Abstract
Bronchopleural fistula and empyema are serious complications after thoracic surgical procedures, and their prevention is paramount. Herein, we review our experience with routine prophylactic use of the pedicled ipsilateral latissimus dorsi muscle flap. From January 2004 through February 2006, 10 surgically high-risk patients underwent intrathoracic transposition of this muscle flap for reinforcement of bronchial-stump closure or obliteration of empyema cavities. Seven of the patients were chronically immunosuppressed, 5 were severely malnourished (median preoperative serum albumin level, 2.4 g/dL), and 5 had severe underlying obstructive pulmonary disease (median forced expiratory volume in 1 second, 44% of predicted level). Three upper lobectomies and 1 completion pneumonectomy were performed in order to treat massive hemoptysis that was secondary to complex aspergilloma. One patient underwent left pneumonectomy due to ruptured-cavitary primary lung lymphoma. One upper lobectomy was performed because of necrotizing, localized Mycobacterium avium-intracellulare infection. One patient underwent right upper lobectomy and main-stem bronchoplasty for carcinoma after chemoradiation therapy. In 3 patients, the pedicled latissimus dorsi muscle was used to obliterate chronic empyema cavities and to buttress the closure of underlying bronchopleural fistulas. No operative deaths or recurrent empyemas resulted. Two patients retained peri-flap air that required no surgical intervention.
We conclude that the use of transposed pedicled latissimus dorsi muscle flap effectively and reliably prevents clinically overt bronchopleural fistula and recurrent empyema. We advocate its routine use in first-time and selected reoperative thoracotomies in patients who are undergoing high-risk lung resection or reparative procedures.
Key words: Bronchial fistula/prevention & control/surgery; empyema, pleural/etiology/prevention & control/surgery; muscle, skeletal/surgery/transplantation; pleural diseases/prevention & control/surgery; pneumonectomy/adverse effects; postoperative complications; reconstructive surgical procedures; risk factors; surgical flaps/methods; thoracic surgical procedures/methods; treatment outcome
Bronchopleural fistula (BPF) and empyema are rare but dangerous complications of pulmonary resections. The incidence of postoperative BPF, reported as 1.5% to 28%,1–4 has been shown in general to relate to the condition's cause and to the surgical technique and experience of the surgeons.5–7 The incidence of empyema after pulmonary resections is between 2% and 16%.8–10 Anatomic lung resections (for example, lobectomy and pneumonectomy) that are performed to treat inflammatory and infectious conditions particularly invite the development of these postoperative complications.
Given the high morbidity and mortality rates of postoperative BPF and recurrent empyema, prevention is paramount. The use of transposed extrathoracic muscle flaps to cover bronchial stumps and to eliminate dead space is a well-established management technique.
We have routinely used the pedicled latissimus dorsi (PLD) muscle flap as our preferred flap in high-risk thoracic surgery patients who have undergone lobectomy, pneumonectomy, or decortication procedures. Here, we review our experience with this technique in 10 patients, and the clinical outcomes thereof.
Patients and Methods
We reviewed the hospital records of all patients who, from January 2004 through February 2006, had undergone posterolateral thoracotomies at our institution for anatomic resection of an inflammatory, infectious, or malignant lung disease, or for decortication for chronic empyema. We noted the characteristics of the patients, the preoperative diagnoses, the timing of the procedures (elective vs urgent), the type of procedure, intraoperative complications, estimated blood loss, blood transfusion requirements, duration of intubation, pulmonary complications, systemic complications (cardiac or renal sequelae, shock, or sepsis), postoperative BPF or empyema, need for reoperation, length of stay (LOS) in the intensive care unit, and LOS in the hospital. We identified 10 surgically high-risk patients (age range, 24–74 yr; 5 men) in whom the ipsilateral PLD muscle flap had been used at the discretion of the primary surgeon (AA). These patients were selected for this review (Table I). Patients were considered to be especially susceptible to BPF and primary or recurrent empyema on the basis of the risk level of the operative intervention (typically, a lobectomy for a chronic infectious disease) and whether they were chronically immunosuppressed or malnourished. Chronic malnutrition was defined as a preoperative serum albumin level lower than 3 g/dL. Severe underlying pulmonary disease was also considered a risk factor.
TABLE I. Characteristics and Risk Factors of the Patients
Seven patients were immunosuppressed, including 3 who were receiving chronic systemic steroidal agents, 2 who had undergone preoperative chemotherapy, and 2 who had tested positive for the human immunodeficiency virus. Five were chronically malnourished (median serum albumin level, 2.4 g/dL). Five had severe underlying pulmonary disease (median forced expiratory volume in 1 second, 44% of predicted level; and median diffusing lung capacity for carbon monoxide, 53% of predicted level).
From the beginning of the study period through the present, we have harvested PLD muscle flaps by means of a simple, step-by-step technique, which we have recently described in detail.11
Results
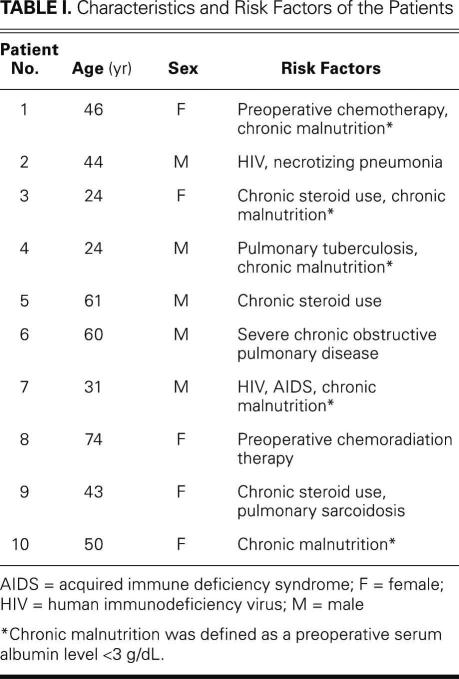
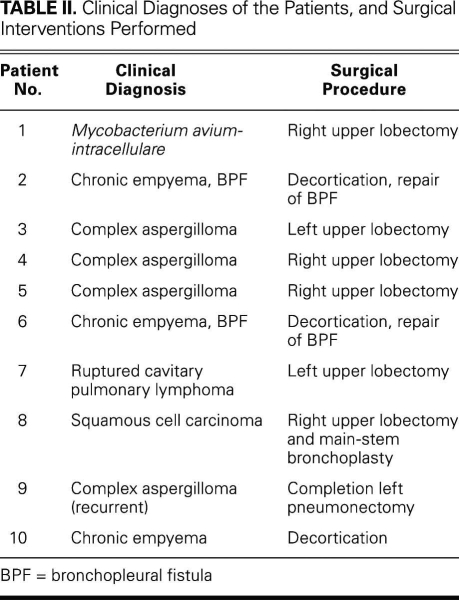
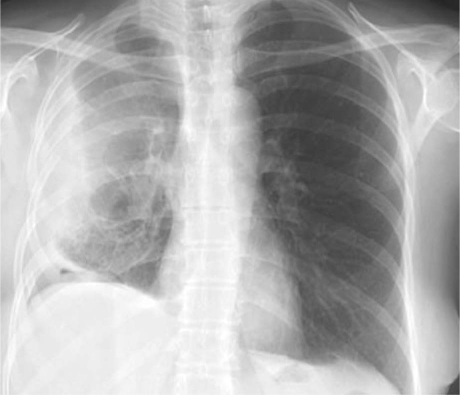
Table II shows the operative indications and procedural characteristics of the 10 patients. Of 4 patients who had complex cavitary aspergillomas and required urgent pulmonary resections for massive hemoptysis, 3 underwent upper lobectomies, and 1 underwent completion pneumonectomy. One patient with acquired immune deficiency syndrome underwent an urgent left pneumonectomy for ruptured-cavitary primary lung lymphoma. The rest of the operations were performed electively. Three patients had chronic empyema. Of these, 1 had chronic primary empyema alone, after prior failed medical therapy for community-acquired pneumonia; the other 2 had previously undergone ipsilateral thoracotomy or thoracoscopy for bullae resection and had developed a loculated empyema cavity with an air–fluid level that suggested occult underlying BPF (Figs. 1 and 2). These 3 patients underwent total pleural decortication and obliteration of the empyema cavity with buttressed closure of the underlying BPF, when one was present, with use of PLD flaps (Fig. 3). One patient underwent an upper lobectomy for necrotizing, localized Mycobacterium avium-intracellulare infection. One patient had been diagnosed with bronchogenic squamous cell carcinoma, for which a right upper lobectomy and main-stem bronchoplasty were performed after induction chemoradiation therapy.
TABLE II. Clinical Diagnoses of the Patients, and Surgical Interventions Performed
Fig. 1 Preoperative chest radiograph of a patient with chronic empyema, apical air–fluid level, and presumed underlying bronchopleural fistula.
Fig. 2 Preoperative chest computed tomogram shows a thick-walled empyema cavity with an air–fluid level (see Fig. 1).
Fig. 3 Postoperative chest radiograph (see Figs. 1 and 2) shows full re-expansion of the right lung. The haziness on the right edge of the pleural space is the transposed pedicled latissimus dorsi muscle flap with complete obliteration of the potential space.
The average estimated blood loss was 495 cc. Six patients required no intraoperative blood transfusion; the other 4 required transfusion of an average of 2 units of packed red blood cells per patient. There were no serious intraoperative complications and no operative deaths.
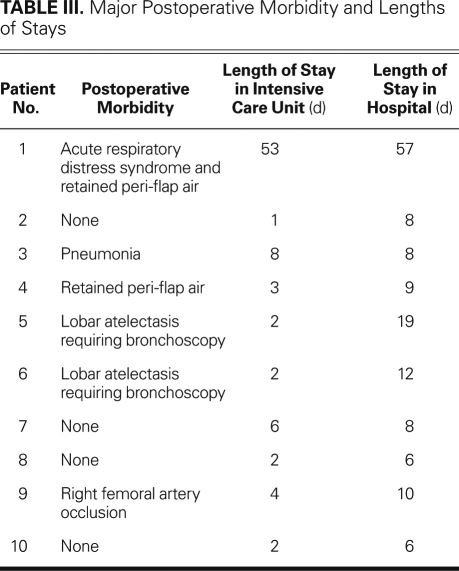
Table III shows a summary of the major postoperative complications and the hospital LOS data. Seven patients were extubated immediately, in the operating room or the recovery room. Neither of the 2 patients who required longer endotracheal intubation developed respiratory complications after extubation. One patient experienced substantial postoperative complications, including line sepsis and severe acute respiratory distress syndrome (ARDS); she required tracheostomy and prolonged ventilatory support. She was successfully weaned from ventilatory support and had her tracheostomy closed 57 days postoperatively. Two patients retained peri-flap air, as was noted on chest radiographs. Neither patient required further surgical intervention, and each was treated successfully via conservative measures and intravenous antibiotics. Other postoperative complications included pneumonia (n=1), lobar atelectasis that required repeated bronchoscopy (n=2), and iatrogenic occlusion of the right common femoral artery (n=1) after failed percutaneous bronchial arterial embolization. There were no primary or recurrent postoperative empyemas. Excluding the patient who developed ARDS and spent 53 days in the intensive care unit, the patients' average LOS in the intensive care unit was 3 days, and the average hospital LOS was 10 days.
TABLE III. Major Postoperative Morbidity and Lengths of Stays
Discussion
In the thoracic surgical literature, several studies show that patients with immunosuppressive disorders, including those resulting from chronic steroid use and recent preoperative chemotherapy, have a higher tendency to develop BPFs and empyemas after pulmonary resection.12,13 Chronic malnutrition, severe chronic obstructive pulmonary disease, and other underlying parenchymal lung diseases are additional risk factors.13 Thoracic surgeons face a different range of challenges when patients present with chronic primary empyema as a result of an inadequately treated parapneumonic effusion, or as a sequela of prior pleural instrumentation or surgical therapy for an unrelated pleural or parenchymal disease. The standard treatment for chronic primary empyema, and often for underlying subtle occult BPF, entails total pleural decortication, obliteration of the empyema cavity, coverage of the raw parenchymal surface with viable tissue, and wide and effective pleural drainage.
Since 1911, when Abrashanoff14 first described the use of intrathoracic muscle transposition in the treatment of BPF, thoracic surgeons have transposed extrathoracic muscle flaps in order to treat difficult pleural-space infections and to buttress the repair of BPF. The chest-wall skeletal muscles, including the latissimus dorsi, pectoralis major, and serratus anterior muscles, have been transposed most frequently,15–19 although the rectus abdominis muscle and pedicled omentum have also been used successfully.18–24 The prophylactic use of an extrathoracic muscle to prevent postoperative BPF has been less commonly practiced. In elective surgery in low-risk patients, transposing an intercostal muscle flap in order to cover a bronchial stump after lobectomy or pneumonectomy is a viable option—and possibly preferred, due to ease of harvest and the avoidance of additional incisions. Indeed, the local intercostal muscle flap is most commonly used in the elective prophylactic coverage of high-risk bronchial stumps.15–17 However, in nearly all chronic infectious or inflammatory pulmonary conditions that require thoracotomies and anatomic resections, severe inflammatory pleural disease and consequent pleural symphysis preclude the use of intercostal muscle flaps. Because these thoracotomies commonly require extrapleural dissection in order to mobilize the underlying lung, preserving an intact, well-vascularized intercostal muscle flap is problematic. Also, intercostal muscle is of inadequate bulk to obliterate the fibrotic, infected bed of resection. Therefore, in these situations, transposition of extrathoracic muscles is preferred.
Several considerations guide the decision to use a particular muscle flap for intrathoracic transposition. Of utmost importance are the reliability and survival of the transposed muscle—outcomes that depend entirely on the surgeon's precise knowledge of the muscle's vascular supply. In addition, the pedicled flap should confer enough length and bulk to reach the desired intrapleural location and successfully obliterate the infected pleural space. As we have discussed in detail previously,11 we believe that the PLD muscle is the most suitable flap and that its use readily satisfies these important factors.
In selecting extrathoracic muscles for use as flaps, one must also consider the form and function of the donor site. The use of some chest-wall muscles may lead to substantial functional loss or contour deformity. As one of several shoulder-girdle muscles, the latissimus dorsi can be sacrificed without affecting a patient's shoulder or arm function. In contrast, the serratus anterior and pectoralis major muscles are not entirely expendable, and functional disability or physical deformity may result if they are harvested for use as flaps. The serratus anterior muscle protracts the scapula by pulling the vertebral border of the scapula anteriorly, which enables full abduction and flexion of the arm.25 Sacrificing the entire muscle will lead to “winging” of the scapula and the patient's resultant inability to elevate an arm above the horizontal plane. Because the pectoralis major muscle adducts and internally rotates the arm, harvesting this muscle for use as a transposition flap will result in the loss of the anterior axillary fold and possibly lead to substantial contour deformity of the chest wall.25
The rectus abdominis muscle and omentum are generally the last flaps to be considered for intrathoracic transposition. Use of these flaps would entail a separate abdominal wound. Furthermore, harvesting an omental flap requires entry into the peritoneal cavity, which incurs inherent risks of bleeding, infection, and injury to visceral organs. Finally, the use of the rectus abdominis muscle as a transposition flap in thin or malnourished patients may result in hernia formation or substantial deformity of the abdominal wall.
In our high-risk patient population, only 2 patients retained peri-flap air. Both did well under conservative management, and neither required reoperation, even though 1 patient developed florid ARDS that required persistent, high-pressure ventilatory support. There were no clinical signs of new or recurrent empyemas in any of the 10 patients. Urgency of operation did not appear to influence outcomes, since all of the patients who underwent urgent operations recovered well. Three reoperations were performed: 1 after a posterolateral thoracotomy that had entailed partial division of the latissimus muscle, 1 after a prior anterior thoracotomy, and 1 after an earlier thoracoscopic procedure. Adequate length and bulk of PLD muscle flap enabled successful intrathoracic transposition in all 3 patients.
Summary
We routinely use the PLD muscle flap in our high-risk thoracic operations as a prophylactic measure, in order to prevent the development of clinically overt BPF and recurrent empyema. In our study, PLD muscle flap afforded enough length and flexibility to reinforce vulnerable bronchial stumps after high-risk pulmonary resections. The use of PLD muscle flap will not diminish shoulder-girdle function or cause physical deformity. To prevent the development of clinically overt BPF, we strongly recommend the use of the PLD muscle flap to buttress the closure of high-risk bronchial stumps or large lung parenchymal defects after pulmonary resections. We further believe that the use of a PLD muscle flap enables the obliteration of chronically infected pleural space and reduces the chance of recurrent empyema. Although our study was retrospective and limited by the size of our patient cohort, we consider the excellent outcomes and low morbidity rate in our high-risk patients to be very encouraging.
Footnotes
Address for reprints: Amir Abolhoda, MD, Division of Cardiothoracic Surgery, Department of Surgery, University of California, Irvine Medical Center, 101 The City Drive, Bldg. 53, Rm. 117, Orange, CA 92868-3298
E-mail: aabolhod@uci.edu
Presented at Chest 2006: Annual Scientific Assembly of The American College of Chest Physicians; Salt Lake City, Utah (October 2006).
References
- 1.McManigle JE, Fletcher GL, Tenholder MF. Bronchoscopy in the management of bronchopleural fistula. Chest 1990;97(5):1235–8. [DOI] [PubMed]
- 2.Cerfolio RJ. The incidence, etiology, and prevention of postresectional bronchopleural fistula. Semin Thorac Cardiovasc Surg 2001;13(1):3–7. [DOI] [PubMed]
- 3.Sirbu H, Busch T, Aleksic I, Schreiner W, Oster O, Dalichau H. Bronchopleural fistula in the surgery of non-small cell lung cancer: incidence, risk factors, and management. Ann Thorac Cardiovasc Surg 2001;7(6):330–6. [PubMed]
- 4.Turk AE, Karanas YL, Cannon W, Chang J. Staged closure of complicated bronchopleural fistulas. Ann Plast Surg 2000; 45(5):560–4. [DOI] [PubMed]
- 5.al-Kattan K, Cattelani L, Goldstraw P. Bronchopleural fistula after pneumonectomy for lung cancer. Eur J Cardiothorac Surg 1995;9(9):479–82. [DOI] [PubMed]
- 6.Conlan AA, Lukanich JM, Shutz J, Hurwitz SS. Elective pneumonectomy for benign lung disease: modern-day mortality and morbidity. J Thorac Cardiovasc Surg 1995;110(4 Pt 1):1118–24. [DOI] [PubMed]
- 7.Sato M, Saito Y, Nagamoto N, Endo C, Usuda K, Takahashi S, et al. An improved method of bronchial stump closure for prevention of bronchopleural fistula in pulmonary resection. Tohoku J Exp Med 1992;168(3):507–13. [DOI] [PubMed]
- 8.Pairolero PC, Trastek VF, Allen MS. Empyema and bronchopleural fistula. Ann Thorac Surg 1991;51(1):157–8. [DOI] [PubMed]
- 9.Deschamps C, Bernard A, Nichols FC 3rd, Allen MS, Miller DL, Trastek VF, et al. Empyema and bronchopleural fistula after pneumonectomy: factors affecting incidence. Ann Thorac Surg 2001;72(1):243–8. [DOI] [PubMed]
- 10.Lopez Pujol J, Alvarez Kindelan A, Algar Algar J, Cerezo Madueno F, Lopez Rivero L, Salvatierra Velazquez A. Perioperative morbimortality in pneumonectomy. Analysis of risk factors [in Spanish]. Arch Bronconeumol 2000;36(5):251–6. [PubMed]
- 11.Abolhoda A, Wirth GA, Bui TD, Milliken JC. Harvest technique for pedicled transposition of latissimus dorsi muscle: an old trade revisited. Eur J Cardiothorac Surg 2008;33(5):928–30. [DOI] [PubMed]
- 12.Sato M, Saito Y, Fujimura S, Usuda K, Takahashi S, Kanma K, et al. Study of postoperative bronchopleural fistulas–analysis of factors related to bronchopleural fistulas [in Japanese]. Nippon Kyobu Geka Gakkai Zasshi 1989;37(3):498–503. [PubMed]
- 13.Sonobe M, Nakagawa M, Ichinose M, Ikegami N, Nagasawa M, Shindo T. Analysis of risk factors in bronchopleural fistula after pulmonary resection for primary lung cancer. Eur J Cardiothorac Surg 2000;18(5):519–23. [DOI] [PubMed]
- 14.Abrashanoff X. Plastische methode des schliessung von fistelgangen, welche von inneren organen kommen [in German]. Zentralbl Chir 1911;38:186–91.
- 15.Cerfolio RJ, Bryant AS, Yamamuro M. Intercostal muscle flap to buttress the bronchus at risk and the thoracic esophageal-gastric anastomosis. Ann Thorac Surg 2005;80(3):1017–20. [DOI] [PubMed]
- 16.Lardinois D, Horsch A, Krueger T, Dusmet M, Ris HB. Mediastinal reinforcement after induction therapy and pneumonectomy: comparison of intercostal muscle versus diaphragm flaps. Eur J Cardiothorac Surg 2002;21(1):74–8. [DOI] [PubMed]
- 17.Yamamoto R, Inoue K, Hori T, Takehara S, Kaji M, Kinoshita H. Intercostal muscle pedicle flap for prophylaxis against bronchopleural fistula after pulmonary resection. Osaka City Med J 1994;40(2):99–105. [PubMed]
- 18.Meyer AJ, Krueger T, Lepori D, Dusmet M, Aubert JD, Pasche P, Ris HB. Closure of large intrathoracic airway defects using extrathoracic muscle flaps. Ann Thorac Surg 2004;77 (2):397–405. [DOI] [PubMed]
- 19.Chan EC, Lee TW, Ng CS, Wan IY, Sihoe AD, Yim AP. Closure of postpneumonectomy bronchopleural fistula by means of single, perforator-based, latissimus dorsi muscle flap. J Thorac Cardiovasc Surg 2002;124(6):1235–6. [DOI] [PubMed]
- 20.Hochberg J, Ardenghy M, Yuen J, Graeber GM, Warden HE, Gonzalez-Cruz R, Conrado RM. Utilization of muscle flaps in the treatment of bronchopleural fistulas. Ann Plast Surg 1999;43(5):484–93. [DOI] [PubMed]
- 21.Arnold PG, Pairolero PC. Intrathoracic muscle flaps. An account of their use in the management of 100 consecutive patients. Ann Surg 1990;211(6):656–62. [PMC free article] [PubMed]
- 22.Dosios T, Papadopoulos O, Mantas D, Georgiou P, Asimacopoulos P. Pedicled myocutaneous and muscle flaps in the management of complicated cardiothoracic problems. Scand J Plast Reconstr Surg Hand Surg 2003;37(4):220–4. [DOI] [PubMed]
- 23.Kondo R, Seki T, Hanamura N, Kobayashi M, Yamanda T, Koike S. Gastric seromuscular and omental pedicle flap for bronchopleural fistula after pneumonectomy. Jpn J Thorac Cardiovasc Surg 2000;48(8):536–9. [DOI] [PubMed]
- 24.Chichevatov D, Gorshenev A. Omentoplasty in treatment of early bronchopleural fistulas after pneumonectomy. Asian Cardiovasc Thorac Ann 2005;13(3):211–6. [DOI] [PubMed]
- 25.Harris SU, Nahai F. Intrathoracic muscle transposition. Surgical anatomy and techniques of harvest. Chest Surg Clin N Am 1996;6(3):501–18. [PubMed]