Abstract
Background
Chronic kidney disease (CKD) and diabetes mellitus (DM) are common comorbidities in heart failure (HF) and each is associated with poor outcomes. However, the effects of multimorbidity related to having both CKD and DM compared to CKD alone have not been well studied in a propensity-matched population of chronic HF patients.
Methods
Of the 7788 ambulatory chronic HF patients in the Digitalis Investigation Group trial, 3527 had CKD, of whom 1095 had DM. Based on the absence or presence of DM, patients were categorized into CKD-only and CKD-DM. Propensity scores for CKD-DM were calculated for each patient and were used to match 987 pairs of CKD-only and CKD-DM patients. Hazard ratios (HR) and 95% confidence intervals (CI) comparing CKD-DM patients with CKD-only patients were estimated using matched Cox regression models.
Results
All-cause mortality occurred in 47.0% (rate, 1783/10000 person-years) of CKD-DM patients and 39.6% (rate, 1414/10000 person-years of follow-up) of CKD-only patients (HR when CKD-DM is compared with CKD-only, 1.25; 95% CI, 1.07–1.46; p=0.006). All-cause hospitalization occurred in 75.4% (rate, 5710/10000 person-years) and 67.8% (rate, 4213/10000 person-years) of CKD-DM and CKD-only patients respectively (HR, 1.32; 95% CI, 1.15–1.52; p<0.0001). Respective HR and 95% CI for other outcomes were: cardiovascular mortality (1.27; 1.06–1.52; p=0.009), HF mortality (1.34; 1.04–1.72; p=0.025); cardiovascular hospitalization (1.29; 1.12–1.49; p=0.001) and HF hospitalization (1.37; 1.16–1.63; p<0.0001).
Conclusions
Compared with comorbidity due to CKD alone, multimorbidity with CKD and DM was associated with poor outcomes in chronic HF patients.
Keywords: heart failure, chronic kidney disease, diabetes mellitus, mortality
1. Introduction
Multimorbidity is common in patients with heart failure (HF) [1]. Chronic kidney disease (CKD) and diabetes mellitus (DM) are common comorbidities in HF and are individually associated with poor outcomes [2, 3]. However, the association of multimorbidity and outcomes in chronic HF has not been well studied. The objective of this study was to test the hypothesis that in patients with chronic HF, compared with the comorbidity due to CKD alone, multimorbidity due to both CKD and DM is associated with increased mortality and hospitalization.
2. Materials and Methods
2.1 Study design and patients
This is a secondary analysis of the Digitalis Investigation Group (DIG) trial, which was a randomized, double-blind placebo-controlled trial of digoxin in chronic HF conducted in the United States (186 centers) and Canada (116 centers) during 1991 to 1995. The design and the results of the DIG are well described in the literature [4–6].
2.2 Patients
All 7788 DIG participants were in normal sinus rhythm, 6800 had left ventricular ejection fraction ≤45%, and over 90% were receiving angiotensin-converting enzyme (ACE) inhibitors. Data on beta-blocker use were not collected. We focused our analysis to 3527 patients who had CKD. We categorized these patients into CKD-DM (n=1095) and CKD-only (n=2432) groups by the presence or absence of DM. CKD was defined as an estimated baseline glomerular filtration rate of <60 ml/min/1.73 m2 body surface area [7, 8]. DM was defined as a history or diagnosis of DM at baseline.
2.3 Outcomes
The primary outcomes were all-cause mortality and all-cause hospitalization during a mean follow up of 32.6 months. Secondary outcomes studied were mortality and hospitalizations due to cardiovascular causes and worsening HF. Data on vital status were 99% complete [9].
2.4 Calculation of propensity scores
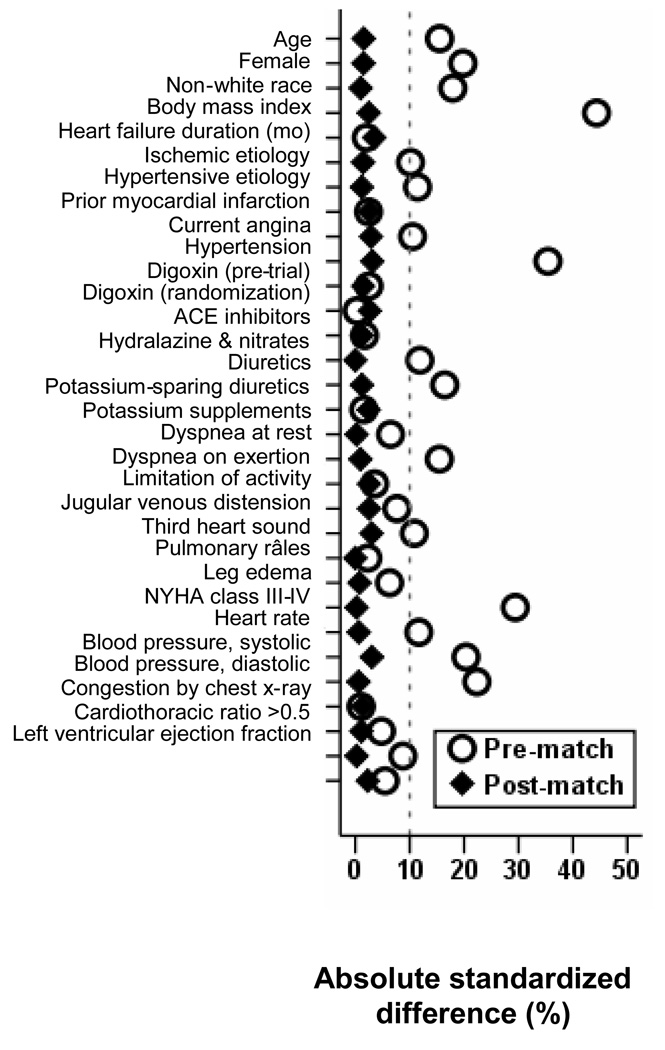
To assemble a cohort in which CKD-only and CKD-DM patients would be well-balanced on all measured baseline covariates, we calculated propensity scores for CKD-DM for each of the 3527 patients using a non-parsimonious multivariable logistic regression model [10, 11]. The propensity score for CKD-DM is the conditional probability of a patient having CKD-DM given that patient's measured covariates. In the model for propensity score, CKD-DM was the dependent variables and all baseline patient characteristics displayed in Figure 1 were used as covariates. We then used propensity scores to match 987 pairs of CKD-only and CKD-DM patients [2, 12].
Figure 1.
Love plot displaying absolute standardized differences for covariates between chronic heart failure patients with comorbidity due to chronic kidney disease alone and those with multimorbidity due to both chronic kidney disease and diabetes mellitus, before and after propensity score matching (ACE=angiotensin-converting enzyme; NYHA=New York Heart Association)
2.5. Assessment of bias reduction: absolute standardized differences
We assessed post-match covariate balance between the two groups by estimating absolute standardized differences and presented those results as Love plots [12]. Absolute standardized differences of <10% are taken to indicate inconsequential bias ([12–14].
2.6. Statistical analysis
For descriptive analyses, we used Pearson Chi square and Wilcoxon rank-sum tests for the prematch, and McNemar's test and paired sample t-test for the post-match comparisons, as appropriate. Kaplan-Meier and matched Cox regression analyses were used to estimate associations of CKD-DM with various outcomes. We confirmed the assumption of proportional hazards by a visual examination of the log (minus log) curves. Homogeneity of the association between CKD-DM and all-cause mortality was further investigated in various subgroups of patients.
Although excellent balance was achieved in our post-match cohort in all measured covariates between the two groups, it is possible that there was bias due to imbalances in unmeasured covariates. Therefore, we conducted a formal sensitivity analysis to quantify the degree of a hidden bias that would need to be present to invalidate our main conclusions. All statistical tests were done using SPSS-15 for Windows [15].
3. Results
3.1 Patient Characteristics
Baseline characteristics for both groups before and after matching are displayed in Table 1 and Figure 1. Values of absolute standardized differences for all covariates were <5% after matching, suggesting substantial bias reduction (Figure 1).
Table 1.
Baseline patient characteristics, before and after propensity score matching
| Before matching | After matching | |||||
|---|---|---|---|---|---|---|
| N (%) or mean (±SD) | CKD | CKD & DM | CKD | CKD & DM | ||
| (N = 2432) | (N=1095) | P | (N = 987) | (N = 987) | P | |
| Age (years) | 69 (±9) | 67 (±8) | <0.0001 | 68 (±10) | 68 (±8) | 0.733 |
| Female | 688 (28%) | 411 (36%) | <0.0001 | 336 (34%) | 343 (35%) | 0.776 |
| Non-white | 175 (7%) | 137 (13%) | <0.0001 | 105 (11%) | 108 (11%) | 0.884 |
| Body mass index, kg/m2 | 26 (±5) | 29 (±6) | <0.0001 | 28 (±5) | 28 (±5) | 0.532 |
| Duration of HF (months) | 30 (±36) | 31 (±38) | 0.580 | 30 (±37) | 31 (±38) | 0.443 |
| Primary cause of HF | ||||||
| Ischemic | 1735 (71%) | 830 (76%) | 738 (75%) | 744 (75%) | ||
| Hypertensive | 226 (9%) | 141 (13%) | 129 (13%) | 125 (13%) | ||
| Idiopathic | 318 (13%) | 96 (9%) | <0.0001 | 93 (9%) | 90 (9%) | 0.667 |
| Others | 153 (6%) | 28 (3%) | 27 (3%) | 28 (3%) | ||
| Prior myocardial infarction | 1591 (65%) | 729 (67%) | 0.503 | 651 (66%) | 663 (67%) | 0.605 |
| Current angina | 661 (27%) | 350 (32%) | 0.004 | 291 (30%) | 304 (31%) | 0.554 |
| Hypertension | 1101 (45%) | 686 (63%) | <0.0001 | 602 (61%) | 587 (60%) | 0.487 |
| Medications | ||||||
| Pre-trial digoxin use | 1025 (42%) | 475 (43%) | 0.493 | 417 (42%) | 424 (43%) | 0.791 |
| Trial use of digoxin | 1205 (50%) | 541 (49%) | 0.938 | 480 (49%) | 493 (50%) | 0.574 |
| ACE inhibitors | 2248 (92%) | 1017 (93%) | 0.643 | 919 (93%) | 916 (93%) | 0.862 |
| Hydralazine & nitrates | 33 (1%) | 34 (3%) | <0.0001 | 26 (3%) | 26 (3%) | 1.000 |
| Diuretics | 1989 (82%) | 960 (88%) | <0.0001 | 861 (87%) | 857 (87%) | 0.838 |
| PS diuretics | 220 (9%) | 104 (10%) | 0.667 | 89 (9%) | 96 (10%) | 0.639 |
| Potassium supplement | 731 (30%) | 362 (33%) | 0.074 | 318 (32%) | 319 (32%) | 1.000 |
| Symptoms and signs of HF | ||||||
| Dyspnea at rest | 504 (21%) | 299 (27%) | <0.0001 | 261 (26%) | 257 (26%) | 0.877 |
| Dyspnea on exertion | 1872 (77%) | 859 (78%) | 0.333 | 785 (80%) | 775 (79%) | 0.621 |
| Limitation of activity | 1867 (77%) | 875 (80%) | 0.038 | 793 (80%) | 783 (79%) | 0.616 |
| Jugular venous distension | 331 (14%) | 192 (18%) | 0.002 | 163 (17%) | 174 (18%) | 0.548 |
| Third heart sound | 600 (25%) | 281 (26%) | 0.529 | 257 (26%) | 257 (26%) | 1.000 |
| Pulmonary râles | 433 (18%) | 222 (20%) | 0.081 | 200 (20%) | 203 (21%) | 0.909 |
| Lower extremity edema | 461 (19%) | 346 (32%) | <0.0001 | 293 (30%) | 292 (30%) | 1.000 |
| NYHA functional class, % | ||||||
| Class I | 298 (12%) | 127 (12%) | 130 (13%) | 118 (12%) | ||
| Class II | 1283 (53%) | 523 (48%) | 461 (47%) | 476 (48%) | ||
| Class III | 789 (32%) | 405 (37%) | 0.008 | 365 (37%) | 360 (37%) | 0.984 |
| Class IV | 62 (3%) | 40 (4%) | 31 (3%) | 33 (3%) | ||
| Heart rate (/minute), | 77 (±13) | 80 (±12) | <0.0001 | 80 (±13) | 79 (±12) | 0.488 |
| Blood pressure (mm Hg) | ||||||
| Systolic | 127 (±21) | 132 (±22) | <0.0001 | 131 (±22) | 131 (±21) | 0.894 |
| Diastolic | 75 (±11) | 74 (±11) | 0.769 | 74 (±12) | 74 (±11) | 0.757 |
| Chest radiograph findings | ||||||
| Pulmonary congestion | 364 (15%) | 183 (17%) | 0.185 | 161 (16%) | 165 (17%) | 0.856 |
| Cardiothoracic ratio >0.5 | 1497 (62%) | 720 (66%) | 0.017 | 641 (65%) | 640 (65%) | 1.000 |
| Ejection fraction | 32 (±13) | 33 (±12) | 0.139 | 33 (±13) | 32 (±12) | 0.620 |
ACE=angiotensin-converting enzyme; CKD=chronic kidney disease; DM=diabetes mellitus; HF=heart failure; NYHA=New York Heart Association; PS=potassium sparing; SD=standard deviation
3.2 CKD-DM and mortality
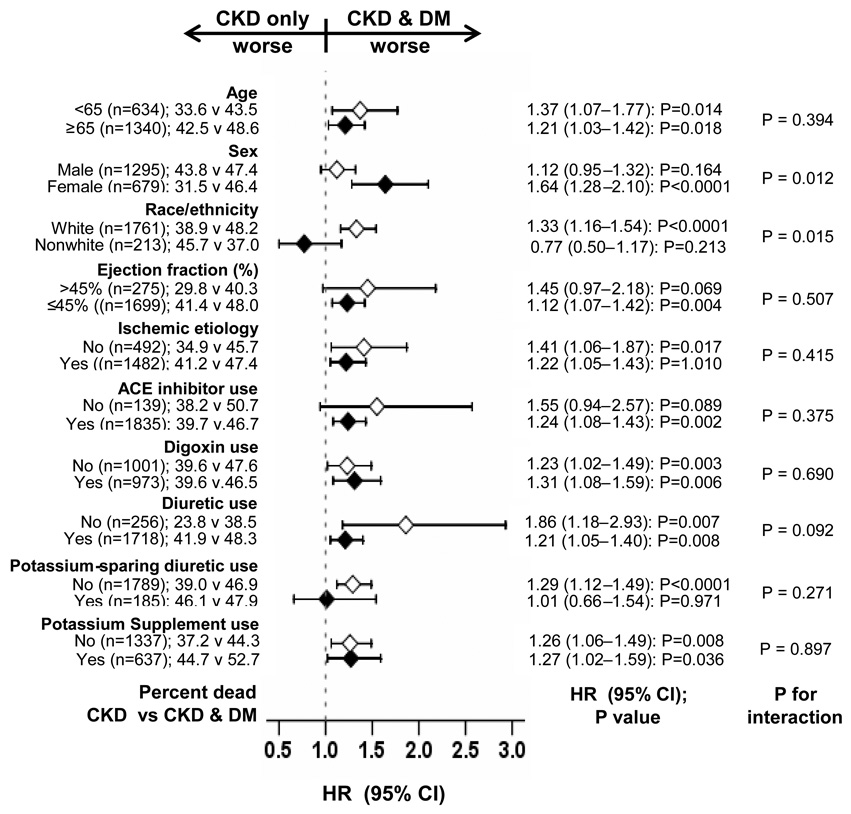
Overall, 855 (43.3%) patients died from all causes. All-cause mortality occurred in 47.0% (rate, 1783/10000 person-years) of CKD-DM patients, and 39.6% (rate, 1414/10000 person-years) of patients with CKD (matched hazard ratio {HR} when CKD-DM was compared with CKD-only, 1.25; 95% confidence interval {CI}, 1.07–1.46; p=0.006; Figure 2a and Table 2). In the absence of a hidden bias, a sign-score test for matched data with censoring provides strong evidence (p=0.005) that CKD-only patients outlived those with CKD-DM. A hidden covariate that is near-perfect predictor of mortality would need to increase the odds of having CKD-DM by 7.08% to potentially explain away this association. The association between CKD-DM and all-cause mortality was homogenous across different subgroups (Figure 3). Associations of CKD-DM with cardiovascular and HF mortality are displayed in Table 2.
Figure 2.
Kaplan-Meier plots for (a) all-cause mortality, and (b) all-cause hospitalization (CI=confidence interval; CKD=chronic kidney disease; DM=diabetes mellitus; HR=hazard ratio)
Table 2.
Association of multimorbidity due to both by chronic kidney disease (CKD) and diabetes mellitus (DM) relative to CKD alone, with mortality and hospitalization
| CKD (N = 987) |
CKD & DM (N = 987) |
Absolute Rate Difference (per 10,000 Person- Years)† |
Matched Hazard Ratio (95% Confidence Interval) |
P Value | |
|---|---|---|---|---|---|
| Outcomes | |||||
| Rate per 10,000 Person-Years (Events / Total Follow-Up Years) |
|||||
| Mortality | |||||
| All-cause | 1414 (391 / 2766) |
1783 (464 / 2603) |
+ 369 | 1.25 (1.07–1.46) | 0.006 |
| Cardiovascular | 1121 (310 / 2766) |
1402 (365 / 2603) |
+ 281 | 1.27 (1.06–1.52) | 0.009 |
| Heart failure | 524 (145 / 2766) |
707 (184 / 2603) |
+ 183 | 1.34 (1.04–1.72) | 0.025 |
| Hospitalization* | |||||
| All-cause | 4213 (669 / 1588) |
5710 (744 / 1303) |
+ 1497 | 1.32 (1.15–1.52) | <0.0001 |
| Cardiovascular | 2764 (521 / 1885) |
3851 (615 / 1597) |
+ 1087 | 1.29 (1.12–1.49) | 0.001 |
| Heart failure | 1409 (325 / 2306) |
2082 (421 / 2022) |
+ 673 | 1.37 (1.16–1.63) | <0.0001 |
Data shown include the first hospitalization of each patient due to each cause.
Absolute differences were calculated by subtracting the percentage of patients dead or hospitalized in the CKD group from the rates of death and hospitalization in the CKD and DM group (before values were rounded)
Figure 3.
Association of multimorbidity due to chronic kidney disease (CKD) and diabetes mellitus (DM) with all-cause mortality in subgroups of propensity-matched chronic heart failure patients (ACE=angiotensin-converting enzyme; CI=confidence interval; HR=hazard ratio)
3.4 CKD-DM and hospitalization
Overall 1413 (71.6%) patients were hospitalized for all causes. All-cause hospitalization occurred in 75.4% (rate, 5710/10000 person-years) and 67.8% (rate, 4213/10000 person-years) of CKD-DM and CKD-only patients, respectively (matched HR when CKD-DM was compared with CKD-only, 1.32; 95% CI, 1.15–1.52; p<0.0001; Figure 2b and Table 2). A sign-score test for matched data with censoring provides strong evidence (p<0.0001) that CKD-only patients had fewer hospitalizations than those with CKD-DM. A hidden covariate would need to increase the odds of hospitalization by 16.00% to potentially explain away this association. Associations of CKD-DM with cardiovascular and HF hospitalization are displayed in Table 2.
4. Discussion
4.1 Key study findings
In the current analysis, we demonstrate that in patients with chronic HF, the risk of adverse outcomes associated with CKD is further increased by the presence of additional comorbidity due to DM. These findings are important as HF is common among older adults who also suffer from multimorbidity. To the best of our knowledge, this is the first report of a propensity-matched study of associations of multimorbidity associated with DM with outcomes in chronic HF patients with CKD.
4.2 Mechanistic insights to study findings
All patients in our study had chronic HF and CKD and thus had a high baseline risk for poor outcomes [2]. The additional risk associated with the presence of DM may be a direct intrinsic effect of DM, a confounding effect by one or more measured covariates, and/or a confounding effect by one or more unmeasured covariates. DM is associated with activation of neurohormonal systems and the direct metabolic effects of DM and hyperglycemia are well known [16, 17]. Patients with DM are at increased risk of developing diabetic cardiomyopathy and diabetic nephropathy, which may indicate poor control or long duration of DM. Therefore, DM in the presence of CKD may be a marker of advanced DM and concomitant vascular disease from oxidative stress, inflammation, and advanced glycation endproducts [18]. The association between multimorbidity and poor outcomes may also be explained by the disease burden. With each additional comorbidity, the overall disease burden is increased, which in turn may increase the risk of disease-disease and disease-treatment interaction, and poor outcomes.
The findings of our study are unlikely to be explained by differences in baseline characteristics, as our matched cohort was well-balanced at baseline on all measured covariates. Results of our sensitivity analysis suggest that our findings may be relatively insensitive to a hidden bias. However, sensitivity analysis cannot determine if such a hidden bias exists or not. More importantly, for an imaginary hidden bias to explain away our findings it should be a near-perfect predictor of mortality and hospitalization, and also should not be strongly correlated with any of the covariates used in our propensity score model.
4.3 Comparison with prior studies
In patients with acute myocardial infarction, the rate of all-cause mortality has been shown to be significantly higher in those with CKD and DM than those with CKD only (40% versus 27%; p <0.001) [19]. However, to the best of our knowledge this is the first report of a propensity-matched study of the association between multimorbidity involving DM and CKD in patients with chronic HF.
4.4 Study limitations
Several limitations of this study need to be acknowledged. Patients in the DIG trial were relatively young, predominantly white and mostly men, with normal sinus rhythm from the pre-beta-blocker era of HF therapy, which may limit generalizability. We also had no data on duration, severity and treatment of DM at baseline or during follow up. Patients without DM at baseline may have developed DM during follow up. This phenomenon, called regression dilution, may have underestimated the true associations observed in our study [20].
4.5 Conclusions
In conclusion, in this propensity-matched study of chronic HF patients with CKD, in which patients with and without DM were well-balanced on all measured baseline characteristics, the presence of DM was associated with significant increases in mortality and hospitalization. These findings highlight the importance of multimorbidity associated with DM and CKD on outcomes relative comorbidity due to CKD in patients with chronic HF. Future studies need to prospectively test preventive and therapeutic interventions to prevent multimorbidity and to reduce multimorbidity-associated poor outcomes in chronic HF.
Acknowledgement
“The Digitalis Investigation Group (DIG) study was conducted and supported by the NHLBI in collaboration with the DIG Investigators. This Manuscript was prepared using a limited access dataset obtained by the NHLBI and does not necessarily reflect the opinions or views of the DIG Study or the NHLBI.”
Funding/Support: Dr. Ahmed is supported by the National Institutes of Health through grants from the National Heart, Lung, and Blood Institute (5-R01-HL085561-02 and P50-HL077100), and a generous gift from Ms. Jean B. Morris of Birmingham, Alabama
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Rosamond W, Flegal K, Furie K, et al. Heart disease and stroke statistics–2008 update: a report from the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Circulation. 2008;117:e25–e146. doi: 10.1161/CIRCULATIONAHA.107.187998. [DOI] [PubMed] [Google Scholar]
- 2.Ahmed A, Aban IB, Vaccarino V, et al. A propensity-matched study of the effect of diabetes on the natural history of heart failure: variations by sex and age. Heart. 2007;93:1584–1590. doi: 10.1136/hrt.2006.113522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kannel WB, Hjortland M, Castelli WP. Role of diabetes in congestive heart failure: the Framingham study. Am J Cardiol. 1974;34:29–34. doi: 10.1016/0002-9149(74)90089-7. [DOI] [PubMed] [Google Scholar]
- 4.Ahmed A, Rich MW, Fleg JL, et al. Effects of digoxin on morbidity and mortality in diastolic heart failure: the ancillary digitalis investigation group trial. Circulation. 2006;114:397–403. doi: 10.1161/CIRCULATIONAHA.106.628347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.The Digitalis Investigation Group. The effect of digoxin on mortality and morbidity in patients with heart failure. N Engl J Med. 1997;336:525–533. doi: 10.1056/NEJM199702203360801. [DOI] [PubMed] [Google Scholar]
- 6.The Digitalis Investigation Group. Rationale, design, implementation, and baseline characteristics of patients in the DIG trial: a large, simple, long-term trial to evaluate the effect of digitalis on mortality in heart failure. Control Clin Trials. 1996;17:77–97. doi: 10.1016/0197-2456(95)00065-8. [DOI] [PubMed] [Google Scholar]
- 7.National Kidney Founation. K/DOQI clinical practice guidelines for chronic kidney disease: evaluation, classification, and stratification. Am J Kidney Dis. 2002;39:S1–S266. [PubMed] [Google Scholar]
- 8.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–470. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
- 9.Collins JF, Howell CL, Horney RA. Determination of vital status at the end of the DIG trial. Control Clin Trials. 2003;24:726–730. doi: 10.1016/j.cct.2003.08.011. [DOI] [PubMed] [Google Scholar]
- 10.Rubin DB. Using propensity score to help design observational studies: Application to the tobacco litigation. Health Services and Outcomes Research Methodology. 2001;2:169–188. [Google Scholar]
- 11.Rosenbaum PR, Rubin DB. The central role of propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 12.Ahmed A, Husain A, Love TE, et al. Heart failure, chronic diuretic use, and increase in mortality and hospitalization: an observational study using propensity score methods. Eur Heart J. 2006;27:1431–1439. doi: 10.1093/eurheartj/ehi890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.D'Agostino RB., Jr Propensity score methods for bias reduction in the comparison of a treatment to a non-randomized control group. Stat Med. 1998;17:2265–2281. doi: 10.1002/(sici)1097-0258(19981015)17:19<2265::aid-sim918>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- 14.Normand ST, Landrum MB, Guadagnoli E, et al. Validating recommendations for coronary angiography following acute myocardial infarction in the elderly: a matched analysis using propensity scores. J Clin Epidemiol. 2001;54:387–398. doi: 10.1016/s0895-4356(00)00321-8. [DOI] [PubMed] [Google Scholar]
- 15.SPSS for Windows, Rel. 15 program] Chicago, IL: SPSS Inc.; 2008. [Google Scholar]
- 16.Lim HS, MacFadyen RJ, Lip GY. Diabetes mellitus, the renin-angiotensin-aldosterone system, and the heart. Arch Intern Med. 2004;164:1737–1748. doi: 10.1001/archinte.164.16.1737. [DOI] [PubMed] [Google Scholar]
- 17.Miller JA. Impact of hyperglycemia on the renin angiotensin system in early human type 1 diabetes mellitus. J Am Soc Nephrol. 1999;10:1778–1785. doi: 10.1681/ASN.V1081778. [DOI] [PubMed] [Google Scholar]
- 18.Son SM. Role of vascular reactive oxygen species in development of vascular abnormalities in diabetes. Diabetes Res Clin Pract. 2007;77 Suppl 1:S65–S70. doi: 10.1016/j.diabres.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 19.Anavekar NS, Solomon SD, McMurray JJ, et al. Comparison of renal function and cardiovascular risk following acute myocardial infarction in patients with and without diabetes mellitus. Am J Cardiol. 2008;101:925–929. doi: 10.1016/j.amjcard.2007.11.037. [DOI] [PubMed] [Google Scholar]
- 20.Clarke R, Shipley M, Lewington S, et al. Underestimation of risk associations due to regression dilution in long-term follow-up of prospective studies. Am J Epidemiol. 1999;150:341–353. doi: 10.1093/oxfordjournals.aje.a010013. [DOI] [PubMed] [Google Scholar]