Abstract
Calpain was recently reported to mediate VEGF-induced angiogenesis. In the current study we investigated detailed molecular mechanisms. VEGF (100 ng/ml) induced a marked increase in endothelial cell production of NO•, specifically detected by electron spin resonance. This response was abolished by inhibition of calpain with ALLN or Calpeptin. Both also diminished membrane-specific calpain activation by VEGF, which was intriguingly attenuated by silencing ezrin with RNAi. A rapid membrane co-localization of calpain and ezrin occurred as short as 10 min after VEGF stimulation. AKT, AMPK and eNOSs1179 phosphorylations in VEGF-stimulated endothelial cells were markedly enhanced, which were however significantly attenuated by either ALLN, Calpeptin, or ezrin siRNA; as well as by Wortmannin or Compound C (respectively for PI3K or AMPK). The latter three also abolished VEGF induction of NO•. These data indicate that AMPK and AKT are both downstream of PI3K, and that AKT activation is partially dependent on AMPK. The interrelationship between AMPK and AKT, though known to be individually important in mediating VEGF activation of eNOS, is clearly characterized. Furthermore, AMPK/AKT/eNOSs1179 was found innovatively downstream of a calpain/ezrin membrane interaction. These data no doubt provide new insights into the long mystified signaling gap between VEGF receptors and PI3K/AKT or AMPK-dependent eNOS activation. In view of the well-established significance of VEGF-dependent angiogenesis, these findings might have broad and important implications in cardiovascular pathophysiology.
Keywords: Calpain, Ezrin, nitric oxide (NO•), vascular endothelial growth factor (VEGF), Angiogenesis, eNOS, AMP-dependent Kinase (AMPK), AKT, PI3K, siRNA, RNA interference (RNAi)
INTRODUCTION
Calpain is a cytosolic cystein protease that translocates to membrane upon activation. Active calpain not only cleaves its substrates but also disrupts protein interactions. Various kinases, phosphatases and cytoskeletal proteins are known substrates for calpain 1–3. Through interactions with its substrates, calpain plays an important role transducing signals of cell migration, differentiation, and proliferation 3–6. Specifically in endothelial cells however, limited knowledge exists regarding how calpain signals to modulate cell functions. Interestingly, a critical role of calpain in mediating VEGF-induced angiogenesis was recently reported, despite unknown molecular mechanisms 7. Because VEGF mediated angiogenesis is at least partially attributed to endothelial cell production of nitric oxide (NO•) 8,9, we became interested in whether some of the undiscovered roles of calpain deal with VEGF production of NO• and angiogenesis. Using the highly specific and sensitive electron spin resonance to measure nitric oxide radical (NO•), we examined for the first time whether calpain is required for VEGF stimulation of endothelial NO• production. Changes in membrane and cytosol-specific activities of calpain, and their relevance to downstream signalings, were studied in depth.
Ezrin is a member of the Ezrin/Radixin/Moesin (ERM) protein complex that is classically involved in cytoskeletal remodeling. Ezrin and other ERM proteins act as linkers between plasma membrane and cytoskeletal proteins 10. Upon conformational changes, ezrin can actively participate in transducing cytoskeletal signals 11,12 to modulate a wide variety of cellular functions besides serving as structural linkers 13,14. However, potential roles of ezrin signaling in modulating endothelial cell function have remained largely unknown. We hypothesized that ezrin exerts its regulatory roles via targeted interaction with calpain. In the present study, ezrin-dependent calpain localization to membrane, and a potential role of ezrin in calpain-mediated endothelial NO• production in response to VEGF, were thoroughly investigated using immuocytochemical, co-immunoprecipitation and RNA interference approaches.
VEGF is one of the most potent angiogenic factors, and its signaling is crucial for both angiogenesis and vasculogenesis 8,15–17. VEGF potently induces NO• production through AKT and/or AMPK-dependent phosphorylation of endothelial nitric oxide synthase (eNOS) 17–26. VEGF-stimulated NO• not only directly enhances endothelial cell migration and proliferation, but also modulates effects of numerous other angiogenic factors that might work in concert with VEGF to promote angiogenesis 16. Indeed, selective inhibition of eNOS prevents VEGF-mediated endothelial migration, proliferation, and capillary-like network formation. On the other hand, a decrease in NO• bioavailability is related to vascular dysfunction 8,9,21,27,28. From these reports, it has been suggested that selective modulation of eNOS activity can be provided as an attractive strategy for regulation of angiogenesis and vascular permeability. Nonetheless, precise signaling mechanisms underlying VEGF activation of eNOS and endothelial NO• production remain incompletely understood. Besides PI3K/AKT and/or AMPK, it has remained unclear whether and how alternative or parallel signaling cascades participate in VEGF activation of eNOS.
Therefore, in the present study we fully characterized innovative roles of ezrin and calpain in VEGF activation of eNOS and the potential interrelated roles of AKT and AMPK in transducing ezrin/calpain signals to eNOS. We found that ezrin-dependent, membrane-specific translocation and activation of calpain by VEGF precedes AMPK and AKT-dependent phosphorylation of eNOSs1179 and production of NO•. AKT is activated downstream of PI3K similarly to AMPK, but is also partially dependent on AMPK. These observations not only provide new information as to how AMPK and AKT interacts to ensure VEGF induction of NO•, but represent first evidences establishing the critical role of ezrin/calpain interaction in modulating endothelial cell function. Since the newly characterized signaling cascade of ezrin/calpain/PI3K/AMPK/eNOS likely has fundamental roles in cell signaling in various other cell types, these innovative observations may have broad applicability to both vascular and cancer pathophysiology.
METHODS AND MATERIALS
Materials
Phospho-AMPK (Thr-172), pan-α-AMPK, and phospho-AKT (Ser473), phosphoeNOS (Ser1179), phospho-p44/p42 (Thr202/Tyr204), PI3K p110, PI3K p85, phospho-PKC (pan; Ser660), phospho-PKC (Thr505), HSP90, ezrin and phospho-eNOS (Thr495) antibodies were purchased from Cell Signaling Technologies (CST, Beverly, MA). Phospho-eNOS (Ser116) antibody was obtained from Upstate (Charlottesville, VA). Anti-M-calpain antibody was purchased from Affinity BioReagent (ABR Inc, Golden, CO). β-actin antibody and secondary antibodies were purchased from Sigma and Bio-Rad respectively. VEGF was purchased from R&D system. Wortmannin, Compound C, STO-609, Calpeptin and ALLN were obtained from Calbiochem (La Jolla, CA). Oligofectamine and Opti-MEM for siRNA transfection were purchased from Invitrogen (Carlsbad, CA). Other chemicals were obtained from Sigma in highest purity.
Cell Culture
Bovine aortic endothelial cells (Cell Systems, Kirkland, WA) of passages 4–6 were cultured in Media 199 (Invitrogen), containing 10% fetal bovine serum to confluence and starved in 5% media overnight before experiments.
Membrane Fractionation
Cells were harvested with ice-cold PBS and centrifuged for 5 min at 1,000 rpm. Cell pellets were re-suspended in homogenization buffer (Tris 50 mmol/L, EDTA 0.1 mmol/L, EGTA 0.1 mmol/L, protease inhibitor cocktail, pH 7.4) and sonicated on ice (5 sec, ×10) to lyze the cells. After centrifugation at 3,500 rpm for 5 min, the supernatant was transferred to a fresh tube and centrifuged for 90 min at 50,000 rpm. The pellets (membrane fraction) were re-suspended in lysis buffer and the cytosolic fraction (supernatant) transferred into a fresh tube.
Calpain Activity Assay
Calpain protease activity was determined using a calpain activity kit (Calbiochem QIA120). Fifty µl each of serially diluted standards, controls or samples were mixed with 5 µl synthetic substrate for calpain, Suc-LLVY-AMC, and 100 µl activation buffer or inhibition buffer, prior to incubation for 15 min at 37°C. The calpain-dependent cleavage product of AMC was measured fluorometrically at excitation and emission wavelengths of 360–380 nm and 440–460 nm respectively. The calculated calpain activity was determined by subtracting the activity readings obtained using the inhibition buffer, from the activity readings detected with the activation buffer. The specific activity is presented as product production at µM/mg protein/min after calculation by the AMC standard curve.
Electron Spin Resonance Detection of Endothelial NO• production
Bioavailable NO• produced by confluent endothelial cells was detected using electron spin resonance (ESR) as we previously published [27, 28].
siRNA Transfection
Proliferating endothelial cells of eighty five to ninety percent confluence were transfected with control siRNA or ezrin siRNA (25 nmol/L, Dharmacon Inc., ezrin sequence: 5’-ACAGCGCCAUGCUGGAAUAUU-3’) using Oligofectamine for 4 hr in Opti-MEM serum-reduced media before addition of growth media containing 10% FBS. Forty-eight hr later, cells were subjected designated assays.
Western Analysis of Protein Phosphorylations
Cells were lysed in ice cold Tris buffer (50 mmol/L Tris pH 7.4, 2 mmol/L EDTA, 1 mmol/L EGTA) containing 1% Triton X-100, 0.1% sodium dodecyl sulfate (SDS), 50 mmol/L NaF, 10 mmol/L Na4P2O7, 1 mmol/L Na3VO4, 1 mmol/L dithiothreitol (DTT), 1 mmol/L phenylmethylfonylsulfonyl fluoride (PMSF), 10 µl/ml of the protease inhibitor cocktail, and 10 µl/ml of the phosphatase inhibitor cocktail stock solution (Sigma, St Louis, MO). Lysate proteins were separated in 7.5% SDS-PAGE. Blots were subjected to incubations with primary antibodies (mostly at 1:1000, β-actin at 1:5000) overnight at 4°C, and peroxidase-conjugated secondary antibodies at RT for 1 hr, and phosphorylated proteins subsequently visualized using ECL. Band intensities were analyzed using Image J program and normalized against β-actin.
Immunocytochemical staining
Cells grown on coverslide were treated with VEGF for 10 min, washed with PBS twice and fixed in cold 4% paraformaldehyde for 20 min and then permeabilized with 0.1% Triton X-100 in PBS for 20 min. After quenching for 10 min with 50 mM NH4Cl, cells were blocked with PBS containing 5% goat serum. Primary antibodies (calpain, 1:250; ezrin, 1:250) were added for 1 hr incubation at RT. After washing, cells were incubated with secondary antibodies at RT for 1 hr (Alexa Fluor 488 for calpain, Alexa Fluor 594 for ezrin in sequence). After washing with PBS for 3 times, cells were mounted on slides using ProLong Antifade (Invtrogen). The fluorescent images were captured using a Leica TCS-SP Confocal Microscope and analyzed with the LCS Lite software.
Immunoprecipitation
For immunoprecipitation (IP), cells were lysed in mild lysis buffer (50 mmol/L Tris-Cl pH 8.0, 150 mmol/L NaCl, 1% NP-40, 0.1 mmol/L phenylmethylsulfonyl fluoride, 5 µg/ml aprotinin and 5 µg/ml leupeptin) and preincubated with protein A/G-agarose beads for 2 hr at 4 °C. Samples were then centrifuged, and supernatants subjected to IP with 2 µg of antibody overnight. The protein A/G−agarose beads (Calbiochem) were added for a further 16 hr incubation at 4°C, and immunoprecipitates used for western blotting.
Statistical Analysis
All data are presented as Means±SEM from four to eight independent experiments. ANOVA was used to compare means of different experimental groups, and Dunnet and Turkey test was used as a post-test. A p value less than 0.05 is considered significant.
RESULTS
A Critical Role of Calpain in Basal and VEGF-stimulated NO• Production from Endothelial Cells
It has been previously established that VEGF activates eNOS to result in an increase in endothelial NO• production. These previous studies either examined mechanisms underlying enzymatic activations of eNOS, or an intermediate role of NO•, in VEGF-provoked therapeutic angiogenesis. Production of NO• was assessed either by its metabolites nitrate and nitrite, or in vitro enzymatic analysis of eNOS. In the present study we measured bioavailable NO• specifically using electron spin resonance (ESR) as previously published 29,30. Indeed, exposure of cells to VEGF (100 ng/ml, 1 hr incubation with NO• -specific spin trap following VEGF treatment) induced a marked and reproducible increase in NO• production (Fig. 1).
Fig. 1. Calpain plays a critical role in basal and VEGF-stimulated endothelial cell production of NO•.
Endothelial cells were pre-incubated with Calpeptin (10 µmol/L, 30 min) or ALLN (30 µmol/L, 30 min) prior to VEGF stimulation (100 ng/ml) and analysis of NO• production using an electron spin resonance (ESR) spectrophotometer. A & B): Representative ESR spectra; C & D): Grouped densitometric data, Means±SEM, ANOVA, *p<0.05, **P<0.01
Intriguingly, in endothelial cells pre-incubated with inhibitors for the cysteine protease calpain, ALLN (30 µmol/L, 30 min) or Calpeptin (10 µmol/L, 30 min), basal and VEGF-stimulated NO• productions were significantly attenuated, as demonstrated by representative ESR spectra and grouped densitometric data (Fig. 1). This calpain-dependent NO• production, as well as calpain-dependent phosphorylations of eNOSs1179, AKT and AMPK, were also reproducible in response to a lower, physiological concentration of VEGF (10 ng/ml, Online Figure I A–C, Online Figure II A–D). These data indicate a novel role of calpain in maintaining basal eNOS activity and mediating VEGF activation of eNOS. Detailed signaling events downstream of VEGF activation of calpain, connecting to eNOS activation, have been studied in depth (see below).
Membrane-specific Activation of Calpain by VEGF and Its Dependence on Co-localization with Ezrin
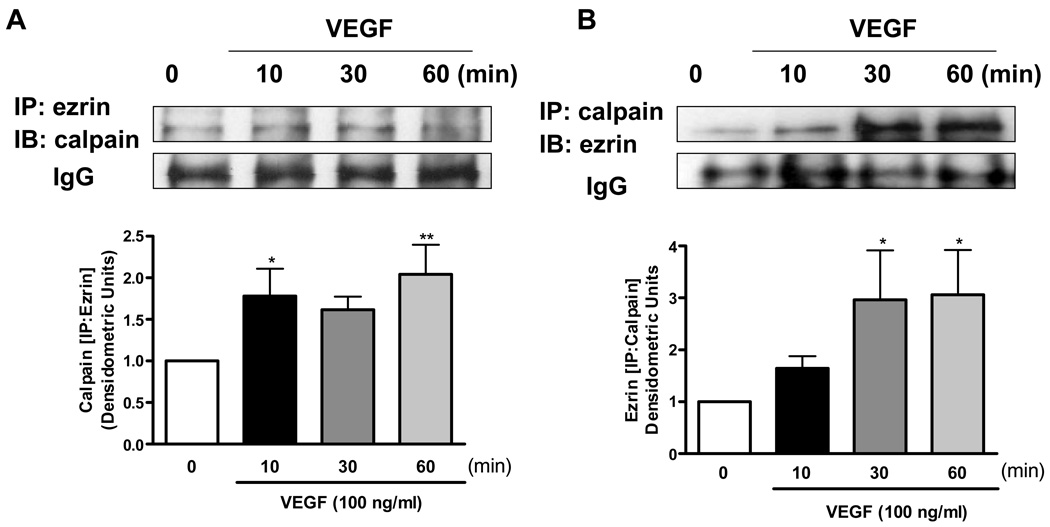
Under basal conditions calpain is predominantly situated in cytosol. In VEGF stimulated endothelial cells, total cellular calpain activity was significantly increased by VEGF treatment (100 ng/ml, 10 min), but abolished by pre-incubation with Calpeptin or ALLN (10 or 30 µmol/L, 30 min) (Online Figure III A). Whereas membrane-specific calpain activity was similarly enhanced by VEGF and attenuated by the inhibitors, cytosolic activity of calpain remained unchanged (Online Figure III B&C). These data seem to suggest that membrane-specific activation of calpain is important for acute VEGF signaling. Interestingly, immunocytochemical staining of VEGF-stimulated cells with calpain, ezrin and secondary fluorescent antibodies revealed enhanced membrane co-localization (Fig. 2A&B). This augmented co-localization was further confirmed by co-immunoprecipitation experiments pulling down calpain or ezrin alternatively (Fig. 3A&B). VEGF induced a time-dependent association between calpain and ezrin, which occurred as early as 10 min, while lysates pulled down with control IgG showed no difference (Fig. 3A&B).
Fig. 2. Colocalization of calpain and ezrin in response to VEGF.
Endothelial cells cultured on coverslips were treated with VEGF for 10 min prior to immunostaining with fluorescent antibodies. Calpain and ezrin were visualized in green and red respectively, whereas nuclear DNA (in blue) was stained with Hoechst 33258. A & B): Representative subcellular localization of calpain and ezrin in un-stimulated and VEGF-stimulated endothelial cells.
Fig. 3. Co-immunoprecipitation of calpain and ezrin.
Endothelial cells were stimulated with 100 ng/ml of VEGF for indicated times. A &B): The cell lysates were subjected to immunoprecipitation with calpain or ezrin antibody, followed by immunoblotting with ezrin or calpain respectively. Grouped quantitative data are presented as Means±SEM (n=4), ANOVA; *p<0.05, **p<0.01
Role of Ezrin in VEGF Stimulation of Endothelial NO• Production
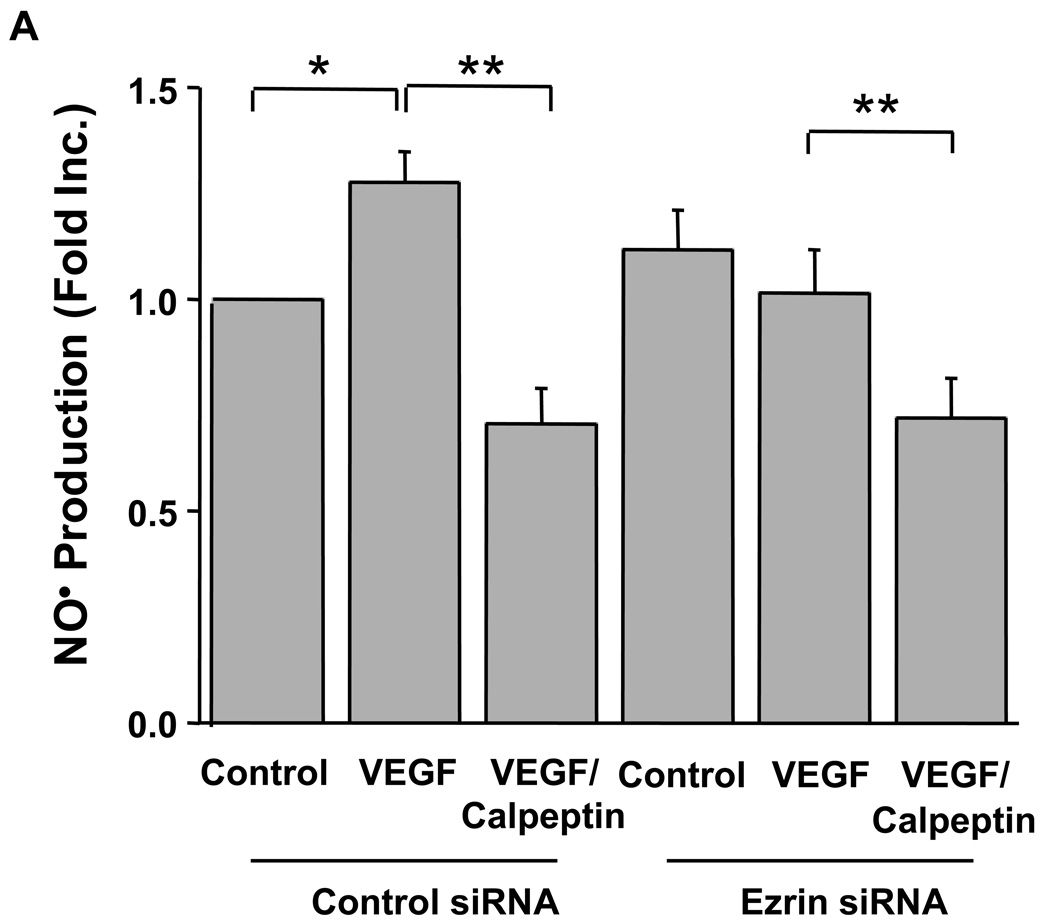
Data described above indicate a critical role of ezrin in VEGF activation of calpain. To investigate a specific role of ezrin in calpain-dependent endothelial NO• production, cells were transfected with ezrin siRNA with or without Calpeptin. In control siRNA transfected cells, VEGF stimulated NO• production similarly as in un-transfected cells, which was abolished by Calpeptin (Fig. 4A). Transfection with ezrin siRNA atteuated NO• production, although Calpeptin was able to further reduce it (Fig. 4A). Ezrin protein expression, eNOSs1179 and AMPK phosphorylations were all attenuated by ezrin siRNA transfection, as shown by representative blots and quantitative data (Fig. 4B–D), whereas the siRNAs had no effects on calpain, eNOS, AKT and AMPK protein levels. Moreover, membrane-specific calpain activity was significantly decreased in VEGF-stimulated, ezrin siRNA-transfected cells (Online Figure IV), confirming a specific effect of ezrin on VEGF-activation of calpain. Take together; these data demonstrate an essential role of ezrin in mediating VEGF activation of calpain, and consequent endothelial NO• production.
Fig. 4. The role of ezrin in calpain-dependent endothelial NO• production in response to VEGF.
Proliferating endothelial cells were transfected with 25 nmol/L control or ezrin siRNA prior to Calpeptin (10 µmol/L, 30 min) and VEGF stimulation (100 ng/ml, 60 min). A): Grouped data of NO• production are presented as Means±SEM, B): Representative western blot from cells transfected with ezrin siRNA, C & D): Ezrin mediated phosphorylation of eNOSs1179 and AKT in response to VEGF, ANOVA; *p<0.05, **p<0.01
Calpain is Required for PI3K/AKT-dependent eNOS Phosphorylation in Response to VEGF
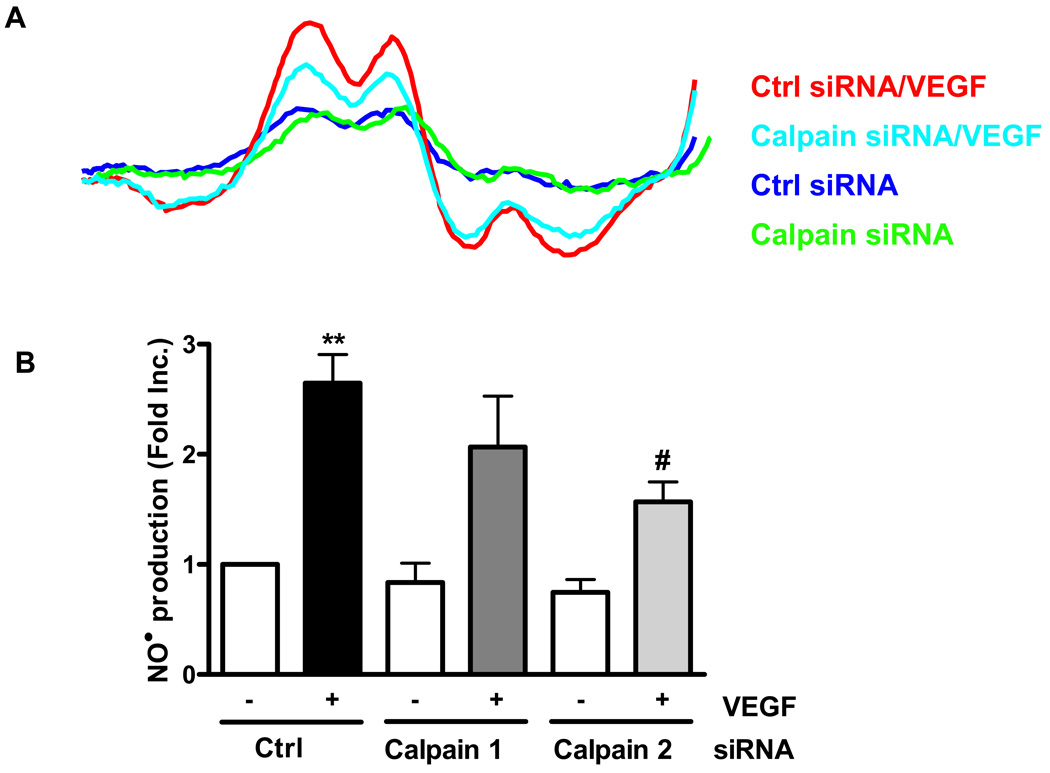
PI3K/AKT is known to mediate VEGF activation of eNOS. Indeed, VEGF increased AKT phosphorylation up to 4.3 fold, which was markedly attenuated by Calpeptin or ALLN (Fig. 5A). Neither VEGF stimulation nor calpain inhibitors affected protein expression of PI3K subunits, indicating absence of genomic effects or calpain-dependent cleavage (data not shown). eNOSs1179 phosphorylation in VEGF-stimulated, Calpeptin or ALLN-pre-incubated endothelial cells mirrored changes in AKT phosphorylation (Fig. 5B). Of note, VEGF stimulation also resulted in an increase in eNOSs116 phosphorylation and a decrease in eNOSt497 phosphorylation, both of which have been previously studied to be functional in modulating eNOS activity. However Calpeptin or ALLN affected neither (Fig. 5C&D). In additional experiments, calpain siRNAs were used to transfect endothelial cells prior to VEGF stimulation. Of note, calpain 2 (M-calpain) siRNA was effective in attenuating VEGF production of NO• (Fig. 6A), as well as calpain expression, AKT, AMPK and eNOSs1179 phosphorylations (Fig. 6B), further confirming a specific role of M-calpain in VEGF activation of eNOS.
Fig. 5. Calpain-dependent phosphorylation of AKT and eNOS in response to VEGF.
Endothelial cells were pre-treated with Calpeptin (10 µmol/L) or ALLN (30 µmol/L) for 30 min prior to VEGF stimulation (100 ng/ml, 10 min). A): AKT phosphorylation; B): eNOSs1179 phosphorylation, C): eNOSs116 phosphorylation, D): eNOSt497 phosphorylation. Representative western blots are shown with quantitative grouped data (Means±SEM, n=5), ANOVA, ***p<0.001 vs control, #p<0.5, ##p<0.01 vs VEGF.
Figure 6. The specific role of calpain-2 on endothelial NO• bioavailability.
BAECs were transfected with 25 nmol/L of control siRNA or 25 nmol/L of calpain 2 siRNA and then exposed to 100 ng/ml of VEGF. A): The bioavailable NO• production was measured by using ESR. B): To investigate the role of calpain-2 in signaling mediating VEGF induced eNOS activation, western blotting was performed using indicated antibodies Grouped data are presented as mean ± SEM. ANOVA; *p<0.05, **p<0.01.
Ezrin/Calpain Interaction is Upstream of Parallel AMPK and AKT-dependent VEGF Activation of eNOS
Intriguingly, in cells pretreated with Compound C (20 µmol/L, 30 min), an inhibitor for AMPK, VEGF-stimulated endothelial NO• production was completely attenuated despite a similarly potent effect of the PI3K antagonist Wortmannin (100 nmol/L, 30 min) (Fig. 7A&B). In consequent experiments, VEGF-induced AMPK phosphorylation was abolished by Wortmannin (Fig. 7C). AKT phosphorylation was partially attenuated by Compound C (Fig. 8B). These data suggest that AMPK has a previously uncharacterized role serving as an intermediate between PI3K and AKT in mediating eNOS activation. Furthermore, markedly increase AMPK phosphorylation in response to VEGF was dramatically attenuated by ALLN or Calpeptin, to a level lower than the baseline (Fig. 7C). On the other hand, calpain activity was unaffected by Compound C or H89, a PKA inhibitor, excluding an upstream role of AMPK or PKA in calpain activation (Fig. 7D). Thus these data establish an innovative role of calpain in VEGF activation of AMPK in endothelial cells. In the same groups of experiments, AMPK phosphorylation was abolished by Compound C, confirming its specificity in endothelial cells (Fig. 8A). eNOSs1179 phosphorylation was consistently inhibited by Calpeptin (Fig. 8C, Fig 6B), and by Wortmannin or Compound C (Fig. 8C), demonstrating that calpain, PI3K, AMPK all lie upstream of VEGF activation of eNOS.
Fig. 7. PI3K and AMPK are required for VEGF induction of NO•.
Endothelial cells were preincubated with an AMPK inhibitor compound C (20 µmol/L, 30min), or a PI3K antagonist wortmannin (100 nmol/L, 30 min), prior to VEGF stimulation (100 ng/ml, 60 min) and analysis of NO• production. A): Representative ESR spectra; B): Grouped densitometric ESR data, Means±SEM (n=4), C): AMPK phosphorylation at thr 172 from BAECs pretreated with calpain inhibitors, calpeptin (10 µmol/L) or ALLN (30 µmol/L) for 30 min prior to VEGF (100 ng/ml, 10 min), D): Calpain activity from cells preincubated with a selective AMPK inhibitor, compound C (20 µmol/L, 30min), or H89 (10 µmol/L, 30 min) prior to 100 ng/ml of VEGF stimulation for 10 min. Grouped data is presented as means ± SEM (n=5). ANOVA; *p<0.05, **p<0.01 vs control
Fig. 8. VEGF activation of eNOS is calpain, PI3K and AMPK-dependent.
Endothelial cells were pre-treated with Compound C (20 µmol/L), wortmannin (100 nmol/L) or Calpeptin (10 µmol/L) for 30 min prior to VEGF stimulation (100 ng/ml, 10 min). Phosphorylation profiles of A): AMPK at thr 172, B): AKT at ser 473, and C): eNOS at ser 1179 are shown with quantitative grouped data (Means±SEM, n=4), ANOVA, *p<0.05 vs control, #p<0.05, ##p<0.01 vs VEGF.
Of note, AKT phosphorylation was significantly inhibited by Wormannin (Fig. 8B), Calpeptin (Fig. 8B, 8A) or Compound C (Fig. 8B). These data confirm the classic pathway of PI3K/AKT/eNOS that is activated upon VEGF stimulation. Its dependency on calpain activation however is a new observation. Furthermore, partial dependence on AMPK of AKT phosphorylation seems to suggest a new intermediate role of AMPK connecting PI3K and AKT, besides its direct effects on eNOS activation.
Calpain Mediates Hydrogen Peroxide Provoked Endothelial NO• production: Potential Link to Redox-sensitivity of VEGF Activation of Calpain
Reactive oxygen species (ROS), particularly superoxide and hydrogen peroxide (H2O2), have been shown to modulate endothelial cell function via interactions with NO pathways 31–34. It has also been demonstrated to mediate some receptor signalings such as VEGFR activation, EGFR transactivation in endothelial cells 32,34–36. VEGF-induced membrane translocation of Rac1 from cytosol activates NADPH Oxidases (NOXs), which require Rac1 as a key regulator for activation 34,35,37,38. NOX-derived ROS can in turn, participate in VEGF-stimulated angiogenesis 34,38,39. Thus, we investigated whether endothelial NO• production, provoked by H2O2, is calpain dependent. As shown in Online Figure V, H2O2 increased NO• production similar to what we found previously 40, and that calpain inhibitors, Calpeptin and ALLN, were effective in completely reversing this response. In contrast, calpain activity was not affected by bradykinin (100 nmol/L for 10 min, Online Figure VI A). The specificity of the compounds in endothelial cells was confirmed (Online Figure VI B). Taken together, these results seem to implicate that calpain-dependent activation of endothelial NO• production is agonist-specific. Whether or not it is a ROS-dependent pathway however, requires further investigations and are beyond the scope of the current study.
We summarized the innovative findings from the current study in a schematic format (Online Figure VII). Upon VEGF stimulation of endothelial cells, membrane-specific activation of calpain occurs ezrin-dependently, which in turn results in PI3K dependent AMPK activation, consequent AKT and eNOS phosphosrylations and increased NO• production. AKT activation seems partially dependent on AMPK, establishing a crosstalk between PI3K/AMPK/eNOS and PI3K/AKT/eNOS pathways. Taken together, these data unravel an innovative ezrin/calpain/PI3K/AMPK/AKT/eNOSs1179 pathway that is critical for VEGF induction of endothelial NO• production, which would in turn mediate VEGF-dependent angiogenesis.
DISCUSSION
The most significant finding of the present study is novel characterization of calpain/ezrin interaction in mediating AMPK dependent eNOS activation in response to VEGF. These data represent first evidence that calpain signaling, via interaction with ezrin, plays a critical role in modulating endothelial cell function, in particular, via regulation of agonist dependent NO• production. Besides the direct activating effect of AMPK on eNOS, our data also revealed a previously uncharacterized intermediate role of AMPK in linking PI3K activation to AKT dependent eNOSs1179 phosphorylation.
In the presence of calcium, purified calpain enzymes were found effective in cleaving eNOS that is pulled down from endothelial cell lysates using a monoclonal antibody for HSP90 41. However, similar procedures indicated that such an incubation did not affect eNOS activity 41. Regardless, these findings may suggest a possible mechanism by which calpain indirectly affects eNOS function, such as, via altering eNOS interactions with chaperon proteins. In our VEGF stimulated endothelial cells however, HSP90 association with eNOS was unaffected (by co-immunoprecipitation experiments, data not shown), seemingly suggesting that such a mechanism is unlikely involved in the calpain-dependent eNOS activation in response to VEGF. By contrast, our data demonstrated an innovative role of cytoskeletal protein ezrin in modulating membrane localization and activation of calpain, thus involved in calpain-dependent eNOS activation. Of interest, in NIH 3T3 cells, inhibition of calpain was found associated with increased ezrin levels and enhanced cell spreading 42. However whether ezrin-dependent membrane translocation of calpain is related to its potential cleaving effects on ezrin, remains to be investigated.
AMPK is a serine/threonine protein kinase that is a critical mediator of energy metabolisms. In endothelial cell, it has been shown that AMPK activates eNOS phosphorylation at ser 1179, subsequently produces NO• in absence of agonist 26. In particular, earlier work has demonstrated an independent role of AMPK in mediating VEGF activation of eNOS via direct augmentation of eNOSs1179 phosphorylation 22. More recent studies indicate that AMPK is redox-sensitively activated by peroxynitrite and H2O2 in endothelial cells 43,44, and is responsible for shear stress activation of eNOS 45,46. Of note, H2O2 activation of eNOS was found dependent on parallel activation of AKT and ERK1/2 40. It is interesting to speculate that AMPK might lie upstream of AKT activation in this cascade. In the present study, we found that AMPK activation is downstream of PI3K, which is consistent with previous observations 44. The findings that AKT activation by VEGF is at least partially AMPK-dependent are consistent with recent reports by Levine and colleagues 25. In addition, AMPK-dependent AKT activation has been reported in adiponectin-induced angiogenesis, 23 and that inhibition of AMPK phosphorylation by insulin-stimulated AKT activation was proposed as a cardioprotective mechanism 24. Despite these controversies, our data clearly demonstrate that in endothelial cells, VEGF activation of eNOS is dependent on parallel PI3K/AMPK/AKT and PI3K/AKT pathways. What remains to be fully elucidated is whether there is a direct interaction between AMPK and AKT, or else whether both their direct and indirect effects on eNOS activation occur at the same signaling domain.
Ezrin is a cytoskeletal linker protein with unclear functional roles in endothelial cell signal transduction. Our present study demonstrated an innovative and critical role of ezrin in regulating membrane-specific activation of calpain and subsequent activation of eNOS. This enhances NO• production in response to VEGF, which would be highly significant in modulating endothelial cell functions. Of note, previous studies have implicated important cytoskeletal participations in regulating eNOS expression at mRNA levels, presumably via uncharacterized actin-actin binding protein(s) complex(s) 48,49. Some cytoskeletal reorganizations have also been found redox-sensitive in endothelial cells, implying potential interactions with NO• signalings 29,50. It has been suggested that NOX-derived ROS not only modulates VEGF expression but also plays a critical role in VEGF-stimulated signalings linked to endothelial cell migration as well as neovascularization 31,32,34,38. In view of recent advances regarding roles of ROS in early VEGF-dependent signal transduction 35,37,38, our data seem to represent a novel mechanism whereby cytoskeletal pathways contribute to redox and NO• -mediated signaling events induced by VEGF.
In summary, new findings in the present study established a critical role of calpain/ezrin interaction in mediating VEGF-dependent signaling to eNOS via AMPK and AKT. While detailed mechanisms underlying ezrin-dependent membrane translocation and activation of calpain, AMPK activation of AKT, as well as calpain activation of PI3K all remain to be fully investigated, our data no doubt have unraveled an important component of acute VEGF signaling at membrane level. Because ezrin and calpain are ubiquitously expressed, these findings may later prove to be broadly applicable and significant in fundamental cell biology, particularly relevant to mitogenic signalings in cardiovascular system. These data represent first evidence that calpain signaling, via interaction with ezrin, plays a critical role in modulating endothelial cell function, in particular, via regulation of agonist-dependent NO• production.
Acknowledgments
SOURCES OF FUNDING
The authors’ work has been supported by National Heart, Lung and Blood Institute (NHLBI) Grants HL077440 (HC), HL081571 (HC), an American Heart Association Grant 0435189N (HC), an American Diabetes Association Award 7-04-RA-16 (HC), a Laubisch Award and a Start-up Fund from University of California Los Angeles (HC).
Footnotes
DISCLOSURES: None.
REFERENCES
- 1.Khorchid A, Ikura M. How calpain is activated by calcium. Nat Struct Biol. 2002;9:239–241. doi: 10.1038/nsb0402-239. [DOI] [PubMed] [Google Scholar]
- 2.Tompa P, Emori Y, Sorimachi H, Suzuki K, Friedrich P. Domain III of calpain is a ca2+regulated phospholipid-binding domain. Biochem Biophys Res Commun. 2001;280:1333–1339. doi: 10.1006/bbrc.2001.4279. [DOI] [PubMed] [Google Scholar]
- 3.Goll DE, Thompson VF, Li H, Wei W, Cong J. The calpain system. Physiol Rev. 2003;83:731–801. doi: 10.1152/physrev.00029.2002. [DOI] [PubMed] [Google Scholar]
- 4.Cuevas BD, Abell AN, Witowsky JA, Yujiri T, Johnson NL, Kesavan K, Ware M, Jones PL, Weed SA, DeBiasi RL, Oka Y, Tyler KL, Johnson GL. MEKK1 regulates calpain-dependent proteolysis of focal adhesion proteins for rear-end detachment of migrating fibroblasts. Embo J. 2003;22:3346–3355. doi: 10.1093/emboj/cdg322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kulkarni S, Saido TC, Suzuki K, Fox JE. Calpain mediates integrin-induced signaling at a point upstream of Rho family members. J Biol Chem. 1999;274:21265–21275. doi: 10.1074/jbc.274.30.21265. [DOI] [PubMed] [Google Scholar]
- 6.Hajimohammadreza I, Raser KJ, Nath R, Nadimpalli R, Scott M, Wang KK. Neuronal nitric oxide synthase and calmodulin-dependent protein kinase IIalpha undergo neurotoxin-induced proteolysis. J Neurochem. 1997;69:1006–1013. doi: 10.1046/j.1471-4159.1997.69031006.x. [DOI] [PubMed] [Google Scholar]
- 7.Su Y, Cui Z, Li Z, Block ER. Calpain-2 regulation of VEGF-mediated angiogenesis. Faseb J. 2006;20:1443–1451. doi: 10.1096/fj.05-5354com. [DOI] [PubMed] [Google Scholar]
- 8.Papapetropoulos A, Garcia-Cardena G, Madri JA, Sessa WC. Nitric oxide production contributes to the angiogenic properties of vascular endothelial growth factor in human endothelial cells. J Clin Invest. 1997;100:3131–3139. doi: 10.1172/JCI119868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu J, deMuinck ED, Zhuang Z, Drinane M, Kauser K, Rubanyi GM, Qian HS, Murata T, Escalante B, Sessa WC. Endothelial nitric oxide synthase is critical for ischemic remodeling, mural cell recruitment, and blood flow reserve. Proc Natl Acad Sci U S A. 2005;102:10999–11004. doi: 10.1073/pnas.0501444102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Wang H, Guo Z, Wu F, Long F, Cao X, Liu B, Zhu Z, Yao X. PKA-mediated protein phosphorylation protects ezrin from calpain I cleavage. Biochem Biophys Res Commun. 2005;333:496–501. doi: 10.1016/j.bbrc.2005.05.143. [DOI] [PubMed] [Google Scholar]
- 11.Louvet-Vallee S. ERM proteins: from cellular architecture to cell signaling. Biol Cell. 2000;92:305–316. doi: 10.1016/s0248-4900(00)01078-9. [DOI] [PubMed] [Google Scholar]
- 12.Khanna C, Wan X, Bose S, Cassaday R, Olomu O, Mendoza A, Yeung C, Gorlick R, Hewitt SM, Helman LJ. The membrane-cytoskeleton linker ezrin is necessary for osteosarcoma metastasis. Nat Med. 2004;10:182–186. doi: 10.1038/nm982. [DOI] [PubMed] [Google Scholar]
- 13.Kishore R, Qin G, Luedemann C, Bord E, Hanley A, Silver M, Gavin M, Yoon YS, Goukassian D, Losordo DW. The cytoskeletal protein ezrin regulates EC proliferation and angiogenesis via TNF-alpha-induced transcriptional repression of cyclin A. J Clin Invest. 2005;115:1785–1796. doi: 10.1172/JCI22849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gimeno L, Corradi A, Cobos I, Consalez GG, Martinez S. Ezrin gene, coding for a membrane-cytoskeleton linker protein, is regionally expressed in the developing mouse neuroepithelium. Gene Expr Patterns. 2004;4:749–754. doi: 10.1016/j.modgep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- 15.Neufeld G, Cohen T, Gengrinovitch S, Poltorak Z. Vascular endothelial growth factor (VEGF) and its receptors. Faseb J. 1999;13:9–22. [PubMed] [Google Scholar]
- 16.Fukumura D, Gohongi T, Kadambi A, Izumi Y, Ang J, Yun CO, Buerk DG, Huang PL, Jain RK. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc Natl Acad Sci U S A. 2001;98:2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Takahashi H, Shibuya M. The vascular endothelial growth factor (VEGF)/VEGF receptor system and its role under physiological and pathological conditions. Clin Sci (Lond) 2005;109:227–241. doi: 10.1042/CS20040370. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, Medhora M, Falck JR, Pritchard KA, Jr, Jacobs ER. Mechanisms of activation of eNOS by 20-HETE and VEGF in bovine pulmonary artery endothelial cells. Am J Physiol Lung Cell Mol Physiol. 2006;291:L378–L385. doi: 10.1152/ajplung.00424.2005. [DOI] [PubMed] [Google Scholar]
- 19.Fulton D, Gratton JP, McCabe TJ, Fontana J, Fujio Y, Walsh K, Franke TF, Papapetropoulos A, Sessa WC. Regulation of endothelium-derived nitric oxide production by the protein kinase Akt. Nature. 1999;399:597–601. doi: 10.1038/21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Michell BJ, Chen Z, Tiganis T, Stapleton D, Katsis F, Power DA, Sim AT, Kemp BE. Coordinated control of endothelial nitric-oxide synthase phosphorylation by protein kinase C and the cAMP-dependent protein kinase. J Biol Chem. 2001;276:17625–17628. doi: 10.1074/jbc.C100122200. [DOI] [PubMed] [Google Scholar]
- 21.Dimmeler S, Fleming I, Fisslthaler B, Hermann C, Busse R, Zeiher AM. Activation of nitric oxide synthase in endothelial cells by Akt-dependent phosphorylation. Nature. 1999;399:601–605. doi: 10.1038/21224. [DOI] [PubMed] [Google Scholar]
- 22.Chen ZP, Mitchelhill KI, Michell BJ, Stapleton D, Rodriguez-Crespo I, Witters LA, Power DA, Ortiz de Montellano PR, Kemp BE. AMP-activated protein kinase phosphorylation of endothelial NO synthase. FEBS Lett. 1999;443:285–289. doi: 10.1016/s0014-5793(98)01705-0. [DOI] [PubMed] [Google Scholar]
- 23.Ouchi N, Kobayashi H, Kihara S, Kumada M, Sato K, Inoue T, Funahashi T, Walsh K. Adiponectin stimulates angiogenesis by promoting cross-talk between AMP-activated protein kinase and Akt signaling in endothelial cells. J Biol Chem. 2004;279:1304–1309. doi: 10.1074/jbc.M310389200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Horman S, Vertommen D, Heath R, Neumann D, Mouton V, Woods A, Schlattner U, Wallimann T, Carling D, Hue L, Rider MH. Insulin antagonizes ischemia-induced Thr172 phosphorylation of AMP-activated protein kinase alpha-subunits in heart via hierarchical phosphorylation of Ser485/491. J Biol Chem. 2006;281:5335–5340. doi: 10.1074/jbc.M506850200. [DOI] [PubMed] [Google Scholar]
- 25.Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK -> Rac1 -> Akt -> endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- 26.Morrow VA, Foufelle F, Connell JM, Petrie JR, Gould GW, Salt IP. Direct activation of AMP-activated protein kinase stimulates nitric-oxide synthesis in human aortic endothelial cells. J Biol Chem. 2003;278:31629–31639. doi: 10.1074/jbc.M212831200. [DOI] [PubMed] [Google Scholar]
- 27.Namba T, Koike H, Murakami K, Aoki M, Makino H, Hashiya N, Ogihara T, Kaneda Y, Kohno M, Morishita R. Angiogenesis induced by endothelial nitric oxide synthase gene through vascular endothelial growth factor expression in a rat hindlimb ischemia model. Circulation. 2003;108:2250–2257. doi: 10.1161/01.CIR.0000093190.53478.78. [DOI] [PubMed] [Google Scholar]
- 28.Stangl V, Lorenz M, Meiners S, Ludwig A, Bartsch C, Moobed M, Vietzke A, Kinkel HT, Baumann G, Stangl K. Long-term up-regulation of eNOS and improvement of endothelial function by inhibition of the ubiquitin-proteasome pathway. Faseb J. 2004;18:272–279. doi: 10.1096/fj.03-0054com. [DOI] [PubMed] [Google Scholar]
- 29.Nguyen A, Cai H. Netrin-1 induces angiogenesis via a DCC-dependent ERK1/2-eNOS feed-forward mechanism. Proc Natl Acad Sci U S A. 2006;103:6530–6535. doi: 10.1073/pnas.0511011103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chalupsky K, Cai H. Endothelial dihydrofolate reductase: critical for nitric oxide bioavailability and role in angiotensin II uncoupling of endothelial nitric oxide synthase. Proc Natl Acad Sci U S A. 2005;102:9056–9061. doi: 10.1073/pnas.0409594102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai H. Hydrogen peroxide regulation of endothelial function: origins, mechanisms, and consequences. Cardiovasc Res. 2005;68:26–36. doi: 10.1016/j.cardiores.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 32.Cai H. NAD(P)H oxidase-dependent self-propagation of hydrogen peroxide and vascular disease. Circ Res. 2005;96:818–822. doi: 10.1161/01.RES.0000163631.07205.fb. [DOI] [PubMed] [Google Scholar]
- 33.Cai H, Harrison DG. Endothelial dysfunction in cardiovascular diseases: the role of oxidant stress. Circ Res. 2000;87:840–844. doi: 10.1161/01.res.87.10.840. [DOI] [PubMed] [Google Scholar]
- 34.Cai H, Griendling KK, Harrison DG. The vascular NAD(P)H oxidases as therapeutic targets in cardiovascular diseases. Trends Pharmacol Sci. 2003;24:471–478. doi: 10.1016/S0165-6147(03)00233-5. [DOI] [PubMed] [Google Scholar]
- 35.Xia C, Meng Q, Liu LZ, Rojanasakul Y, Wang XR, Jiang BH. Reactive oxygen species regulate angiogenesis and tumor growth through vascular endothelial growth factor. Cancer Res. 2007;67:10823–10830. doi: 10.1158/0008-5472.CAN-07-0783. [DOI] [PubMed] [Google Scholar]
- 36.Chen K, Vita JA, Berk BC, Keaney JF., Jr c-Jun N-terminal kinase activation by hydrogen peroxide in endothelial cells involves SRC-dependent epidermal growth factor receptor transactivation. J Biol Chem. 2001;276:16045–16050. doi: 10.1074/jbc.M011766200. [DOI] [PubMed] [Google Scholar]
- 37.Ushio-Fukai M, Nakamura Y. Reactive oxygen species and angiogenesis: NADPH oxidase as target for cancer therapy. Cancer Lett. 2008;266:37–52. doi: 10.1016/j.canlet.2008.02.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abid MR, Spokes KC, Shih SC, Aird WC. NADPH oxidase activity selectively modulates vascular endothelial growth factor signaling pathways. J Biol Chem. 2007;282:35373–35385. doi: 10.1074/jbc.M702175200. [DOI] [PubMed] [Google Scholar]
- 39.Ushio-Fukai M, Tang Y, Fukai T, Dikalov SI, Ma Y, Fujimoto M, Quinn MT, Pagano PJ, Johnson C, Alexander RW. Novel role of gp91(phox)-containing NAD(P)H oxidase in vascular endothelial growth factor-induced signaling and angiogenesis. Circ Res. 2002;91:1160–1167. doi: 10.1161/01.res.0000046227.65158.f8. [DOI] [PubMed] [Google Scholar]
- 40.Cai H, Li Z, Davis ME, Kanner W, Harrison DG, Dudley SC., Jr Akt-dependent phosphorylation of serine 1179 and mitogen-activated protein kinase kinase/extracellular signal-regulated kinase 1/2 cooperatively mediate activation of the endothelial nitric-oxide synthase by hydrogen peroxide. Mol Pharmacol. 2003;63:325–331. doi: 10.1124/mol.63.2.325. [DOI] [PubMed] [Google Scholar]
- 41.Averna M, Stifanese R, De Tullio R, Salamino F, Bertuccio M, Pontremoli S, Melloni E. Proteolytic degradation of nitric oxide synthase isoforms by calpain is modulated by the expression levels of HSP90. Febs J. 2007;274:6116–6127. doi: 10.1111/j.1742-4658.2007.06133.x. [DOI] [PubMed] [Google Scholar]
- 42.Potter DA, Tirnauer JS, Janssen R, Croall DE, Hughes CN, Fiacco KA, Mier JW, Maki M, Herman IM. Calpain regulates actin remodeling during cell spreading. J Cell Biol. 1998;141:647–662. doi: 10.1083/jcb.141.3.647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Nagata D, Takeda R, Sata M, Satonaka H, Suzuki E, Nagano T, Hirata Y. AMP-activated protein kinase inhibits angiotensin II-stimulated vascular smooth muscle cell proliferation. Circulation. 2004;110:444–451. doi: 10.1161/01.CIR.0000136025.96811.76. [DOI] [PubMed] [Google Scholar]
- 44.Zou MH, Hou XY, Shi CM, Nagata D, Walsh K, Cohen RA. Modulation by peroxynitrite of Akt-and AMP-activated kinase-dependent Ser1179 phosphorylation of endothelial nitric oxide synthase. J Biol Chem. 2002;277:32552–32557. doi: 10.1074/jbc.M204512200. [DOI] [PubMed] [Google Scholar]
- 45.Fleming I, Fisslthaler B, Dixit M, Busse R. Role of PECAM-1 in the shear-stress-induced activation of Akt and the endothelial nitric oxide synthase (eNOS) in endothelial cells. J Cell Sci. 2005;118:4103–4111. doi: 10.1242/jcs.02541. [DOI] [PubMed] [Google Scholar]
- 46.Zhang Y, Lee TS, Kolb EM, Sun K, Lu X, Sladek FM, Kassab GS, Garland T, Jr, Shyy JY. AMP-activated protein kinase is involved in endothelial NO synthase activation in response to shear stress. Arterioscler Thromb Vasc Biol. 2006;26:1281–1287. doi: 10.1161/01.ATV.0000221230.08596.98. [DOI] [PubMed] [Google Scholar]
- 47.Bertrand L, Horman S, Beauloye C, Vanoverschelde JL. Insulin signalling in the heart. Cardiovasc Res. 2008;79:238–248. doi: 10.1093/cvr/cvn093. [DOI] [PubMed] [Google Scholar]
- 48.Searles CD, Miwa Y, Harrison DG, Ramasamy S. Posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res. 1999;85:588–595. doi: 10.1161/01.res.85.7.588. [DOI] [PubMed] [Google Scholar]
- 49.Searles CD, Ide L, Davis ME, Cai H, Weber M. Actin cytoskeleton organization and posttranscriptional regulation of endothelial nitric oxide synthase during cell growth. Circ Res. 2004;95:488–495. doi: 10.1161/01.RES.0000138953.21377.80. [DOI] [PubMed] [Google Scholar]
- 50.Cai H, Liu D, Garcia JG. CaM Kinase II-dependent pathophysiological signalling in endothelial cells. Cardiovasc Res. 2008;77:30–34. doi: 10.1093/cvr/cvm010. [DOI] [PubMed] [Google Scholar]