Abstract
In this descriptive prospective study, we evaluate the outcomes of surgery in 98 patients who were scheduled to undergo minimally invasive aortic valve replacement. These patients were compared with a group of 50 patients who underwent scheduled aortic valve replacement through a full sternotomy.
The 30-day mortality rate for the 98 patients was zero, although 14 of the 98 mini-sternotomies had to be converted to complete sternotomies intraoperatively due to technical problems. Such conversion doubled the operative time over that of the planned full sternotomies. In the group of patients whose operations were completed as mini-sternotomies, 4 died later of noncardiac causes. The aortic cross-clamp and perfusion times were significantly different across all groups (P < 0.001), with the intended full-sternotomy group having the shortest times.
In conclusion, the mini-aortic valve replacement is an excellent operation in selected patients, but its true advantages over conventional aortic valve replacement (other than a smaller scar) await evaluation by means of randomized clinical trial. The “extended mini-aortic valve replacement” operation, on the other hand, is a risky procedure that should be avoided by better preoperative evaluation of patients. In any event, the decision to extend a mini-sternotomy to a full sternotomy should be made early in the course of operation, before cardiopulmonary bypass is instituted.
Key words: Aortic valve/surgery; cardiopulmonary bypass; intraoperative period; heart valve diseases/surgery; heart valve prosthesis implantation/methods; postoperative complications; sternum/surgery; surgical procedures, minimally invasive; treatment outcome
Aortic valve replacement (AVR) has developed into an operation that carries very little risk for the patient and yields excellent long-term results. Conventional AVR has proved safe and effective over time.1 Although the minimally invasive approach for AVR (mini-AVR) has been around for more than a decade and large series of these operations have been performed at some cardiac surgical centers in the United States and Europe, thorough scientific evaluation of the method has been lacking. The only obvious outcome after mini-AVR is that the scar is merely one third the length of a scar left by a full sternotomy.1–4
As the term applies to valve surgery, “minimally invasive” refers only to exposure of the heart by a small incision; total cardiopulmonary bypass (CPB) is still necessary in mini-AVR, and CPB is not minimally invasive. The proposed benefits of mini-AVR include less trauma, less pain, decreased transfusion requirements, better cosmetic results, equivalent efficacy, earlier return to productivity, and decreased cost.5,6 Nevertheless, Cooley1 has cautioned that mini-AVR may entail higher mortality and morbidity rates than conventional AVR and has argued that the main stimulus for mini-AVR may be economic.
Beginning with the hypothesis that mini-AVR is beneficial to patients and can be implemented at no additional risk, we sought to evaluate the outcomes of mini-AVRs performed in 98 patients at a single center in Scandinavia.
Patients and Methods
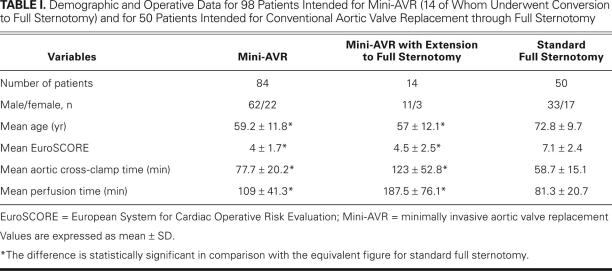
From 2003 through 2007, 98 patients underwent elective mini-AVR through a partial upper sternotomy. At the same time, 50 patients were selected for conventional AVR through a full sternotomy (Table I). Patients were selected at the discretion of the surgeon. Those selected for mini-AVR were typically younger, more slender, and more likely to be free of severe lung disease. Decreased left ventricular function was not a criterion for exclusion from mini-AVR, and even patients with an ejection fraction as low as 0.20 were offered the procedure.
TABLE I. Demographic and Operative Data for 98 Patients Intended for Mini-AVR (14 of Whom Underwent Conversion to Full Sternotomy) and for 50 Patients Intended for Conventional Aortic Valve Replacement through Full Sternotomy
This prospective study was approved by our departmental ethics committee, and patients gave informed consent to participate. No cases involving emergency operation, reoperation, or concomitant coronary artery bypass grafting were included in any of the groups. All patients received the same anesthesia. All patients were operated upon by the senior author, except for 12 patients in the mini-AVR group, who underwent operation by 2 colleagues, supervised by the senior author. The valves were from St. Jude Medical, Inc. (St. Paul, Minn) and were either mechanical or biological stented valves (Biocor®) or biological unstented valves (Toronto®).
Surgical Technique
Minimally Invasive Aortic Valve Replacement (Reversed-L Partial Upper Sternotomy). The mini-AVR operations were performed as described by Cosgrove and colleagues.7 The patient was anesthetized in the supine position and intubated with a single-lumen endotracheal tube. Defibrillator patches were placed on the patient's back and anterior left chest wall. A transesophageal echocardiographic Doppler probe was placed intraoperatively to evaluate the anatomy of the diseased aortic valve and to assist in visualizing the effect of procedures for removing air from the cardiac chambers at the completion of the procedure. An 8-cm incision was made beginning 1 cm above the angle of Louis. The incision was carried down to the sternum by means of electrocautery. The sternum was opened from the sternal notch to the 3rd or 4th intercostal space and subsequently was extended rightward, severing the sternum. To reduce the potential for air emboli, a cannula was sewn to the wound edge to enable flooding of the surgical field with carbon dioxide. To enable the use of smaller venous cannulae, we used vacuum-assisted venous drainage. A triple-stage venous cannula was placed in the superior vena cava. The aorta was cannulated for arterial return at the pericardial reflection. Normothermic CPB and aortic cross-clamping with a flexible clamp were used.
In the first 20 patients, we administered cold crystalloid (St. Thomas) cardioplegic solution retrograde. However, intubation of the coronary sinus is time-consuming and carries some risk, so we administered cardioplegic solution antegrade from that point forward. To facilitate myocardial protection, we used cold Bretschneider's histidine-tryptophan-ketoglutarate-solution (Custodiol® HTK), because it needs to be given only once. This infusion was preceded by fibrillation of the heart in cases of aortic insufficiency. Later in the study period, we used cold-blood cardioplegic solution, which was given every 20 minutes by direct infusion into the coronary ostia.
In replacing the aortic valve, we used sutures with pledgets. Before completing closure of the aorta, we inflated the lungs to expel air from the left ventricle and aorta. The completeness of air removal was monitored by means of echocardiography. At the completion of the procedure, the patient was decannulated. Two atrial and ventricular pacing wires were placed. A straight chest tube was inserted behind the sternum. The sternum was closed with wires, and the wound was closed in layers.
Full-Sternotomy Aortic Valve Replacement. We approached via a traditional median sternotomy, which was followed by aortic and right atrial cannulation with vacuum suction. Cold-crystalloid or cold-blood cardioplegic solution was administered antegrade, and then retrograde every 20 minutes after opening the aorta.
Minimally Invasive Aortic Valve Replacement Extended to a Full Sternotomy. The reversed-L partial upper sternotomy was extended to a full sternotomy. In most cases, we used slight hypothermia (systemic cooling of the patient to 32°C).
After the operation, all patients were transferred to the intensive care unit. Tracheal extubation was performed in accordance with the standard criteria.
Statistical Analysis
Values are generally given as mean ± SD. One-way analysis of variance was performed in order to evaluate overall significance as appropriate. One-way analysis of variance was followed by a Tukey studentized range test, in order to locate the possible differences. This 2-step procedure controlled the experiment-wise error rate at a 5% significance level despite multiple comparisons (that is, 3 operative categories).8 Analyses were performed using GraphPad InStat version 3.01 (GraphPad Software, Inc.; La Jolla, Calif) on a Windows 2003 platform.
Results
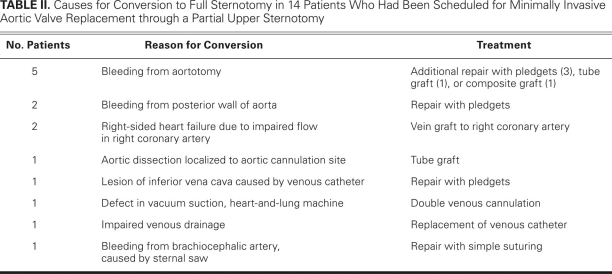
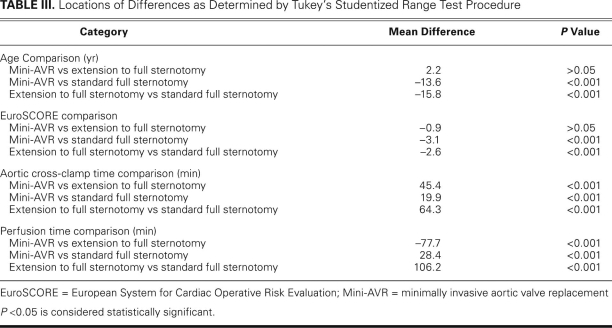
The 30-day mortality rate for the 98 patients intended for mini-AVR was zero. Fourteen of the 98 mini-AVRs were converted to conventional surgeries (with complete sternotomies) during the operation. Conversions were necessary due to technical problems or bleeding (Table II). The complications leading to a full sternotomy were not clustered at the beginning of the series but spread out evenly over time. The demographic and operative data for the 84 patients whose procedures were completed as mini-AVRs, the 14 patients whose procedures were extended to full sternotomies, and the 50 patients who underwent conventional AVRs through full sternotomies (as intended) are shown in Table I. The locations of the differences observed, as determined by the Tukey studentized range test, are shown in Table III.
TABLE II. Causes for Conversion to Full Sternotomy in 14 Patients Who Had Been Scheduled for Minimally Invasive Aortic Valve Replacement through a Partial Upper Sternotomy
TABLE III. Locations of Differences as Determined by Tukey's Studentized Range Test Procedure
In the group of patients whose operations were completed as mini-AVRs, 4 died later of noncardiac causes: a 68-year-old man died 6 weeks postoperatively of a generalized systemic mycotic infection; a 71-year-old man died 3 months postoperatively of bleeding from a cerebral aneurysm; a 76-year-old woman died 7 months postoperatively of colon cancer complicated by strangulation of the large intestine; and a 78-year-old man died 3 years postoperatively of bladder cancer. Two patients, including 1 with a myocardial infarction, had postoperative heart failure that required an intra-aortic balloon pump. One of these patients required hemodialysis. Two patients with mechanical valves underwent reoperation within the 1st year for valve dysfunction (impaired mobility of a leaflet, in both cases). Both of these patients needed additional bypass grafting, as well as a new valve. One patient who had a stented biological valve required reoperation after 18 months due to tissue overgrowth and severe stenosis of the prosthesis. Ten patients developed late pericardial effusions that needed drainage. One patient acquired a superficial wound infection that developed into a sternal fistula. No patient developed sternal dehiscence or mediastinitis.
In the group of mini-AVR patients who underwent extension to a full sternotomy, no patient died. One patient sustained a major complication in the form of a cerebral infarction, followed by full remission of symptoms. Another patient had postoperative heart failure that necessitated an intra-aortic balloon pump and hemodialysis for a week.
No significant differences in late death or major complications were found between patients intended for full-sternotomy AVR and those intended for mini-AVR, even when the mini-AVR patients underwent intraoperative extension to a complete sternotomy.
Discussion
In this study, which constitutes the largest reported series of mini-AVR procedures from Scandinavia to date, we were able to implement this technique in selected patients without perioperative death. The mean EuroSCORE (European System for Cardiac Operative Risk Evaluation) in our mini-AVR group was 4, which means that our expected loss would have been 4 patients. One patient, a 68-year-old man, died 6 weeks postoperatively of a fungal infection that was surgically related.
Elective, conventional AVR performed through a complete sternotomy is nowadays a safe procedure that carries low mortality and morbidity rates. Although the implementation of a new operative technique for AVR with a worse outcome would appear to be unacceptable, no studies to date have shown significant differences between mini-AVR and conventional AVR with regard to death and major complications.4,9–11 However, the only long-term benefits of mini-AVR are a smaller scar (8 cm vs 25–30 cm) and, theoretically, the presence of an intact distal pericardium with fewer adhesions, which could facilitate subsequent cardiac operations. Because of the lack of controlled clinical studies and thorough scientific evaluation, the benefits of mini-AVR in comparison with conventional AVR are unproved. However, several observational and nonrandomized studies have indicated that mini-AVR patients might experience less postoperative pain, better respiratory function with fewer atelectases, and shorter hospital stay.5,10,12 The drawback is that even uncomplicated mini-AVR is technically more difficult and requires longer perfusion and cross-clamp times.1,3,13
In our hands, 84 of 98 patients intended for mini-AVR underwent operation as planned. Most of these patients had a smooth perioperative course characterized by little bleeding, reduced pain, fewer atelectases, and early mobilization (all in comparison with full sternotomy). However, the operations in 14 patients who were intended for mini-AVR had to be converted to full sternotomies due to technical or bleeding problems during surgery (Table II). Although it might be assumed that these 14 cases were somehow associated with a learning curve, it should be observed that the conversions to full sternotomy were not clustered at the beginning of the series but spread out evenly over time. We believe that such a high number of conversions was the consequence of limited surgical access and technical difficulties. Such patients are the “losers” of mini-AVR, because they have long operative times and are more likely to experience bleeding, blood transfusions, and prolonged intensive-care and hospital stays. Mini-AVR patients who underwent extension to full sternotomy experienced aortic cross-clamp and perfusion times that were more than twice the length of times experienced by patients who underwent conventional AVR performed electively via full sternotomy (Table I). The duration of CPB is clearly a risk factor for morbidity and death after cardiac surgery.14 We believe that the lengthy aortic cross-clamp and perfusion times (>2 hrs and >3 hrs, respectively) associated with our 14 converted procedures are hazardous. In all likelihood, all 14 of these patients survived because of their relative youth (mean age, 57 ± 12.1 yr) and quite low mean EuroSCORE (4.5 ± 2.5). Conversion to a full sternotomy before CPB could have reduced the extremely long bypass times in this group of patients. Bleeding from the aortotomy in 5 of the 14 patients is unacceptable; therefore, we have started reinforcing the proximal edge of fragile aortic incisions with Teflon felt strips and fibrin sealant in order to secure hemostasis.
The percentage of patients intended for mini-AVR but converted to full sternotomy must be reduced before we can find it reasonable, in future, to offer mini-AVR to all patients in lieu of full-sternotomy AVR—which has proved safe and effective over time. Computed tomographic scanning might in future improve the determination of preoperative indications by establishing the anatomy of the diseased ascending aorta and by enabling 3-dimensional computer simulation of the actual operation in each individual patient. Patients who are technically difficult or impossible to operate upon through partial sternotomy—those with deep chests or with dilated or calcified ascending aortas—could then be eliminated from consideration, thereby reducing the number of patients who need intraoperative conversion to full sternotomy.15
To clarify the results of mini-AVR, there should be full disclosure (in published studies) of failed attempts to perform the procedure. With few exceptions,2 most authors do not reveal, when reporting the benefits of mini-AVR over conventional AVR, whether their mini-AVR patients who had to be converted to full sternotomy are included in their mini-AVR group or in their full-sternotomy group—or indeed whether these patients are included in the accounting at all. We believe that this is a cause for concern regarding the accuracy and completeness of the literature published on this subject. We are also aware of individual surgeons and entire departments that have abandoned the mini-AVR technique, because the approach was found to entail higher morbidity and mortality rates than does conventional AVR. We agree with Cooley that minimizing access implies maximizing technical difficulty.1 Because of increased technical difficulty, limited-access valve procedures can heighten the surgeon's mental stress. In our unit, 4 of 5 cardiac surgeons did not feel comfortable with the mini-AVR technique, and this uncertainty is probably representative: therefore, mini-AVR has not become a very common procedure, even after more than 10 years of promotion in the medical literature and lay press. Conventional AVR is faster, the surgeon has direct access to the heart if complications arise, and air can be evacuated both from the aorta and from the apex of the heart. Moreover, late pericardial effusions (sometimes weeks after the operation) follow mini-AVR operations in 10% of cases and are dangerous; these emphasize the need for close follow-up because the pericardium must be drained before frank tamponade develops.10
In conclusion, mini-AVR is an excellent operation in selected patients, but its true advantages over conventional AVR (other than a smaller scar) await evaluation by means of randomized clinical trial. The “extended mini-AVR” operation, on the other hand, is a risky procedure that should be avoided by better preoperative evaluation of patients. In any event, the decision to extend a mini-sternotomy to a full sternotomy should be made early in the course of operation, before CPB is instituted.
Footnotes
Address for reprints: Henrik K. Kjaergard, MD, Department of Cardiothoracic Surgery, Rigshospitalet, University of Copenhagen, Blegdamsvej 9, Copenhagen 2100, Denmark
E-mail: henrik@dadlnet.dk
References
- 1.Cooley DA. Antagonist's view of minimally invasive heart valve surgery. J Card Surg 2000;15(1):3–5. [DOI] [PubMed]
- 2.Detter C, Deuse T, Boehm DH, Reichenspurner H, Reichart B. Midterm results and quality of life after minimally invasive vs. conventional aortic valve replacement. Thorac Cardiovasc Surg 2002;50(6):337–41. [DOI] [PubMed]
- 3.Masiello P, Coscioni E, Panza A, Triumbari F, Preziosi G, Di Benedetto G. Surgical results of aortic valve replacement via partial upper sternotomy: comparison with median sternotomy. Cardiovasc Surg 2002;10(4):333–8. [DOI] [PubMed]
- 4.Sharony R, Grossi EA, Saunders PC, Schwartz CF, Ribakove GH, Baumann FG, et al. Propensity score analysis of a six-year experience with minimally invasive isolated aortic valve replacement. J Heart Valve Dis 2004;13(6):887–93. [PubMed]
- 5.Estrera AL, Reardon MJ. Current approaches to minimally invasive aortic valve surgery. Curr Opin Cardiol 2000;15(2):91–5. [DOI] [PubMed]
- 6.Mihaljevic T, Cohn LH, Unic D, Aranki SF, Couper GS, Byrne JG. One thousand minimally invasive valve operations: early and late results. Ann Surg 2004;240(3):529–34. [DOI] [PMC free article] [PubMed]
- 7.Hearn CJ, Kraenzler EJ, Wallace LK, Starr NJ, Sabik JF, Cosgrove DM. Minimally invasive aortic valve surgery: anesthetic considerations. Anesth Analg 1996;83(6):1342–4. [DOI] [PubMed]
- 8.Benjamini Y, Braun H. John W. Tukey's contributions to multiple comparisons. Ann Statist 2002;30(6):1576–94.
- 9.Vanoverbeke H, Van Belleghem Y, Francois K, Caes F, Bove T, Van Nooten G. Operative outcome of minimal access aortic valve replacement versus standard procedure. Acta Chir Belg 2004;104(4):440–4. [PubMed]
- 10.Bakir I, Casselman FP, Wellens F, Jeanmart H, De Geest R, Degrieck I, et al. Minimally invasive versus standard approach aortic valve replacement: a study in 506 patients. Ann Thorac Surg 2006;81(5):1599–604. [DOI] [PubMed]
- 11.Bonacchi M, Prifti E, Giunti G, Frati G, Sani G. Does ministernotomy improve postoperative outcome in aortic valve operation? A prospective randomized study. Ann Thorac Surg 2002;73(2):460–6. [DOI] [PubMed]
- 12.Candaele S, Herijgers P, Demeyere R, Flameng W, Evers G. Chest pain after partial upper versus complete sternotomy for aortic valve surgery. Acta Cardiol 2003;58(1):17–21. [DOI] [PubMed]
- 13.Farhat F, Lu Z, Lefevre M, Montagna P, Mikaeloff P, Jegaden O. Prospective comparison between total sternotomy and ministernotomy for aortic valve replacement. J Card Surg 2003;18(5):396–403. [DOI] [PubMed]
- 14.Kirklin JK, Westaby S, Blackstone EH, Kirklin JW, Chenoweth DE, Pacifico AD. Complement and the damaging effects of cardiopulmonary bypass. J Thorac Cardiovasc Surg 1983;86(6):845–57. [PubMed]
- 15.Ammar R, Porat E, Eisenberg DS, Uretzky G. Utility of spiral CT in minimally invasive approach for aortic valve replacement. Eur J Cardiothorac Surg 1998;14 Suppl 1:S130–3. [DOI] [PubMed]