Abstract
Eosinophilic myocarditis is characterized by progressive myocardial damage that results in heart failure and death. Herein, we present the case of a 54-year-old man who presented with symptoms of acute myocardial infarction. Normal coronary angiographic results and the presence of elevated levels of peripheral-blood eosinophilia prompted an endomyocardial biopsy that revealed acute eosinophilic myocarditis. The early initiation of steroid therapy resulted in the patient's substantial clinical improvement and survival.
Early diagnosis of eosinophilic myocarditis and its treatment with steroid agents in some patients can lead to a favorable outcome. We discuss the challenge of diagnosing and identifying the characteristics of this variant of necrotizing eosinophilic myocarditis before the condition proves fatal.
Key words: Acute disease; biopsy; diagnosis, differential; eosinophilia/etiology/pathology/therapy; heart failure/etiology; immunosuppressive agents/therapeutic use; myocarditis/classification/diagnosis/drug therapy/etiology/pathology; necrosis; prednisone/therapeutic use
Early-stage eosinophilic myocarditis that presents with necrosis follows a rapidly deteriorating course. Swift diagnosis and the treatment of some patients with steroid agents can lead to a favorable outcome. Here, we report the case of a 54-year-old man who presented with acute eosinophilic myocarditis that mimicked myocardial infarction. We discuss the diagnosis and treatment of this condition and the challenge of promptly identifying the characteristics of this variant of necrotizing eosinophilic myocarditis.
Case Report
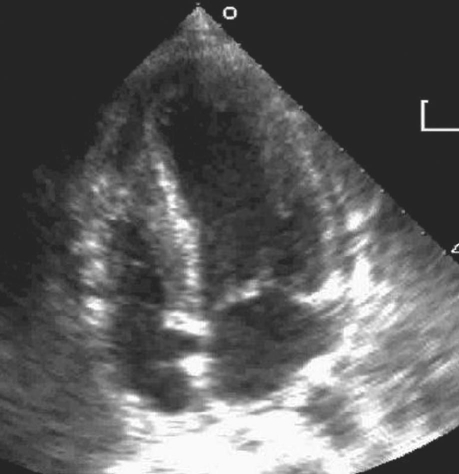
In February 2008, a 54-year-old white man was admitted to our hospital with typical angina symptoms of 1 week's duration. He had no previous history of coronary artery disease. His medical history included gastric bypass surgery a year earlier (secondary to morbid obesity), type 2 diabetes mellitus, and obstructive sleep apnea. He was a nonsmoker and was not taking any medication known to cause hypereosinophilia. Due to atypical chest pain 6 months earlier, he had undergone a treadmill imaging test; the results were negative. Four days before the current presentation, the patient experienced sudden and painless loss of vision in his right eye; this was diagnosed as central retinal artery occlusion. At that time, the reported results of carotid Doppler ultrasonography, transthoracic echocardiography (TTE), and magnetic resonance imaging of the brain were normal. At the current presentation, physical examination revealed normal cardiac sounds and bilateral lower-lung crackles. Electrocardiography revealed sinus rhythm with anterior and inferior J-point elevation, but no acute ischemic changes. Upon his admission to the hospital, TTE revealed a left ventricular ejection fraction (LVEF) of 0.55 with septal wall hypokinesis and increased left ventricular (LV) wall thickness—results that were not shown on an echocardiogram of 1 week earlier. Laboratory tests revealed a white blood cell count of 18,120/mm3 (normal range, 4,500–10,000/mm3), elevated levels of cardiac troponins, and an absolute eosinophil count (AEC) of 8,350/mm3 (normal range, 50–350/mm3)—this last in contrast with the normal results of an AEC that had been performed 6 months previously. A computed tomographic scan of the chest revealed multifocal pulmonary opacities with a tree-in-bud pattern that was consistent with bronchopneumonia. The patient's condition was initially stabilized; however, he developed severe, recurrent chest pain on the 2nd day of hospitalization. Because an electrocardiogram showed new anterolateral ST-segment elevation, he was taken for emergency coronary angiography, which revealed nonobstructive disease. Later that day, another TTE revealed worsening LV function (LVEF, 0.30–0.35), severe septal hypokinesis, and moderate inferior and anteroapical hypokinesis. Spontaneous echocontrast was noted in the LV. Moderate pericardial effusion with no evidence of cardiac tamponade, along with the progressive thickening of the LV wall, suggested an infiltrative disorder (Fig. 1).
Fig. 1 Transthoracic echocardiography of the left ventricle shows increased septal wall thickness.
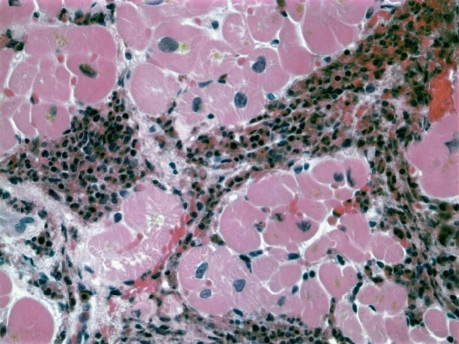
The patient was immediately started on intravenous methylprednisolone (100 mg, 3 times daily) and was treated with angiotensin-converting enzyme inhibitors and β-blockers. Laboratory tests revealed mildly elevated serum inflammatory markers (sedimentation rate, 22 mm/sec; C-reactive protein level, 5.4 mg/dL), an elevated serum immunoglobulin E (IgE) level of 998 IU/L, no evidence of human immunodeficiency virus, normal thyroid and iron levels, and no ova or parasites in the stool. A bone marrow biopsy disclosed no leukemic blasts. Bronchoscopy showed no endobronchial lesions or eosinophilic necrosis. On day 3, an endomyocardial biopsy revealed eosinophilic infiltration of the myocardium with extensive necrosis, confirmed upon microscopic staining (Fig. 2). After a week's treatment with high dosages of intravenous steroids, the patient's symptoms continued to improve, and his LVEF improved slightly. He was discharged from the hospital with instructions to take 60 mg of prednisone once daily. One week later, his AEC was normal, and the dosage of prednisone was reduced. Six months later, the prednisone was discontinued, and his LVEF had normalized with normal AEC.
Fig. 2 Histographic endomyocardial biopsy shows eosinophilic infiltration into the myocardial interstitium (H & E, orig. ×1,000).
Discussion
Cardiac involvement in eosinophilia has been well studied.1,2 Although Loeffler's endocarditis is documented, reports are scarce regarding myocarditis that is due to eosinophilic degranulation. Eosinophilic myocarditis can present as an acute condition, or chronic, as in connective-tissue diseases, hypereosinophilic syndrome, eosinophilic leukemia, and drug-induced or infection-related settings. The connective-tissue diseases (systemic lupus erythematosus and polymyositis) can be associated with myocarditis and pericarditis; however, the myocarditis is usually mild.3 The reported duration of illness in chronic myocarditis ranges from 1 to 11 years from diagnosis until death.3 Patients with chronic eosinophilic myocarditis generally present with symptoms of congestive heart failure, which usually respond to glucocorticoids and cytotoxic agents.
In contrast, acute necrotizing eosinophilic cardiomyopathy (NEC) follows a more rapidly deteriorating cardiac course with less systemic involvement, and the clinical severity does not correspond with the level of peripheral eosinophilia. Glucocorticoids have not been found to be effective, primarily because they have been introduced late in the clinical course.4 Although previous reports describe an association of eosinophilic myocarditis with exposure to drugs or with a preceding viral infection, acute NEC differs in important ways. Hypersensitivity myocarditis (HSM) from drug exposure is characterized by less necrosis, more evidence of perivascular infiltrate, vasculitis, and more hepatic involvement.5 A viral infection with accompanying cardiomyopathy has a chiefly mononuclear infiltrate, as opposed to the predominantly eosinophilic infiltrate in NEC. Eosinophilic myocarditis associated with hypereosinophilic syndrome is characterized by peripheral eosinophilia greater than 1,500/μL over 6 months, without a known cause.6 This variant of eosinophilic myocarditis is typically more indolent than NEC and evolves over at least 6 months. Conversely, in NEC, the patient is usually healthy and experiences a rapid, acute progression to systolic failure and hemodynamic compromise.4 The diagnosis of NEC is usually confirmed only upon autopsy, which reveals extensive myocardial necrosis.6,7 Degranulation of eosinophils with release of major basic protein is implicated in the pathologic processes of this disease.8 Major basic protein is also a potent stimulator of platelets that result in cardiac thrombi.
Currently, endomyocardial biopsy is the method of choice for diagnosis of NEC.9 A rapid decline in cardiac function despite optimal medical care indicates a need to perform endomyocardial biopsy, and to initiate immunosuppression if infection is not suspected. Endomyocardial biopsy is a class I recommendation of the American Heart Association/American College of Cardiology in patients who have experienced less than 2 weeks of symptoms, because the results can distinguish giant-cell myocarditis (GCM) (which has the poorest prognosis without cardiac transplantation) from both lymphocytic myocarditis (which has a better prognosis) and NEC (for which early immunosuppression can be life-saving).9 Endomyocardial biopsy is also a class I recommendation for patients who, for 2 weeks to 3 months, have exhibited symptoms of heart failure—such as arrhythmias and heart block—that have not responded to usual care. Typically, GCM occurs in the 4th or 5th decade of life and is associated with conduction-system disease and with ventricular tachycardia that is due to infiltration of the fascicles and myocardium; however, ST-segment elevation is rare in comparison with its frequency in eosinophilic myocarditis.7 Moreover, LV dilation without hypertrophy and segmental hypokinesis is usually seen in GCM, whereas LV wall thickening is prominent in eosinophilic myocarditis. Pericardial effusion is also unlikely in GCM. In GCM, there is marked necrosis with giant cells and macrophage and eosinophil infiltration, whereas in NEC there are no giant cells except for a few in the regions of necrosis.2 Early immunosuppressive therapy offers the only chance of survival for patients with NEC; our patient's condition improved upon the high-dose administration of steroid agents. Although HSM also responds to steroid therapy, the presence of this condition was less likely in our patient due to the extensive eosinophilic infiltrate, myocardial necrosis, low degree of extracardiac involvement, and absence of an offending medication. The clinical course of HSM is also self-limiting: patients generally present with fever, rash, arrhythmias, and only mild heart-failure symptoms that resolve spontaneously after the offending agent is removed. Finally, ST-segment elevation is very unlikely in HSM.5
Eosinophilic myocarditis has 3 stages.1 Stage 1 is the acute necrotic stage, which is due to the infiltration and extracellular deposition of eosinophils and consequently interleukin-5–mediated injury. Stage 2, the thrombotic stage, is characterized by layered thrombus along damaged endocardium due to an activation of tissue factor by the eosinophils. Stage 3 is the fibrotic stage, characterized by myocardial fibrosis. Patients can present with acute coronary syndromes during any of the stages.3 Stage 1 lasts from 2 to 3 weeks. Cerebral thromboemboli are very common during the thrombotic stage, and, unfortunately, anticoagulative therapy may not be entirely protective. Good prognostic signs in our patient were the suppressed AEC upon steroid therapy, an elevated serum IgE level, and the absence of leukemic changes.7 The goal in patients with NEC is to taper the dosage of steroid agents to the lowest level necessary for eosinophil suppression. Biannual echocardiographic follow-up is recommended, because end-organ involvement is not related to the eosinophilia level.7
In conclusion, acute NEC is a medical emergency that is usually fatal, and the condition is difficult to diagnose before a patient's death. The swift initiation of high-dose corticosteroids can impart dramatic improvement, as in our patient, before the final diagnosis of eosinophilic myocarditis can be confirmed. If there is a strong clinical suspicion of eosinophilic myocarditis, the example of our patient reinforces the need to perform a prompt endomyocardial biopsy in order to arrive at a diagnosis and to initiate high-dosage corticosteroid therapy without waiting for the diagnosis.
Footnotes
Address for reprints: Senthil Thambidorai, MD, 3006 Webster Street, Omaha, NE 68131
E-mail: skt73723@creighton.edu
References
- 1.Aretz HT, Billingham ME, Edwards WD, Factor SM, Fallon JT, Fenoglio JJ Jr, et al. Myocarditis. A histopathologic definition and classification. Am J Cardiovasc Pathol 1987;1(1):3–14. [PubMed]
- 2.Herzog CA, Snover DC, Staley NA. Acute necrotising eosinophilic myocarditis. Br Heart J 1984;52(3):343–8. [DOI] [PMC free article] [PubMed]
- 3.Janin A. Eosinophilic myocarditis and fibrosis. Hum Pathol 2005;36(5):592–3. [DOI] [PubMed]
- 4.Cooper LT, Zehr KJ. Biventricular assist device placement and immunosuppression as therapy for necrotizing eosinophilic myocarditis. Nat Clin Pract Cardiovasc Med 2005;2(10):544–8. [DOI] [PubMed]
- 5.Hawkins ET, Levine TB, Goss SJ, Moosvi A, Levine AB. Hypersensitivity myocarditis in the explanted hearts of transplant recipients. Reappraisal of pathologic criteria and their clinical implications. Pathol Annu 1995;30(Pt 1):287–304. [PubMed]
- 6.Al Ali AM, Straatman LP, Allard MF, Ignaszewski AP. Eosinophilic myocarditis: case series and review of literature. Can J Cardiol 2006;22(14):1233–7. [DOI] [PMC free article] [PubMed]
- 7.Ginsberg F, Parrillo JE. Eosinophilic myocarditis. Heart Fail Clin 2005;1(3):419–29. [DOI] [PubMed]
- 8.deMello DE, Liapis H, Jureidini S, Nouri S, Kephart GM, Gleich GJ. Cardiac localization of eosinophil-granule major basic protein in acute necrotizing myocarditis. N Engl J Med 1990;323(22):1542–5. [DOI] [PubMed]
- 9.Cooper LT, Baughman KL, Feldman AM, Frustaci A, Jessup M, Kuhl U, et al. The role of endomyocardial biopsy in the management of cardiovascular disease: a scientific statement from the American Heart Association, the American College of Cardiology, and the European Society of Cardiology. Endorsed by the Heart Failure Society of America and the Heart Failure Association of the European Society of Cardiology. J Am Coll Cardiol 2007;50(19):1914–31. [DOI] [PubMed]