Abstract
Following treatment of hepatitis B virus (HBV) infection with nucleos(t)ide reverse transcriptase inhibitors (NRTIs) there is a biphasic clearance of HBV, similar to that seen following treatment of HIV-1 and hepatitis C virus. Little is known about the impact of combination NRTIs and HIV-1 co-infection on HBV viral kinetic parameters following the initiation of HBV-active highly active antiretroviral therapy (HAART). HIV-1-HBV co-infected patients (n=21) were enrolled in a viral kinetics sub-study of the Tenofovir in HIV-1-HBV Coinfection study (TICO). TICO was a randomized (1:1:1) trial of tenofovir disoproxil fumarate (TDF, 300mg) vs lamivudine (LMV, 300mg) vs TDF/LMV within an efavirenz based HAART regimen initiated in HIV-1-HBV co-infected antiretroviral naïve individuals in Thailand. HBV DNA was measured frequently over the first 56 days. To fit the viral load data, we used a model of HBV kinetics that allows the estimation of treatment effectiveness, viral clearance and infected cell loss. We observed a biphasic decline in HBV DNA in almost all patients. We did not observe any significant differences in HBV viral dynamic parameters between the three treatments groups. Overall, median (IQR) HBV treatment effectiveness was 98% (95%–99%), median HBV virion half-life was 1.2 days (0.5–1.4 days), and median infected cell half-life was 7.9 days (6.3–11.0 days). When we compared HBeAg-positive and HBeAg-negative individuals, we found a significantly longer infected cell half-life in HBeAg-positive individuals (6.2 vs. 9.0 days, p=0.02). Conclusion: HBV viral dynamic parameters are similar following anti-HBV NRTI monotherapy and dual combination therapy in the setting of HIV-1-HBV coinfection. HIV-1 co-infection has minimal effect on HBV viral dynamics, even in the setting of advanced HIV-1-related immunosuppression.
Keywords: viral dynamics, combination therapy, antiviral effectiveness
Introduction
Co-infection with HIV-1 alters the natural history of chronic hepatitis B virus (HBV) infection with higher serum HBV DNA, lower alanine aminotransferase (ALT) levels and higher rates of cirrhosis, particularly in those with low CD4+ T cell counts (1, 2). Liver-related mortality has now become the leading cause of non-AIDS related death and a significant proportion of this is attributed to co-infection with HBV or hepatitis C virus (HCV) (3).
The treatment of HBV infection has significantly improved with the introduction of nucleoside analogues such as lamivudine (LMV) and entecavir and the nucleotide analogues adefovir dipivoxil and tenofovir disoproxil fumarate (TDF) (4–7). Although LMV can suppress HBV DNA (4, 8–10), the prevalence of antiviral resistance increases with time and overall is higher in HIV-1-HBV co-infected individuals (4, 11–14). LMV and TDF are commonly used in combination as a component of highly active antiretroviral therapy (HAART) for the treatment of HIV-1-HBV co-infection, however, it is unknown if TDF in combination with LMV is superior to the use of TDF alone (15, 16).
The availability of potent antiviral drugs for the treatment of viral diseases and the ability to accurately quantify viral load in vivo, has facilitated the mathematical analysis of the dynamics of HIV-1, HBV, HCV and simian immunodeficiency virus (SIV). Studies of HBV viral dynamics have demonstrated a biphasic clearance of HBV DNA with an initial rapid first phase decline representing clearance of free virions and a slower second phase representing clearance of infected hepatocytes (17–19, 20). These modeling studies also allowed an estimation of the effectiveness of treatment in stopping viral production . However, little is known about the impact of HIV-1 infection on HBV viral dynamics following initiation of HBV-active HAART. HIV-1 co-infection may reduce HBV-specific T cell activity and therefore reduce clearance of infected hepatocytes (21, 22). Alternatively, HIV-1 and HBV may be cleared by similar mechanisms and therefore compete for clearance by the reticuloendothelial system which could significantly change the first phase decay of HBV. Recent data on HCV viral dynamics in HIV-1-HCV co-infected individuals treated with interferon demonstrated that the HCV virion half-life was longer in HIV-1-HCV co-infected individuals compared with HCV mono-infected individuals (23, 24).
We therefore aimed to examine the HBV viral dynamic parameters in treatment naïve HIV-1-HBV co-infected individuals who initiated HBV-active HAART as part of a randomized, prospective clinical study. In addition, we aimed to determine the effect of combination anti-HBV treatment on HBV DNA decay and treatment effectiveness.
Methods
Subjects
The Tenofovir in HIV-1-HBV Coinfection study (TICO) was a randomized (1:1:1) trial of TDF (300mg/day) and zidovudine (AZT 250mg bid) vs LMV (300 mg/day) and AZT vs TDF/LMV within an efavirenz (EFV) based HAART regimen initiated in antiretroviral naïve HIV-1-HBV co-infected individuals in Thailand (25). Inclusion criteria included informed consent; documented HIV-1 infection by ELISA; age > 18 years; HBV DNA > 2 × 105 IU/ml at screening; HBV surface antigen (HBsAg) positive for > 6 months; ALT < 10 × upper limit of normal; Creatinine < 2.0mg/dl; Platelet count ≥ 50,000/mm3; HCV antibody negative. Patients were not tested for Hepatitis delta virus. Exclusion criteria included prior treatment with LMV, TDF, adefovir dipivoxil therapy or anti-HIV-1 therapy; active opportunistic infection; concurrent malignancy requiring cytotoxic chemotherapy; or Child’s C cirrhosis. Twenty one HIV-1-HBV co-infected individuals were enrolled in a viral kinetics sub-study. All subjects gave informed written consent and the study was approved by the relevant institutional review boards in Thailand and Australia. HBV DNA was measured at treatment initiation, days 1, 2, 3, 7, 10, 14, 17, 21, 28, 42, and 56. The baseline characteristics according to treatment arm are summarised in Table 1.
Table 1.
Demographic details and HBV viral dynamic parameters. LAM=lamivudine; TDF=tenofovir; m=male; f = female; t1/2=half life; ALT = alanine transferase; V0 = viral load at baseline; IQR = interquartile range; SD = standard deviation
| Therapy | No | Age, years | gender | HIV RNA, copies/ml | CD4, cells/ml | Genotype | HBeAg | ALT, IU/ml | HBV V0, IU/ml | log drop HBV DNA, W12 | efficacy | t1/2 1st phase, hours | t1/2 2nd phase, days | τ, delay, days |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| LAM | P03 | 30 | m | 3.9E+05 | 7 | C | + | 95 | 5.3E+07 | 5.1 | 98% | 21 | 9 | 0 |
| LAM | P06 | 35 | m | 9.2E+03 | 15 | C | + | 44 | 3.0E+07 | 5.8 | 92% | 17 | 4.8 | 0 |
| LAM | P10 | 48 | f | 4.9E+04 | 19 | B | + | 36 | 5.9E+08 | 3.2 | 97% | 9 | 14.9 | 0.8 |
| LAM | P21 | 40 | m | 2.7E+04 | 225 | C | + | 43 | 3.8E+07 | 3.8 | 96% | 28 | 11.2 | 0 |
| LAM | P24 | 33 | m | 3.1E+04 | 233 | C | − | 88 | 3.4E+07 | 4.5 | 99% | 34 | 7.7 | 0 |
| LAM | P30 | 47 | m | 5.5E+03 | 204 | C | + | 54 | 1.3E+07 | 0.7 | 97% | 20 | 9.0 | 0 |
| LAM | P37 | 28 | m | 4.1E+04 | 6 | C | + | 38 | 4.7E+07 | 2.6 | 98% | 32 | 12.5 | 0 |
| median | 35 | 3.1E+04 | 19 | 44 | 3.8E+07 | 3.8 | 97% | 21 | 9.0 | 0 | ||||
| IQR | 32–44 | 1.8E+04−4.5E+04 | 11–215 | 41–71 | 3.2E+07−5.0E+07 | 2.9–4.8 | 97%–98% | 19–30 | 8.4–11.9 | 0–0 | ||||
| TDF | P04 | 36 | m | 1.6E+04 | 15 | C | − | 74 | 1.1E+09 | 4.2 | 88% | 11 | 6.3 | 0.8 |
| TDF | P07 | 23 | m | 7.2E+04 | 59 | G | + | 130 | 3.7E+09 | 5.4 | 100% | 84 | 17.5 | 0 |
| TDF | P09 | 28 | f | 1.3E+05 | 22 | C | + | 22 | 1.8E+08 | 4.2 | 99% | 42 | 6.9 | 0 |
| TDF | P19 | 52 | m | 1.8E+05 | 88 | C | − | 106 | 1.1E+09 | 6.3 | 99% | 29 | 7 | 6.1 |
| TDF | P25 | 39 | f | 2.1E+05 | 212 | C | − | 36 | 8.1E+03 | ND | 95% | 10 | 6.2 | 0.4 |
| TDF | P35 | 21 | m | 3.8E+05 | 8 | B | + | 36 | 1.7E+07 | 3.9 | 97% | 30 | 8.7 | 2 |
| TDF | P38 | 34 | m | 1.2E+04 | 14 | C | − | 54 | 1.1E+08 | 6.2 | 98% | 30 | 4.4 | 1.6 |
| median | 34 | 1.3E+05 | 22 | 54 | 1.8E+08 | 4.8 | 98% | 30 | 6.9 | 0.8 | ||||
| IQR | 26–38 | 4.4E+04−2.0E+05 | 14.5–73.5 | 36–90 | 6.5E+07−1.1E+09 | 4.2–6.0 | 96%–99% | 20–36 | 6.3–7.9 | 0.2–1.8 | ||||
| LAM/TDF | P05 | 34 | m | 4.3E+05 | 39 | C | + | 71 | 7.2E+06 | 4.6 | 100% | 45 | 95.2 | 0 |
| LAM/TDF | P14 | 31 | f | 1.3E+05 | 31 | C | + | 24 | 6.3E+05 | 3.1 | 76% | 37 | 7.1 | 0 |
| LAM/TDF | P17 | 50 | f | 4.8E+04 | 61 | C | − | 26 | 2.1E+04 | 1.9 | 97% | 8 | 15.9 | 0.5 |
| LAM/TDF | P23 | 30 | f | 3.1E+03 | 230 | C | + | 20 | 4.9E+08 | 5.6 | 100% | 33 | 8.3 | 0 |
| LAM/TDF | P27 | 36 | m | 3.3E+04 | 216 | C | − | 26 | 3.3E+03 | ND | 69% | 6 | 1.3 | 0 |
| LAM/TDF | P36 | 56 | m | 7.9E+04 | 102 | B | − | 118 | 5.4E+10 | ND | 87% | 14 | 4.1 | 1.4 |
| LAM/TDF | P39 | 29 | f | 2.2E+04 | 19 | B | + | 19 | 7.0E+08 | 4.4 | 99% | 8 | 7.9 | 2 |
| median | 34 | 4.8E+04 | 61 | 26 | 7.2E+06 | 4.4 | 97% | 14 | 7.9 | 0 | ||||
| IQR | 31–43 | 2.7E+04−1.0E+05 | 35–159 | 22–49 | 3.3E+05−5.9E+08 | 3.1–4.6 | 81%–100% | 8–35 | 5.6–12.1 | 0–1 | ||||
| Overall | ||||||||||||||
| Median | 34 | 4.8E+04 | 39 | 43 | 4.7E+07 | 4.3 | 97% | 28 | 7.9 | 0 | ||||
| IQR | 30–40 | 2.2E+04−1.3E+05 | 15–204 | 26–74 | 1.3E+07−5.9E+08 | 3.3–5.3 | 95%−99% | 11–33 | 6.3–11.2 | 0–0.8 | ||||
| p-values | 0.69 | 0.43 | 0.4 | 0.28 | 0.46 | 0.32 | 0.84 | 0.61 | 0.36 | |||||
| Mean | 36 | 1.1E+05 | 87 | 55 | 2.9E+09 | 4.2 | 94% | 26 | 12.7 | 0.7 | ||||
| SD | 10 | 1.3E+05 | 90 | 34 | 1.2E+10 | 1.5 | 8% | 18 | 19.3 | 1.4 | ||||
HBV DNA and HIV-1 RNA quantification
HBV DNA was measured using both the bDNA assay (Bayer, Tarrytown, NJ; lower limit of detection, LLOD, 357 IU/ml) and COBAS Taqman assay (Roche, Branchburg, NJ; LLOD 15 IU/ml). HIV-1 RNA was quantified using the COBAS Amplicor assay (Roche; LLOD 50 copies/ml). HBV genotype was determined by PCR and sequencing of the polymerase gene as previously described (26)
Liver biopsies
Liver biopsies were performed prior to initiation of HBV-active HAART and after 48 weeks of therapy. A 5mm core was snap frozen in liquid nitrogen. Total liver HBV DNA and covalently closed circular (ccc)DNA was quantified by real-time PCR as previously described (27).
Description of the model
To fit the HBV DNA data, we used our previously published model of HBV kinetics that allows the estimation of treatment effectiveness, viral clearance and infected cell loss (19). Briefly, we modeled the dynamics of infected cells (I) and free virions (V), following the standard principles of viral dynamics modeling (19, 28). The solution of our model predicts the evolution of HBV DNA over time – V(t) – as:
where λ1, λ2 are given by ½(c+δ+θ) and ½(c+δ-θ), respectively, and . Here c is the free virus clearance rate, δ is the infected cell loss rate, ϵ is the effectiveness of treatment in preventing viral production and η=0.5 corresponds to any effect of drug in reducing viral infection of uninfected hepatocytes (see (19) for a full discussion of this model). We defined the drug effictiveness, ϵ, such that the virion production rate under therapy is (1 - ϵ)p. Therefore, a drug that has 100% effectiveness (ϵ = 1) results in complete suppression of new virion production; while a drug with effectiveness of 98% (ϵ = 0.98) results in suppression of new virion production to 2% of the original value. We note that this concept is different from clinical efficacy defined in terms of response rates over long periods of treatment. The parameter τ is a delay, corresponding to the time it takes between the initiation of drug treatment and its effect in reducing HBV DNA (eg. pharmacokinetic delays). The solution is valid for t > τ. For t < τ, the solution is V(t)=V0, where V0 is the initial HBV DNA.
Statistical methods
The model was fitted to the data using non-linear least squares regression to find the parameters that best describe the data. From the fits we obtain the parameters, δ, c, ϵ and τ. From these we calculate the infected cell and virion half-lives (t½), given by ln(2)/δ and ln(2)/c, respectively, where the natural logarithm of 2, ln(2)≈0.693. The nonparametric Kruskal-Wallis was used to compare differences between the treatment groups. The Wilcoxon rank test was used to compare HBeAg-positive and HBeAg-negative disease . The nonparametric Spearman test was used to assess the correlation between continuous variables. Nominal values were compared by the Fisher’s Exact test if the sample contained a sub-population that was less than 4. Significance was assessed at the α=0.05 level.
Results
Baseline patient characteristics
This viral kinetics sub-study included seven individuals in each of three treatment arms: TDF vs. LMV vs. TDF/LMV, within EFV-containing HAART regimens. As shown in Table 1, baseline characteristics were similar across treatment arms. Both HBeAg positive (n=13; 62%) and HBeAg-negative (n=8; 38%) individuals were included in the viral kinetic substudy. The majority of individuals were infected with genotype C virus (n=15; 66%). The remainder were genotype B (n=5) and genotype G (n=1). At baseline, overall median (interquartile range (IQR)) HBV DNA was 2.0×108 (0.22–34×108) IU/ml, median HIV-1 RNA was 4.8×104 (2.2–13×105) copies/ml, median CD4+ T-cell count was 39 (15–204) cells/µl, and median ALT was 59 (30–102) IU/ml.
Biphasic decay in HBV DNA following HBV-active HAART
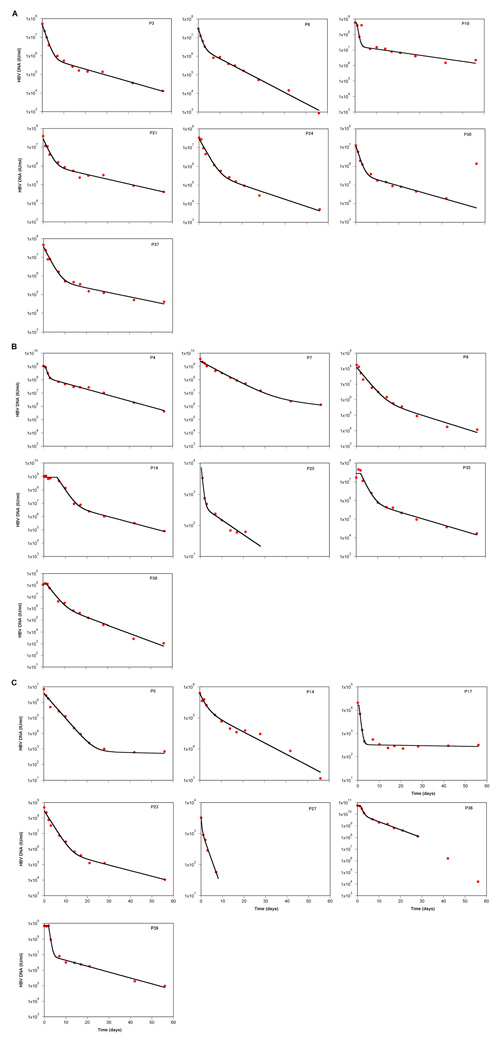
We observed a biphasic decline in HBV DNA in most individuals during the first 8 weeks of treatment (n=19; see Figure 1). In two individuals, P5 and P17 (both on combination LMV/TDF), no second phase decay was observed and the HBV DNA stayed at 500 and 250 IU/ml, respectively, over several weeks. Neither of these individuals had virus with sequence changes in HBV polymerase consistent with drug resistance (data not shown). In one other individual, P30 (on LMV), HBV DNA increased at day 56 of treatment. This was thought to be due to non-compliance as HBV DNA remained elevated until week 24, when the patient was lost to follow up. Again, no mutations were identified in the HBV polymerase in virus from this patient at day 56. Despite the late increase in HBV DNA in P30, we were able to fit the model in all the individuals (Figure 1). Indeed, the early decay (prior to 56 days) in P30 followed a very typical pattern, and the parameters obtained were similar to those of the other patients. Thus, we kept this patient in all analyses.
Figure 1.
Results of fitting the model to the data. The data points for HBV DNA are represented by the symbols, and the lines show the best fit of the mathematical model to the data; the best estimates for the fitted parameters are shown in Table 1. Data points are not shown once HBV DNA reached the lower limit of detection (<30 IU/ml; P25 and P27). All individuals were treated with HBV-active HAART containing efavirenz and (A) lamivudine (LMV), (B) tenofovir (TDF) or (C) combination LMV/TDF
Relationship of HBV viral dynamic parameters, treatment and immunosuppression
The HBV viral dynamic parameters are summarised in Table 1. We did not observe any differences in HBV dynamic parameters between the three treatments groups. The treatment effectiveness for LMV, TDF and LMV/TDF was 97%, 98% and 97% respectively (p=0.8). Overall, median (IQR) HBV treatment effectiveness was 97% (95%–99%), median HBV virion half-life was 1.2 days (0.5–1.4 days), and median infected cell half-life was 7.9 days (6.3–11 days) with no differences amongst the 3 treatment arms (p=0.84, 0.61 and 0.36 respectively). There was significant inter-patient variability within each treatment group. By week 12, the overall median drop in HBV DNA from baseline was 4.3 log IU/ml (3.3 – 5.3 log IU/ml), again with no difference between treatment arms (p=0.32). Moreover, there was no association between the viral dynamic parameters, including infected cell loss rate, and baseline HBV DNA, ALT, CD4+ T-cell count; HIV-1 RNA or BMI.
Because HBeAg status has been associated with a potentially impaired immune response (29, 30) we compared the two groups (HBeAg-positive (n=13) vs. HBeAg-negative (n=8)) for the different parameters. There were no differences in any of the baseline parameters (median): CD4+ T-cell (95 vs. 22 cells/µl, for HBeAg-negative vs. HBeAg-positive, respectively, p=0.17); ALT (85 vs. 44 IU/ml, p=0.10); HIV-1 RNA (4.6 vs. 4.7 log10copies/ml; p=0.92); not even in baseline HBV DNA (8.5 vs. 8.3 log10 copies/mL, p>0.99), which is often higher in HBeAg-positive individuals. However, we did find a difference in infected cell half-life, which was longer in HBeAg-positive individuals (6.2 vs. 9.0 days, p=0.02).
Liver biopsies and kinetic parameters
In five of the subjects (P27, P35, P37, P38, and P39) two liver biopsies were conducted, at baseline and at 48 weeks post-therapy. In two additional subjects (P19, P25) a liver biopsy was obtained at baseline only, whereas in 7 other individuals (P3, P5, P6, P7, P9, P14, P21) biopsy was performed only at 48 weeks. The median (IQR) cccDNA and total liver HBV DNA was 0.45 (0.25 – 1.1) and 11 (1.2 – 26) copies per genome equivalent (GEq) at baseline (n=7) and these dropped to 0.08 (0.03 – 0.28) and 0.74 (0.22 – 1.3) at 48 weeks post-therapy (n=12) respectively. We found no correlations between the drop in cccDNA per GEq or the drop in total liver HBV DNA per GEq and the loss rate of infected cells, nor any correlation between baseline levels of those quantities and δ.
Comparison of viral dynamic parameters between HBeAg positive HBV mono-infected and HIV-1-HBV co-infected individuals
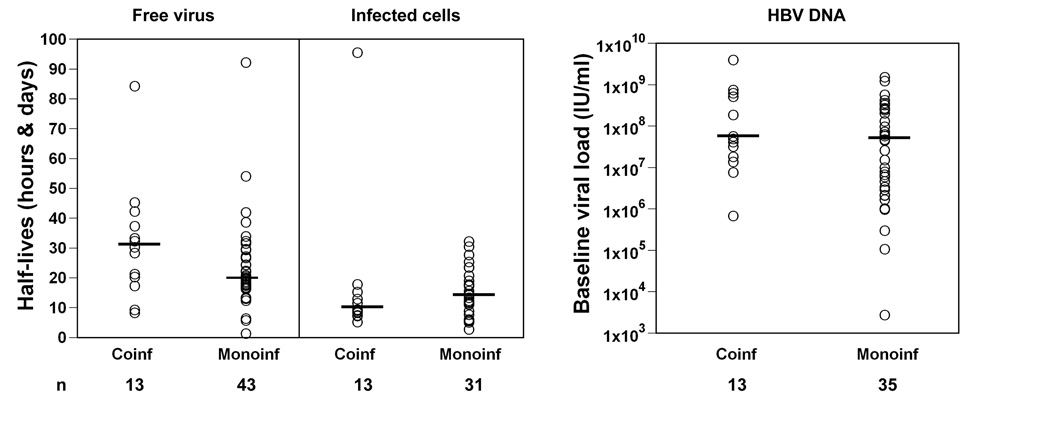
We compared HBV viral dynamic parameters in our HBeAg positive HIV-1-HBV co-infected individuals with HBV mono-infection using historical data obtained by us and others following NRTI in HBV mono-infection (Figure 2). Using kinetic historical data on 43 HBeAg-positive individuals (19, 20, 31, 32), treated with either LMV, a combination of LMV and famciclovir, adefovir or entecavir we did not find a difference in HBV virion clearance or infected cell loss rates – median half-life of virus: 1. 2 days HIV-1-HBV co-infected vs. 0.8 days HBV-infected (p=0.11, n=43 for historical data); median half-life of infected cells: 9 days HIV-1-HBV co-infected vs. 13 days HBV-infected (p=0.17, n=31 for historical data).
Figure 2.
Viral kinetic parameters (left panel) and baseline HBV DNA (right panel) from HBeAg positive HIV-1-HBV co-infected individuals in this study and HBV mono-infected individuals using historical data obtained by us and others following NRTI in HBV mono-infection ((19, 20, 31, 32). HBV mono-infected individuals were treated with either LMV, a combination of LMV and famciclovir, adefovir or entecavir. Half-life of free virus is given in hours and half-life for infected cells is in days. The horizontal line represents the median.
Discussion
This is the first randomized study to examine HBV viral dynamics in HIV-1-HBV co-infected individuals naïve to treatment for HBV and HIV-1. Following HBV-active HAART, we found a biphasic decline in HBV DNA in HIV-1-HBV co-infected individuals. In addition, we surprisingly found no difference in effectiveness, first or second phase decay between the three treatment arms. In addition, clearance of HBV free virions and infected hepatocytes was not dependent on the degree of HIV-1-related immunosuppression. Rather clearance of infected hepatocytes was slower in HBeAg-positive individuals. Finally, we found similar first and second phase decay rates of HBV in HIV-1-HBV co-infection to that previously reported by us and others in HBV mono-infection. These results suggest that HBV viral dynamic parameters are similar following anti-HBV NRTI monotherapy and combination therapy in the setting of HIV-1-HBV co-infection.
We were surprised to not find any differences in effectiveness between LMV, TDF or the combination LMV /TDF. These findings are, however, entirely consistent with the findings of the parent study TICO where there was no significant difference between the treatment arms in relation to the primary endpoint of mean area under curve HBV DNA decline over 48 weeks (25). There was, however, a significantly higher proportion of patients with HBV DNA above 1,000 copies/ml at week 48 in the LMV only arm and the only cases of viral rebound and HBV resistance were seen in this arm. Therefore, the benefit of TDF is likely to be related to protection against viral breakthrough or rebound due to a high genetic barrier to resistance rather than greater antiviral effectiveness per se (33–36). Another recent randomized study of HBeAg-positive individuals (n=115) that compared LMV with LMV and adefovir also showed no difference in the time-weighted average change in HBV DNA in the first 16 weeks however this study was only performed in HIV-negative individuals (37).
Prior viral kinetic studies of combination anti-HBV therapy have only been evaluated in HBV mono-infection. In these studies, LMV was used at the recommended but lower anti-HBV dose of 100mg/day (here we used 300mg/day). Most previous viral kinetic studies showed superior effectiveness of combination therapy to monotherapy, including LMV and famciclovir compared with LMV alone (19) emtricitabine and adefovir compared with adefovir alone (38, 39) and pegylated interferon and LMV compared with either pegylated interferon alone or LMV alone (40). A small randomized study of 600mg vs 100mg of LMV in chronic HBV infection demonstrated a faster second phase decline and greater overall change in HBV DNA of 4.06 vs 1.52 log10 copies/ml with 600mg vs 100 mg LMV respectively (32). Therefore, it is possible that prior viral dynamic studies were able to show enhanced effectiveness with combination therapy, even using agents with very modest anti-HBV activity such as famciclovir, because LMV was used at a sub-optimal and less effective dose. In this study, using a higher dose of LMV we found little difference in effectiveness compared with both TDF and combination TDF/LMV. Another explanation for not finding any difference in effectiveness across the three arms may have been the small patient cohort with significant inter-patient variability within treatment arms. However, our study had similar patient numbers and variability in viral kinetic parameters to other previous viral dynamic studies of combination anti-HBV treatment (19, 38). Finally, close to half of the individuals in this cohort were HBeAg negative and this also may have had an impact on viral kinetic parameters (40–43).
There have been limited detailed viral kinetic studies of TDF in individuals with chronic HBV infection naïve to antivirals. To date all studies of TDF have been performed in small HIV-1-HBV co-infected cohorts, often with high rates of LMV resistance (44, 45). In the only viral dynamic study of TDF treatment of HIV-1-HBV co-infected naïve individuals (n=5), the authors calculated the effectiveness, ϵ, for TDF as ~ 96% (44). The effectiveness of TDF in the current study of naïve individuals was higher at 97–98%. The differences may possibly have been related to HBV genotype. Although genotype is not mentioned by de Vries-Sluijs et al. (44), the study was performed in the Netherlands and therefore likely to include predominantly individuals infected with genotype A or D. In our study, all individuals were recruited in Thailand and were predominantly infected with either genotype B or C. Two other studies have examined the effectiveness of TDF in LMV-experienced HIV-1-HBV co-infected individuals and both found a lower effectiveness of TDF of ~88 % (44) and 93% (45). There have been no formal viral dynamic studies of TDF in HBV mono-infection although superior clinical efficacy has recently been demonstrated when compared to adefovir (46, 47).
Most HBV viral kinetic studies have been performed on HBeAg positive HBV mono-infected individuals (17–20, 31, 32, 38). However, two prior studies of pegylated interferon with and without lamivudine in HBeAg negative mono-infected patients demonstrated viral kinetic parameters similar to HBeAg positive individuals, although direct comparisons between HBeAg positive and HBeAg negative individuals were not made in these studies (40, 41). Our findings of a significantly longer second phase decline in HBeAg positive individuals compared with HBeAg negative individuals support the data that HBeAg may impair both the adaptive and innate immune response to HBV therefore leading to a more prolonged second phase decline in HBV DNA (29, 30 , 42). It would be worth examining these differences in future larger studies that also incorporate quantification of HBeAg into the mathematical model (48).
We found no significant differences between the first and second phase clearance of HBV in HBeAg positive HIV-1-HBV co-infected and HBV mono-infected individuals treated with NRTIs (17–20, 31). A significant limitation of this comparison was that we were only able to compare data with historical controls and from HBeAg positive individuals. It would have been preferable in this study to have included HBV mono-infected individuals randomized to the same treatment arms, however, this was not possible. We were surprised to not find altered HBV viral kinetics in the HIV-1-HBV co-infected individuals given the advanced immunosuppression of our patient cohort (median CD4 T-cell count was 72 cells/µl) and our expectation that recovery of HBV-specific CD4+ T-cells and therefore clearance of infected cells would be impaired (21).
In conclusion, this study demonstrated similar HBV viral dynamic parameters and drug effectiveness using three separate HBV-active HAART regimens including combination anti-HBV therapy. In addition, the viral dynamic parameters in HIV-1-HBV co-infected individuals were similar to that seen in HBV mono-infection. Although there may be a significant benefit in the use of tenofovir and/or combination therapy for the long term treatment of HBV in reducing the development of drug resistance, there is little difference in the initial anti-HBV effectiveness of nucleos(t)ide monotherapy and dual combination therapy in this setting of HIV-1-HBV co-infection, as judged by the kinetics of viral decay.
Acknowledgments
Financial support:
This study was sponsored by Gilead Sciences, Inc. and was part funded by NIH R21 AI055379-01 A1 (SRL, SAL, GJD). SRL is an Australian NHMRC Practitioner Fellow (#251651). The National Centre in HIV Epidemiology and Clinical Research is affiliated with the Faculty of Medicine, University of New South Wales and funded by the Commonwealth Department of Health and Ageing. KR is part funded by the National Center for Genetic Engineering and Biotechnology (BIOTEC), and the National Science and Technology Development Agency (NSTDA), Bangkok, Thailand. Part of this work was done under the auspices of the US Department of Energy and supported by NIH grants R01-RR06555 and R37-AI28433 (ASP) and P20-RR18754 (RMR).
Abbreviations
- HBV
Hepatitis B Virus
- HIV-1
human immunodeficiency virus
- HAART
highly active antiretroviral therapy
- ALT
alanine aminotransferase
- HBeAg
precore antigen
- HBsAg
HBV surface antigen
- HCV
Hepatitis C virus
REFERENCES
- 1.Thio CL, Seaberg EC, Skolasky R, Jr, Phair J, Visscher B, Munoz A, Thomas DL. HIV-1, hepatitis B virus, and risk of liver-related mortality in the Multicenter Cohort Study (MACS) Lancet. 2002;360:1921–1926. doi: 10.1016/s0140-6736(02)11913-1. [DOI] [PubMed] [Google Scholar]
- 2.Colin JF, Cazals-Hatem D, Loriot MA, Martinot-Peignoux M, Pham BN, Auperin A, Degott C, et al. Influence of human immunodeficiency virus infection on chronic hepatitis B in homosexual men. Hepatology. 1999;29:1306–1310. doi: 10.1002/hep.510290447. [DOI] [PubMed] [Google Scholar]
- 3.Weber R, Sabin CA, Friis-Moller N, Reiss P, El-Sadr WM, Kirk O, Dabis F, et al. Liver-related deaths in persons infected with the human immunodeficiency virus: the D:A:D study. Arch Intern Med. 2006;166:1632–1641. doi: 10.1001/archinte.166.15.1632. [DOI] [PubMed] [Google Scholar]
- 4.Lai CL, Chien RN, Leung NW, Chang TT, Guan R, Tai DI, Ng KY, et al. A one-year trial of lamivudine for chronic hepatitis B. Asia Hepatitis Lamivudine Study Group. N Engl J Med. 1998;339:61–68. doi: 10.1056/NEJM199807093390201. [DOI] [PubMed] [Google Scholar]
- 5.Cooper D, Cheng A, Coakley D, Sayre J, Zhong L, Chen S, Westland C, et al. Ninth Conference on Retroviruses and Opportunistic Infection. Seattle, WA: 2002. Anti-HBV activity of tenofovir disoproxil fumarate (TDF) in lamivudine experienced HIV/HBV co-infected. 2002. p. abstract 124. [Google Scholar]
- 6.Nelson M, Portsmouth S, Stebbing J, Atkins M, Barr A, Matthews G, Pillay D, et al. An open-label study of tenofovir in HIV-1 and Hepatitis B virus co-infected individuals. Aids. 2003;17:F7–F10. doi: 10.1097/00002030-200301030-00002. [DOI] [PubMed] [Google Scholar]
- 7.de Man RA, Wolters LM, Nevens F, Chua D, Sherman M, Lai CL, Gadano A, et al. Safety and effectiveness of oral entecavir given for 28 days in patients with chronic hepatitis B virus infection. Hepatology. 2001;34:578–582. doi: 10.1053/jhep.2001.26815. [DOI] [PubMed] [Google Scholar]
- 8.Dienstag JL, Perrillo RP, Schiff ER, Bartholomew M, Vicary C, Rubin M. A preliminary trial of lamivudine for chronic hepatitis B infection. N Engl J Med. 1995;333:1657–1661. doi: 10.1056/NEJM199512213332501. [DOI] [PubMed] [Google Scholar]
- 9.Benhamou Y, Katlama C, Lunel F, Coutellier A, Dohin E, Hamm N, Tubiana R, et al. Effects of lamivudine on replication of hepatitis B virus in HIV-infected men. Ann Intern Med. 1996;125:705–712. doi: 10.7326/0003-4819-125-9-199611010-00001. [DOI] [PubMed] [Google Scholar]
- 10.Dore G, Cooper D, Barrett C, Goh L, Thakrar B, Atkins M, CAESAR ft, et al. Dual effectiveness of lamivudine treatment in human immunodeficiency virus/hepatitis B virus-coinfected persons in a randomized, controlled study (CAESAR) J Infect Dis. 1999;180:607–613. doi: 10.1086/314942. [DOI] [PubMed] [Google Scholar]
- 11.Benhamou Y, Bochet M, Thibault V, Di Martino V, Caumes E, Bricaire F, Opolon P, et al. Long-term incidence of hepatitis B virus resistance to lamivudine in human immunodeficiency virus-infected patients. Hepatology. 1999;30:1302–1306. doi: 10.1002/hep.510300525. [DOI] [PubMed] [Google Scholar]
- 12.Pillay D, Cane P, Ratcliffe D, Atkins M, Cooper D. Evolution of lamivudine-resistant hepatitis B virus and HIV-1 in co-infected individuals: an analysis of the CAESAR study. AIDS. 2000;14:1111–1116. doi: 10.1097/00002030-200006160-00007. [DOI] [PubMed] [Google Scholar]
- 13.Cooley L, Ayers A, Bartholomeusz A, Lewin S, Crowe S, Mijch A, Locarnini S, et al. Prevalence and characterisation of lamivudine-resistant Hepatitis B virus (HBV) mutations in HIV and HBV co-infected individuals. AIDS. 2003;27:1649–1657. doi: 10.1097/00002030-200307250-00009. [DOI] [PubMed] [Google Scholar]
- 14.Matthews GV, Bartholomeusz A, Locarnini S, Ayres A, Sasaduesz J, Seaberg E, Cooper DA, et al. Characteristics of drug resistant HBV in an international collaborative study of HIV-HBV-infected individuals on extended lamivudine therapy. Aids. 2006;20:863–870. doi: 10.1097/01.aids.0000218550.85081.59. [DOI] [PubMed] [Google Scholar]
- 15.Rockstroh JK, Bhagani S, Benhamou Y, Bruno R, Mauss S, Peters L, Puoti M, et al. European AIDS Clinical Society (EACS) guidelines for the clinical management and treatment of chronic hepatitis B and C coinfection in HIV-infected adults. HIV Med. 2008;9:82–88. doi: 10.1111/j.1468-1293.2007.00535.x. [DOI] [PubMed] [Google Scholar]
- 16.Panel on Antiretroviral Guidelines for adults and Adolescents. Guidelines for the use of antiretrovirals in HIV-1-infected adults and adolescents. Department of Health and Human Services. 2008 Jan 29;:1–128. [Google Scholar]
- 17.Nowak MA, Bonhoeffer S, Hill AM, Boehme R, Thomas HC, McDade H. Viral dynamics in hepatitis B virus infection. Proc Natl Acad Sci U S A. 1996;93:4398–4402. doi: 10.1073/pnas.93.9.4398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lau GK, Tsiang M, Hou J, Yuen S, Carman WF, Zhang L, Gibbs CS, et al. Combination therapy with lamivudine and famciclovir for chronic hepatitis B-infected Chinese patients: a viral dynamics study. Hepatology. 2000;32:394–399. doi: 10.1053/jhep.2000.9143. [DOI] [PubMed] [Google Scholar]
- 19.Lewin S, Ribeiro R, Walters T, Lau G, Bowden S, Locarnini S, Perelson A. Analysis of hepatitis B viral load decline under potent therapy: complex decay profiles observed. Hepatology. 2001;34:1012–1020. doi: 10.1053/jhep.2001.28509. [DOI] [PubMed] [Google Scholar]
- 20.Tsiang M, Rooney JF, Toole JJ, Gibbs CS. Biphasic clearance kinetics of hepatitis B virus from patients during adefovir dipivoxil therapy. Hepatology. 1999;29:1863–1869. doi: 10.1002/hep.510290626. [DOI] [PubMed] [Google Scholar]
- 21.Chang J, Wightman F, Bartholomeusz A, Ayres A, Kent S, Sasadeusz J, Lewin S. Reduced HBV-specific CD4+ T-cell responses in HIV-1-HBV co-infected individuals receiving HBV-active antiretroviral therapy. J Virol. 2005;79:3038–3051. doi: 10.1128/JVI.79.5.3038-3051.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lascar RM, Gilson RJ, Lopes AR, Bertoletti A, Maini MK. Reconstitution of hepatitis B virus (HBV)-specific T cell responses with treatment of human immunodeficiency virus/HBV coinfection. J Infect Dis. 2003;188:1815–1819. doi: 10.1086/379896. [DOI] [PubMed] [Google Scholar]
- 23.Torriani FJ, Ribeiro RM, Gilbert TL, Schrenk UM, Clauson M, Pacheco DM, Perelson AS. Hepatitis C virus (HCV) and human immunodeficiency virus (HIV) dynamics during HCV treatment in HCV/HIV coinfection. J Infect Dis. 2003;188:1498–1507. doi: 10.1086/379255. [DOI] [PubMed] [Google Scholar]
- 24.Talal AH, Ribeiro RM, Powers KA, Grace M, Cullen C, Hussain M, Markatou M, et al. Pharmacodynamics of PEG-IFN alpha differentiate HIV/HCV coinfected sustained virological responders from nonresponders. Hepatology. 2006;43:943–953. doi: 10.1002/hep.21136. [DOI] [PubMed] [Google Scholar]
- 25.Matthews GV, Avihingsanon A, Lewin SR, Amin J, Rerknimitr R, Petcharapirat P, Marks P, et al. A randomized trial of combination hepatitis B therapy in HIV/HBV coinfected antiretroviral naive individuals in Thailand. Hepatology. 2008;11:11. doi: 10.1002/hep.22462. [DOI] [PubMed] [Google Scholar]
- 26.Ayres A, Locarnini S, Bartholomeusz A. HBV genotyping and analysis for unique mutations. Methods Mol Med. 2004;95:125–149. doi: 10.1385/1-59259-669-X:125. [DOI] [PubMed] [Google Scholar]
- 27.Werle B, Wursthorn K, Petersen J, Bowden S, Locarnini S, Lau G, Trepo C, et al. Quantitative analyses of hepatic HBV ccc DNA during the natural history of chronic hepatitis B and adefovir dipivoxil therapy: an International, Multicenter study. Hepatology. 2002;36:296A. [Google Scholar]
- 28.Neumann AU, Lam NP, Dahari H, Gretch DR, Wiley TE, Layden TJ, Perelson AS. Hepatitis C viral dynamics in vivo and the antiviral effectiveness of interferon-alpha therapy. Science. 1998;282:103–107. doi: 10.1126/science.282.5386.103. [DOI] [PubMed] [Google Scholar]
- 29.Milich D, Chen M, Hughes J, Jones J. The secreted hepatitis B precore antigen can modulate the immune response to the nucleocapsid: a mechanism for persistence. J Immunol. 1998;160:2013–2021. [PubMed] [Google Scholar]
- 30.Visvanathan K, Skinner NA, Thompson AJ, Riordan SM, Sozzi V, Edwards R, Rodgers S, et al. Regulation of Toll-like receptor-2 expression in chronic hepatitis B by the precore protein. Hepatology. 2007;45:102–110. doi: 10.1002/hep.21482. [DOI] [PubMed] [Google Scholar]
- 31.Wolters LM, Hansen BE, Niesters HG, DeHertogh D, de Man RA. Viral dynamics during and after entecavir therapy in patients with chronic hepatitis B. J Hepatol. 2002;37:137–144. doi: 10.1016/s0168-8278(02)00115-0. [DOI] [PubMed] [Google Scholar]
- 32.Wang CC, Holte S, Huang ML, Sacks SL, Engelberg R, Ferrenberg J, Shuhart M, et al. Kinetics of hepatitis B viral load during 48 weeks of treatment with 600 mg vs 100 mg of lamivudine daily. J Viral Hepat. 2004;11:443–447. doi: 10.1111/j.1365-2893.2004.00523.x. [DOI] [PubMed] [Google Scholar]
- 33.Dienstag J. Hepatitis B virus infection. N Engl J Med. 2008;359:1486–1500. doi: 10.1056/NEJMra0801644. [DOI] [PubMed] [Google Scholar]
- 34.Benhamou Y, Fleury H, Trimoulet P, Pellegrin I, Urbinelli R, Katlama C, Rozenbaum W, et al. Anti-hepatitis B virus effectiveness of tenofovir disoproxil fumarate in HIV-infected patients. Hepatology. 2006;43:548–555. doi: 10.1002/hep.21055. [DOI] [PubMed] [Google Scholar]
- 35.Audsley J, Arrifin N, Yuen L, Ayres A, Crowe S, Bartholomeusz A, Locarnini S, et al. Prolonged use of Tenofovir in HIV-HBV co-infected individuals does not lead to HBV polymerase mutations and is associated with persistence of Lamivudine HBV polymerase mutations. HIV Med. 2008 doi: 10.1111/j.1468-1293.2008.00675.x. in press. [DOI] [PubMed] [Google Scholar]
- 36.Dore GJ, Cooper DA, Pozniak AL, DeJesus E, Zhong L, Miller MD, Lu B, et al. Effectiveness of tenofovir disoproxil fumarate in antiretroviral therapy-naive and -experienced patients coinfected with HIV-1 and hepatitis B virus. J Infect Dis. 2004;189:1185–1192. doi: 10.1086/380398. Epub 2004 Mar 1112. [DOI] [PubMed] [Google Scholar]
- 37.Sung JJ, Lai JY, Zeuzem S, Chow WC, Heathcote EJ, Perrillo RP, Brosgart CL, et al. Lamivudine compared with lamivudine and adefovir dipivoxil for the treatment of HBeAg-positive chronic hepatitis B. J Hepatol. 2008;48:728–735. doi: 10.1016/j.jhep.2007.12.026. Epub 2008 Feb 2029. [DOI] [PubMed] [Google Scholar]
- 38.Lau GK, Cooksley H, Ribeiro RM, Powers KA, Shudo E, Bowden S, Hui CK, et al. Impact of early viral kinetics on T-cell reactivity during antiviral therapy in chronic hepatitis B. Antivir Ther. 2007;12:705–718. [PubMed] [Google Scholar]
- 39.Hui CK, Zhang HY, Bowden S, Locarnini S, Luk JM, Leung KW, Yueng YH, et al. 96 weeks combination of adefovir dipivoxil plus emtricitabine vs. adefovir dipivoxil monotherapy in the treatment of chronic hepatitis B. J Hepatol. 2008;48:714–720. doi: 10.1016/j.jhep.2007.10.013. Epub 2007 Dec 2031. [DOI] [PubMed] [Google Scholar]
- 40.Colombatto P, Civitano L, Bizzarri R, Oliveri F, Choudhury S, Gieschke R, Bonino F, et al. A multiphase model of the dynamics of HBV infection in HBeAg-negative patients during pegylated interferon-alpha2a, lamivudine and combination therapy. Antivir Ther. 2006;11:197–212. [PubMed] [Google Scholar]
- 41.Sypsa VA, Mimidis K, Tassopoulos NC, Chrysagis D, Vassiliadis T, Moulakakis A, Raptopoulou M, et al. A viral kinetic study using pegylated interferon alfa-2b and/or lamivudine in patients with chronic hepatitis B/HBeAg negative. Hepatology. 2005;42:77–85. doi: 10.1002/hep.20738. [DOI] [PubMed] [Google Scholar]
- 42.Ribeiro R, Germanidis G, Powers K, Pellegrin B, Perelson A, Pawlotsky J. HBV viral kinetics under antiviral therapy help understand the anti-HBV immune response in HBeAg-negative patients with chronic hepatitis B. Hepatology. 2005;42:721A. [Google Scholar]
- 43.Fang CT, Chen PJ, Chen MY, Hung CC, Chang SC, Chang AL, Chen DS. Dynamics of plasma hepatitis B virus levels after highly active antiretroviral therapy in patients with HIV infection. J Hepatol. 2003;39:1028–1035. doi: 10.1016/s0168-8278(03)00416-1. [DOI] [PubMed] [Google Scholar]
- 44.de Vries-Sluijs TE, van der Eijk AA, Hansen BE, Osterhaus AD, de Man RA, van der Ende ME. Wild type and YMDD variant of hepatitis B virus: no difference in viral kinetics on lamivudine/tenofovir therapy in HIV-HBV co-infected patients. J Clin Virol. 2006;36:60–63. doi: 10.1016/j.jcv.2005.12.004. Epub 2006 Jan 2018. [DOI] [PubMed] [Google Scholar]
- 45.van der Eijk AA, Hansen BE, Niesters HG, Janssen HL, van de Ende M, Schalm SW, de Man RA. Viral dynamics during tenofovir therapy in patients infected with lamivudine-resistant hepatitis B virus mutants. J Viral Hepat. 2005;12:364–372. doi: 10.1111/j.1365-2893.2005.00620.x. [DOI] [PubMed] [Google Scholar]
- 46.Heathcote E, Gane E, DeMan R. A Randomized, Double-Blind, Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg Positive Chronic Hepatitis B (CHB): Study GS-US-174–0103. 58th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD 2007); Boston. 2007. [Google Scholar]
- 47.Marcellin P, Buti M, Krastev Z, Germanidis G, Kaita K, Kotzev I, Buggisch P, et al. Randomized, Double-Blind, Comparison of Tenofovir DF (TDF) versus Adefovir Dipivoxil (ADV) for the Treatment of HBeAg-Negative Chronic Hepatitis B (CHB): Study GS-US-174–0102. 58th Annual Meeting of the American Association for the Study of Liver Diseases (AASLD 2007); Boston. 2007. [Google Scholar]
- 48.Fried MW, Piratvisuth T, Lau GK, Marcellin P, Chow WC, Cooksley G, Luo KX, et al. HBeAg and hepatitis B virus DNA as outcome predictors during therapy with peginterferon alfa-2a for HBeAg-positive chronic hepatitis B. Hepatology. 2008;47:428–434. doi: 10.1002/hep.22065. [DOI] [PubMed] [Google Scholar]