-fluorouracil and F-beta-alanine following oral administration of S-1, a dihydropyrimidine dehydrogenase inhibitory fluoropyrimidine, as compared with protracted venous infusion of 5-fluorouracil. Br J Cancer 89: 816–820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zatloukal P, Petruzelka L, Zemanova M, Havel L, Janku F, Judas L, Kubik A, Krepela E, Fiala P, Pecen L (2004) Concurrent vs sequential chemoradiotherapy with cisplatin and vinorelbine in locally advanced non-small cell lung cancer: a randomized study. Lung Cancer 46: 87–98 [DOI] [PubMed] [Google Scholar]