Abstract
Alzheimer's disease (AD) is characterized by accumulation and deposition of Aβ peptides in the brain. Aβ deposition in cerebral vessels occurs in many AD patients and results in cerebral amyloid angiopathy (AD/ CAA). Aβ deposits evoke neuro- and neurovascular inflammation contributing to neurodegeneration. In this study, we found that exposure of cultured human brain endothelial cells (HBEC) to Aβ1–40 elicited expression of inflammatory genes MCP-1, GRO, IL-1β and IL-6. Up-regulation of these genes was confirmed in AD and AD/CAA brains by qRT-PCR. Profiling of 54 transcription factors indicated that AP-1 was strongly activated not only in Aβ-treated HBEC but also in AD and AD/CAA brains. AP-1 complex in nuclear extracts from Aβ-treated HBEC bound to AP-1 DNA-binding sequence and activated the reporter gene of a luciferase vector carrying AP-1-binding site from human MCP-1 gene. AP-1 is a dimeric protein complex and supershift assay identified c-Jun as a component of the activated AP-1 complex. Western blot analyses showed that c-Jun was activated via JNK-mediated phosphorylation, suggesting that as a result of c-Jun phosphorylation, AP-1 was activated and thus up-regulated MCP-1 expression. A JNK inhibitor SP600125 strongly inhibited Aβ-induced c-Jun phosphorylation, AP-1 activation, AP-1 reporter gene activity and MCP-1 expression in cells stimulated with Aβ peptides. The results suggested that JNK-AP1 signaling pathway is responsible for Aβ-induced neuroinflammation in HBEC and Alzheimer's brain and that this signaling pathway may serve as a therapeutic target for relieving Aβ-induced inflammation.
Keywords: Alzheimer's disease, Aβ peptides, Brain endothelial cells, Inflammatory gene expression, Signaling pathway, JNK-AP1
Introduction
Alzheimer's disease (AD) is a multi-factorial neurodegenerative disease characterized by progressive synaptic loss and neuronal death with gradual cognitive decline (Selkoe, 2001). However, the pathogenic factors and mechanisms of Alzheimer's disease are still not fully understood. The pathological characteristics of Alzheimer's disease include accumulation and deposition of β-amyloid (Aβ) peptides in brain parenchyma (senile plaques) and cerebral vessels and the formation of neurofibrillary tangles (NFTs) (Selkoe, 2001). One of the main hypotheses about the pathogenesis of Alzheimer's disease, the beta-amyloid hypothesis, is supported by a number of epidemiological, genetic and experimental studies. Deposition of Aβ peptides in the brain and cerebral vessels results in neuroinflammation and neurovascular inflammation (Suo et al., 1998; McGeer and McGeer 2001, 2004; Craft et al., 2006). Microglial cells are activated by Aβ peptides and release inflammatory cytokines. Aβ-stimulated neuroinflammation contributes to neurodegeneration in Alzheimer's disease (McGeer and McGeer 2001, 2004; Craft et al., 2006).
The amyloid-neuroinflammation hypothesis originated from histopathologic studies demonstrating the presence of activated microglia at sites of amyloid deposition in AD brain and that anti-inflammatory therapy relieves Alzheimer's symptoms (Haga et al., 1989; Itagaki et al.,1989; Rozemuller et al.,1989; Mattiacce et al.,1990; Perlmutter et al., 1992; Rogers et al., 1992, 2002). Further studies demonstrated that many cytokines and complement proteins are detectable in AD brain in association with microglia (Rogers et al.,1992 Griffin et al., 1995). Therefore, amyloid peptide was suggested to stimulate microglia to produce a variety of neurotoxic substances, such as reactive oxygen and nitrogen species, pro-inflammatory cytokines, chemokines, complement, and other inflammatory mediators that bring about neurodegenerative changes in Alzheimer's disease (Akiyama et al., 2000; Eikelenboom et al., 2002; McGeer and McGeer, 2001, 2004; Rogers et al., 2002). It was suggested that NFκB and p38 kinase signaling pathways are involved in Aβ-induced responses in microglial and astroglial cells (O'Neill and Kaltschmidt, 1997; Akama and Van Eldik, 2000; Hensley et al., 1999; Zhu et al., 2001a,b; Walker and Lue, 2003).
A large number of Alzheimer's patients have deposits of Aβ peptides in cerebral vessels and develop cerebral amyloid angiopathy (CAA). CAA involves both small and large brain vessels, including microvessels and capillaries. Aβ deposition in cerebrovascular structures induces significant damage to endothelial cells and may alter cellular redox state (i.e. hypoxia), which triggers down stream kinase cascades leading to neurovascular inflammation (Grammas and Ovase 2001; Malinski, 2007). Although many inflammatory factors have been detected in AD brain suggesting that inflammatory reactions play an important role in the pathogenesis of AD (Hull et al., 1996; Ho et al., 2005; Tuppo and Arias, 2005; Neuroinflammation working group, 2000), little is known about the molecular mechanisms of Aβ-induced neurovascular and neuroinflammation.
In the current study, we demonstrate that the JNK-AP1 signaling pathway is responsible for increased expression of inflammatory genes induced by Aβ peptides in human brain endothelial cells and in Alzheimer's brain.
Materials and methods
Chemical and biochemical reagents
Dulbecco's modified Eagle's medium (DMEM) was purchased from Invitrogen Inc., (N.Y., US). EBM-2 media (Clonetics Co./Lonza, Basel, Switzerland), growth factor and supplements were purchased from Bio Whittaker Inc. Fetal bovine serum (FBS) was purchased from Hyclone Inc (NY, USA). Synthetic Aβ1–40 peptide and scrambled Aβ40–1 peptide at 1 mg/vial was purchased from Biopeptides Co., Inc. (San Diego, CA, USA) and non-specific peptide polyarginine was purchased from Sigma (St. Louis, MO, USA). The peptides were dissolved in a small volume of 10 mM NaOH and further rehydrated in 2 mM NaOH to a final concentration of 231 µM. Dissolved peptides were aggregated overnight at 37 °C and stored at −80 °C until use. The stock solutionwas diluted to a desired concentration in plain medium immediately before the use. Western blot showed that Aβ1–40 peptides formed oligomers during this process (data not shown). TranSignal Protein/DNA Array I (Cat# MA1010), TranSignal RayBio Human Cytokine Antibody Array 3 (Cat# MA6020), and AP-1 reporter gene luciferase constructs were obtained from Panomics Inc (Redwood City, CA). Lipofectamine™ 2000 reagent was purchased from Invitrogen Inc. SP600125, an anthrapyrazolone inhibitor of C-Jun N-terminal kinase (JNK), was obtained from Calbiochem Inc/EMD Biosciences. PhosphoPlus(R) c-Jun (Ser63) II and c-Jun (Ser73) Antibody kit (Cat# 9260) was obtained from Cell Signaling Technology (Danvers, MA, USA).
Cell cultures
Primary human brain endothelial cell (HBEC) cultures were generously provided by Dr. Alexander Prat at the Montreal Neurological Institute (Montreal, CA) and maintained as described previously (Zhang et al., 1999, 2000, 2003). Passages 4 to 6 were used in this study. Due to rare availability of primary HBEC cultures, an immortalized HBEC hCMEC/D3 was obtained from Dr. P-O. Couraud (Paris, France) and used in the experiments. The biological properties of iHBEC cells were well characterized and similar to those of primary HBEC cultures (Weksler et al., 2005). However, higher concentrations of Aβ1–40 peptides (~20 µM) were needed to stimulate the cells to express inflammatory genes as compared to primary HBEC cells. The iHBEC cell line was obtained at passage 29 (Weksler et al., 2005) and were maintained in EBM-2 media supplemented with 2.5% FBS, hydrocortisone, VEGF, hFGF, R3-IGF-I, ascorbic acid, heparin and gentamycin. iHBEC were plated on rat tail collagen type I-coated culture dishes (100 µg/ml) and media were changed every second day. Human embryonic kidney epithelial 293 cells (HEK293) were maintained in 10% FBS in DMEM. No coating was required on culture dishes and media were changed every second day.
Human brain tissue samples
The use of human brain tissues in this work was approved by the Research Ethics Board of National Research Council of Canada (NRCREB). The brain tissue samples of Alzheimer's disease (AD), AD with cerebral amyloid angiopathy (AD/CAA), and age-matched non-demented controls (ND) were obtained from the Brain and Body Donation Program at the Sun Health Research Institute (Sun City, Arizona, USA). The Consent form for Participation in the Program was approved by the Sun Health Institutional Review Board (IRB). Brain samples (occipital lobes) of 13 AD patients with CAA pathology (AD/CAA), 13 AD patients (without histopathological CAA finding), and 12 age-matched non-demented (ND) controls were used in this study. The patients were examined and diagnosed by neurologists, and post-mortem brain samples were examined and diagnosed by neuropathologists. The diagnosis of cerebral amyloid angiopathy pathology was made according to the presence of Aβ deposition in leptomeningeal or superficial cortical blood vessels as described (Olichney et al., 1996).
RNA isolation, RT-PCR, and real-time quantitative PCR
Total RNA was isolated from cultured cells or tissues using TRIzol reagent (Invitrogen Inc.) following the manufacturer's instructions. RNA pellets were resuspended in DEPC-treated H2O and heated to 55 °C for 10 min. RNA concentration was determined in DEPC-treated H2O and reading at 260 OD values for each sample using Beckman DU 530 Life Science UV/Vis Spectrophotometer. RT-PCR and real-time quantitative PCR were performed as described previously (Zhang et al., 2003). PCR primers were designed according to published sequences in GenBank and are listed in Table 1. All primers were synthesized by Alpha DNA (Montreal, Quebec). For semi-quantitative PCR, the DNA bands on gels were analyzed by using a densitometer with Kodak 1D 3.6 Version program.
Table 1.
Primers used for RT-PCRa and real-time qPCR
| Gene | Protocol | Sense primer | Anti-sense primer |
|---|---|---|---|
| MCP-1 | RT-PCR | 5′-GCTCGCTCAGCCAGATGCAAT-3′ | 5′-TGGGTTGTGGAGTGAGTGTTC-3′ |
| IL-8 | RT-PCR | 5′-ATGACTTCCAAGCTGGCCGTG-3′ | 5′-CTCCACAACCCTCTGCACCCA-3′ |
| IL-6 | RT-PCR | 5′-CAAGCCAGAGCTGTGCAGATG-3′ | 5′-GCAGCCACTGGTTCTGTGCC-3′ |
| IL-1β | RT-PCR | 5′-TCCCTTCATCTTTGAAGAAGA-3′ | 5′-GAGGCCCAAGGCCACAGG-3′ |
| GRO-α/β/γ | RT-PCR | 5′-CAATCCCCGGCTCCTGCG-3′ | 5′-TTCACACTTTGGATGTTCTTG-3′ |
| β-actin | RT-PCR | 5′-GACTATGACTTAGTTGCGTTA-3′ | 5′-GCCTTCATACATCTCAAGTTG-3′ |
| MCP-1 | qPCR | 5′-GACCATTGTGGCCAAGGAGAT-3′ | 5′-TGCTTGTCCAGGTGGTCCAT-3′ |
| IL-6 | qPCR | 5′-ATGTAGCCGCCCCACACA-3′ | 5′-CCGTGCAGGATGTACCGAATT-3′ |
| IL-1β | qPCR | 5′-TCTTCGACACATGGGATAACGA-3′ | 5′-TCCCGGAGCGTGCAGTT-3′ |
| GRO-β/γ | qPCR | 5′-CAAGAATGGGAAGAAAGCTTGTC-3′ | 5′-CCTTGTTCAGTATCTTTTCGATGATTT-3′ |
| GAPDH | qPCR | 5′-CCACCCATGGCAAATTCC-3′ | 5′-TGGGATTTCCATTGATGACAAG-3′ |
RT-PCR using the above primers generates PCR DNA fragments as following: a 257 bp fragment for MCP-1; a 289 bp fragment for IL-8; a 144 bp fragment for IL-6; a 275 bp fragment for IL-1β; a 146 bp fragment for GRO-α, β, and γ, and a 504 bp fragment for β-actin.
Western blot analyses
Confluent HBEC grown in 60 mm culture dishes were treated with Aβ1–40, scrambled Aβ40–1 or control peptides for 4 and 8 h. Cells were lysed in buffer A (100 mM HEPES, pH 7.9; 100 mM KCl and 100 mM EDTA) (Panomics Inc.), 100 mM DTT, protease inhibitor cocktail, and IGEPAL (Sigma). The cell lysates were centrifuged at 15,000 rpm for 3 min at 4 °C. Supernatant was separated and stored at −80 °C until use, while nuclear pellet was dissolved in buffer B mixture containing buffer B (100mM HEPES; ph 7.9; 2M NaCl; 5mM EDTA; 50% glycerol), protease inhibitor cocktail and 100 mM DTT. The pellet was lysed for 2 h on rocking platform at 200 rpm, and the lysate was centrifuged at 15,000 g for 5 min at 4 °C. Due to high salt concentration, buffer C mixture [20 mM Tris, pH 7.9; 20% (w/v) glycerol, 0.1 M KCl, 0.2 mM EDTA and 0.5 mM DTT] was added to collected supernatant from the nuclear extract. Protein concentration was determined using BioRad DC Protein Assay kit (BioRad Laboratories Inc., CA). Loading buffer was added to 10 µg of nuclear extract protein samples and the proteins were resolved on 10% SDS-polyacrylamide gels. Proteins were transferred to nitrocellulose membrane. The blots were reacted with primary polyclonal rabbit IgG antibodies of anti-c-Jun, anti-Ser73 or anti-Ser63-Phospho-c-Jun (1:500 dilution) and then with a secondary antibody (1:3000 dilution) conjugated with horseradish peroxidase (donkey anti-rabbit IgG). Blots were developed using ECL Plus reagents (Amersham Pharmacia Biotech, Montreal, Quebec) and exposed to X-ray films.
Cytokine array blots
Supernatant media were harvested from control or Aβ-treated HBEC cells at different time points. TranSignal RayBio Human Cytokine Antibody Array 3 (Panomics Inc., Fremont, CA) was used to detect the levels of 42 cytokines released from the cells into culture media. The array analyses were carried out following the manufacturer's instructions (Callaghan et al., 2007). The blots were exposed to X-ray films and the levels of cytokines were analyzed by using a densitometer with Kodak 1D 3.6 Version program. The density of each cytokine spot was subtracted with an average background on the blot. Six spots of the same cytokine were collected and used for the comparison between controls and Aβ-treated HBEC cells.
TranSignal™ protein/DNA array
Primary HBEC were plated on 60 mm culture dishes and grown to confluence. Cells were then treated with 5 µM Aβ1–40, 5 µM scrambled Aβ40–1 or 2 mM NaOH (vehicle) for 8 h, and nuclear extracts were prepared from the cells as described above. The arrays were performed following the manufacturer's instructions (Panomics Inc., Fremont, CA). Briefly, nuclear extract proteins and TranSignal probe mix (TF-Probe mix) were mixed and incubated for 30 min at 15 °C. TF-Probe mix was passed through a spin column previously washed with 1x Column Incubation Buffer, and centrifuged at 7000 rpm for 30 s at 4 °C. The spin column was washed with wash buffer for three times, and the column was spun at 7000 rpm after each wash. Elution buffer was added to spin column to elute TF-bound probe. The probes were denatured at 95 °C for 3 min, chilled on ice and subsequently added to array membranes, previously pre-warmed at 42 °C water bath, and hybridized at 42 °C overnight in rotating hybridization membrane bottles. On next day, the membranes were washed twice with pre-warmed hybridization wash buffer for 20 min in rotating hybridization tubes in a hybridization oven. The membranes were placed in 1x Blocking Buffer at room temperature for 15 min with gentle shaking. For signal detection, membranes were incubated with Streptavidin-HRP conjugate 1:1000 made in 1× blocking buffer for 15 min at room temperature. This was followed by washing the membranes for three times with the wash buffer. Detection buffer was then added to each membrane and the membranes were incubated at room temperature for 5 min. The membranes were exposed to an X-ray film. The levels of activated TFs on the blots were analyzed by using a densitometer with Kodak 1D 3.6 Version program.
There are two AP-1 DNA binding sequences spotted on the TF array blot for detecting AP-1 activation, i.e., AP-1(1): 5′-TGAGTCA-3′ and AP-1(2): 5′-TGACTAA-3′. There is only one base difference between the two sequences. Upon different subunit components, activated AP-1 may prefer binding to AP-1(1) or AP-1(2) sequence or both.
Electrophoretic mobility shift assay (EMSA) and Supershift Assay
Primary HBEC cultures were grown to confluence in 100 mm dishes and treated with 5 µM Aβ1–40, 5 µM scrambled Aβ40–1 or 2 mM NaOH (vehicle). Nuclear extracts were prepared from the cells using a Panomics Inc kit following the manufacturer's instructions. Protein concentration was determined by BioRad DC protein assay reagents. Ten micrograms of protein sample was used in the reaction. Synthetic double-strand nucleotides containing AP-1-binding site were labeled with 50 µCi [γ-32P]-ATP using T4 polynucleotide kinase and separated from free [γ-32P]-ATP by gel filtration using a G-25 sephadex column (Armesham Pharmacia Biotech Inc., Montreal). Double-strand nucleotide sequences used for EMSA were as follows: wild-type AP-1(2): 5′- CGC TTG ATG ACT CAG CCG GAA-3′ and AP-1 mutant: 5′-CGC TTG ATG ACT TGG CCG GAA-3′, synthesized by Alpha DNA (Montreal, Quebec). Prior to addition of [32P]-labeled oligonucleotides (25,000 cpm), 10 µg of nuclear extract was mixed with DNA binding buffer [4% glycerol, 1 mM EDTA, 1 mM DTT, 100 mM NaCl, 10 mM Tris–HCl (pH 7.5)], herring sperm DNA and poly (dI–dC), mixed and kept at room temperature for 10 min. For supershift assay, an anti-c-Jun antibody was added to the reaction. Subsequently, [32P]-labeled nucleotides were added to nuclear extract reaction mix, and the reaction was incubated for 20 min at room temperature. Gel loading buffer was added to the reaction, and the samples were loaded to 5% poly-acrylamide gel in 1× Tris–Glycine buffer. Gel was run at 200 V for 2 h, then dried for 1 h under vacuum and exposed to X-ray film overnight for radiography.
Cloning and AP-1 luciferase reporter gene assay
AP-1 binding sequence (70 bp) from the promoter region of human MCP-1 gene (5′-AGATTTAACAGCCCACTTATCACTCATGGAA-GATCCCTCCTCCTGGTTGACTCCGCCCTCTCTCCCTCTG- 3) was cloned in a pGL3 promoter reporter vector (Promega Corp., Madison, WI). The cloned sequence was verified by restriction digestion with BamHI and HindIII for correct size of fragment and sequenced for accuracy. Plasmid DNA was prepared using a QIAGEN kit following the manufacture's instructions. Due to low transfection efficiency in iHBEC cells (<15%), HEK293 cells were instead used for plasmid transfection and reporter gene assays. HEK293 cells were grown to 80–90% confluence and were transiently transfected with AP-1 luciferase reporter gene vectors that carry either a classic AP-1-binding site (Panomics Inc. Redwood, CA) or an AP-1-binding fragment cloned from human MCP-1 gene using LipoFectamine transfection reagent (at 2:1 ratio of reagent in µl to plasmid in µg). The transfection efficiency was ~75% (data not shown). After a 48-h recovery period at 37 °C, transfected cells were treated with 5 or 10 µM Aβ1–40 peptide, control peptides, vehicle or TPA (or LPS) for 2 and 4 h. Luciferase assay was preformed using a Promega kit following the manufacturer's instructions (Cat# E1500, Promega Inc, Madison, WI) and luminescence units were determined using FLUOstar OPTIMA (BMG Laboratories Inc). Luminescence units were normalized to protein in µg per sample using BioRad DC protein assay reagents (BioRad Laboratories, Hercules, CA). Each reaction was duplicated, and the experiments were repeated at least three times.
Statistical analysis
Data were presented as mean±SD. Statistical analysis for single comparison was performed by Student's t-test where each experiment was repeated at least 3 times (n=3). For multiple comparisons, one-way ANOVA analysis was performed. The criterion for statistical significance was p < 0.05.
Results
Aβ1–40 induces inflammatory gene expression in HBEC
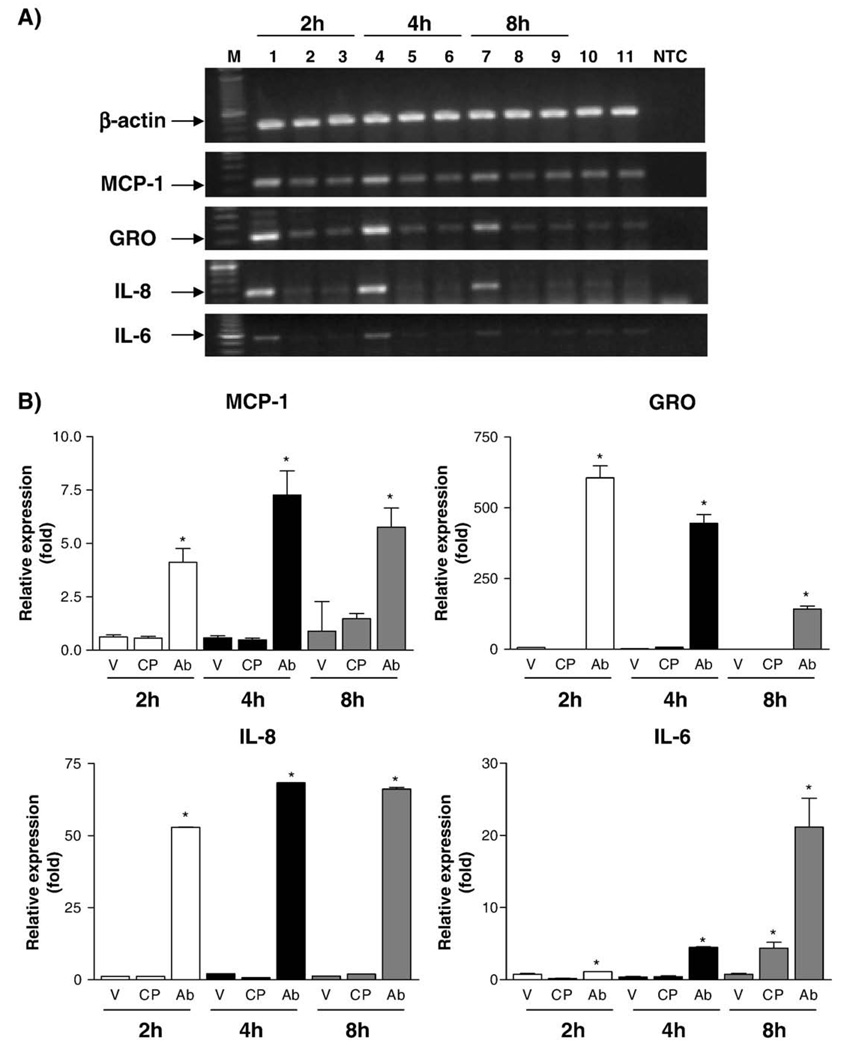
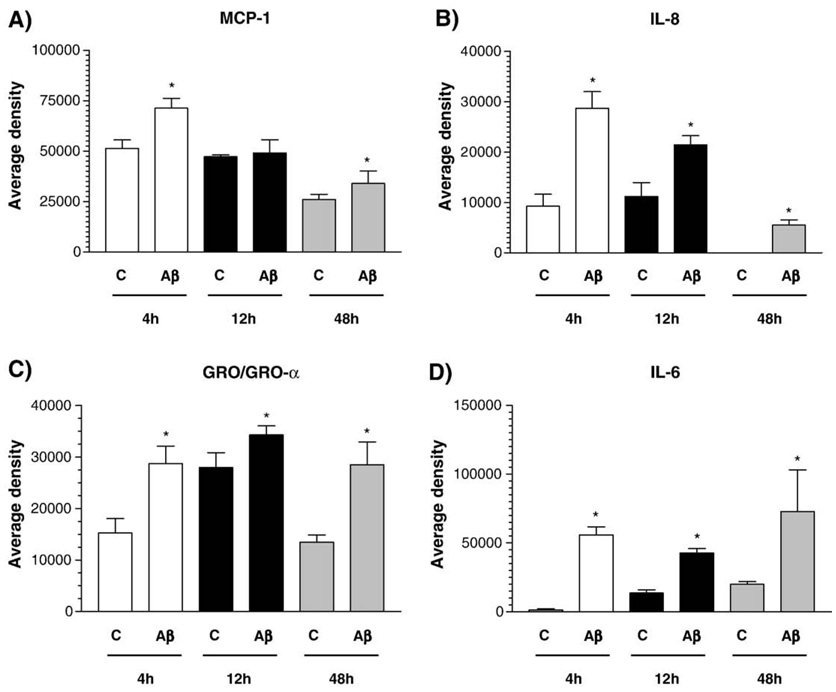
The exposure of primary HBEC to 5 µM Aβ1–40 for 2, 4, and 8 h resulted in increased expression of MCP-1, IL-6, IL-8 and GRO (GRO-α, GRO-β/MIP-2α, and GRO-γ/MIP-2β) genes, normalized to β-actin and compared to control treatments (scrambled Aβ40–1 or vehicle) (Fig. 1A). Increased expression of IL-1β was also observed in Aβ-treated HBEC as we reported previously (Callaghan et al., 2007). Increased expression of these inflammatory genes in Aβ-treated HBEC was confirmed by real-time qRT-PCR (Fig. 1B). Cytokine array analyses showed that the levels of chemokines, MCP-1, IL-6, IL-8 and GRO, released from Aβ-treated HBEC into culture media were significantly increased at 4, 12 and 48 h compared to controls (Fig. 2) with the exception of MCP-1 at 12 h. These results demonstrate that the expression of MCP-1, IL-6, IL-8 and GRO was significantly increased at both the mRNA and/or protein levels in Aβ-treated HBEC compared to controls.
Fig. 1.
The effects of aggregated Aβ1–40 peptides on inflammatory gene expression in HBEC. Panel A: Primary HBEC cultures were treated with 5 µM Aβ1–40 (lanes #1, 4, and 7), 5 µM control peptides (lanes #2, 5, and 8) or 2 mM NaOH (vehicle) (lanes #3, 6, and 9) over 2, 4 and 8 h. The expression of MCP-1, GRO, IL-8 and IL-6 was determined by RT-PCR. The experiment was repeated three times with consistent results. Lane# 10 was cells treated with stale HBEC media. Lane# 11 were cells treated with DMSO in which control peptide was resuspended. NTC: negative control for PCR. Panel B: Real-time qRT-PCR analyses of inflammatory gene expression in HBEC treated with Aβ1–40 peptides. Ab, V, and CP represent cells treated with Aβ, vehicle or control peptides, respectively (one-way ANOVA, p < 0.001).
Fig. 2.
Cytokine array analysis of inflammatory gene expression in HBEC treated with Aβ1–40 peptides. Cytokines and chemokines secreted into media from primary HBEC exposed to Aβ1–40 peptides were analyzed by cytokine arrays at 4,12 and 48 h post-exposure and quantified by using a Kodak Image 1000 system. Panels A–D show quantitative results (average densities) of MCP-1, IL-8, GRO/GRO-α, and IL-6, respectively (one-way ANOVA, *p < 0.001).
The expression of inflammatory genes was up-regulated in AD brain
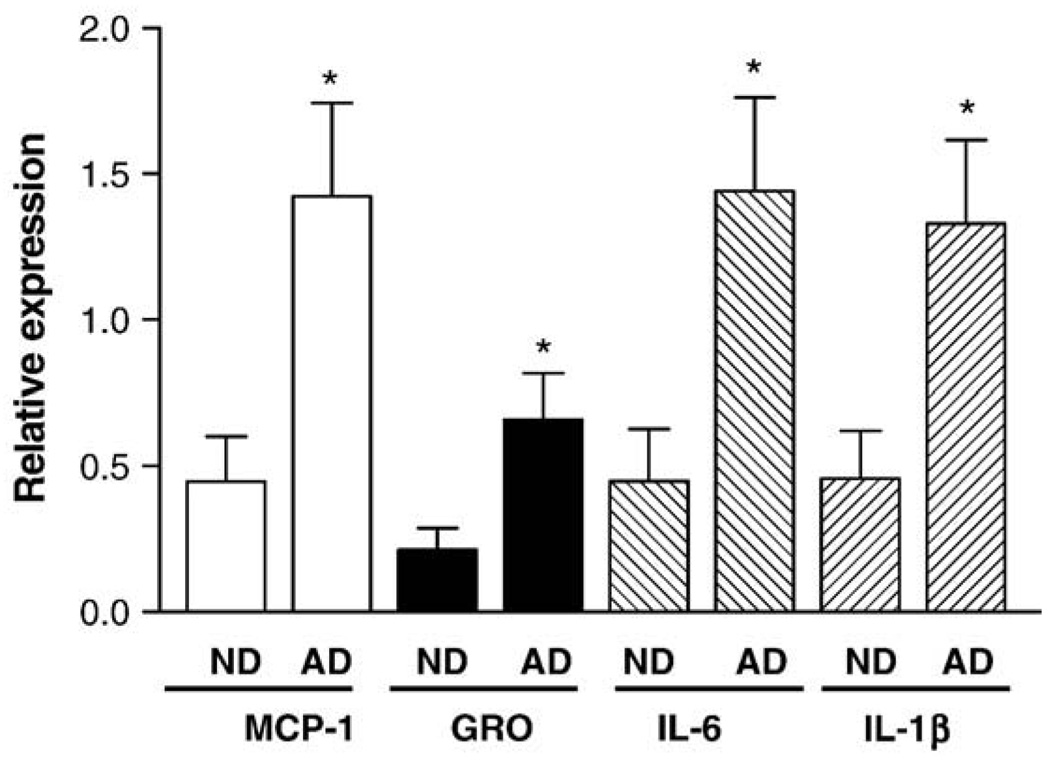
To examine whether genes stimulated by Aβ in HBEC cells were also up-regulated in Alzheimer's brains, RNA samples were isolated from ND, AD and AD/CAA brain tissues and real-time qRT-PCR was performed. The expression of MCP-1, GRO, IL-6, and IL-1β was significantly increased in AD and AD/CAA brains compared to the levels of the genes in ND brains (one-way ANOVA, p < .0021) (Fig. 3). The “AD” samples used in Fig. 3 included both AD and AD/CAA samples. Although variation was observed among different human samples, the expression of the four genes was on average 2–3 fold higher in AD and AD/CAA brains than those in ND brains. The levels of these inflammatory genes were slightly higher in AD than those in AD/CAA but there is no statistical difference (data not shown). The results from cultured HBEC and AD brains demonstrate that the expression of these inflammatory genes was increased in Aβ-treated HBEC, AD and AD/CAA brains.
Fig. 3.
Real-time qRT-PCR analysis of inflammatory gene expression in Alzheimer's brain. The expression of MCP-1, GRO/GRO-α, IL-6 and IL-1β was determined in AD and AD/CAA brains compared to those in age-matched non-demented controls (ND) as described in Materials and methods (one-way ANOVA, *p < 0.0021). The AD brain samples used in the qRT-PCR included both AD and AD/CAA brains.
AP-1 was activated in Aβ-treated HBEC cells and in Alzheimer's brain
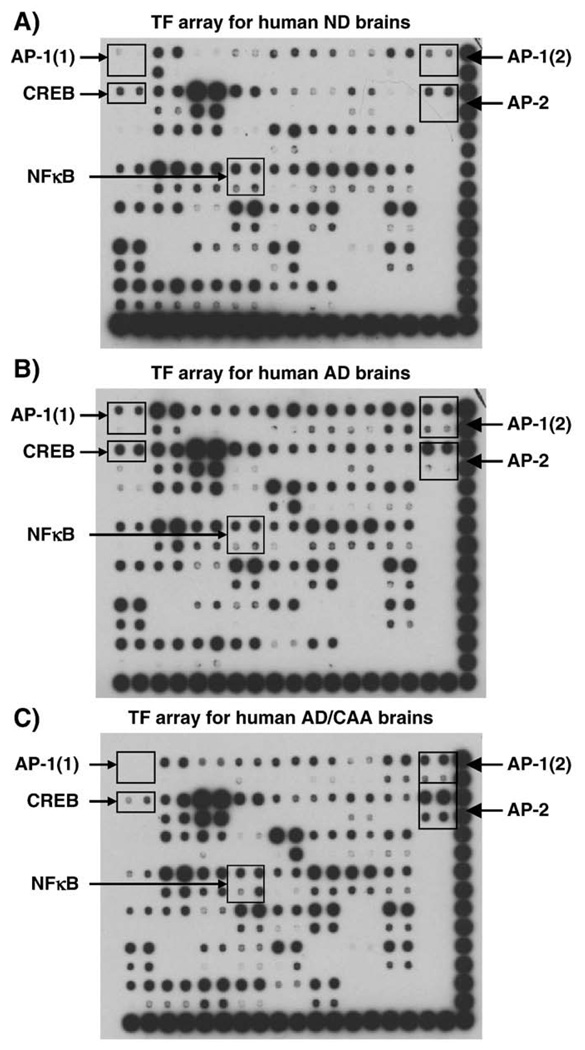
The expression of inflammatory genes is regulated at the transcriptional level by many different transcription factors (TFs). To identify the transcription factors responsible for regulating inflammatory gene expression, TranSignal™ Protein/DNA Array blots were used to profile 54 different TFs in HBEC treated with 5 µM Aβ1–40, 5 µM scrambled Aβ40–1 or vehicle (2 mM NaOH) for 8 h. The arrays show that Aβ treatment of HBEC resulted in over two-fold increase in the activities of AP-1, CREB, NFATc, GRE and GATA when compared to cells treated with control peptide or vehicle (Fig. 4). Interestingly, NFκB was not activated in HBEC cells treated with Aβ peptides (Fig. 4). To validate whether any of these five TFs is also activated in AD brains, the activities of the TFs were profiled in AD, AD/CAA, and ND brain samples. The profiling revealed that AP-1 activity in human AD brains [AP-1(1)] was significantly increased (Fig. 5B) when compared to ND brain samples (Fig. 5A). The TF array blot can also detect the activity of AP-1 binding to a 2nd AP-1 DNA sequence [AP-1(2)]. The difference between the two AP-1 oligos is only one nucleotide as described above. TF array shows that AP-1 was strongly activated in AD (Fig. 5B) and AD/CAA brains [AP-1(2)] (Fig. 5C) compared to ND samples (Fig. 5A). AP-2 was activated in both AD and AD/CAA compared to ND samples (Fig. 5). AP-2 expression in rat primary afferent neurons was induced during acute inflammation, but its role in inflammatory response is still unclear (Donaldson et al., 1995). CREB was activated in AD but not in AD/CAA compared to ND brain samples (Fig. 5). The data indicate that AP-1 was strongly activated in Aβ-treated HBEC cells as well as in AD and AD/CAA brains compared to controls and thus may be involved in the regulation of inflammatory gene expression in HBEC cells and AD brain induced by Aβ peptides. It should be noted that NFκB was not activated in either AD or AD/CAA brains (Fig. 5) as well as in Aβ-treated HBEC (Fig. 4) and thus may not be the principal TF involved in the regulation of inflammatory gene expression in Aβ-induced inflammation.
Fig. 4.
The effects of Aβ1–40 on transcription factor activation in HBEC. Primary HBEC cultures were treated with 5 µM Aβ1–40, 5 µM scrambled Aβ peptides or 2 mM NaOH (vehicle) for 8 h. The levels of five transcription factors AP-1, CREB, GATA, GRE, and NFATc were increased over two-fold in Aβ-treated HBEC as compared to controls and scrambled Aβ-treated cells
Fig. 5.
Activation of transcription factors in AD and AD/CAA brains. Nuclear extracts were isolated from 6 ND, 6 AD, and 6 AD/CAA brain samples, respectively. Equal amounts of nuclear extracts from each ND, AD or AD/CAA were pooled, respectively. The levels of AP-1 and AP-2 activities were increased over two-fold in AD and AD/CAA brains as compared to ND brains. CREB was activated in AD brains but not in AD/CAA brains as compared to ND samples. NFκB was not activated in the any of the brain samples.
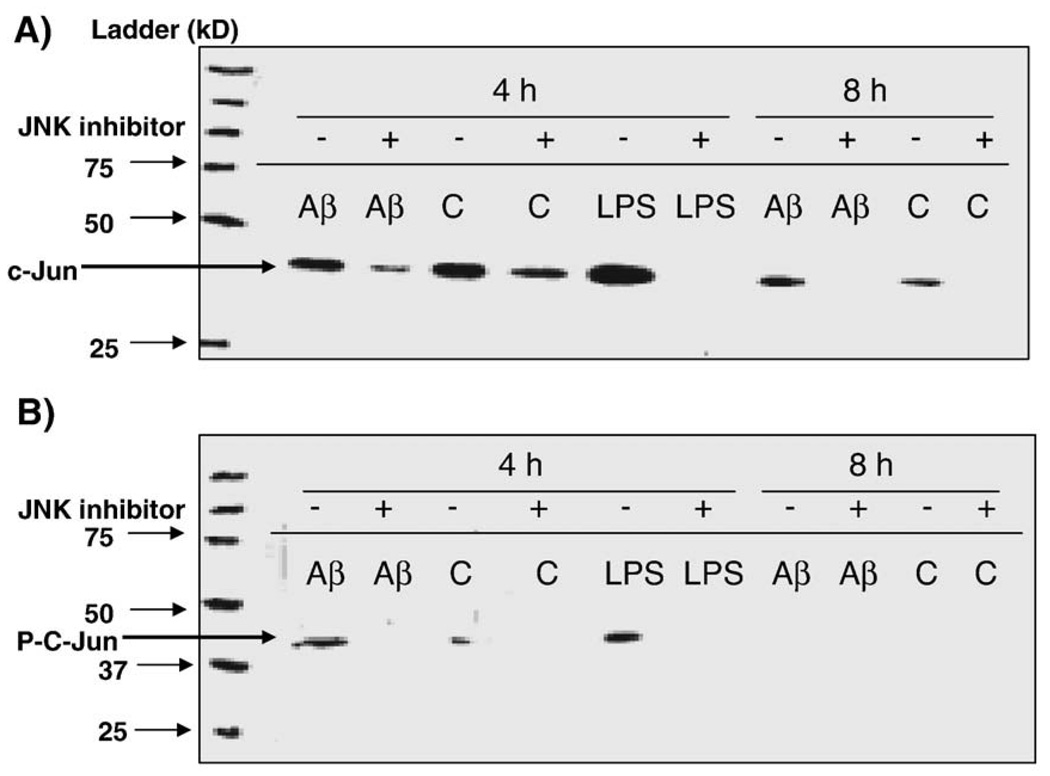
C-Jun expression and activation in HBEC treated with Aβ1–40
AP-1 is a dimeric protein complex composed of Jun (v-Jun, c-Jun, Jun-B, Jun-D), Fos (v-Fos, c-Fos, Fos-B, Fra-1, Fra2) or ATF subunits (Karin et al., 1997). Since c-Jun is a common component of AP-1 protein complex and AP-1 is usually activated as a result of c-Jun phosphorylation (Thakur et al., 2007), we analyzed the effects of Aβ peptides on c-Jun expression and c-Jun phosphorylation in hCMEC/D3 treated with Aβ1–40 peptides. The level of total c-Jun was moderately increased in hCMEC/D3-treated with Aβ at 8 h compared to vehicle-treated cells (Fig. 6A). C-Jun was strongly phosphorylated at serine 73 in Aβ-treated cells (Fig. 6B). The phosphorylation of c-Jun at serine 63 was less affected (data not shown). Since c-Jun N-terminal kinase (JNK) is responsible for activation of c-Jun by phosphorylation, a JNK inhibitor SP600125 was used to test whether c-Jun expression and phosphorylation are inhibited. The inhibitor prevents phosphorylation of JNK substrates by blocking ATP-binding domain of JNKs. As the dual phosphorylation motif of JNK remains unaffected, the inhibitory effects of SP600125 can only be seen by reduction of phosphorylation of JNK substrates, i.e., c-Jun (Duyckaerts et al., 2008). Aβ-induced increase in c-Jun protein was inhibited by SP600125 (Fig. 6A). JNK-mediated phosphorylation of c-Jun at Ser-73was completely inhibited by the JNK inhibitor SP600125 (Fig. 6B). These results suggest that phosphorylation of c-Jun at Ser-73 is responsible for AP-1 activation and validates the direct involvement of JNK signaling pathway in the inflammatory response of iHBEC cells to Aβ peptides.
Fig. 6.
c-Jun expression and phosphorylation in Aβ-treated hCMEC/D3. hCMEC/D3 were pre-incubated with 30 µM JNK inhibitor SP600125 for 60 min and then treated with Aβ1–40, vehicle (2 mM NaOH) or LPS (1:500, positive control) for 4 and 8 h. Nuclear extracts were prepared and used in Western blots. Panel A: Polyclonal rabbit c-Jun antibody (1:500 dilution) was used to detect total c-Jun. LPS was used as a positive control. Panel B: Polyclonal rabbit antibody was used to detect phosphorylated c-Jun at serine 73 (1:500 dilution).
Activated AP-1 complex interacts with AP-1 DNA sequence and activates AP-1 reporter gene activity
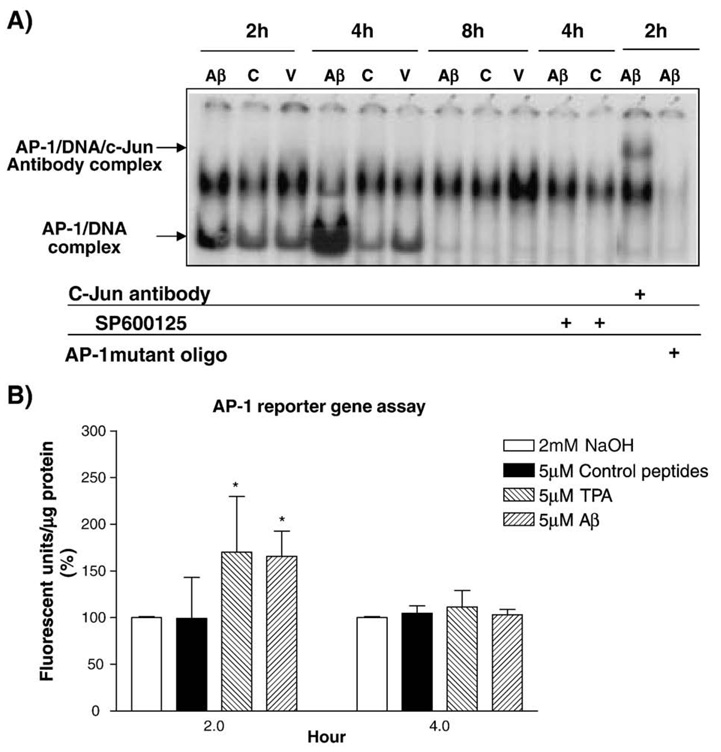
EMSA was used to test the binding of Aβ-activated AP-1 complex to AP-1 DNA-binding sequence. The assay shows that AP-1 in the nuclear extracts isolated from HBEC treated with Aβ for 2 and 4 h was strongly activated and formed an AP-1/DNA complex with the AP-1 binding sequence when compared to 5 µM scrambled Aβ40–1 or vehicle-treated HBEC (Fig. 7A). To further demonstrate that c-Jun is a component of AP-1 complex, a c-Jun antibody was used in the supershift assays by incubating with Aβ-induced HBEC nuclear samples for 30 min. The binding of c-Jun antibody to AP-1/DNA complex shifted the band upward in the gel (Fig. 7A). This analysis confirmed that c-Jun is a component of activated AP-1 protein complex. JNK inhibitor SP600125 was also used to test whether JNK and c-Jun are involved in AP-1 activation. HBEC were pre-incubated with 30 µMSP600125 followed by Aβ-induction for 4 h. EMSA showed that AP-1 activation and DNA binding were completely inhibited by SP600125 (Fig. 7A). The results indicate that AP-1 activation in response to Aβ treatment results from JNK-mediated c-Jun phosphorylation and that JNK signaling pathway is likely involved in Aβ-induced inflammatory gene expression in HBEC.
Fig. 7.
EMSA and reporter gene assays for AP-1 activation stimulated by Aβ peptides. Panel A shows EMSA for physical binding of activated AP-1 protein complex to AP-1 binding DNA sequence. Primary HBEC were treated with 5 µM Aβ1–40, 5 µM control peptide or vehicle (2 mM NaOH) for 2, 4 and 8 h. Some of the HBEC cultures were pre-incubated with 30 µM JNK inhibitor (SP600125) for 60 min before adding Aβ. EMSA and Supershift assays were performed as described in Materials and methods. AP-1 was strongly activated in Aβ-treated HBEC at 2 and4h.AP-1 activation was inhibited by the JNK inhibitor SP600125. Panel B shows reporter gene assays. Cloned AP-1 binding site from human MCP-1 gene in a luciferase pGL-3 promoter reporter gene vector or an empty vector was transfected intoHEK293 cells. The transfectedHEK293 cells were treated with 20 µM TPA, 5µM Aβ1–40, control peptides or vehicle (2 mM NaOH). Luciferase assays shows that AP-1 reporter gene was strongly activated at 2 h in cells treated with TPA or Aβ1–40 peptides and AP-1 activation was strongly inhibited by the JNK inhibitor (t test, p < 0.05).
To demonstrate that the binding of AP-1 complex to AP-1 DNA sequence activates transcription of a target gene, a luciferase reporter gene assay was used. There are two typical AP-1 binding sites (TPA-response elements, TREs) in the promoter region of the human MCP-1 gene. This promoter region was cloned into pGL3 promoter vector. Since the transfection efficiency of iHBEC is extremely low (5–15%), the construct was transiently transfected into HEK293 cells using LipoFectamine. The transfection efficiency of HEK293 cells was 75% (data not shown). The cells were recovered overnight and subsequently treated with 5 µM Aβ1–40, 5 µMcontrol peptide or 2mMNaOH (vehicle) for 2 or 4 h. Aβ peptides significantly induced AP-1 reporter gene activity in HEK293 cells when compared to control peptide- or vehicle-treated cells at 2 h post treatment (Fig. 7B) (p < 0.05). No significant effect was seen at 4 h post treatment (Fig. 7B).
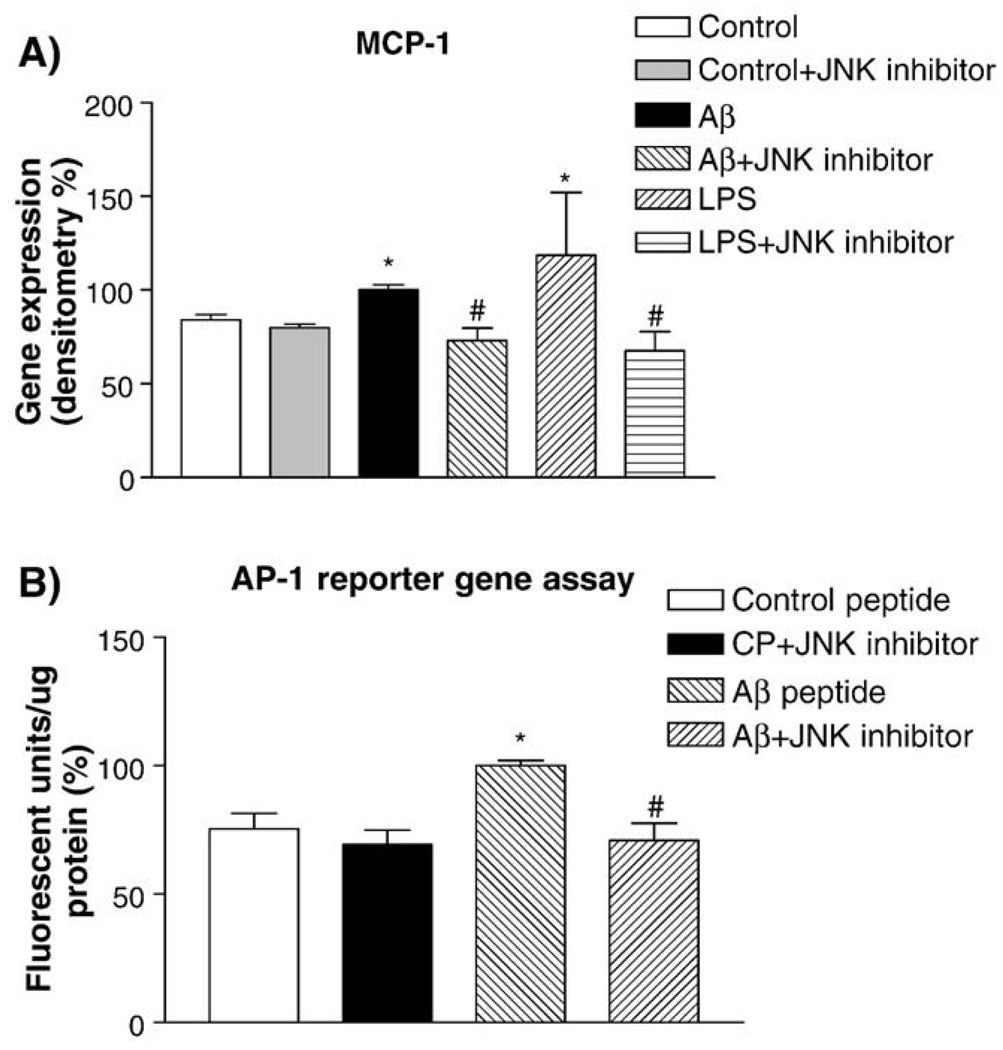
JNK inhibitor SP600125 significantly reduces MCP-1 gene expression in HBEC cells treated with Aβ1–40 peptides
To further test the involvement of JNK signaling pathway in AP-1-mediated regulation of inflammatory gene expression, hCMEC/D3 cells were treated with Aβ1–40 peptides in the presence of the JNK inhibitor. The cells were pre-incubated with 30 µM SP600125 and then treated with 20 µM Aβ1–40 or control peptides for 2 or 4 h. Semi-quantitative RT-PCR analyses showed that MCP-1 gene expression was increased in Aβ-treated hCMEC/D3 when compared to controls (Fig. 8A). The Aβ-stimulated MCP-1 gene expression in hCMEC/D3 was inhibited by SP600125 (Fig. 8A). Densitometry analysis of RT-PCR demonstrated that the MCP-1 gene expression in hCMEC/D3 treated with Aβ was significantly increased compared to vehicle (p < 0.009) and that SP600125 significantly reduced Aβ-stimulated MCP-1 gene expression (p < 0.004) (Fig. 8A). When transfected HEK293 cells were pre-incubated with 30 µM SP600125 and then treated with Aβ peptides, AP-1 reporter gene activity was also significantly reduced (p < 0.05) (Fig. 8B). Inhibitors for p38 kinase were tested and did not affect any of the gene expression (data not shown).
Fig. 8.
The effects of a JNK inhibitor on MCP-1 expression and AP-1 reporter gene activity induced by Aβ peptides. Panel A: hCMEC/D3 cultures were treated with Aβ1–40 or control peptides for 4 h in the presence or absence of the JNK inhibitor SP600125 (30 µM). Lipopolysaccharide (LPS) was used as a positive control. MCP-1 expression in hCMEC/D3 induced by Aβ1–40 or LPS was inhibited by JNK (*p < 0.01 as compared controls; #p < 0.01 as compared to Aβ- or LPS treated cells). Panel B: The effect of the JNK inhibitor SP600125 on AP-1 reporter gene assays in HEK293 cells treated with Aβ1–40 peptides (*p < 0.01 as compared to control; # p < 0.01 as compared to Aβ-treated cells).
Discussion
Alzheimer's disease is a multifaceted neurodegenerative disease. One of the important mechanisms leading to neurodegenerative changes in Alzheimer's brain is neuroinflammation, including neurovascular inflammation. Up-regulation of inflammatory mediators has been found in AD brain (McGeer and McGeer, 2001, 2004). However, the molecular mechanisms of the inflammation in AD brain still remain largely unknown. We have demonstrated in this study that Aβ1–40 peptides up-regulate the expression of inflammatory genes in HBEC and these genes are also up-regulated in AD brain and that this Aβ-stimulated up-regulation of inflammatory gene expression in HBEC and AD brain is mediated by the JNK-AP1 signaling pathway. This is supported by the following evidence from our study: 1) application of Aβ1–40 peptides to HBEC cells triggered the JNK signaling pathway resulting in phosphorylation of c-Jun; 2) c-Jun is a component of the activated AP-1 protein complex in Aβ-treated HBEC cells, and phosphorylation of c-Jun by JNK activates AP-1, which binds to AP-1-binding DNA sequence and activates AP-1 reporter gene activity (the vector carries AP-1-binding site from human MCP-1 gene); 3) AP-1was activated in AD and AD/CAA brains and in Aβ-treated HBEC cells; 4) activated AP-1 up-regulated the expression of inflammatory genes (such as MCP-1) in cells; 5) up-regulation of inflammatory genes (MCP-1, GRO, IL-6 and IL-1β) was found in AD and AD/CAA brains and in Aβ-treated HBEC cells; 6) many inflammatory genes (MCP-1, IL-8, IL-6 and GRO) carry AP-1-binding sites in their promoter regions (Ben-Baruch et al., 1995; Kick et al., 1995; Murayama et al., 1997; Walpen et al., 2001); and 7) the JNK inhibitor SP600125 strongly inhibited c-Jun phosphorylation/AP-1 activation, MCP-1 expression and AP-1 reporter gene activity in cells treated with Aβ peptides.
Accumulation and deposition of Aβ peptides in the brain is a hallmark of Alzheimer's disease. Aβ peptides aggregate to form fibrillar deposits, the principal component of senile plaques, which triggers inflammatory reactions and activates microglia in AD brain. In vitro and in vivo studies have suggested that the resident phagocytes, microglia, are the major players of Aβ-triggered inflammation in AD brain. Microglia activated by small doses of aggregated Aβ1–42 in vitro secrete inflammatory cytokines, including MCP-1, TNF-α, IL-8 and IL- 1β (Araujo and Cotman, 1992; Meda et al., 1995; Chao et al., 1994; Walker and Lue, 2003; Walker et al., 2001, 2006; Walker and Lue, 2005). Similarly, activated microglia are consistently associated with senile plaques in AD brain (Mackenzie et al., 1995). Microglia also respond to Aβ deposits in brain through activation of tyrosine kinase-based intracellular signal transduction cascades involving Lyn, Syk, FAK, and Pyk2 (McDonald et al., 1997, 1998; Combs et al., 1999, 2000) leading to induction of pro-inflammatory gene expression, such as TNF-α and IL-6 (Combs et al., 2000; Davis, 2000), and production of reactive oxygen and nitrogen species. As a result, these inflammatory products, acting in concert, produce neuronal toxicity and death (Bamberger and Landreth, 2001). In vitro studies show that Aβ peptides generate oxidative stress in neurons by activating NFκB and inducing expression of macrophage-colony stimulating factor (M-CSF) (Yan et al., 1997). M-CSF released by neurons stimulates its receptors, c-fms, on microglia inducing activation of macrophage scavenger receptor and ApoE (Yan et al., 1997). Aβ1–42 peptides also activate astrocytes resulting in activation of NFκB and production of iNOS (Davis, 2000). Astrocytes in AD brains secrete IL-1, IL-6 and transforming growth factor β (TGF-β) (Ata et al., 1997; Del Bo et al., 1995). It appears that NFκB and the relevant signaling pathways are activated by Aβ peptides in cultured microglia, neuronal cells and astrocytes to trigger inflammatory responses. In contrast, TF array analyses performed in this study revealed that NFκB was not activated either in AD and AD/CAA brains or in cultured HBEC treated with Aβ peptides. Interestingly, these inflammatory genes (MCP-1, GRO, IL-6 and IL-8) up-regulated in AD brains and Aβ-treated HBEC cells carry NFκB-binding sites in their promoter regions (Ben-Baruch et al., 1995; Kick et al., 1995; Murayama et al., 1997;Walpen et al., 2001). Our data suggests that NFκB is not a major transcription factor responsible for up-regulating the expression of these inflammatory genes in AD brain and HBEC stimulated by Aβ peptides. There are several explanations about the differences between our and others' observations: 1) the differences of cultured microglial cells vs. human Alzheimer's brain tissues; 2) treatment of cultured microglial cells with Aβ peptides (usually with Aβ1–42 peptides) results in an acute inflammatory response, while the inflammatory response in Alzheimer's brain is a chronic and possibly mild process; 3) Since the peptides deposited in cerebral vessels are mostly Aβ1–40 peptides, we used Aβ1–40 peptides in this study. Aβ1–42 peptides form high-molecular aggregates, while Aβ1–40 peptides form low-molecular weight oligomers. Aβ1–42 is much stronger than Aβ1–40 in stimulating inflammatory response. Thus, AP-1 may be more responsive to mild and chronic stimulus, while NFκB may be more responsive to stronger and acute stimulus.
The majority of AD patients have Aβ deposition in cerebral microvessels, which affects vascular function and results in vascular inflammation. Brain endothelial cells, like microglia and astrocytes, are also involved in the inflammation observed in AD (Griffin and Stanley, 1993). Little is done, however, on characterization of brain endothelial cells for their involvement if any in the inflammatory response. Suo et al. (1998) attempted to study the effect of Aβ peptides in brain endothelial cells by using a cell line from human aortic endothelial cells and by manipulating it with different factors, such as bovine brain extract to mimic brain environment. This model has many variable parameters and limitations to validate the true effect of Aβ1–40 on brain endothelial cells (BEC). Instead, we have used both primary and immortalized HBEC cultures as an in vitro model and treated the cells with Aβ peptides. These HBEC cultures have been well characterized and described previously (Zhang et al., 1999, 2000, 2003; Weksler et al., 2005). Deposition of Aβ peptides on HBEC cells stimulated the expression of MCP-1, GRO, IL-1β, IL-6, and IL-8. Up-regulation of MCP-1, GRO, IL-1β, and IL-6 has been confirmed in both AD and AD/CAA brain samples. This demonstrates that the inflammatory response induced by Aβ peptides in HBEC is similar to that in Alzheimer's brain. Neuroinflammation in Alzheimer's disease is a chronic inflammatory response to aggregated Aβ peptides and amyloid plaques. It appears that MCP-1 is a key player in this Aβ-induced inflammatory response since the expression of MCP-1 is significantly increased in Alzheimer's brain and HBEC treated with Aβ peptides. MCP-1 attracts monocytes from peripheral blood to transmigrate across the BBB to the inflammatory site in the brain and plays an important part in Alzheimer's inflammatory response (Nagele et al., 2004; Britschgi and Wyss-Coray 2007; El Khoury et al., 2007). These monocytes are converted to microglia at the inflammatory site (Nagele et al., 2004; El Khoury et al., 2007). In contrast, IL-1β is a key pro-inflammatory mediator in Aβ-induced inflammatory response. IL-1β is significantly up-regulated in Alzheimer's brain and Aβ-treated HBEC (Callaghan et al., 2007). IL-1β is capable of up-regulating the expression of MCP-1 in HBEC and astrocytes (Zhang et al., 1999, 2000).
Transcription factors are known to be located at the end of signaling pathways and once activated, bind to the promoter regions of target genes and regulate their expression in response to various stimuli by either increasing or decreasing gene transcription. In contrast to NFκB, AP-1 was strongly activated in Aβ-treated HBEC cells and in both AD and AD/CAA brains. Inflammatory genes found to be up-regulated by Aβ in HBEC and in AD brain (including MCP-1, IL-8, IL-6 and GRO) carry both AP-1 and NFκB binding sites in their promoter regions (Ben-Baruch et al., 1995; Kick et al., 1995; Murayama et al., 1997; Walpen et al., 2001). Both AP-1 and NFκB can regulate the expression of these genes, but only AP-1 was found to be activated. CREB (cyclic-AMP response element binding protein) activity was also increased in Aβ-treated HBEC and AD brain but not in AD/CAA brain. CREB is known to be activated by various extracellular stimuli and regulate the expression of genes important to cell proliferation, differentiation, adaptation, and survival in many cell types. Some of the genes involving inflammatory process (such as COX-2) are regulated by CREB. CREB may be thus a minor player in the inflammatory response evoked by Aβ peptides. Since only AP-1 was activated in Aβ-treated HBEC and in AD and AD/CAA brain, it suggests that AP-1 is a principal transcription factor involved in the regulation of inflammatory gene expression in Aβ-induced Alzheimer's neuroinflammation and neurovascular inflammation.
Various studies support the importance of AP-1 in inflammatory responses (Cho et al., 2002;Wang et al.,1999; Neff et al., 2001; Swantek et al.,1997; Tyt et al.,1999). AP-1 is a dimeric protein complex. Multiple signaling pathways are known to activate AP-1, including ERK-1/2, JNK, p38 kinase, and PI-3 kinase pathways. Evidence from this study shows that c-Jun is a component of the activated AP-1 complex and that c-Jun phosphorylation activates AP-1 suggests that the JNK signaling pathway is responsible for AP-1 activation. This was supported by the use of a JNK-specific inhibitor, SP600125, which inhibited AP-1 activation and MCP-1 expression. The application of p38 kinase inhibitors did not affect MCP-1 expression in Aβ-treated HBEC in this study (data not shown). Hensley et al. (1999) reported that p38 kinase is activated in Alzheimer's brain. AP-1 is located at the end of p38 kinase signaling pathway. The fact that p38 kinase inhibitors did not affect MCP-1 expression in Aβ-treated HBEC cells does not mean that p38 kinase signaling pathway is not activated in Alzheimer's brain. Further research work is needed to investigate whether activation of p38 kinase signaling pathway in Alzheimer's brain is one of the factors responsible for AP-1 activation.
JNK is a major cellular stress response protein induced by oxidative stress and plays an important role in Alzheimer's disease (Zhu et al., 2001a). Several lines of evidence indicate the involvement of JNK in Alzheimer's disease: 1) Aβ peptides induce JNK signaling which mediates Aβ toxicity and adverse effects on long-term potentiation in the hippocampus (Bozyczko-Coyne et al., 2001; Morishima et al., 2001; Troy et al., 2001; Wei et al., 2002; Minogue et al., 2003); 2) JNK phosphorylates tau protein in a manner similar to that of paired helical filaments (PHF)-tau in AD (Reynolds et al., 2000). Activated JNK was found in the hippocampal and cortical regions of individuals with severe AD and localized with neurofibrillar alterations (Zhu et al., 2001a, 2001b). JNK activation is considered an early event in Alzheimer's disease (Zhu et al., 2001a). Activated JNK is located in nucleus in mild AD cases, but is exclusively in cytoplasm in more advanced stages of AD, suggesting that activation and re-distribution of JNK correlates with the progress of Alzheimer's disease (Zhu et al., 2001a, b). Thework of Reynolds et al. and Zhu et al. suggested that JNK activation was related to the tau-pathology of neurofibrillary tangles; 3) JNK's upstream activator JKK1 is activated in vulnerable neurons in AD (Zhu et al., 2003); and 4) Marcus et al. reported that there were c-Jun-positive and c-Fos-positive neurons in nearly all AD hippocampal regions (Marcus et al., 1998). However, there was no indication in the literature that the JNK-AP1 signaling pathway is involved in Aβ-induced Alzheimer's neuroinflammation. The observation of Zhu et al. (2003) that JKK1 is activated in AD supports our finding that JNK-AP1 signaling pathway is activated in AD and JNK inhibitor blocks the signaling pathway. Giri et al. (2003) showed that Aβ peptides at physiological concentration triggered cellular signaling pathway in THP-1 monocytes and increased the gene expression of specific pro-inflammatory factors, such as TNF-α, IL-1, IL-8, and MCP-1. This signaling pathway involved activation of tyrosine kinase and extracellular signal-regulated kinase (ERK-1 and ERK-2), but not p38. The activation of JNK results in phosphorylation of c-Jun on residues Ser63 and Ser73 (Whitmarsh and Davis, 1996). Phosphorylation on these sites also leads to inhibition of c-Jun ubiquitination and degradation. There are three JNKs (JNK1, 2, and 3) in cells, among which JNK2 shows the highest affinity for c-Jun since it contains the putative loop region which interacts with JNK-docking site on c-Jun. JNK is activated by environmental insults, such as, osmotic shock, ultraviolet (UV) irradiation, pH changes and reactive oxygen species (ROS), inflammatory stimuli (antigens, cytokines and infection), and growth factors (Derijard et al., 1994; Waetzig et al., 2005). Differential phosphorylation of serine residues on c-Jun protein in response to various stimuli was observed (Thakur et al., 2007). Thakur et al. found that Ser73-phosphorylated c-Jun is more prominent than Ser63-phosphorylated c-Jun; but others reported that Ser63 and not Ser73 is phosphorylated (Pearson et al., 2006). These studies looked at cell death but not inflammation. Our study shows that interaction of Aβ peptides with cells activates JNK, which in turn phosphorylates c-Jun at Ser73, leading to AP-1 activation and MCP-1 expression.
It is known that oxidative stress evokes inflammatory response in cells. Deposition of Aβ peptides generates oxidative stress (ROS) which may activate JNK signaling in cells. Aβ peptides may also interact with RAGE (receptor for advanced glycation end products) on cell membrane leading to generation of oxidative stress (Giri et al., 2000). We observed RAGE expression in iHBEC cells by Western blots (data not shown). Oxidative stress generated by Aβ deposition or Aβ interaction with RAGE likely occurs prior to inflammation observed in Alzheimer's brain (Nunomura et al., 2001; Odetti et al., 1998). The involvement of the JNK-AP1 signaling pathway in Aβ-induced inflammatory response is supported by the observation from our study that JNK inhibitor inhibited c-Jun phosphorylation, AP-1 activation and MCP-1 expression. The involvement of the JNK-AP1 in Aβ-evoked Alzheimer's neuroinflammation may have therapeutic relevance for an anti-inflammatory strategy.
In summary, this study yielded several important findings: 1) AP-1, but not NF-κB, is activated in Aβ-treated HBEC and in AD and AD/CAA brains; 2) AP-1 is activated as a result of c-Jun phosphorylation; 3) c- Jun phosphorylation is mediated by JNK; 4) activation of the JNK-AP1 signaling pathways results in increased expression of inflammatory genes, such as MCP-1; and 5) JNK inhibitor inhibited Aβ-induced c-Jun phosphorylation, AP-1 activation and MCP-1 expression in HBEC. The results suggest that activation of the JNK-AP1 signaling pathway is a common mechanism of inflammatory response in HBEC and Alzheimer's brain evoked by Aβ peptides. These findings may provide potential therapeutic targets in controlling Aβ-induced neuroinflammation in Alzheimer's disease.
Acknowledgments
The authors would like to thank Dr. A. Prat at the Montreal Neurological Institute for providing the primary HBEC, Aimee Jones for maintaining the HBEC cultures, Dr. Huaqi Xiong for helping with the plasmid DNA preparation, and Dr. Stewart Whitman (University of Ottawa Heart Institute) for his helpful comments on the work. This study was supported in part by funding from the Canadian Research Program “Vascular Health and Dementia” sponsored by the Heart and Stroke Foundation of Canada, Canadian Institute of Health Research, Alzheimer Society of Canada, and Pfizer and by funding from the National Research Council of Canada. The research work in Dr. Lih-Fen Lue's laboratory is supported by a NIH RO1 grant (NS049075-01A1).We are grateful to the Sun Health Research Institute Brain Donation Program of Sun City, Arizona for the provision of human brain tissues. The Brain Donation Program is supported by the National Institute on Aging (P30 AG19610 Arizona Alzheimer's Disease Core Center), the Arizona Department of Health Services (contract 211002, Arizona Alzheimer's Research Center), the Arizona Biomedical Research Commission (contracts 4001, 0011 and 05-901 to the Arizona Parkinson's Disease Consortium) and the Prescott Family Initiative of the Michael J. Fox Foundation for Parkinson's Research.
References
- Akama KT, Van Eldik LJ. B-amyloid stimulation of inducible nitric-oxide synthase in astrocytes is interleukin-1β- and tumor necrosis factor-α (TNF-α)-dependent, and involves a TNF-α receptor-associated factor- and NF-κB-inducing kinase-dependent signaling mechanism. J. Biol. Chem. 2000;275:7918–7924. doi: 10.1074/jbc.275.11.7918. [DOI] [PubMed] [Google Scholar]
- Akiyama H, Barger S, Barnum S, Bradt B, Bauer J, Cole GM, Cooper NR, Eikelenboom P, Emmerling M, Fiebich B, Finch CE, Frautschy S, Griffin WST, Hampel H, Hull M, Landreth G, Lue LF, Mrak R, Mackenzie IR, McGeer PLO, Banion MK, Patcher J, Pasinetti G, Plata-Salaman C, Rogers J, Rydel R, Shen Y, Streit W, Strohmeyer R, Tooyama I, Van Muiswinkel FL, Veerhuis R, Walker D, Webster S, Wegrzyniak B, Wenk G, Wyss-Corat T. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo DM, Cotman CW. Beta-amyloid stimulates glial cells in vitro to produce growth factors that accumulate in senile plaques in Alzheimer's disease. Brain Res. 1992;569:141–145. doi: 10.1016/0006-8993(92)90380-r. [DOI] [PubMed] [Google Scholar]
- Ata AK, Funa K, Ollsson Y. Expression of various TGF-β isoforms and type I receptor in necrotizing human brain lesions. Acta Neuropathol. 1997;93:326–333. doi: 10.1007/s004010050623. [DOI] [PubMed] [Google Scholar]
- Bamberger ME, Landreth GE. Microglial interaction with beta-amyloid: implications for the pathogenesis of Alzheimer's disease. Microsc. Res. Tech. 2001;54:59–70. doi: 10.1002/jemt.1121. [DOI] [PubMed] [Google Scholar]
- Ben-Baruch A, Michiel DF, Oppenheim JJ. Signals and receptors involved inrecruitments of inflammatory cells. J. Biol. Chem. 1995;270:11703–11706. doi: 10.1074/jbc.270.20.11703. [DOI] [PubMed] [Google Scholar]
- Britschgi M, Wyss-Coray T. Immune cells may fend off Alzheimer disease. Nat. Med. 2007;13:408–409. doi: 10.1038/nm0407-408. [DOI] [PubMed] [Google Scholar]
- Bozyczko-Coyne D, O'Kane TM, Wu ZL, Dobrzanski P, Murthy S, Vaught JL, Scott RW. CEP-1347/KT-7515, an inhibitor of SAPK/JNK pathway activation, promotes survival and blocks multiple events associated with Aβ-induced cortical neuron apoptosis. J. Neurochem. 2001;77:849–863. doi: 10.1046/j.1471-4159.2001.00294.x. [DOI] [PubMed] [Google Scholar]
- Callaghan D, Vukic V, Jones A, Walker D, Lue LF, Woulfe J, Beach TG, Sue L, Stanimirovic D, Zhang W. Expression and regulation of inflammatory genes in human cerebrovascular endothelial cells induced by Aβ peptides. In: Iqbal K, Winblad B, Avila J, Medimond SRL, editors. Alzheimer's Disease: New Advances. Bologna, Italy: 2007. pp. 83–87. [Google Scholar]
- Chao CC, Hu S, Kravitz FH, Tsang M, Anderson WR, Peterson PK. Transforming growth factor-beta protects human neurons against beta-amyloid-induced injury. Mol. Chem. Neuropathol. 1994;23:159–178. doi: 10.1007/BF02815409. [DOI] [PubMed] [Google Scholar]
- Cho NH, Seong SY, Huh M-S, Kim N-H, Choi M-S. Induction of the gene encoding macrophage chemoattractant protein-1 by orientia tsutsugamushi in human endothelial cells involves activation of transcription factor activator protein 1. Infection and Immunity. 2002;70:4841–4850. doi: 10.1128/IAI.70.9.4841-4850.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DJ, Cannady SB, Lehman TM, Landreth GE. Identification of microglial signal transduction pathways mediating a neurotoxic response to amyloidogenic fragments of β-amyloid and prion proteins. J. Neurosci. 1999;19:928–939. doi: 10.1523/JNEUROSCI.19-03-00928.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Combs CK, Johnson DE, Karlo JC, Cannady SB, Landreth GE. Inflammatory mechanisms in Alzheimer's disease: inhibition of beta-amyloid stimulated proinflammatory responses and neurotoxicity by PPARgamma agonists. J. Neurosci. 2000;20:558–567. doi: 10.1523/JNEUROSCI.20-02-00558.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Craft JM, Watterson DM, Van Eldik LJ. Human amyloid beta-induced neuroinflammation is an early event in neurodegeneration. Glia. 2006;53:484–490. doi: 10.1002/glia.20306. [DOI] [PubMed] [Google Scholar]
- Davis R. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- Del Bo R, Angeretti N, Lucca E, De Simoni MG, Forloni G. Reciprocal control of inflammatory cytokines, IL-1 and IL-6, and β amyloid production in cultures. Neurosci. Lett. 1995;188:70–74. doi: 10.1016/0304-3940(95)11384-9. [DOI] [PubMed] [Google Scholar]
- Derijard B, Hibi M, Wu I-H, Barrett T, Su B, Deng T, Karin M, Davis RJ. JNK1: a protein kinase stimulated by UV light and H-Ras that binds and phosphorylates the c-Jun activation domain. Cell. 1994;76:1020–1028. doi: 10.1016/0092-8674(94)90380-8. [DOI] [PubMed] [Google Scholar]
- Donaldson LF, McQueen DS, Seckl JR. Induction of transcription factor AP2 mRNA expression in rat primary afferent neurons during acute inflammation. Neurosci. Lett. 1995;196:181–184. doi: 10.1016/0304-3940(95)11870-3. [DOI] [PubMed] [Google Scholar]
- Duyckaerts C, Potier MC, Delatour B. Alzheimer's disease model and neuropathology: similarities and differences. Acta Neuropathol. 2008;115:5–38. doi: 10.1007/s00401-007-0312-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eikelenboom P, Bate C, Van Gool WA, Hoozemans JJ, Rozemuller JM, Veerhuis R, Williams A. Neuroinflammation in Alzheimer's disease and prion disease. Glia. 2002;40:232–239. doi: 10.1002/glia.10146. [DOI] [PubMed] [Google Scholar]
- El Khoury J, Toft M, Hickman SE, Means TK, Terada K, Geula C, Luster AD. Ccr2 deficiency impairs microglial accumulation and accelerates progression of Alzheimer-like disease. Nat. Med. 2007;13:432–438. doi: 10.1038/nm1555. [DOI] [PubMed] [Google Scholar]
- Giri R, Shen Y, Stins M, Du Yan S, Schmidt AM, Stern D, Kim KS, Zlokovic B, Kalra VY. β-amyloid-inducedmigration ofmonocytes across human brain endothelial cells involves RAGE and PECAM-1. Am. J. Physiol. Cell. Physiol. 2000;279:1772–1781. doi: 10.1152/ajpcell.2000.279.6.C1772. [DOI] [PubMed] [Google Scholar]
- Giri RK, Selvaraj SK, Kalra VK. Amyloid peptide-induced cytokine and chemokine expression in THP-1 monocytes is blocked by small inhibitory RNA duplexes for early growth response-1 messenger RNA. J. Immun. 2003;170:5281–5294. doi: 10.4049/jimmunol.170.10.5281. [DOI] [PubMed] [Google Scholar]
- Grammas P, Ovase R. Inflammatory factors are elevated in brain microvessels in Alzheimer's disease. Neurobiol. Aging. 2001;22:837–842. doi: 10.1016/s0197-4580(01)00276-7. [DOI] [PubMed] [Google Scholar]
- Griffin WST, Stanley LC. In: Biology and Pathology of Astrocyte-Neuron Interactions. Federoff S, Juurlink BHJ, Doucette R, Burkholder G, editors. New York: Plenum Press; 1993. pp. 359–381. [Google Scholar]
- Griffin WS, Sheng JG, Roberts GW, Mrak RE. Interleukin-1 expression in different plaque types in Alzheimer's disease: significance in plaque evolution. J. Neuropathol. Exp. Neurol. 1995;54:276–281. doi: 10.1097/00005072-199503000-00014. [DOI] [PubMed] [Google Scholar]
- Haga S, Akai K, Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. Acta Neuropathol. 1989;77:569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- Hensley K, Floyd RA, Zheng NY, Nael R, Robinson KA, Nguyen X, Pye QN, Stewart CA, Geddes J, Markesbery WR, Patel E, Johnson GV, Bing G. kinase is activated in the Alzheimer's disease brain. J. Neurochem. 1999;72:2053–2058. doi: 10.1046/j.1471-4159.1999.0722053.x. [DOI] [PubMed] [Google Scholar]
- Ho GJ, Drego R, Hakimian E, Masliah E. Mechanisms of cell signaling and inflammation in Alzheimer's disease. Curr. Drug Targets Inflamm. Allergy. 2005;4:247–256. doi: 10.2174/1568010053586237. [DOI] [PubMed] [Google Scholar]
- Hull M, Strauss S, Berger M, Volk B, Bauer J. The participation of interleukin-6, a stress-inducible cytokine, in pathogenesis of Alzheimer's disease. Behav. Brain Res. 1996;78:37–41. doi: 10.1016/0166-4328(95)00213-8. [DOI] [PubMed] [Google Scholar]
- Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J. Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- Karin M, Liu Z-G, Zandi I. AP-1 function and regulation. Curr. Opin. Cell. Biol. 1997;9:240–246. doi: 10.1016/s0955-0674(97)80068-3. [DOI] [PubMed] [Google Scholar]
- Kick G, Messer G, Goetz A, Plewig G, Kind P. Photodynamic therapy induces expression of interleukin 6 by activation of AP-1 but not NF-B DNA binding. Cancer Res. 1995;55:2373–2379. [PubMed] [Google Scholar]
- Mackenzie IR, Hao C, Munoz DG. Role of microglia in senile plaque formation. Neurobiol. Aging. 1995;16:797–804. doi: 10.1016/0197-4580(95)00092-s. [DOI] [PubMed] [Google Scholar]
- Malinski T. Nitric oxide and nitroxidative stress in Alzheimer's disease. J. Alzheimers Dis. 2007;11:207–218. doi: 10.3233/jad-2007-11208. [DOI] [PubMed] [Google Scholar]
- Marcus DL, Strafaci JA, Miller DC, Masia S, Thomas CG, Rosman J, Hussain S, Freedman ML. Quantitative neuronal c-fos and c-jun expression in Alzheimer's disease. Neurobiol. Aging. 1998;19:393–400. doi: 10.1016/s0197-4580(98)00077-3. [DOI] [PubMed] [Google Scholar]
- Mattiace LA, Davies P, Dickson DW. Detection of HLA-DR on microglia in the human brain is a function of both clinical and technical factors. Am. J. Pathol. 1990;136:1101–1114. [PMC free article] [PubMed] [Google Scholar]
- McDonald DR, Bruden KR, Landreth GE. Amyloid fibrils activate tyrosinekinase dependent signaling and superoxide production in microglia. J. Neurosci. 1997;17:2284–2294. doi: 10.1523/JNEUROSCI.17-07-02284.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald S, Bamberger M, Combs C, Landreth G. B-amyloid fibrils activate parallel mitogen-activated protein kinase pathways in microglia and THP-1 monocytes. J. Neurosci. 1998;18:4451–4460. doi: 10.1523/JNEUROSCI.18-12-04451.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation, autotoxicity and Alzheimer's disease. Neurobiol. Aging. 2001;22:799–809. doi: 10.1016/s0197-4580(01)00289-5. [DOI] [PubMed] [Google Scholar]
- McGeer PL, McGeer EG. Inflammation and degenerative disease of aging. Ann. N. Y. Acad. Sci. 2004;1035:104–116. doi: 10.1196/annals.1332.007. [DOI] [PubMed] [Google Scholar]
- Meda L, Cassatella MA, Szendrei GI, Otvos L, Baron P, Vilalba M, Ferrari D, Rossi F. Activation of microglial cells by β-amyloid protein and γ-interferon. Nature. 1995;374:647–650. doi: 10.1038/374647a0. [DOI] [PubMed] [Google Scholar]
- Minogue AM, Schmid AW, Fogarty MP, Moore AC, Campbell VA, Herron CE, Lynch MA. Activation of the JNK signaling cascade mediates the effect of amyloid-beta on LTP and cell death in hippocampus: a role for IL-1β. J. Biol. Chem. 2003;278:27971–27980. doi: 10.1074/jbc.M302530200. [DOI] [PubMed] [Google Scholar]
- Morishima Y, Gotoh Y, Zieg J, Barrett T, Takano H, Flavell R, Davis RJ, Shirasaki Y, Greenberg ME. Beta-amyloid induces neuronal apoptosis via a mechanism that involves the c-Jun N-terminal kinase pathway and the induction of Fas ligand. J. Neurosci. 2001;21:7551–7560. doi: 10.1523/JNEUROSCI.21-19-07551.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murayama T, Ohara Y, Obuchi M, Khabar KS, Higashi H, Mukaida N, Matsushima K. Human cytomegalovirus induces interleukin-8 production by a human monocytic cell line, THP-1, through acting concurrently on AP-1- and NF-kappaB-binding sites of the interleukin-8 gene. J. Virol. 1997;71:5692–5695. doi: 10.1128/jvi.71.7.5692-5695.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagele RG, Wegiel J, Venkataraman V, Imaki H, Wang KC, Wegiel J. Contribution of glial cells to the development of amyloid plaques in Alzheimer's disease. Neurobiol. Aging. 2004;25:663–674. doi: 10.1016/j.neurobiolaging.2004.01.007. [DOI] [PubMed] [Google Scholar]
- Neff L, Zeiss M, Sibilia J, Scholler-Guinard M, Klein JP, Wachsmann D. NF-kappaB and MAP kinases/AP-1 pathways are both involved in interleukin-6 and interleukin-8 expression in fibroblast-like synoviocytes stimulated by protein I/II, a modulin from oral streptococci. Cell. Microbiol. 2001;3:703–712. doi: 10.1046/j.1462-5822.2001.00148.x. [DOI] [PubMed] [Google Scholar]
- Nunomura A, Perry G, Aliev G, Hirai K, Takeda A, Balraj EK, Jones PK, Ghanbari H, Wataya T, Shimohama S, Chiba S, Atwood CS, Petersen RB, Smith MA. Oxidative damage is the earliest event in Alzheimer disease. J. Neuropathol. Exp. Neurol. 2001;60:759–767. doi: 10.1093/jnen/60.8.759. [DOI] [PubMed] [Google Scholar]
- Odetti P, Angelini G, Dapino D, Zaccheo D, Garibaldi S, Dagna-Bricarelli F, Piombo G, Perry G, Smith M, Traverso N, Tabaton M. Early glycoxidation damage in brains from Down's syndrome. Biochem. Biophys. Res. Commun. 1998;243:849–851. doi: 10.1006/bbrc.1998.8186. [DOI] [PubMed] [Google Scholar]
- Olichney JM, Hansen LA, Galasko D, Saitoh T, Hofstetter CR, Katzman R, Thal LJ. The apolipoprotein E epsilon 4 allele is associated with increased neuritic plaques and cerebral amyloid angiopathy in Alzheimer's disease and Lewy body variant. Neurology. 1996;47:190–196. doi: 10.1212/wnl.47.1.190. [DOI] [PubMed] [Google Scholar]
- O'Neill LAJ, Kaltschmidt C. NF-κB: a crucial transcription factor for glial and neuronal cell function. Trends Neurosci. 1997;20:252–258. doi: 10.1016/s0166-2236(96)01035-1. [DOI] [PubMed] [Google Scholar]
- Neuroinflammation Working Group. Inflammation and Alzheimer's disease. Neurobiol. Aging. 2000;21:383–421. doi: 10.1016/s0197-4580(00)00124-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson AG, Byrne UT, MacGibbon GA, Faull RL, Dragunow M. Activated c-Jun is present in neurofibrillary tangles in Alzheimer's disease brains. Neurosci. Lett. 2006;398:246–250. doi: 10.1016/j.neulet.2006.01.031. [DOI] [PubMed] [Google Scholar]
- Perlmutter LS, Scott SA, Barrón E, Chui HC. MHC class II-positive microglia in human brain: association with Alzheimer lesions. J. Neurosci. Res. 1992;33:549–558. doi: 10.1002/jnr.490330407. [DOI] [PubMed] [Google Scholar]
- Reynolds CH, Betts JC, Blackstock WP, Nebreda AR, Anderton BH. Phosphorylation sites on tau identified by nanoelectrospray mass spectrometry: differences in vitro between the mitogen-activated protein kinases ERK2, c-Jun N-terminal kinase and P38, and glycogensynthase kinase-3β. J. Neurochem. 2000;74:1587–1595. doi: 10.1046/j.1471-4159.2000.0741587.x. [DOI] [PubMed] [Google Scholar]
- Rogers J, Cooper NR, Webster S, Schultz J, McGeer PL, Styren SD, Civin WH, Brachova L, Bradt B, Ward P, Lieberburg I. Complement activation by β-amyloid in Alzheimer disease. Proc. Natl. Acad. Sci. U.S.A. 1992;89:10016–10020. doi: 10.1073/pnas.89.21.10016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rogers J, Strohmeyer R, Koveleowski CJ, Li R. Microglia and inflammatory mechanisms in the clearance of amyloid b peptide. Glia. 2002;40:260–269. doi: 10.1002/glia.10153. [DOI] [PubMed] [Google Scholar]
- Rozemuller JM, Eikelenboom P, Pals ST, Stam FC. Microglial cells around amyloid plaques in Alzheimer's disease express leucocyte adhesion molecules of the LFA-1 family. Neurosci. Lett. 1989;101:288–292. doi: 10.1016/0304-3940(89)90547-8. [DOI] [PubMed] [Google Scholar]
- Selkoe D. Alzheimer's disease: genes, proteins, therapy. Physiol. Rev. 2001;81(2):742–760. doi: 10.1152/physrev.2001.81.2.741. [DOI] [PubMed] [Google Scholar]
- Suo Z, Tan J, Placzekm A, Crawford F, Fang C, Mullan M. Alzheimer's β-amyloid peptides induce inflammatory cascade in human vascular cells: the roles of cytokines. Brain Res. 1998;807:110–117. doi: 10.1016/s0006-8993(98)00780-x. [DOI] [PubMed] [Google Scholar]
- Swantek JL, Cobb MH, Geppert TD. Jun N-terminal kinase/stress-activated protein kinase (JNK/SAPK) is required for lipopolysaccharide stimulation of tumor necrosis factor alpha (TNF-α) translation: glucocorticoids inhibit TNF-a translation by blocking JNK/SAPK. Mol. Cell. Biol. 1997;17:6274–6282. doi: 10.1128/mcb.17.11.6274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thakur A, Wang X, Siedlack SL, Perry G, Smith MA, Xhu X. C-Jun phosphorylation in Alzheimer's disease. J. Neurosci. Res. 2007;85:1668–1673. doi: 10.1002/jnr.21298. [DOI] [PubMed] [Google Scholar]
- Troy CM, Rabacchi SA, Xu Z, Maroney AC, Connors TJ, Shelanski ML, Greene LA. Beta-Amyloid-induced neuronal apoptosis requires c-Jun N-terminal kinase activation. J. Neurochem. 2001;77:157–164. doi: 10.1046/j.1471-4159.2001.t01-1-00218.x. [DOI] [PubMed] [Google Scholar]
- Tuppo EE, Arias HR. The role of inflammation in Alzheimer's disease. Int. J. Biochm. Cell. Biol. 2005;37:289–305. doi: 10.1016/j.biocel.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Tyt LM, Doker WH, BirkenKamp K, Koopmans SB, Lummen C, Kruijer W, Vellenga E. Extracellular-regulated kinase 1/2, Jun N-terminal kinase, and c-Jun are involved in NF-kappaB-dependent IL-6 expression in human monocytes. J. Immunol. 1999;162:4893–4902. [PubMed] [Google Scholar]
- Waetzig V, Czeloth K, Hidding U, Mielke K, Kanzow M, Brecht S, Goetz M, Lucius R, Herdegen T, Hanisch UK. C-Jun N-terminal kinases (JNKs) mediate proinflammatory actions of microglia. Glia. 2005;50:235–246. doi: 10.1002/glia.20173. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue L-F. Microglial response in Alzheimer's disease. In: Wood PL, editor. Neuroinflammation: Mechanism and Management. Totowa, NJ: Humana Press; 2003. pp. 267–282. [Google Scholar]
- Walker DG, Lue L-F, Beach TG. Gene expression profiling of amyloid beta peptide-stimulated human post-mortem brain microglia. Neurobiol. Aging. 2001;22:57–966. doi: 10.1016/s0197-4580(01)00306-2. [DOI] [PubMed] [Google Scholar]
- Walker DG, Lue L-F. Investigations with cultured human microglia on pathogenic mechanisms of Alzheimer's disease and other neurodegenerative diseases. J. Neurosci. Res. 2005;81(3):412–425. doi: 10.1002/jnr.20484. [DOI] [PubMed] [Google Scholar]
- Walker DG, Link J, Lue L-F, Dalsing-Hrnandez JE, Boyes BE. Gene expression changes by amyloid beta peptide-stimulated human post mortem brain microglia identify activation of multiple inflammatory processes. J. Leukoc. Biol. 2006;79:589–610. doi: 10.1189/jlb.0705377. [DOI] [PubMed] [Google Scholar]
- Walpen S, Beck KF, Schaefer L, Raslik I, Eberhardt W, Schaefer RM, Pfeilschifter J. Nitric oxide induces MIP-2 transcription in rat renal mesangial cells and in a rat model of glomerulonephritis. FASEB J. 2001;15(3):571–573. doi: 10.1096/fj.00-0518fje. [DOI] [PubMed] [Google Scholar]
- Wang N, Verna L, Hardy S, Forsayeth J, Zhu Y, Stemerman MB. Adenovirus-mediated overexpression of c-jun and c-fos induces intercellular adhesion molecule-1 and monocyte chemoattractant protein-1 in human endothelial cells. Arterioscler. Thromb. Vasc. Biol. 1999;19:2078–2084. doi: 10.1161/01.atv.19.9.2078. [DOI] [PubMed] [Google Scholar]
- Wei W, Wang X, Kusiak JW. Signaling events in amyloid betapeptide-induced neuronal death and insulin-like growth factor I protection. J. Biol. Chem. 2002;277:17649–17656. doi: 10.1074/jbc.M111704200. [DOI] [PubMed] [Google Scholar]
- Weksler BB, Subileau EA, Perriere N, Charneau P, Holloway K, Leveque M, Tricoire-Leignel H, Nicotra A, Bourdoulous S, Turowski P, Male DK, Roux F, Greenwood J, Romero JA, Couraud PO. Blood-brain barrier-specific properties of a human adult brain endothelial cell line. FASEB J. 2005;19:1872–1874. doi: 10.1096/fj.04-3458fje. [DOI] [PubMed] [Google Scholar]
- Whitmarsh AJ, Davis RJ. Transcription factor AP-1 regulation by mitogenactivated protein kinase signal transduction pathways. J. Mol. Med. 1996;74:589–607. doi: 10.1007/s001090050063. [DOI] [PubMed] [Google Scholar]
- Yan S-D, Zhu H, Fu J, Yan SF, Roher A, Tourtellotte WW, Rajavashitsh T, Chen X, Godman GC, Stem D, Schmidt AM. Amyloid-beta-peptide receptor for advanced glycation endproducts interaction elicits neuronal expression of macrophage- colony stimulating factor: a proinflammatory pathway in Alzheimer's disease. Proc. Natl. Acad. U.S.A. 1997;94:5296–5301. doi: 10.1073/pnas.94.10.5296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W, Smith C, Shapiro A, Monette R, Hutchison J, Stanimirovic D. Increases expression of bioactive chemokines in human cerebromicrovascular endothelial cells and astrocytes subjected to stimulated ischemia in vitro. J. Neuroimmunol. 1999;101:148–160. doi: 10.1016/s0165-5728(99)00137-x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Smith C, Howlett C, Stanimirovic D. Inflammatory activation of human brain endothelial cells by hypoxic astrocytes in vitro is mediated by IL-1β. J. Cereb. Blood Flow. Metab. 2000;20:967–978. doi: 10.1097/00004647-200006000-00009. [DOI] [PubMed] [Google Scholar]
- Zhang W, Mojsilovic-Petrovic J, Andrade M, Zhang H, Ball M, Stanimirovic D. Expression and functional characterization of ABCG2 in brain endothelial cells and vessels. FASEB J. 2003;17:2085–2087. doi: 10.1096/fj.02-1131fje. [DOI] [PubMed] [Google Scholar]
- Zhu X, Raina AK, Rottkamp CA, Aliev G, Perry G, Boux H, Smith MA. Activation and redistribution of c-jun N-terminal kinase/stress activated protein kinase in degenerating neurons in Alzheimer's disease. J. Neurochem. 2001a;76:435–441. doi: 10.1046/j.1471-4159.2001.00046.x. [DOI] [PubMed] [Google Scholar]
- Zhu X, Castellani RJ, Takeda A, Nunomura A, Atwood CS, Perry G, Smith MA. Differential activation of neuronal ERK, JNK/SAPK and p38 in Alzheimer disease: the ‘two hit’ hypothesis. Mech. Ageing Dev. 2001b;123:39–46. doi: 10.1016/s0047-6374(01)00342-6. [DOI] [PubMed] [Google Scholar]
- Zhu X, Ogawa O, Wang Y, Perry G, Smith MA. JKK1, an upstream activator of JNK/SAPK, is activated in Alzheimer's disease. J. Neurochem. 2003;85:87–93. doi: 10.1046/j.1471-4159.2003.01645.x. [DOI] [PubMed] [Google Scholar]