Abstract
Hydrogen peroxide (H2O2) is a central modulator of stomatal closure. It remains unknown, however, how the upstream regulation of H2O2 homeostasis operates. In this issue of Genes & Development, Huang and colleagues (pp. 1805–1817) report that a novel C2H2-type transcription factor, drought and salt tolerance (DST), mediates H2O2-induced stomatal closure and abiotic stress tolerance.
Keywords: Rice, zinc finger protein, drought tolerance, salt tolerance, stomatal aperture, hydrogen peroxide
As sessile organisms, plants have evolved a wide variety of strategies to respond to, struggle with, and ultimately adapt to environmental adversities such as drought and salt stresses. At the molecular level, many transcription factors (TFs) have evolved to function as crucial mediators and/or modulators of diverse signaling pathways to help plants respond to various forms of abiotic stress. However, drought and salt stresses still pose severe threats to plant growth and agricultural production worldwide (Mahajan and Tuteja 2005; Ren et al. 2005;Karaba et al. 2007).
Both drought and salinity require an osmotic response. Since stomata play crucial roles in optimizing water use efficiency, stomatal opening or closing is important not only for CO2 uptake but also for the response to drought and salt stresses. Reactive oxygen species (ROS) including hydrogen peroxide (H2O2) have been known as important signaling components controlling stomatal movement (Bienert et al. 2006), although the molecular mechanism of ROS-mediated stomatal closure remains poorly understood. In this issue of Genes & Development, a research team led by Dr. Hong-Xuan Lin (Huang et al. 2009) reveals a novel signaling pathway for H2O2-mediated stomatal closure. They cloned and characterized the drought and salt tolerance (dst) gene, and show that it encodes a zinc finger TF that controls the expression of genes involved in H2O2 homeostasis. These findings provide new insight into the process of stomatal movement, as well as a novel strategy for engineering drought and salt tolerance in crops.
Abscisic acid (ABA)-mediated stomatal closure via H2O2
Stomata are small pores distributed in the epidermis of plant leaves, which are surrounded by a pair of guard cells. Through the stomata, CO2 in the atmosphere enters the plant as the carbon source for photosynthesis, and water vapor is released into the atmosphere in a process called transpiration (Hetherington and Woodward 2003). The opening or closing of stomata is controlled by turgor pressure in guard cells, and an increase or decrease in the guard cell turgor pressure enhances or reduces stomatal aperture, respectively (Assmann 1994; Hetherington 2001). Under drought or highly saline conditions, stomatal closure provides an adaptive strategy to mitigate transpiration-mediated water loss (Hugouvieux et al. 2001).
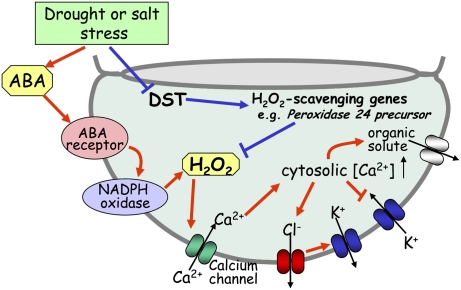
It is widely accepted that the plant stress hormone ABA induces stomatal closure. ABA is a weak acid that is inductively synthesized in plants subjected to a wide variety of abiotic stresses. It is able to diffuse through cell membranes, and thus triggers a signaling cascade in guard cells (Davies and Jones 1991; Assmann 1994). The signaling cascade mediated by ABA causes the efflux of K+ and Cl− and the removal of organic solutes from guard cells, thereby reducing cellular turgor and leading to rapid stomatal closing to prevent water loss by transpiration (Fig. 1; MacRobbie 1998; Blatt 2000; Schroeder et al. 2001; Cominelli et al. 2005). In the process of this ABA-mediated stomatal closure, H2O2 plays a vital role as a secondary messenger by elevating calcium levels in guard cells through the activation of plasma membrane calcium channels. In this context, there is some evidence to show that H2O2 and calcium are important factors in ABA-mediated stomatal closing. For example, Pei et al. (2000) reported that the application of exogenous ABA results in H2O2 generation in guard cells of Arabidopsis. They also showed the activation of calcium channels in guard cell plasma membrane by H2O2, and such H2O2-activated calcium channels mediate increases in the calcium level in the cytosol of guard cells. Furthermore, double-knockout Arabidopsis mutants of NADPH oxidase catalytic subunits, AtrbohD and AtrbohF, which are involved in the production of H2O2 in guard cells of Arabidopsis, showed failure in ABA-promoted H2O2 production, calcium channel activation, and stomata closure (Kwak et al. 2003). To date, the signaling pathway related to abiotic stress tolerance that leads to stomatal closure via H2O2 as a secondary messenger is widely known as being induced by ABA.
Figure 1.
Schematic model for abiotic stress-triggered stomatal closure mediated by H2O2. The net ion release and turgor reduction lead to stomatal closing. ABA-dependent process (red arrows and clamps): Under conditions of drought or salt stress, ABA induces H2O2 production via the activation of plasma membrane NADPH oxidases. Then, H2O2 activates plasma membrane calcium channels resulting in cytosolic Ca2+ elevation. This cytosolic Ca2+ elevation activates outward Cl− channels and inactivates inward K+ channels, causing membrane depolarization. Membrane depolarization activates outward K+ channels and facilitates the efflux of organic solutes. ABA-independent process (blue arrow and clamp): The expression of DST is negatively regulated by drought or salt stress. DST positively regulates the expression of H2O2 scavenger genes; e.g., peroxidase 24 precursor.
Novel ABA-independent stomatal closure mechanism
The DST-mediated pathway operates in an ABA-independent pattern (Huang et al. 2009), indicative of a novel system linking the regulation of H2O2 accumulation to stomatal closure and abiotic tolerance in plants. The finding of this ABA-independent signaling mechanism was triggered by the isolation of a rice mutant, dst, which shows markedly reduced wilting during drought treatment and enhanced tolerance to high-salt stresses. The microscopic examination of the stomatal status of the mutant leaves at normal conditions revealed a higher occurrence of stomatal closure. Consequently, stomatal conductance, which is determined by the opening and density of stomata, is lower in the mutant than in its wild-type counterpart under both normal and stressed conditions. Based on these results, Huang et al. (2009) performed a series of physiological analyses and found that the leaves of the dst mutant have a better capacity to maintain relative water content and osmolality under dehydration stress, while the shoots are able to reduce cation (Na+ and K+) concentration upon high NaCl treatment. As such, it is reasonable to infer that the reduction of the steady state of stomatal aperture in the dst mutant causally confers on plants enhanced tolerance to drought and salt stresses. Interestingly, the endogenous level of ABA in the dst mutant was not different from that in the wild-type plant, indicating that the increased stomatal closure did not relate to ABA accumulation. As mentioned previously, stomatal closure under abiotic stress condition has been considered to be ABA-dependent, and therefore the stomatal closure in the dst mutant may depend on an unknown mechanism.
In the process of ABA-mediated stomatal closure, H2O2 activates plasma membrane calcium channels in guard cells and triggers stomatal closure. Interestingly, the dst mutant plants also contain higher levels of H2O2 in their guard cells. The higher level of H2O2 in the dst guard cells correlates with increased stomatal closure at normal conditions and enhanced abiotic tolerance. Thus, the H2O2-mediated signaling pathway for abiotic stress tolerance may also function in this mutant. Although Huang et al. (2009) did not examine the activity of calcium channels or the level of calcium in guard cells, it is possible that the increased H2O2 in dst mutants may also function as an effector for stomatal closure like in the ABA-mediated system. Hence, the possibility of the cross-talk between the DST-mediated pathway and the ABA-dependent signaling network on H2O2 accumulation exists. In this context, the fact that NADPH oxidases essentially function to produce H2O2 in the ABA-mediated stomatal closure pathway while DST functions to induce some scavengers of H2O2, may be significant (see below). This leads us to speculate that the ABA-dependent pathway regulates stomatal closure mainly via an H2O2-producing enzyme, such as the NADPH oxidase, while the ABA-independent pathway might depend on H2O2 scavenging enzymes. Differences in experimental design may underlie observed differences in H2O2 homeostatic mechanisms: Most of the experiments on ABA-dependent stomatal closure have been carried out in Arabidopsis, whereas an ABA-independent DST-mediated pathway has been studied in rice. However, Arabidopsis mutants for cytosolic and chloroplastic ascorbate peroxidase, which are responsible for H2O2 removal, show more tolerance to salinity stress (Miller et al. 2007), indicating that H2O2 removal by scavenging enzymes is also an available strategy to gain elevated abiotic stress tolerance in Arabidopsis. Nevertheless, further research into the mechanisms of H2O2 homeostasis regulation in both Arabidopsis and rice plants is surely welcomed.
DST: a new face in abiotic stress tolerance
Studies over the past decade have revealed that various types of TFs mediate plants' responses to abiotic stress (Hu et al. 2006). Two well-characterized examples of the involvement of TFs in abiotic tolerance are the DREB (CBF) and AREB (ABF) bZIP TFs, which interact with the drought responsible cis-acting element (DRE) and the ABA-responsive element (ABRE), respectively (Oeda et al. 1991; Yamaguchi-Shinozaki and Shinozaki 1994). Identification of these TFs has largely relied on molecular biological approaches via the identification and characterization of cis-elements in stress-responsive genes. Only recently has the involvement of the TFs in H2O2-mediated stomatal closure been elucidated. For example, a NAC-type TF in rice, SNAC1 (STRESS-RESPONSIVE NAC1), is expressed predominantly in guard cells, and its overexpression results in increased stomatal closure and improved drought and salt tolerance at the vegetative stage, as well as increased sensitivity to ABA (Hu et al. 2006). Two Arabidopsis guard cell-specific MYB TFs, AtMYB60 and AtMYB61, are functionally characterized as important modulators of stomatal aperture and plant drought tolerance. Whereas the former is negatively regulated by drought and reduces stomatal opening in its null mutant, the latter positively modulates stomatal closure, and loss of its activity resulted in more-open stomata (Cominelli et al. 2005; Liang et al. 2005). Although these observations suggest that some TFs are involved in stomatal movement, how these TFs are involved in stomatal movement has not been clarified. In this context, the study on DST by Huang et al. (2009) provides new insight.
Positional cloning and transgenic experiments revealed that the causal gene for the dst mutation encodes a previously unknown C2H2-type zinc finger-containing protein (Huang et al. 2009). The C2H2-type zinc finger is one of the most common DNA-binding motifs for eukaryotic TFs, and it typically contains two or more finger domains for DNA interaction (Wolfe et al. 2000). Sometimes such zinc fingers are also involved in protein–protein contact, interacting directly with other TFs, but they have never been shown to function as transcriptional activation domains (Lin et al. 2007). Previous studies have revealed that zinc finger TFs are often involved in response to various abiotic stresses. For example, Arabidopsis ZPT2-related proteins, which contain two canonical C2H2-type zinc finger motifs, function as transcription repressors under drought, cold, and high-salinity stress conditions (Sakamoto et al. 2004). The DST protein is unique in that its single zinc finger motif is required for both its DNA binding and transcriptional activation (Huang et al. 2009). These findings indicate that DST is a new type of C2H2-type zinc finger TF, and prompt our reconsideration of the previous knowledge about this type of TF.
How does DST regulate stomata aperture?
As mentioned previously, the biosynthetic enzyme NADPH oxidase regulates the H2O2 homeostasis in the ABA-dependent pathway. Then, how does the ABA-independent, DST-mediated pathway increase the level of H2O2 under abiotic stress conditions? To answer this question, Huang et al. (2009) searched some putative target genes of DST, whose products may be related to the homeostasis of H2O2. First, they identified the nucleotide sequences interacting with DST by a bacterial one-hybrid system and electrophoretic mobility shift assay. Then, they performed expression profile analysis by microarray and screened 60 genes, the expression levels of which were either significantly up-regulated or down-regulated in the dst mutant background compared with that of wild type. Of these genes, five were selected based on three criteria: the expected relevance of the gene product to H2O2 homeostasis, down-regulated expression in the mutant, and DST-interacting sequences in the promoter. Chromatin immunoprecipitation (ChIP) assay confirmed that DST binds directly to the interacting sequences of each of these genes.
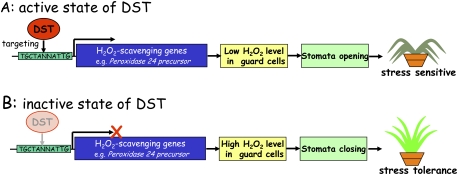
Huang et al. (2009) focused specifically on the gene encoding peroxidase 24 precursor, as they expected this gene to directly scavenge H2O2. Biochemical experiments confirmed that the product of this gene has in vitro peroxidase activity, catalyzing the reduction of H2O2 (Huang et al. 2009). They also compared the expression pattern between DST and peroxidase 24 precursor and found that both genes share a similar expression pattern; that is, both are preferentially expressed in guard cells and responded to salt and drought stresses. Based on these observations, Huang et al. (2009) proposed a model for the role of DST in the regulation of abiotic stress (Fig. 2). In the active state, DST interacts directly with its target sequences located in the promoter regions of H2O2 homeostasis genes to enhance their expression. Enhanced expression of H2O2 homeostasis genes, including peroxidase 24 precursor, decreases the H2O2 level in guard cells and consequently opens the stomata. Under such a condition, plants become sensitive to salt and drought stresses (Fig. 2A). Under saline or drought conditions, the expression of DST in the wild-type plant or in the dst mutant is suppressed or failed, resulting in the down-regulation of H2O2 homeostasis genes. Consequently, the H2O2 level is increased, promoting stomatal closure and thereby enhancing drought and salt tolerance (Fig. 2B).
Figure 2.
Role of DST in abiotic stress tolerance via stomatal movement. (A) In its active state , DST binds to its interacting sequences (TGCTANNATTG) located in the promoter regions of H2O2 scavenging genes (e.g., Peroxidase 24 precursor) to induce gene expression, decrease H2O2 levels, and induce stomatal opening to heighten sensitivity to stress. (B) In its inactive state (either in dst mutant or in wild-type plants under stress), DST cannot activate the expression of H2O2 scavenging genes, resulting in high levels of H2O2, stomatal closure, and enhanced drought and salt tolerance.
Huang et al. (2009) also examined the relationship between the DST-mediated H2O2 homeostasis and ABA-dependent stress response pathway. Exogenous ABA treatment did not significantly affect the DST expression, and no significant difference in stomatal opening was observed among the wild type, dst mutant, complementation line, or RNAi line under ABA treatment. These observations confirmed the independence of DST-mediated H2O2 homeostasis from the ABA-dependent pathway. However, cross-talk between these two pathways remains an important avenue for future investigation.
Application of the dst mutation to crop breeding
An unexpected finding was that the dst mutant plant shows normal grain productivity. Since the opening or closing of stomata controls both photosynthesis and transpiration (Hetherington and Woodward 2003), the higher occurrence of stomatal closure in the mutant would have been expected to have adverse effects on photosynthesis, plant growth, and grain productivity. However, Huang et al. (2009) found that this is not the case with the dst mutant. The mutant shows only prominent drought and salt tolerance, without significant deficiencies in grain productivity. Based on these observations, Huang et al. (2009) proposed a strategy for engineering abiotic stress tolerance using the knockout allele of DST. This strategy seems very attractive for producing abiotic stress-hardy crops. However, we may have to be careful in adapting this strategy in practical breeding. Huang et al. (2009) discussed, based on the phenotypic analysis of dst, that smaller stomatal opening and lower stomatal density in the dst plant are sufficient for CO2 influx and growth. Why, then, does the rice plant maintain the DST gene in an active state? If the loss-of-function allele of DST is more suitable for surviving stress conditions without any negative effects, such a mutant allele would ultimately take the place of the functional allele during the long breeding history of rice. In this context, Huang et al. (2009) observed pleiotropic effects of this dst mutation on stomatal density and leaf width. This suggests that dst is involved not only in the upstream regulation of H2O2 homeostasis in guard cells but also in regulating the development of tissues and/or organs. Further detailed phenotypic analyses of the dst mutant and clarification of how DST regulates stomatal density and leaf width are necessary to evaluate the feasibility of the dst mutation in practical crop breeding.
Acknowledgments
We acknowledge Rosalyn B. Angeles-Shim for helpful comments on the manuscript. Our research is supported by a grant from the Ministry of Agriculture, Forestry, and Fisheries of Japan (Genomics for Agricultural Innovation, IPG -0003).
Footnotes
Article is online at http://www.genesdev.org/cgi/doi/10.1101/gad.1834509.
References
- Assmann SM. Ins and outs of guard cell ABA receptors. Plant Cell. 1994;6:1187–1190. [Google Scholar]
- Bienert GP, Schjoerring JK, Jahn TP. Membrane transport of hydrogen peroxide. Biochim Biophys Acta. 2006;1758:994–1003. doi: 10.1016/j.bbamem.2006.02.015. [DOI] [PubMed] [Google Scholar]
- Blatt MR. Cellular signaling and volume control in stomatal movements in plants. Annu Rev Cell Dev Biol. 2000;16:221–241. doi: 10.1146/annurev.cellbio.16.1.221. [DOI] [PubMed] [Google Scholar]
- Cominelli E, Galbiati M, Vavasseur A, Conti L, Sala T, Vuylsteke M, Leonhardt N, Dellaporta SL, Tonelli C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr Biol. 2005;15:1196–1200. doi: 10.1016/j.cub.2005.05.048. [DOI] [PubMed] [Google Scholar]
- Davies WJ, Jones HG. Abscisic acid. Bios Scientific Publishers; Oxford, UK: 1991. [Google Scholar]
- Hetherington AM. Guard cell signaling. Cell. 2001;107:711–714. doi: 10.1016/s0092-8674(01)00606-7. [DOI] [PubMed] [Google Scholar]
- Hetherington AM, Woodward FI. The role of stomata in sensing and driving environmental change. Nature. 2003;424:901–908. doi: 10.1038/nature01843. [DOI] [PubMed] [Google Scholar]
- Hu H, Dai M, Yao J, Xiao B, Li X, Zhang Q, Xiong L. overexpressing a NAM, ATAF, and CUC (NAC) transcription factor enhances drought resistence and salt tolerance in rice. Proc Natl Acad Sci. 2006;103:12987–12992. doi: 10.1073/pnas.0604882103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X-Y, Chao D-Y, Gao J-P, Zhu M-Z, Shi M, Lin H-X. A previously unknown zinc finger protein, DST, regulates drought and salt tolerance in rice via stomatal aperture control. Genes & Dev. 2009 doi: 10.1101/gad.1812409. (this issue) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hugouvieux V, Kwak JM, Schroeder JI. An mRNA cap binding protein, ABH1, modulates early abscisic acid signal transduction in Arabidopsis. Cell. 2001;106:477–487. doi: 10.1016/s0092-8674(01)00460-3. [DOI] [PubMed] [Google Scholar]
- Karaba A, Dixit S, Greco R, Aharoni A, Trijatmiko KR, Marsch-Martinez N, Krishnan A, Nataraja KN, Udayakumar M, Pereira A. Improvement of water use efficiency in rice by expression of HARDY, an Arabidopsis drought and salt tolerance gene. Proc Natl Acad Sci. 2007;104:15270–15275. doi: 10.1073/pnas.0707294104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak JM, Mori IC, Pei ZM, Leonhardt N, Torres MA, Dangl JL, Bloom RE, Bodde S, Jones JDG, Schroeder JI. NADPH oxidase AtrbohD and AtrbohF genes function in ROS-dependent ABA signaling in Arabidopsis. EMBO J. 2003;22:2623–2633. doi: 10.1093/emboj/cdg277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang YK, Dubos C, Dodd IC, Holroyd GH, Hetherington AM, Campbell MM. AtYMB61, an R2R3-YMB transcription factor controlling stomatal aperture in Arabidopsis thaliana. Curr Biol. 2005;15:1201–1206. doi: 10.1016/j.cub.2005.06.041. [DOI] [PubMed] [Google Scholar]
- Lin RC, Ding L, Casola C, Ripoll DR, Feschotte C, Wang HY. Transposase-derived transcription factors regulate light signaling in Arabidopsis. Science. 2007;318:1302–1305. doi: 10.1126/science.1146281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacRobbie EA. Signal transduction and ion channels in guard cells. Philos Trans R Soc Lond B Biol Sci. 1998;353:1475–1488. doi: 10.1098/rstb.1998.0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: An overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Miller G, Suzuki N, Rizhsky L, Hegie A, Koussevitzky S, Mittler R. Double mutants deficient in cytosolic and thylakoid ascorbate peroxidase reveal a complex mode of interaction between reactive oxygen species, plant development, and response to abiotic stresses. Plant Physiol. 2007;144:1777–1785. doi: 10.1104/pp.107.101436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oeda K, Salinas J, Chua NH. A tobacco bZIP transcriptional activator (TAF-1) binds to a G-box-like motif conserved in plant genes. EMBO J. 1991;10:1793–1802. doi: 10.1002/j.1460-2075.1991.tb07704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei ZM, Murata Y, Benning G, Thomine S, Klusener B, Allen GJ, Grill E, Schroeder JI. Calcium channels activated by hydrogen peroxide mediate abscisic acid signaling in guard cells. Nature. 2000;406:731–734. doi: 10.1038/35021067. [DOI] [PubMed] [Google Scholar]
- Ren ZH, Gao JP, Li LG, Cai XL, Huang W, Chao DY, Zhu MZ, Wang ZY, Luan S, Lin HX. A rice quantitative trait locus for salt tolerance encodes a sodium transporter. Nat Genet. 2005;37:1141–1146. doi: 10.1038/ng1643. [DOI] [PubMed] [Google Scholar]
- Sakamoto H, Maruyama K, Sakuma Y, Meshi T, Iwabuchi M, Shinozaki K, Yamaguchi-Shinozaki K. Arabidopsis Cys2/His2-type zinc-finger proteins function as transcription repressors under drought, cold, and high-salinity stress conditions. Plant Physiol. 2004;136:2734–2746. doi: 10.1104/pp.104.046599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder JI, Kwak JM, Allen GJ. Guard cell abscisic acid signaling and engineering drought hardiness in plants. Nature. 2001;410:327–330. doi: 10.1038/35066500. [DOI] [PubMed] [Google Scholar]
- Wolfe SA, Nekludova L, Pabo CO. DNA recognition by Cys2His2 zinc finger proteins. Annu Rev Biophys Biomol Struct. 2000;29:183–212. doi: 10.1146/annurev.biophys.29.1.183. [DOI] [PubMed] [Google Scholar]
- Yamaguchi-Shinozaki K, Shinozaki K. A novel cis-acting element in an Arabidopsis gene is involved in responsiveness to drought, low-temperature, or high-salt stress. Plant Cell. 1994;6:251–264. doi: 10.1105/tpc.6.2.251. [DOI] [PMC free article] [PubMed] [Google Scholar]