Abstract
Type 2 diabetes is associated with microvascular complications. We hypothesized that the sustained elevated EGFR phosphorylation produces structural wall remodeling and altered mechanical properties of mesenteric resistance artery (MRA) in type 2 diabetes.
Freshly isolated MRA (80–100 µm diameter) from type 2 diabetic (db−/db−, diabetic) and non-diabetic (db−/db+, control) mice were subjected to pressure-passive diameter and wall thickness relationships; western blot analysis and immunohistology.
Data indicated that MRA from diabetic mice have a smaller passive diameter than MRA from control mice under intraluminal pressure range from 25 to 125 mmHg. Measurements of wall thickness:lumen diameter ratios (21±1.8 vs. 14±1.2 at 75 mmHg diabetic vs. control respectively), wall thickness and remodeling index (38±5% vs. control) revealed eutrophic structural remodeling of MRA from diabetic mice, which was strengthened with histology. Mechanical properties revealed a great strain-stress relationship in MRA from control vs. diabetic mice indicating increased stiffness in MRA from diabetic mice. Western blot analysis showed increased collagen type 1 content in a freshly isolated MRA from type 2 diabetic mice compared to control. Diabetic mice treated with EGFR inhibitor (AG1478, 10 mg/kg/day) for two weeks reduced EGFR phosphorylation, wall thickness, collagen type 1 content, and improved the altered mechanical properties of MRA.
Conclusion and implications
These data provide evidence regarding the role of EGFR in morphological wall remodeling and altered mechanical properties of MRA from type 2 diabetic mice. This may identify new therapeutic targets for the control of vascular structure and therefore have important implications in type 2 diabetes.
Keywords: Resistance artery remodeling, collagen type 1, stiffness and type 2 diabetes
INTRODUCTION
Clinical and experimental studies have demonstrated microvascular complication in type 2 diabetes. Type 2 diabetes occurs when beta-cell function fails to compensate for insulin resistance (1–3). Although the multi-factorial effects of diabetes on the regulatory mechanisms that govern vessel diameter are not well understood, it is likely that altered vascular structure is involved. Limited studies of the relationship between diabetes and altered vascular structure have been conducted in the microvasculature from diabetic models. It has been reported that a deteriorating glucose tolerance status is associated with an increased cardiovascular disease risk; and the mechanisms responsible remain unclear but are likely to involve functional and structural macrovascular and microvascular changes (4; 5). Moreover, a recent study reported an induction of carotid arterial remodeling from patient with type 2 diabetes (6). Wall thickening and diameter widening characterize arterial structural remodeling (7; 8). Generally structural wall remodeling can include hypertrophy and/or hyperplasia of smooth muscle cells and changes in the content of extracellular matrix leading to alteration of mechanical properties of resistance arteries and subsequently tissue perfusion.
Resistance arteries undergo structural changes (vascular remodeling) in metabolic and cardiovascular diseases. These involve media thickness, lumen diameter and extracellular matrix accumulation changes. The vascular wall is an active, flexible, and integrated organ made up of cellular (endothelial cells, smooth muscle cells, adventitia cells, and fibroblasts) and noncellular (extracellular matrix) components, which in a dynamic way change shape or number. These components can also reorganize in response to physiological and pathological stimuli, maintaining the integrity of the vessel wall in physiological conditions or participating in the vascular changes in metabolic and cardiovascular diseases.
In a previous study we showed EGFR as key element in resistance artery function dictating the development of myogenic tone (9). Therefore we propose that type 2 diabetes-induced structural remodeling and altered mechanical properties of resistance artery is mediated by sustained increased EGFR phosphorylation. To test this hypothesis, we treated diabetic mice with EGFR tyrosine kinase inhibitor for two weeks and determined the characteristics and mechanical properties of resistance arteries.
METHODS
Animal Model
Homozygote obese type 2 diabetic (db−/db−, diabetic) and heterozygote non-diabetic (db+/db−, control) adult (12 to 14 weeks old) male mice were obtained from Jackson Laboratory. Mice were divided into 4 groups: 1) control mice (n=7); 2) diabetic mice (n=7); control mice treated with EGFR inhibitor (AG1478, 10 mg/Kg/day, Sigma) for two weeks; and 4) diabetic mice treated with EGFR inhibitor (AG1478, 10 mg/Kg/day) for two weeks. The treatment was delivery using an osmotic mini-pump (Alzet) implemented in the animal. These studies conformed to the principles of the National Institutes of Health “Guide for the Care and Use of Laboratory Animals”, and were approved by the LSU Institutional Animal Care and Use Committee.
Blood glucose measurements
We used an ACCU-CHEK Compact Plus System (Roche Diagnostic) for measuring blood glucose level in all group after a 5 hr fast (n=6).
In vivo blood pressure measurements
Diabetic and control mice were anesthetized with ketamine/xylazine and a catheter, connected to a pressure sensor (Living System Instrumentation, Burlington, Vermont), was inserted into left carotid. After surgery, the mice were subjected to a 20-minutes equilibration period before mean arterial pressure was measured.
Resistance artery
Mice were anesthetized by intraperitoneal injection of a mixture of 100-mg/kg ketamine and 20-mg/kg xylazine. Freshly mesenteric resistance arteries (80–100 µm) from mice were isolated and mounted onto two glass cannulas in a vessel chamber and then pressurized to 100 mmHg by using a pressure-servo-control perfusion (Living System Instrumentation) in order to stretch the artery and set a constant arterial length. Vessel diameter was continuously monitored via a video image analyzer as previously described (9–11). The cannulated arterial segments were submerged in 2 ml of physiological salt solution (pH 7.4), oxygenated (10%O2- 5%CO2 and 85%N2). Next, after a 45-minute equilibration period, resistance artery was perfused with high concentration of nitric oxide donor (SNP, 100 µM), 2 mM of EGTA and diameter changes were then measured when intraluminal pressure was increased from 25 to 125 mmHg (active diameter) by 25 mmHg for each step. The time difference between each step was set at 3–5 min. At each step of intraluminal pressure, internal, external diameter and wall thickness were measured. Stiffness and remodeling index of mesenteric resistance arteries were calculated as previously published (12–14).
Histology
Freshly isolated mesenteric resistance arteries, from diabetic and control mice perfused with 4% of formalin, were placed into freezing mold Tissues® OCT snap frozen with liquid N2, sectioned on a cryostat, placed on slides and subjected to bright field staining using a fluorescent microscope.
Western blot analysis
Freshly isolated mesenteric resistance arteries from all groups were immediately frozen with liquid nitrogen and stored at −80°C. Frozen vessel segments were pulverized and re-suspended in ice-cold lysis buffer (20 mM Tris-HCl, pH 7.5, 5 mM EGTA, 150 mM NaCl, 20 mM glycerophosphate, 10 mMNaF, 1 mM sodium orthovanadate, 1% Triton X-100, 0.1% Tween-20, 1 µg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM N-tosyl-L-phenylalanine chloromethyl ketone, and 0.5 mmol/L N-p-tosyl-L-lysine chloromethyl ketone). Extracts were incubated on ice for 15 minutes and then centrifuged (12000 xg for 15 minutes at 4°C). The detergent-soluble supernatant fractions were retained, and protein concentrations in samples were determined using a Pierce Protein Assay. Tissue lysates were subjected to immunoblotting with collagen type 1 specific antibodies overnight and then to the secondary for 1 hour at room temperature. Membranes were subjected to horseradish peroxidase-conjugated secondary antibodies. Immunoreactive bands were visualized using enhanced chemiluminescence reagents (ECL, Amersham) exposed to Hyperfilm at the linear range of film density. Films were scanned and densitometry analysis performed with NIH image software.
Statistical analysis
Results are expressed as mean±sem. Significance of the differences between groups was determined by repeated 1- or 2-factor ANOVA, where appropriate, followed by Bonferroni posthoc analysis (InStat). The 2-tailed Student’s t-test was also used to compare the variables “mean arterial pressure and body weight” between the two groups. Differences were considered significant at P<0.05.
RESULTS
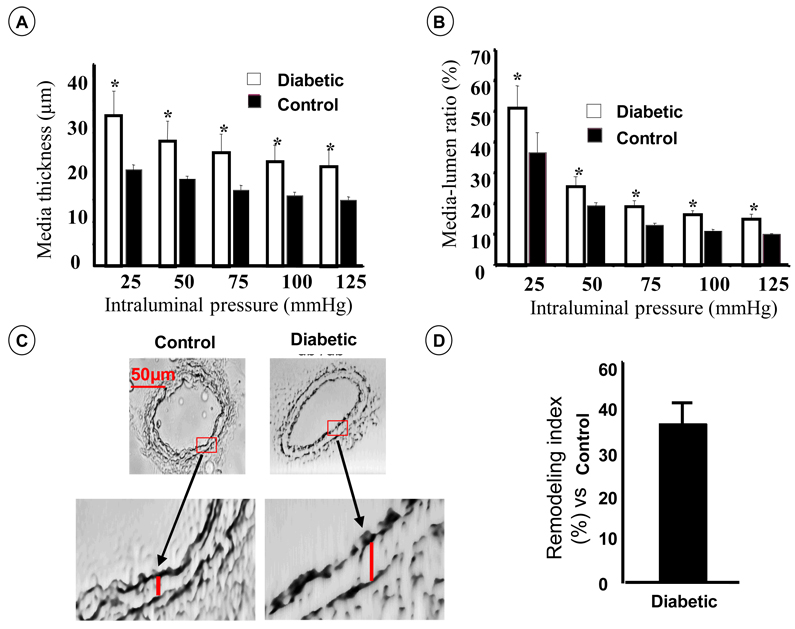
Adult diabetic mice develop hyperglycemia compared to control (353±12 vs. 119±10.3 mg/dL in diabetic compared to control mice respectively, p<0.05). Mean arterial pressure was similar in all groups (96±3 vs. 98±5 in diabetic and control mice respectively, p>0.05). The treatment of diabetic mice with AG1478 had no effect of blood glucose and blood pressure (data not shown). To determine the structural wall remodeling and mechanical properties of mesenteric resistance artery from each group, we performed intraluminal pressure-passive diameter relationship under perfused and superfused arteries with high concentration of nitric oxide donor (SNP, 100 µM) and 2 mM of EGTA. Measurements of lumen diameter, wall thickness (media) and remodeling index under full intraluminal pressure range revealed significant increases in wall thickness and lumen:media ratio, which in combination with smaller luminal diameter lead to eutrophic structural remodeling of mesenteric resistance artery from diabetic compared to control mice (Figures 1A–B). Data also showed an increase of remodeling index strengthen the induction of structural remodeling in mesenteric resistance artery from diabetic mice (Figure 1D).
Figure 1.
Structural artery wall remodeling of mesenteric resistance artery from 12-week old type 2 diabetic and control mice. A: Media thickness measurements; B: Media/lumen ratio; C: Cross section of mesenteric resistance arteries from diabetic and control mice perfused in vivo with 4% of formalin, 60x magnification; D: Remodeling index (percentage of difference between internal diameters of diabetic and control vessels not attributable to growth), n=5–7; p<0.05 statistically different between diabetic vs. control. A–B. Resistance arteries were mounted in arteriograph for measurements of intraluminal and external diameter, and wall thickness under range of pressure from 25 to 125 mmHg.
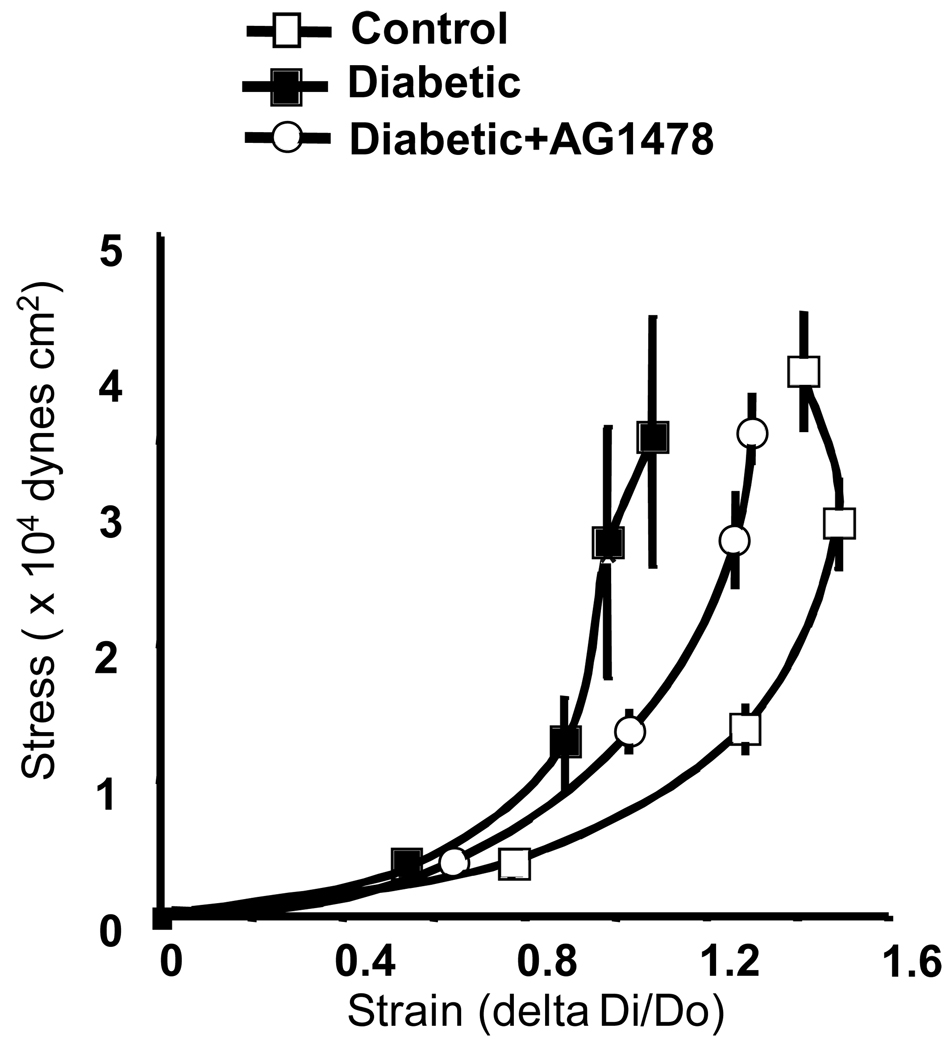
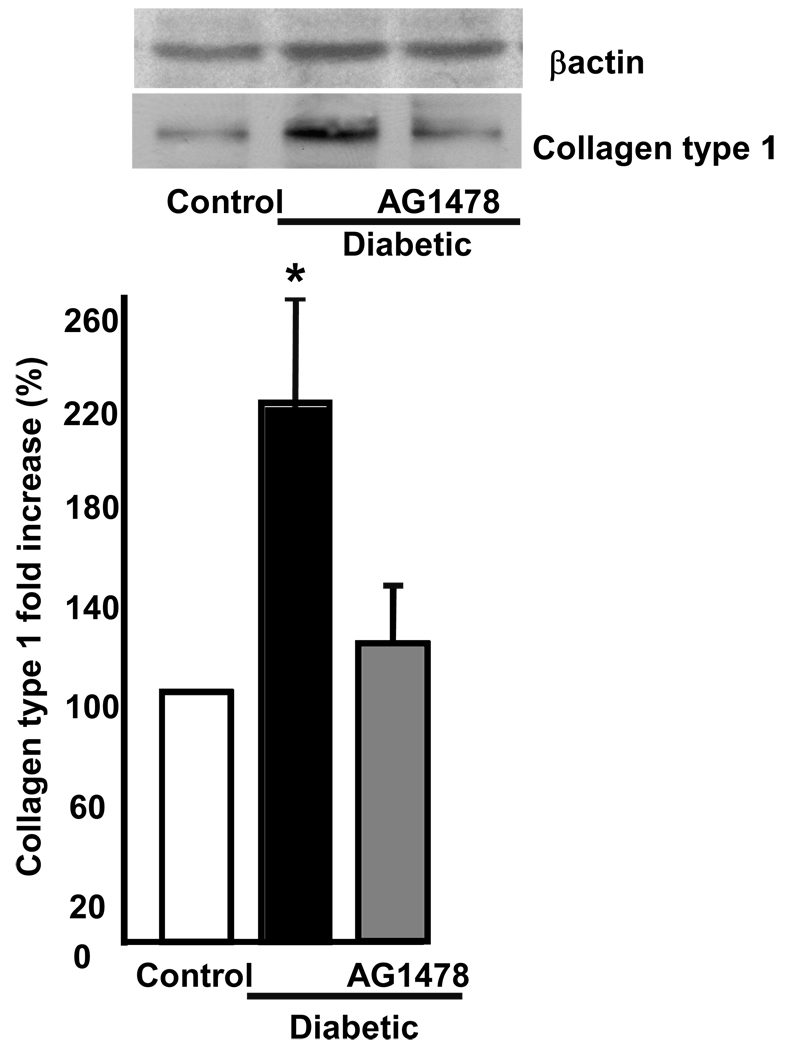
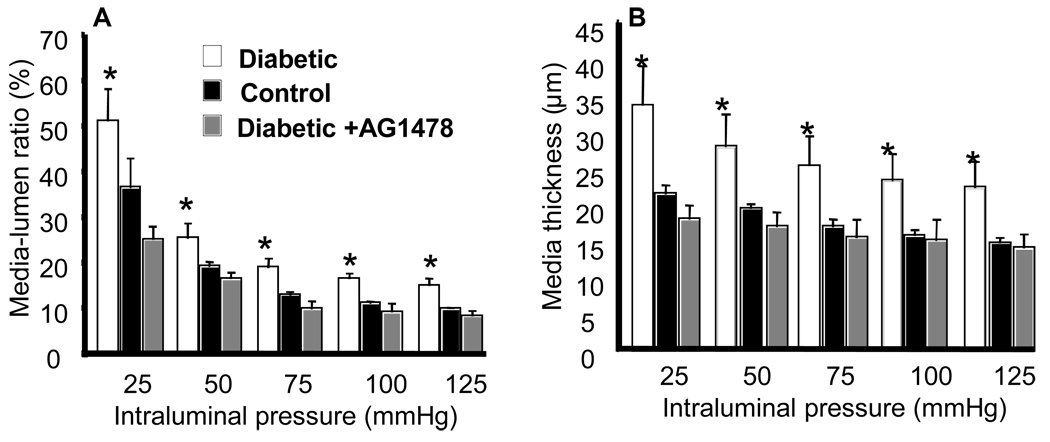
Freshly isolated mesenteric resistance arteries from diabetic and control mice were placed into freezing mold Tissues® OCT snap frozen with liquid N2, sectioned on a cryostat, placed on slides and subjected to bright field staining using a fluorescent microscope. Data revealed an increase of wall thickness associated with small lumen diameter (Figure 1C). There has been much recent interest in the relationship between arterial stiffness and cardiovascular disease. As shown in Figures 2, mesenteric resistance artery from diabetic mice showed an increase in stiffness, assessed with stress-strain relationship. To determine whether increased arterial stiffness was associated with abnormal ECM accumulation, freshly isolated mesenteric resistance arteries were subjected to western blot analysis, using collagen type 1 specific antibody, and found an increase in collagen type 1 content of mesenteric resistance arteries from diabetic and control mice (Figure 3). Diabetic mice treated with EGFR tyrosine kinase inhibitor (10 mg/Kg/day, for two weeks) showed a normalization of wall thickness, lumen diameter and a significant reduction in collagen type 1 content (Figure 2–Figure 4). In a recent study, we showed the increased EGFR phosphorylation in mesenteric resistance arteries from diabetic mice compared to control, which was significantly reduced after treatment with AG1478(15).
Figure 2.
Strain-stress relationship of mesenteric resistance arteries isolated from diabetic, diabetic + AG1478 and control mice, n=6 for each experiment.
Figure 3.
Western blot analysis and cumulative data of collagen type 1 in freshly isolated mesenteric resistance arteries from diabetic, diabetic + AG1478 and control mice, n=6, p<0.05 is statistically different between diabetic vs. diabetic + AG1478 and control.
Figure 4.
Media:lumen ratio (A) and wall thickness (B) of mesenteric resistance arteries from diabetic, diabetic + AG1478 and control mice, n=6–7, p<0.05 is statistically different between diabetic vs. diabetic + AG1478 and control.
DISCUSSION
Our analysis yielded five important observations regarding mesenteric resistance artery complications in a model for obesity and type 2 diabetes: 1) alteration of structural remodeling; 2) increased wall thickness and media:lumen ratios; 3) increased stiffness; 4) abnormal extracellular matrix deposition, especially collagen type 1; 5) EGFR tyrosine kinase is a key mechanism dictating the structural remodeling and mechanical properties since its inhibition improves the characteristics and mechanical properties of mesenteric resistance artery from type 2 diabetic mice. All structural remodeling changes and mechanical properties alteration are independent of hypertension since blood pressure was normal as previously published by our group (15). These morphological and mechanical changes represent a hallmark of resistance arteries complications in type 2 diabetes.
Resistance arteries play a crucial role in protecting capillaries from high blood pressure, governing local blood flow and tissue perfusion. Resistance arteries tone is mainly regulated by mechanical factors (pressure and shear stress) (16; 17). Generally shear stress induces endothelium-dependent vasodilation (18; 19). On the other hand, intraluminal pressure induces myogenic tone, (16) which is generally modulated by shear stress through endothelial cells activation by shear stress (10; 20). It is well established that structural remodeling of arterial wall is related to sustained mechanical and hormonal modification. Our data revealed induction of structural wall remodeling and alteration of mechanical properties in mesenteric resistance artery from type 2 diabetic mice. These data indicate that metabolic factors (likely hyperglycemia and insulin resistance) could play a major role in the structural remodeling of microvessels. Recent studies in human and animal model showed mal-adaptative structural remodeling of carotid artery from diabetic model (6). Our study showed eutrophic structural remodeling of mesenteric resistance artery from diabetic mice characterized by increased wall thickness and collagen type 1 content associated with small lumen diameter similar to that seen in hypertensive human and animal models. In addition, it has been reported in diabetic-streptozotocin rats the structural changes of preglomerular vessels independently of blood pressure changes (21). These findings support our data regarding structural remodeling of resistance artery from diabetic mice independently of blood pressure elevation.
The morphological studies of small arteries in diabetes revealed a fundamental abnormality, which needs to be considered against what is known about the effects of hypertension on the vasculature. Thus, it has been reported in vessels from type 2 diabetic patients, whether hypertensive or not: Lumen diameter was unchanged compared with control values, and wall thickness was significantly increased with an increased cross-sectional area. The findings were similar in type 2 diabetes and type 2 diabetes+Hypertension, and calculations of growth and remodeling indexes clearly point to the vessels having undergone hypertrophy, a feature noted in previous investigations in diabetes (22). Our studies revealed an increase of collagen type 1 content in resistance arteries from diabetic compared to samples from control mice. The increased collagen type 1 was associated with enhanced stiffness. In recent years, studies on rodents have shown that, particularly in old age, diabetes mellitus, and/or in subjects on a high-sodium diet, arterial calcifications and attachment molecules (between VSM cells, or VSM cells and extracellular matrix proteins, or between collagen fibers), contribute per se to stiffen the vascular wall (22). Cross-links may stabilize collagen fibrils, preventing slippage of adjacent molecules under applied tensile stress and contributing to increase in arterial stiffness through formation of end glycation products (23).
Our recent study showed the involvement of EGFR tyrosine kinase in the development of myogenic tone in response to pressure changes (9). Immunostaining revealed a sustained EGFR tyrosine kinase phosphorylation increase of mesenteric resistance arteries from diabetic mice compared to control(15). Thus, the treatment of diabetic mice for two weeks with EGFR tyrosine kinase inhibitor normalized wall thickness, lumen diameter and significantly, reduced collagen type 1 content and stiffness(15). These data strongly indicate that EGFR tyrosine kinase as key element involved in structural remodeling and altered mechanical properties of mesenteric resistance artery from diabetic mice. Further studies are needed to determine the upstream signaling leading to increased EGFR tyrosine kinase phosphorylation and downstream signaling responsible for structural remodeling and altered mechanical properties of mesenteric resistance artery in type 2 diabetes.
In type 2 diabetes, the pathological consequences of microvascular structure complications could be extremely important: Impaired structural remodeling has been implicated as an early sign of microvascular disease, and diabetics are certainly prone to this upstream in resistance arteries. An alteration in the microvascular structure might lead to local blood flow autoregulation failure and subsequently tissue damage.
Study enigma
It is crucial to mention that the treatment of diabetic mice with EGFR tyrosine kinase for two weeks normalize structural remodeling and significantly improves stiffness of resistance arteries. On one hand, we strongly believe in the involvement of EGFR tyrosine kinase in resistance artery structural complication. On the other hand, EGFR tyrosine kinase is not the only pathway leading to structural remodeling induction of resistance artery from diabetic mice. Multiple potential pathways could exist dictating structural remodeling. Similar to our concept Dr. Zhang (24) laboratory reported that endothelial dysfunction in type 2 diabetes was completely normalized when mice were treated with TNFα specific antibody for 3 days. In addition, it is important to mention that in vivo studies of mesenteric arteries structure have shown attenuation of the type 1 diabetes (different from type 2 diabetes) related changes with aminoguanidine (25) and also with ACE inhibition (26) and sodium hydrogen exchange inhibition (27). All together, these studies strongly emphasize a myriad of signaling that could be independent or dependent of each other. These data revealed the complications of in vivo study and deeper investigations should be taken to dissolve this lake of knowledge.
In conclusion, the present study provides novel and significant insight into basic mechanisms that control resistance artery structural remodeling and mechanical properties in type 2 diabetes. The sustained increased EGFR tyrosine kinase phosphorylation is linked to the structural remodeling and altered mechanical properties of resistance artery in type 2 diabetes. These results may identify new therapeutic targets for the control of vascular structure and therefore have important implications in type 2 diabetes.
ACKNOWLEDGEMENTS
This work was supported by the SDG National American Heart Association, 0430278N (PI: Dr. K Matrougui), Enhancement Research Phase II Award Tulane University (PI: Dr. K Matrougui); Tulane COBRE in Hypertension and Renal Biology (2P20RR017659-06, NIH/NCRR, PI. Dr. L Navar, Co-PI. K Matrougui)
REFERENCES
- 1.DeFronzo RA. Lilly lecture 1987. The triumvirate: beta-cell, muscle, liver. A collusion responsible for NIDDM. Diabetes. 1988;37:667–687. doi: 10.2337/diab.37.6.667. [DOI] [PubMed] [Google Scholar]
- 2.Simonson DC, Tappy L, Jequier E, Felber JP, DeFronzo RA. Normalization of carbohydrate-induced thermogenesis by fructose in insulin-resistant states. Am J Physiol. 1988;254:E201–E207. doi: 10.1152/ajpendo.1988.254.2.E201. [DOI] [PubMed] [Google Scholar]
- 3.Ferrannini E, Simonson DC, Katz LD, Reichard G, Jr, Bevilacqua S, Barrett EJ, Olsson M, DeFronzo RA. The disposal of an oral glucose load in patients with non-insulin-dependent diabetes. Metabolism. 1988;37:79–85. doi: 10.1016/0026-0495(88)90033-9. [DOI] [PubMed] [Google Scholar]
- 4.Terry JG, Tang R, Espeland MA, Davis DH, Vieira JL, Mercuri MF, Crouse JR., 3rd Carotid arterial structure in patients with documented coronary artery disease and disease-free control subjects. Circulation. 2003;107:1146–1151. doi: 10.1161/01.cir.0000051461.92839.f7. [DOI] [PubMed] [Google Scholar]
- 5.Mathieu-Costello O, Kong A, Ciaraldi TP, Cui L, Ju Y, Chu N, Kim D, Mudaliar S, Henry RR. Regulation of skeletal muscle morphology in type 2 diabetic subjects by troglitazone metformin: relationship to glucose disposal. Metabolism. 2003;52:540–546. doi: 10.1053/meta.2002.50108. [DOI] [PubMed] [Google Scholar]
- 6.Henry RM, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Kamp O, Bouter LM, Stehouwer CD. Carotid arterial remodeling: a maladaptive phenomenon in type 2 diabetes but not in impaired glucose metabolism the Hoorn study. Stroke. 2004;35:671–676. doi: 10.1161/01.STR.0000115752.58601.0B. [DOI] [PubMed] [Google Scholar]
- 7.Ward MR, Jeremias A, Huegel H, Fitzgerald PJ, Yeung AC. Accentuated remodeling on the upstream side of atherosclerotic lesions. Am J Cardiol. 2000;85:523–526. doi: 10.1016/s0002-9149(99)00804-8. [DOI] [PubMed] [Google Scholar]
- 8.Pasterkamp G, Fitzgerald PF, de Kleijn DP. Atherosclerotic expansive remodeled plaques: a wolf in sheep's clothing. J Vasc Res. 2002;39:514–523. doi: 10.1159/000067204. [DOI] [PubMed] [Google Scholar]
- 9.Lucchesi PA, Sabri A, Belmadani S, Matrougui K. Involvement of metalloproteinases 2/9 in epidermal growth factor receptor transactivation in pressure-induced myogenic tone in mouse mesenteric resistance arteries. Circulation. 2004;110:3587–3593. doi: 10.1161/01.CIR.0000148780.36121.47. [DOI] [PubMed] [Google Scholar]
- 10.Matrougui K, Loufrani L, Heymes C, Levy BI, Henrion D. Activation of AT(2) receptors by endogenous angiotensin II is involved in flow-induced dilation in rat resistance arteries. Hypertension. 1999;34:659–665. doi: 10.1161/01.hyp.34.4.659. [DOI] [PubMed] [Google Scholar]
- 11.Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- 12.Intengan HD, Schiffrin EL. Structure and mechanical properties of resistance arteries in hypertension: role of adhesion molecules and extracellular matrix determinants. Hypertension. 2000;36:312–318. doi: 10.1161/01.hyp.36.3.312. [DOI] [PubMed] [Google Scholar]
- 13.Baumbach GL, Heistad DD. Remodeling of cerebral arterioles in chronic hypertension. Hypertension. 1989;13:968–972. doi: 10.1161/01.hyp.13.6.968. [DOI] [PubMed] [Google Scholar]
- 14.Park JB, Schiffrin EL. Small artery remodeling is the most prevalent (earliest?) form of target organ damage in mild essential hypertension. J Hypertens. 2001;19:921–930. doi: 10.1097/00004872-200105000-00013. [DOI] [PubMed] [Google Scholar]
- 15.Belmadani S, Palen DI, Gonzalez-Villalobos RA, Boulares HA, Matrougui K. Elevated Epidermal Growth Factor Receptor Phosphorylation Induces Resistance Artery Dysfunction in Diabetic db/db mice. Diabetes. 2008 doi: 10.2337/db07-0739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev. 1999;79:387–423. doi: 10.1152/physrev.1999.79.2.387. [DOI] [PubMed] [Google Scholar]
- 17.Bevan JA, Joyce EH. Flow-induced resistance artery tone: balance between constrictor and dilator mechanisms. Am J Physiol. 1990;258:H663–H668. doi: 10.1152/ajpheart.1990.258.3.H663. [DOI] [PubMed] [Google Scholar]
- 18.Osol G. Mechanotransduction by vascular smooth muscle. J Vasc Res. 1995;32:275–292. doi: 10.1159/000159102. [DOI] [PubMed] [Google Scholar]
- 19.Herrmann J, Lerman A. The endothelium: dysfunction and beyond. J Nucl Cardiol. 2001;8:197–206. doi: 10.1067/mnc.2001.114148. [DOI] [PubMed] [Google Scholar]
- 20.Bolla M, Matrougui K, Loufrani L, Maclouf J, Levy B, Levy-Toledano S, Habib A, Henrion D. p38 mitogen-activated protein kinase activation is required for thromboxane-induced contraction in perfused and pressurized rat mesenteric resistance arteries. J Vasc Res. 2002;39:353–360. doi: 10.1159/000065547. [DOI] [PubMed] [Google Scholar]
- 21.Turoni CM, Reynoso HA, Maranon RO, Coviello A, Peral de Bruno M. Structural changes in the renal vasculature in streptozotocin-induced diabetic rats without hypertension. Nephron Physiol. 2005;99:p50–p57. doi: 10.1159/000083136. [DOI] [PubMed] [Google Scholar]
- 22.Longhini CS, Scorzoni D, Bazzanini F, Manservigi D, Fratti D, Gilli G, Musacci GF. The structural arteriolar changes in diabetes mellitus and essential hypertension. The relative contribution of ageing and high blood pressure. Eur Heart J. 1997;18:1135–1140. doi: 10.1093/oxfordjournals.eurheartj.a015409. [DOI] [PubMed] [Google Scholar]
- 23.Susic D, Varagic J, Ahn J, Frohlich ED. Crosslink breakers: a new approach to cardiovascular therapy. Curr Opin Cardiol. 2004;19:336–340. doi: 10.1097/01.hco.0000127135.73849.4f. [DOI] [PubMed] [Google Scholar]
- 24.Gao X, Belmadani S, Picchi A, Xu X, Potter BJ, Tewari-Singh N, Capobianco S, Chilian WM, Zhang C. Tumor necrosis factor-alpha induces endothelial dysfunction in Lepr(db) mice. Circulation. 2007;115:245–254. doi: 10.1161/CIRCULATIONAHA.106.650671. [DOI] [PubMed] [Google Scholar]
- 25.Rumble JR, Cooper ME, Soulis T, Cox A, Wu L, Youssef S, Jasik M, Jerums G, Gilbert RE. Vascular hypertrophy in experimental diabetes. Role of advanced glycation end products. J Clin Invest. 1997;99:1016–1027. doi: 10.1172/JCI119229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hulthen UL, Cao Z, Rumble JR, Cooper ME, Johnston CI. Vascular hypertrophy and albumin permeability in a rat model combining hypertension and diabetes mellitus. Effects of calcium antagonism, angiotensin converting enzyme inhibition, and angiotensin II-AT1-receptor blockade. Am J Hypertens. 1996;9:895–901. doi: 10.1016/s0895-7061(96)00177-x. [DOI] [PubMed] [Google Scholar]
- 27.Jandeleit-Dahm K, Hannan KM, Farrelly CA, Allen TJ, Rumble JR, Gilbert RE, Cooper ME, Little PJ. Diabetes-induced vascular hypertrophy is accompanied by activation of Na(+)-H(+) exchange and prevented by Na(+)-H(+) exchange inhibition. Circ Res. 2000;87:1133–1140. doi: 10.1161/01.res.87.12.1133. [DOI] [PubMed] [Google Scholar]