Abstract
In experimental research, progesterone has been found to be beneficial to the central nervous and cardiovascular systems; however, its potential role in preventing atherosclerosis in elderly men remains unclear. In this prospective study, we analyzed data in 385 older men and women from 6 communities in Beijing, China, in order to discover whether progesterone is associated with carotid intima–media thickness and plaque occurrence. Intima–media thickness and atherosclerotic plaques were determined by use of ultrasonography. Sex-hormone levels were measured by immunoassay. The data were analyzed via analysis of covariance and logistic regression analysis; P < 0.05 was considered statistically significant.
We found a negative association between mean intima–media thickness and progesterone concentration in men, before and after adjustments for such traditional risk factors of atherosclerosis as age, triglyceride levels, total and low-density lipoprotein cholesterol levels, high-sensitivity C-reactive protein, systolic blood pressure, waist-to-hip ratio, and body mass index (analysis of covariance, P = 0.007 and P = 0.015, respectively). However, no such association was found in women (P = 0.304 and P = 0.247, respectively). In the logistic regression model that was adjusted for the confounding factors of atherosclerosis, men with progesterone levels in the lowest quartile (<1.87 nmol/L) had more risk of higher intima–media thickness (odds ratio, 2.15; P = 0.042). Although further experimental and prospective studies are warranted in order to determine the mechanism of progesterone's function in atherosclerosis prevention, we conclude that progesterone concentrations are negatively associated with carotid artery atherosclerosis in northern Chinese men 60 years of age or older.
Key words: Aging/blood; analysis of variance; atherosclerosis/prevention & control; carotid arteries/pathology/physiology/ultrasonography; cohort studies; muscle, smooth, vascular/physiopathology; progesterone/physiology; risk factors; sex characteristics
Atherosclerosis is the primary cause of death among the elderly in Western countries, and it is a major determinant of chronic disability.1,2 It has been suggested that, as people age, inflammation and endogenous sex-hormone levels have an increasing independent effect on the risk of atherosclerosis.3 Whether changes in endogenous sex-hormone levels in men have an effect on atherosclerosis has remained unclear; more attention has been paid to the importance of testosterone and estrogens in the cause, prevention, and treatment of male atherosclerosis.4,5 Evidence increasingly indicates that progesterone beneficially regulates coronary artery reactivity in monkeys6 and human beings.7–9 However, the mechanisms of the beneficial effect of progesterone on atherosclerosis in men are largely unknown, and the effect of progesterone still seems contradictory.10
Carotid intima–media thickness (IMT) is a widely accepted noninvasive measurement of preclinical atherosclerosis and an independent predictor of future adverse cardiovascular and cerebrovascular events.11–13 To the best of our knowledge, no study has previously been conducted to determine the relationship between progesterone levels and atherosclerosis in older northern Chinese. We sought to investigate whether serum progesterone is associated with atherosclerosis, as measured by IMT of the carotid artery in a population-based cohort of 385 independently living Chinese men and women 60 years of age or older.
Participants and Methods
The 385 study participants were recruited for this cross-sectional study from 6 communities in Beijing, China. They were required to be Beijing residents of Chinese origin and 60 years of age or older. Height and weight were measured as participants stood erect and wore light clothing without shoes. Body mass index (BMI) (kg/m2) was then calculated. Waist and hip circumferences were measured at the umbilical line in accordance with written protocol. All participants completed self-administered questionnaires that included queries about physical activity and medical history; these were reviewed by trained nurses. Cardiovascular disease was defined as a history of myocardial infarction, angina pectoris, or stroke. A physical activity score was calculated from the questionnaire results by adding the number of hours during which participants undertook light or vigorous physical activity; the hours of vigorous activity received double weight. By use of a table sphygmomanometer, blood pressure was measured in the right arm as participants sat after a 5-minute rest and with their bladders empty. Systolic blood pressure was recorded precisely to 2 mmHg. Systolic blood pressure was defined as the point of the appearance (Korotkoff I) and disappearance (Korotkoff V) of Korotkoff sounds. The average of 2 measurements was used. All participants provided written informed consent that was approved by our hospital's ethics committee. All procedures were performed in accordance with institutional guidelines.
Carotid Ultrasonography
High-resolution B-mode carotid ultrasonography (iU22, Koninklijke Philips Electronics N.V.; Eindhoven, The Netherlands) with a 7.5- to 10-MHz linear array was used. All measurements were performed with the participants in supine position with head elevation of up to 45° and a side tilt of 30° to the right and then to the left. Carotid IMT was measured bilaterally at the common carotid artery (1 cm proximal to the dilation of the carotid bulb), at the bifurcation (the 1-cm segment proximal to the flow divider), and at the internal carotid artery (the 1-cm segment in the internal branch distal to the flow divider). Contiguous measurements (1 mm each) were attempted in each of the 6 segments. Intima–media thickness was defined as the distance between the luminal–endothelial interface and the junction between the tunica media and the adventitia. Measurements were performed over 3 cardiac cycles by a single investigator, and the results were expressed as arithmetic means. Images were also visually inspected for plaque, defined as a localized echo structure that encroached into the arterial lumen by at least 0.5 mm or 50% of the surrounding IMT value, or that demonstrated a thickness of ≥1.5 mm beyond the interface between the lumen and the tunica intima. Mean IMT (defined as the mean of IMT at standard sites on the right and left carotid arteries) was used in the analysis. The IMT examination was performed and interpreted by an investigator who was unaware of the laboratory evaluations. The IMT scores were highly reproducible: the estimate of correlations between scans that were performed by different readers (inter-reader reliability) was 0.96. The same type of ultrasonography and the same method were used to examine plaque formation.
Clinical Measurements
Fasting venous blood was collected for the serologic measurements. All blood samples were drawn between 7:30 and 8:30 AM, and serum samples were stored at −80°C. Levels of total cholesterol, low-density-lipoprotein (LDL) cholesterol, and triglycerides were determined, and the results were standardized. Levels of serum high-sensitivity C-reactive protein (hsCRP) were measured in the General Hospital of PLA by use of a particle-enhanced, immuno-turbidometric latex agglutination assay. Serum concentrations of progesterone (nmol/L) were measured by use of commercial radioimmunoassay kits (Diagnostic Systems Laboratories, Inc.; Webster, Tex).
Statistical Analysis
Statistical results were expressed as mean ± SD. The male and female participants were categorized into quartiles in accordance with progesterone levels. Characteristics among the 4 groups were compared by using 1-way analysis of variance, the Kruskal-Wallis test, or the χ2 test, as appropriate. Post hoc multiple comparison was performed via the Bonferroni test. Mean differences in IMT measurements were compared among the 4 progesterone-level groups via analysis of covariance, with adjustment for age, triglycerides, total cholesterol, LDL cholesterol, systolic blood pressure, waist-to-hip ratio, and BMI. The independent relationship between IMT and atherosclerosis risk factors, age, and progesterone levels was also calculated, by means of logistic regression analysis. Risk factors were dichotomized according to the following cutpoints: BMI, >24 kg/m2; waist-to-hip ratio, >0.90; systolic blood pressure, >140 mmHg; diastolic blood pressure, >90 mmHg; triglycerides, >1.70 mmol/L; total cholesterol, >5.2 mmol/L; LDL cholesterol, >3.64 mmol/L; and lack of physical exercise, <3 hr/wk. Age was used as a continuous variable, and the progesterone cutpoint was in the lowest progesterone quartile (<1.87 nmol/L). All statistical tests were 2-tailed, with statistical significance defined as P < 0.05. The data were analyzed with the SPSS version 15.0 statistical package (SPSS Inc.; Chicago, Ill).
Results
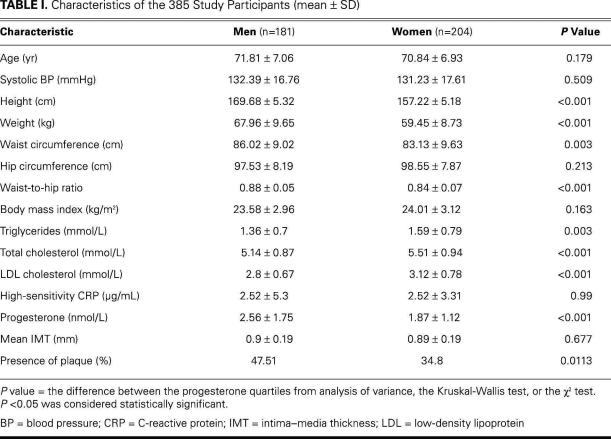
The characteristics of the 385 participants are shown in Table I. The mean age of the men was 71.81 ± 7.06 yr, and the mean age of the women was 70.84 ± 6.93 yr. The numbers of participants who undertook light physical exercise (<3 hr/wk), moderate exercise (3–7 hr/wk), and vigorous exercise (>7 hr/wk) were 100 (25.97%), 118 (30.65%), and 167 (43.38%), respectively. No significant differences between the men and the women were found in age, systolic blood pressure, hip circumference, BMI, or hsCRP. The progesterone levels of the men were much higher than those of the women (2.56 ± 1.75 vs 1.87 ± 1.12 nmol/L, P < 0.001). The mean IMTs were similar between the 2 groups (0.9 ± 0.19 vs 0.89 ± 0.19 mm, P = 0.677), whereas the incidence of plaque was higher in the men than in the women (Table I).
TABLE I. Characteristics of the 385 Study Participants (mean ± SD)
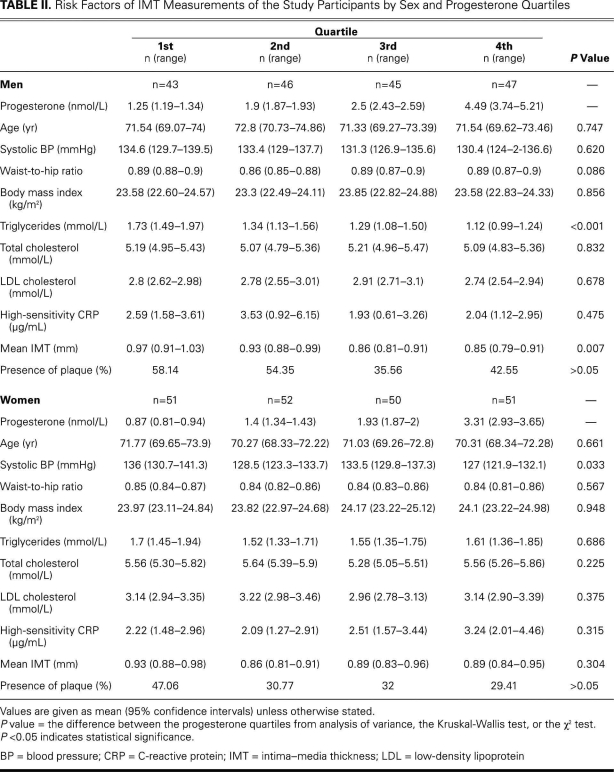
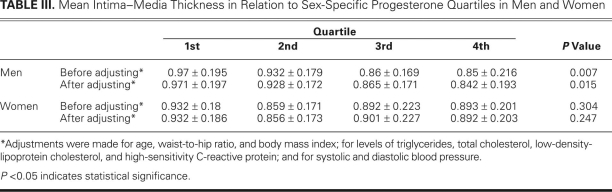
The median levels (ranges) of progesterone of the quartiled groups were 1.25 (1.19–1.34), 1.9 (1.87–1.93), 2.5 (2.43–2.59) and 4.49 (3.74–5.21) nmol/L in the men; and 0.87 (0.81–0.94), 1.4 (1.34–1.43), 1.93 (1.87–2), and 3.31 (2.93–3.65) nmol/L in the women (Table II). Because of the statistically significant difference in progesterone levels between the men and women (P < 0.001) (Table I), further analyses were carried out in the different sexes separately. There was a negative association between progesterone and mean IMT in men (P = 0.007). After adjustment for age, triglycerides, total cholesterol, LDL cholesterol, hsCRP, systolic blood pressure, waist-to-hip ratio, and BMI, the association remained statistically significant (P = 0.015); however, no association was found in women before adjustment (P = 0.304) or after adjustment (P = 0.247) (Tables II and III). No significant differences of plaque incidence were found in the quartiled groups (Table II).
TABLE II. Risk Factors of IMT Measurements of the Study Participants by Sex and Progesterone Quartiles
TABLE III. Mean Intima–Media Thickness in Relation to Sex-Specific Progesterone Quartiles in Men and Women
The traditional risk factors of atherosclerosis and the IMT measurements in relation to the progesterone quartiles are shown in Table II. Although no association was found between progesterone and age, systolic blood pressure, waist-to-hip ratio, BMI, total cholesterol, LDL cholesterol, hsCRP, and plaque incidence, progesterone was negatively associated with triglycerides in men. In women, there was a significant difference in systolic blood pressure among the progesterone quartiles; however, no correlation between progesterone and the other risk factors was found.
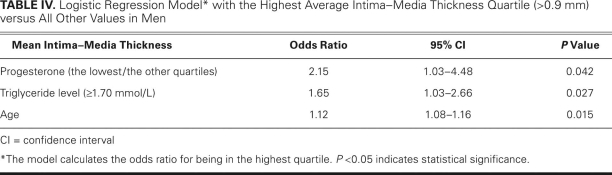
Logistic regression analysis showed that men with a progesterone level in the lowest quartile (<1.87 nmol/L) were more likely (odds ratio = 2.15, P = 0.042) to have a higher average IMT (>0.9 mm), independent of age and the other risk factors. Age (odds ratio = 1.12, P = 0.015) and triglyceride levels >1.70 mmol/L (odds ratio = 1.65, P = 0.027) were also independently associated with carotid IMT (Table IV).
TABLE IV. Logistic Regression Model* with the Highest Average Intima–Media Thickness Quartile (>0.9 mm) versus All Other Values in Men
Discussion
We found that progesterone levels were significantly related to carotid IMT in our study population of older northern Chinese men. This observation was surprising, because it contradicts the conclusion that most existing studies suggested.14–16 However, in our study, the association remained statistically significant after adjustments for age, physical exercise, and common risk factors. We also found that lower levels of progesterone were associated with increased IMT independently of age or other risk factors, and that age and higher levels of triglycerides were independently associated with higher average IMT. Notably, there was no association between mean IMT and progesterone levels in women before or after adjustment. We also observed no significant differences in plaque incidence in the quartiled groups. Progesterone levels had a considerable effect on carotid IMT in men—higher than the effect of such well-known major risk factors as age and triglyceride levels. However, because this was a cross-sectional study, it was not possible to determine either the possible causal role of progesterone in atherosclerosis or the mechanisms behind the association that we found.
To our knowledge, few studies had been conducted concerning the association between progesterone and the risks of atherosclerosis. However, evidence has begun to support the concept of a beneficial role of progesterone in the central nervous and cardiovascular systems. Progesterone and synthetic progestin are highly associated with relaxation in rat aortas.17 Aggarwal and colleagues18 reported that intraperitoneal progesterone administration (15 mg/kg) significantly reduced the size of cerebral infarcts that resulted from the suppression of inflammatory responses and induced nitric oxide synthase expression in a model of bilateral common carotid artery occlusion. Progesterone can also attenuate the estrogen-mediated inhibition of neointimal formation after balloon injury in the carotid artery of rats19 and can attenuate coronary artery atherosclerosis in female monkeys.20 Low-dose intraperitoneal progesterone (2 mg/kg) significantly reduced inflammatory reactivity, enhanced free-radical scavenging ability, improved contractile function of the myocardium, and stabilized electrocardiographic values (decreased ST-segment elevation and incidences of ventricular tachycardia) in rats that had experienced ischemia/reperfusion injury.21
In men, the mechanisms of the beneficial effect of progesterone on atherosclerosis are largely unknown. However, animal studies may provide indications. In ischemic rats, research indicates that progesterone significantly reduced tumor necrosis factor-a levels, increased superoxide dismutase activity, reduced glutathione hormone and malondialdehyde levels, decreased myeloperoxidase activity, suppressed creatine kinase activity, and attenuated DNA fragmentation.21 Progesterone also repressed thromboxane A2 receptor expression in ovariectomized monkeys, which beneficially regulated coronary artery reactivity.18 The other possible mechanism may involve regulation of smooth-muscle-cell proliferation inhibition. Lee and associates22 showed in rats that progesterone, at physiologic levels, inhibits DNA synthesis and decreased cell numbers in cultured aortic smooth-muscle cells in a dose-dependent manner. Also in rats, progesterone inhibits the proliferation of aortic smooth-muscle cells by increasing the levels of p21 and p27 proteins, which in turn inhibits cyclin-dependent kinase-2 activity and interrupts the cell cycle.23
Numerous studies have reported conflicting observations about the susceptibility of men and women to cardiovascular disorders; however, this susceptibility may be directly or indirectly interlinked to sex hormones.24,25 Investigators have suggested that progesterone is a paradoxical hormone that exhibits either growth-stimulatory or growth-inhibitory effects.21,26,27 Lee and colleagues23 reported that progesterone at physiologic levels (5–500 nM) inhibited DNA synthesis in cultured rat aortic smooth-muscle cells. In rhesus monkeys with normal menstrual cycles, the levels of progesterone that proved protective were in the low physiologic range.6 Experimental studies also reported that synthetic (rather than natural) progestins interfered with estrogen's protective effects against vasoconstriction.7 It follows that the disparate effects of progesterone depend on different types and dosages. Experiments and well-conducted prospective studies are needed to determine the role of progesterone in atherosclerosis.
Conclusion
In summary, we found that low progesterone levels were negatively associated with atherosclerosis in northern Chinese men 60 years of age or older, independent of other cardiovascular risk factors. Our findings suggest potential applications of progesterone to atherosclerosis.
Footnotes
Address for reprints: Xiangmei Chen, MD, PhD, Institute of Nephrology, General Hospital of PLA, 28 Fuxing Road, Beijing 100853, PRC
E-mail: xmchen301@126.com
Drs. Ma and Sun contributed equally to this paper and are first co-authors.
This study was supported by local funds from the Major State Basic Research Development Program of China (2007CB507405).
References
- 1.Ostberg JE, Storry C, Donald AE, Attar MJ, Halcox JP, Conway GS. A dose-response study of hormone replacement in young hypogonadal women: effects on intima media thickness and metabolism. Clin Endocrinol (Oxf) 2007;66(4):557–64. [DOI] [PubMed]
- 2.Muller M, van den Beld AW, Bots ML, Grobbee DE, Lamberts SW, van der Schouw YT. Endogenous sex hormones and progression of carotid atherosclerosis in elderly men. Circulation 2004;109(17):2074–9. [DOI] [PubMed]
- 3.Abbott RD, Curb JD, Rodriguez BL, Masaki KH, Yano K, Schatz IJ, et al. Age-related changes in risk factor effects on the incidence of coronary heart disease. Ann Epidemiol 2002; 12(3):173–81. [DOI] [PubMed]
- 4.Alexandersen P, Haarbo J, Christiansen C. The relationship of natural androgens to coronary heart disease in males: a review. Atherosclerosis 1996;125(1):1–13. [DOI] [PubMed]
- 5.Khaw KT, Barrett-Connor E. Endogenous sex hormones, high density lipoprotein cholesterol, and other lipoprotein fractions in men. Arterioscler Thromb 1991;11(3):489–94. [DOI] [PubMed]
- 6.Minshall RD, Stanczyk FZ, Miyagawa K, Uchida B, Axthelm M, Novy M, Hermsmeyer K. Ovarian steroid protection against coronary artery hyperreactivity in rhesus monkeys. J Clin Endocrinol Metab 1998;83(2):649–59. [DOI] [PubMed]
- 7.Miyagawa K, Rosch J, Stanczyk F, Hermsmeyer K. Medroxyprogesterone interferes with ovarian steroid protection against coronary vasospasm. Nat Med 1997;3(3):324–7. [DOI] [PubMed]
- 8.Rosano GM, Panina G. Cardiovascular pharmacology of hormone replacement therapy. Drugs Aging 1999;15(3):219–34. [DOI] [PubMed]
- 9.Taddei S, Virdis A, Ghiadoni L, Mattei P, Sudano I, Bernini G, et al. Menopause is associated with endothelial dysfunction in women. Hypertension 1996;28(4):576–82. [DOI] [PubMed]
- 10.Badimon L, Bayes-Genis A. Effects of progestogens on thrombosis and atherosclerosis. Hum Reprod Update 1999;5(3):191–9. [DOI] [PubMed]
- 11.Bots ML, Hoes AW, Hofman A, Witteman JC, Grobbee DE. Cross-sectionally assessed carotid intima-media thickness relates to long-term risk of stroke, coronary heart disease and death as estimated by available risk functions. J Intern Med 1999;245(3):269–76. [DOI] [PubMed]
- 12.Mack WJ, LaBree L, Liu C, Selzer RH, Hodis HN. Correlations between measures of atherosclerosis change using carotid ultrasonography and coronary angiography. Atherosclerosis 2000;150(2):371–9. [DOI] [PubMed]
- 13.O'Leary DH, Polak JF, Kronmal RA, Manolio TA, Burke GL, Wolfson SK Jr. Carotid-artery intima and media thickness as a risk factor for myocardial infarction and stroke in older adults. Cardiovascular Health Study Collaborative Research Group. N Engl J Med 1999;340(1):14–22. [DOI] [PubMed]
- 14.Rocha A, Azevedo I, Soares R. Anti-angiogenic effects of imatinib target smooth muscle cells but not endothelial cells. Angiogenesis 2007;10(4):279–86. [DOI] [PubMed]
- 15.Hillebrand U, Hausberg M, Stock C, Shahin V, Nikova D, Riethmuller C, et al. 17beta-estradiol increases volume, apical surface and elasticity of human endothelium mediated by Na+/H+ exchange. Cardiovasc Res 2006;69(4):916–24. [DOI] [PubMed]
- 16.Zitzmann M, Erren M, Kamischke A, Simoni M, Nieschlag E. Endogenous progesterone and the exogenous progestin norethisterone enanthate are associated with a proinflammatory profile in healthy men. J Clin Endocrinol Metab 2005;90(12):6603–8. [DOI] [PubMed]
- 17.Glusa E, Graser T, Wagner S, Oettel M. Mechanisms of relaxation of rat aorta in response to progesterone and synthetic progestins. Maturitas 1997;28(2):181–91. [DOI] [PubMed]
- 18.Aggarwal R, Medhi B, Pathak A, Dhawan V, Chakrabarti A. Neuroprotective effect of progesterone on acute phase changes induced by partial global cerebral ischaemia in mice. J Pharm Pharmacol 2008;60(6):731–7. [DOI] [PubMed]
- 19.Levine RL, Chen SJ, Durand J, Chen YF, Oparil S. Medroxyprogesterone attenuates estrogen-mediated inhibition of neointima formation after balloon injury of the rat carotid artery. Circulation 1996;94(9):2221–7. [DOI] [PubMed]
- 20.Adams MR, Register TC, Golden DL, Wagner JD, Williams JK. Medroxyprogesterone acetate antagonizes inhibitory effects of conjugated equine estrogens on coronary artery atherosclerosis. Arterioscler Thromb Vasc Biol 1997;17(1):217–21. [DOI] [PubMed]
- 21.Dhote VV, Balaraman R. Gender specific effect of progesterone on myocardial ischemia/reperfusion injury in rats. Life Sci 2007;81(3):188–97. [DOI] [PubMed]
- 22.Lee WS, Harder JA, Yoshizumi M, Lee ME, Haber E. Progesterone inhibits arterial smooth muscle cell proliferation. Nat Med 1997;3(9):1005–8. [DOI] [PubMed]
- 23.Lee WS, Liu CW, Juan SH, Liang YC, Ho PY, Lee YH. Molecular mechanism of progesterone-induced antiproliferation in rat aortic smooth muscle cells. Endocrinology 2003;144(7):2785–90. [DOI] [PubMed]
- 24.Butterworth J, James R, Prielipp R, Cerese J, Livingston J, Burnett D. Female gender associates with increased duration of intubation and length of stay after coronary artery surgery. CABG Clinical Benchmarking Database Participants. Anesthesiology 2000;92(2):414–24. [DOI] [PubMed]
- 25.Abramov D, Tamariz MG, Sever JY, Christakis GT, Bhatnagar G, Heenan AL, et al. The influence of gender on the outcome of coronary artery bypass surgery. Ann Thorac Surg 2000;70(3):800–6. [DOI] [PubMed]
- 26.Groshong SD, Owen GI, Grimison B, Schauer IE, Todd MC, Langan TA, et al. Biphasic regulation of breast cancer cell growth by progesterone: role of the cyclin-dependent kinase inhibitors, p21 and p27(Kip1). Mol Endocrinol 1997;11(11):1593–607. [DOI] [PubMed]
- 27.Musgrove EA, Swarbrick A, Lee CS, Cornish AL, Sutherland RL. Mechanisms of cyclin-dependent kinase inactivation by progestins. Mol Cell Biol 1998;18(4):1812–25. [DOI] [PMC free article] [PubMed]