Abstract
Background
Preclinical studies suggest that the antiretroviral agent, nelfinavir mesylate (NFV), may have antineoplastic properties. The relationship between NFV and cancer incidence among HIV-infected patients is unknown.
Methods
We evaluated the impact of NFV on cancer development in a large cohort of HIV-infected persons with 108 cancer events during 13,421 person-years of follow-up. Using multivariate time-updated Cox proportional hazard models, the risk of cancer among those receiving NFV were compared to those on non-NFV antiretroviral regimens.
Results
The risk of cancer among those receiving NFV was similar to those on non-NFV antiretroviral regimens (hazard ratio 1.0, 95% confidence interval 0.5, 1.7, P=0.90). We also examined AIDS-defining and non-AIDS-defining cancers separately and found no significant associations between NFV use and cancer risk. Antiretroviral use, with or without a protease inhibitor (PI) component, was associated with a reduced risk of AIDS-defining cancers compared with no antiretroviral therapy; however, the risk of cancer was the same among those using PI or PI-sparing regimens.
Discussion
Despite reports that NFV may have tumoricidal activity, we found no significant relationship between NFV or PI use compared with other antiretrovirals and the risk of developing cancer among a large cohort of HIV-infected persons. Nelfinavir mesylate (NFV) is a protease inhibitor (PI) approved by the Food and Drug Administration (FDA) in March of 1997 and widely used as a component of early antiretroviral regimens. Recently, NFV has been evaluated for potential repositioning as part of cancer therapy (1). PI side effects, such as insulin resistance and hyperlipidemia, were noted to be similar to those observed from the blockade of phosphoinositide 3-kinase/Akt, a known survival pathway for cancer cells (2). Recent data suggest that PIs, particularly NFV, inhibit tumor growth via Akt inhibition (1) as well as by other mechanisms.
Keywords: HIV, cancers, protease inhibitor, nelfinavir
NFV appears to be the most potent anticancer PI, having demonstrated activity against 60 different cancer cell lines (1, 3–5). NFV’s antineoplastic activity occurs through the induction of cancer cell death by apoptotic and non-apoptotic pathways. The non-apoptotic pathway is thought to occur via the induction of endoplasmic reticulum stress and autophagy (1, 3). Furthermore, NFV appears to have the greatest Akt inhibition activity among PIs (1).
Prior in vitro and in vivo studies suggest that NFV has activity against Kaposi’s sarcoma, skin cancer, prostate cancer, multiple myeloma, as well as a variety of other types of malignancies (6–9). Phase 1 clinical trials are currently underway to study the effect of NFV as part of cancer therapies for HIV-uninfected patients (10).
Malignancies are an important cause of morbidity and mortality among HIV-infected persons who have a two- to three-fold higher risk of cancers than the general population (11–14). We and others have previously shown that use of HAART is protective of development of AIDS-defining cancers (15–18), but no study to date has evaluated the potential impact of NFV on the risk of cancer among HIV-infected patients who used NFV as part of their antiretroviral regimen. We evaluated the receipt of NFV and its potential effect on cancer rates.
Methods
We retrospectively analyzed subjects who were participants in a prospective, multicenter U.S. HIV Natural History Study. The study included subjects enrolled since 1984 who had follow-up during the time of the availability of nelfinavir (after March 14, 1997). Malignancies were classified as AIDS-defining cancers (ADCs), which included Kaposi’s sarcoma, non-Hodgkin’s lymphoma, or invasive cervical carcinoma; other cancers were categorized as non-AIDS-defining cancers (NADCs). ART use was defined as receipt of one or more antiretroviral medications. HAART was defined as: two or more nucleoside reverse transcriptase inhibitors (NRTIs) in combination with at least one PI or one non-nucleoside reverse transcriptase inhibitors (NNRTI); one NRTI in combination with at least one PI and at least one NNRTI; or an abacavir or tenofovir containing regimen of three or more NRTIs.
Descriptive statistics were used to compare the characteristics at time of HIV diagnosis and at HAART initiation for those who did and did not receive NFV during follow-up. Medians are given with interquartile ranges (IQR). Univariate logistic regression analyses were used to ascertain characteristics between those who received NFV or did not receive NFV during follow-up. Multivariate Cox proportional hazards models were used to assess the association between NFV use and cancer. In those models, time was measured from the date of NFV availability (March 14, 1997), and participants who did not experience a cancer event were censored at their last study visit through December 31, 2006. Time-updated indicators were used to capture episodes of NFV use, non-NFV ART, and no-ART; the models were further adjusted for age, race (Caucasian versus other), gender, time-updated CD4 cell count, and a time-updated indicator of having a non-cancer AIDS-defining event. For the time-updated variables, all available measurements from baseline through the event or censoring date were used. Models utilized any ART use to increase the sensitivity in detecting an effect of NFV. Analyses were repeated for receipt of indinavir (IDV) to detect the effect of an alternative PI (which has been shown to have limited anti-cancer activity compared to NFV) on cancer rates, and again for both the receipt of any PI and for any ART regimen. Hazard ratios (HR) are compared to those on the drug of interest (NFV, IDV, any PI, or any ART), and are reported with 95% confidence intervals (CI). Analyses were performed using SAS version 9.1 (SAS Institute Inc., Cary, NC).
Results
The study population consisted of 2,499 HIV-infected persons with a total 13,421 person-years (PYs) of follow-up. The study cohort had a median age at HIV diagnosis of 29 years (IQR 24–34), 91% were male, and race was 47% African American, 42% Caucasian and 12% other (Table 1). The median CD4 cell count was 506 cells/mm3 (IQR 361–664) and the median HIV RNA was 4.4 log10 copies/ml (IQR 3.7–4.9) at HIV diagnosis. Seventy-five percent of the cohort utilized HAART sometime during follow-up. At the time of HAART initiation, the median age was 34 years (IQR 29–39), median CD4 cell count was 340 cells/mm3 (IQR 211–475), and 6% had experienced a non-cancer AIDS event prior to HAART. During the study period, there were a total of 108 cancer events; 39 ADCs (25 cases of Kaposi’s sarcoma, 13 of non-Hodgkin’s lymphoma, and one case of cervical carcinoma) and 69 NADCs (28 cases of skin cancer, 12 of anal cancer, six Hodgkin’s, five prostate, three renal, and 15 other cancer types).
Table 1.
Characteristics of the Study Population and Patients Receiving Nelfinavir (NFV) During Follow-up
| Characteristic | Patients Receiving NFV* | Patients Not Receiving NFV | Overall Population |
|---|---|---|---|
| N=839 | N=1660 | N=2499 | |
| Total Person-Years of Follow-up | 5,621 | 7,800 | 13,421 |
| Person-Years on NFV | 1,780 | --- | 1,780 |
| Cancer Events, number, % | 45 (5.4%) | 63 (3.8%) | 108 (4.3%) |
| Age, median (IQR) | |||
| At HIV diagnosis | 28.7 (24.3, 33.7) | 28.4 (24.0, 34.0) | 28.5 (24.1, 33.9) |
| At HAART Initiation | 34.1 (29.7, 39.3) | 34.2 (29.0, 39.1) | 34.2 (29.2, 39.1) |
| Gender, male, number, % | 744 (88.7%) | 1529 (92.1%) | 2273 (91.0%) |
| Race, number, % | |||
| Caucasian | 357 (42.6%) | 680 (41%) | 1037 (41.5%) |
| African American | 372 (44.3%) | 791 (47.7%) | 1163 (46.5%) |
| Other | 110 (13.1%) | 189 (11.4%) | 299 (12.0%) |
| Receipt of HAART, yes, number, % | 836 (99.6%) | 1036 (62.0%) | 1872 (74.9%) |
| CD4 cell count, cells/mm3 median (IQR)** | |||
| At HIV diagnosis | 486 (336, 640) | 515 (377, 682) | 506 (361, 664) |
| At HAART Initiation | 339 (182, 486) | 340 (225, 468) | 340 (211, 475) |
| Log10 HIV Viral Load, copies/ml median (IQR)** | |||
| At HIV diagnosis | 4.5 (3.9, 4.9) | 4.3 (3.7, 4.8) | 4.4 (3.7, 4.9) |
| At HAART Initiation | 4.4 (3.6, 5.0) | 4.5 (3.8, 4.9) | 4.5 (3.7, 4.9) |
| AIDS event***prior to HAART, yes, number, % | 85 (10.1%) | 74 (4.5%) | 159 (6.4%) |
HAART, highly active antiretroviral therapy; IQR, interquartile range; NFV, nelfinavir.
Receipt of NFV sometime during the study follow-up.
CD4 cell count was available for 2003 patients at HIV diagnosis and 1558 at the time of HAART initiation. Viral load was available for 1130 patients at HIV diagnosis and 1482 at the time of HAART initiation. Of those receiving HAART, 80% of those on NFV had a CD4 count at HAART initiation compared to 83% not on NFV (p=0.07); at HAART initiation HIV viral load was available for 74% of those on NFV compared to 81% (p<0.001). Differences in the availability of CD4 counts and viral loads were likely due to enrollment date differences.
AIDS defining event per the 1993 CDC Criteria excluding CD4 cell count <200 cells/mm3 or cancer event.
As shown in Table 1, NFV was utilized by 839 (34%) of the study population for a total of 1780 PYs of NFV use. Among those who received NFV during follow-up, the median duration of NFV use was 17 months (IQR 7–36). Their median age at HAART initiation was 34 years (IQR 30–39), 44% were African American, and 89% were male (Table 1). Their median CD4 cell count at HIV diagnosis was 486 (IQR 336–640) and was 339 (IQR 182–486) at HAART initiation and 10% had a prior AIDS event prior to HAART. Forty-five patients who received NFV had a subsequent diagnosis of cancer (16 ADC and 29 NADC events). The duration of NFV use did not differ between those who did and did not develop cancer (p=0.06).
HIV patients who were less likely to receive NFV included males (OR 0.67; 95% CI 0.51–0.89), those with higher CD4 cell counts at HIV diagnosis (per 50 cell increase, OR 0.98, 95% CI 0.96–1.0), and those without an AIDS event prior to HAART initiation (OR 0.41, 95% CI 0.30–0.57).
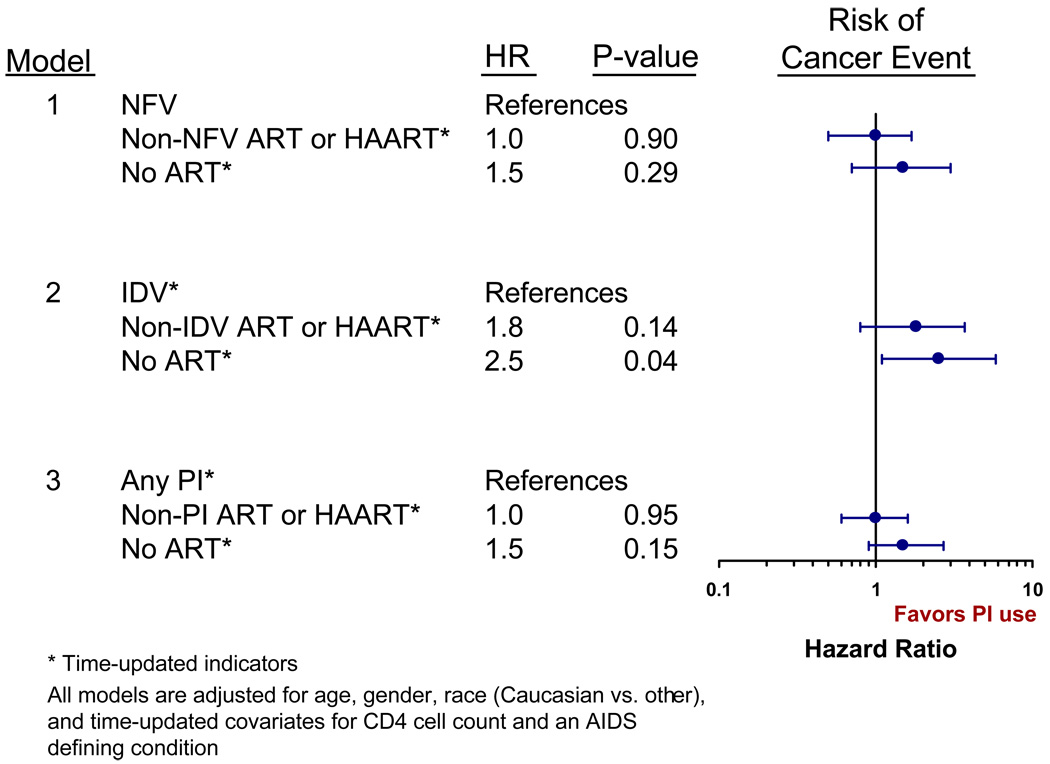
From the adjusted multivariate proportional hazards model, compared to those on NFV, the risk of cancer was similar for those receiving non-NFV ART (HR 1.0, 95% CI 0.5–1.7, Table 2 and Figure 1), while those not on any ART were 1.5 times more likely to develop cancer (95% CI 0.7–3.0). We also used a CD4<200 cells/mm3 and then a CD4<350 cells/mm3 time-updated indicator in our models without a change in the effect of NFV on cancer events. No significant protective effect was noted among those receiving NFV when ADCs and NADCs were examined separately (Table 2). Analyses were repeated for using time intervals of early HAART (1997–1999) and later HAART (2000–2006) with similar results (data not presented).
Table 2.
Multivariate Cox Proportional Hazard Ratios for Cancer Development by Protease Inhibitor Receipt, 1997–2006
| Any Cancer | ADC | NADC | |||||
|---|---|---|---|---|---|---|---|
| Model | Drug | HR*(95% CI) | P-value | HR*(95% CI) | P-value | HR*(95% CI) | P-value |
| 1 | NFV** | 1.0 | 1.0 | 1.0 | |||
| No ART** | 1.5 (0.7, 3.0) | 0.29 | 1.5 (0.5, 4.8) | 0.18 | 1.4 (0.6, 3.6) | 0.47 | |
| Non-NFV ART** | 1.0 (0.5, 1.7) | 0.90 | 0.7 (0.3, 2.0) | 0.55 | 1.1 (0.5, 2.3) | 0.80 | |
| 2 | IDV** | 1.0 | 1.0 | 1.0 | |||
| No ART** | 2.5 (1.1, 5.8) | 0.04 | 2.9 (0.7, 11.2) | 0.13 | 2.4 (0.8, 7.1) | 0.13 | |
| Non-IDV ART** | 1.8 (0.8, 3.7) | 0.14 | 1.6 (0.5, 5.4) | 0.49 | 2.0 (0.8, 5.1) | 0.15 | |
| 3 | Any PI** | 1.0 | 1.0 | 1.0 | |||
| No ART** | 1.5 (0.9, 2.7) | 0.15 | 1.5 (0.7, 3.6) | 0.34 | 1.5 (0.7, 3.2) | 0.33 | |
| Non-PI ART** | 1.0 (0.6, 1.6) | 0.95 | 0.4 (0.1, 1.2) | 0.10 | 1.3 (0.8, 2.2) | 0.35 | |
ADC, AIDS-defining cancer; ART, antiretroviral therapy; HR, hazards ratio; IDV, indinavir; NADC, non-AIDS-defining cancer; NFV, nelfinavir; PI, protease inhibitor.
Model adjusted for age, race (Caucasian vs. other), gender, time updated CD4 cell count, and time-updated non-cancer AIDS event.
Time-updated
Figure 1.
Risk of Cancer by Receipt of Nelfinavir, Indinavir, or Protease Inhibitors in Multivariate Cox Proportional Hazard Models (1997–2006).
We also examined the effect of indinavir which was utilized by 862 (34%) of the study population during a total of 1804 PYs of IDV use. The median duration of IDV use was 16 months (IQR 8–34). Of those who received IDV, 42 developed cancer (17 ADC and 25 NADC events). The results from the adjusted Cox proportional hazard models are shown in Table 2 and Figure 1. Compared to those on IDV, those not receiving ART were significantly more likely to develop cancer (HR 2.5, 95% CI 1.1–5.8), while the risk of a cancer event was not significantly different for those on non-IDV ART (HR 1.8, 95% CI 0.8–3.7).
PIs were used by 1,492 (60%) of the study cohort for a total of 5415 PYs of PI use, with the median duration of usage of 34 months (IQR 16–66). Cancer developed in 77 patients who were receiving a PI (30 ADC and 47 NADC events). Compared to those on a PI, those not receiving ART were more likely to develop cancer (HR 1.5, 95% CI 0.9–2.7), although the increased risk was not statistically significant. The risk of a cancer event was similar for those on non-PI ART (HR 1.0, 95% CI 0.6 – 1.6).
Finally, we examined the effect of any ART regimen on cancer events. Compared to those without ART, those receiving ART were less likely to develop any cancer (HR 0.7, 95% CI 0.4, 1.1), an ADC (HR 0.2, 95% CI 0.1, 0.5) and a NADC (HR 0.5, 95% CI 0.2, 1.0).
Discussion
This study shows that despite reports that NFV may reduce the risk of cancer (1–9), we found no specific relationship between NFV use and cancer occurrences among HIV-infected persons who used NFV as part of their antiretroviral regimen. Similar to what other studies have found (15–17), our results suggest that the receipt of antiretroviral therapy may reduce the risk of AIDS-defining cancers. However, this effect was likely due to immune reconstitution, rather than NFV or protease inhibitors themselves, since the risk of cancer was not different based on the antiretroviral regimen.
Although studies have suggested NFV’s efficacy against several cancer types, our study did not find such an effect. Several reasons may account for our study not having found a protective relationship between NFV and cancer. First, it is possible that an association may not exist between NFV or PI-based antiretroviral therapy and a reduced risk of cancer. Alternative explanations may include that NFV is protective against cancer, but that higher doses of NFV are needed for cancer suppression compared to HIV protease inhibition for virologic control in the setting of HIV infection. The lack of association may also have been related to the need for a larger cohort to detect an effect. Although we studied a large HIV cohort, we had a limited number of cancer events (n=108); hence a potential protective effect may have been missed. However, our study cohort represents one of the largest HIV cohorts with detailed antiretroviral medication information over the course of the HIV epidemic; as such, one may have expected some effect to have been seen. In addition, our study included a wide array of cancer types, which may have missed a protective effect of NFV on a specific cancer type. As such, the effect of NFV on individual cancer types in our study could not be fully assessed due to the variety of different cancer types as well as the limited number of cancer events which are occurring during the HAART era. Studies have suggested that NFV has anticancer effects on both ADCs such as Kaposi’s sarcoma as well as a variety of NADCs (3–9). Despite examining ADC and NADC events separately, we did not find a relationship between NFV use and reduced rates of cancer; however, NFV may be protective against a subset of cancers which may not have been fully evaluated in our study cohort.
Additional studies using large registries and post-marketing surveillance are needed to provide further data on the possible relationship between NFV and other PIs and cancer inhibition among HIV-infected persons. In the meantime, clinical trials are underway among HIV-uninfected patients to examine the potential new role for nelfinavir in the treatment of cancer.
Acknowledgments
Support for this work was provided by the Infectious Disease Clinical Research Program (IDCRP), Uniformed Services University of the Health Sciences (USUHS), Bethesda, MD, of which the TriService AIDS Clinical Consortium (TACC) is a component. The IDCRP is a DoD tri-service program executed through USUHS and the Henry M. Jackson Foundation for the Advancement of Military Medicine in collaboration with HHS/NIH/NIAID/DCR through Interagency Agreement HU0001-05-2-0011.
Footnotes
This work is original and has not been published elsewhere. The data in this report were presented in part at the International AIDS Conference, Mexico City, Mexico, August 3- 8, 2008.
The opinions or ascertains contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of the Departments of the Army, Navy, or Air Force, or the Department of Defense. The authors have no commercial or other association that might pose a conflict of interest in this work.
References
- 1.Gills JJ, LoPiccolo J, Tsurutani J, et al. Nelfinavir, a lead HIV protease inhibitor, is a broad-spectrum, anticancer agent that induces endoplasmic reticulum stress, autophagy, and apoptosis In vitro and In vivo. Clin Cancer Res. 2007;13:5183–5194. doi: 10.1158/1078-0432.CCR-07-0161. [DOI] [PubMed] [Google Scholar]
- 2.Gupta AK, Cerniglia GJ, Mick R, et al. HIV protease inhibitors block Akt signaling and radiosensitize tumor cells both In vitro and In vivo. Cancer Res. 2005;65:8256–8265. doi: 10.1158/0008-5472.CAN-05-1220. [DOI] [PubMed] [Google Scholar]
- 3.Pyrko P, Kardosh A, Wang W, et al. HIV-1 protease inhibitors nelfinavir and atazanavir induce malignant glioma death by triggering endoplasmic reticulum stress. Cancer Res. 2007;67:10920–10928. doi: 10.1158/0008-5472.CAN-07-0796. [DOI] [PubMed] [Google Scholar]
- 4.Yang Y, Ikezoe T, Nishioka C, et al. NFV, an HIV-protease inhibitor, induces growth arrest, reduced Akt signalling, apoptosis and docetaxel sensitisation in NSCLC cell lines. Br J Cancer. 2006;95:1653–1662. doi: 10.1038/sj.bjc.6603435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Srirangam A, Mitra R, Wang M, et al. Effects of HIV protease inhibitor ritonavir on Akt-regulated cell proliferation in breast cancer. Clin Cancer Res. 2006;12:1883–1896. doi: 10.1158/1078-0432.CCR-05-1167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jiang W, Mikochik PJ, Ra JH, et al. HIV protease inhibitor Nelfinavir inhibits growth of human melanoma cells by induction of cell cycle arrest. Cancer Res. 2007;67:1221–1227. doi: 10.1158/0008-5472.CAN-06-3377. [DOI] [PubMed] [Google Scholar]
- 7.Yang Y, Ikezoe T, Takeuchi T, et al. HIV-1 protease inhibitor induces growth arrest and apoptosis of human prostate cancer LNCaP cells in vitro and in vivo in conjunction with blockade of androgen receptor STAT3 and AKT signaling. Cancer Sci. 2005;96:425–433. doi: 10.1111/j.1349-7006.2005.00063.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ikezoe T, Saito T, Bandobashi K, et al. HIV-1 protease inhibitor induces growth arrest and apoptosis of human multiple myeloma cells via inactivation of signal transducer and activator of transcription 3 and extracellular signal-regulated kinase 1/2. Mol Cancer Ther. 2004;3:473–479. [PubMed] [Google Scholar]
- 9.Sgadari C, Barillari G, Toschi E. HIV protease inhibitors are potent antiangiogenic molecules and promote regression of Kaposi sarcoma. Nat Med. 2002;8:225–232. doi: 10.1038/nm0302-225. [DOI] [PubMed] [Google Scholar]
- 10.NCI. [December 1, 2007];A Phase I trial of nelfinavir (Viracept®) in adults with solid tumors. at http://bethesdatrials.cancer.gov/solid_tumor/nci07c0047/default.asp.
- 11.Engels EA, Pfeiffer RM, Goedert JJ, et al. for the HIV/AIDS Cancer Match Study. Trends in cancer risk among people with AIDS in the United States 1980–2002. AIDS. 2006;20:1645–1654. doi: 10.1097/01.aids.0000238411.75324.59. [DOI] [PubMed] [Google Scholar]
- 12.Clifford GM, Polesel J, Rickenbach M, et al. Swiss HIV Cohort. Cancer risk in the Swiss HIV Cohort Study: associations with immunodeficiency, smoking, and highly active antiretroviral therapy. J Natl Cancer Inst. 2005;97:425–432. doi: 10.1093/jnci/dji072. [DOI] [PubMed] [Google Scholar]
- 13.Goedert JJ, Coté TR, Virgo P, et al. Spectrum of AIDS-associated malignant disorders. Lancet. 1998;351:1833–1839. doi: 10.1016/s0140-6736(97)09028-4. [DOI] [PubMed] [Google Scholar]
- 14.AIDS-Cancer Match Registry Study Group. Frisch M, Biggar RJ, Engels EA, et al. Association of cancer with AIDS-related immunosuppression in adults. JAMA. 2001;285:1736–1745. doi: 10.1001/jama.285.13.1736. [DOI] [PubMed] [Google Scholar]
- 15.International Collaboration on HIV and Cancer. Highly active antiretroviral therapy and incidence of cancer in human immunodeficiency virus-infected adults. J Natl Cancer Inst. 2000;92:1823–1830. doi: 10.1093/jnci/92.22.1823. [DOI] [PubMed] [Google Scholar]
- 16.Grulich AE, Li Y, McDonald AM, Correll PK, Law MG, Kaldor JM. Decreasing rates of Kaposi’s sarcoma and non-Hodgkin’s lymphoma in the era of potent combination anti-retroviral therapy. AIDS. 2001;15:629–633. doi: 10.1097/00002030-200103300-00013. [DOI] [PubMed] [Google Scholar]
- 17.Jacobson LP, Yamashita TE, Detels R, et al. Impact of potent antiretroviral therapy on the incidence of Kaposi’s sarcoma and non-Hodgkin’s lymphomas among HIV-1 infected individuals. Multicenter AIDS Cohort Study. J Acquire Immune Defic Syndr Hum Retroviral. 1999;21:S34–S41. [PubMed] [Google Scholar]
- 18.Crum-Cianflone N, Hullsiek KH, Marconi V, et al. Trends in the incidence of cancers among HIV-infected persons and the impact of antiretroviral therapy: a 20-year cohort study. AIDS. 2009;23:41–50. doi: 10.1097/QAD.0b013e328317cc2d. [DOI] [PMC free article] [PubMed] [Google Scholar]