Abstract
Cardiovascular problems are a major cause of morbidity and mortality in patients with autosomal-dominant polycystic kidney disease (ADPKD). Hypertension is a common early symptom of ADPKD, and occurs in approximately 60% of patients before renal function has become impaired. Hypertension is associated with an increased rate of progression to end-stage renal disease and is the most important potentially treatable variable in ADPKD. Left ventricular hypertrophy, which is a powerful, independent risk factor for cardiovascular morbidity and mortality, also occurs frequently in patients with ADPKD. Both hypertension and left ventricular hypertrophy have important roles in cardiovascular complications in these individuals. Moreover, biventricular diastolic dysfunction, endothelial dysfunction, increased carotid intima-media thickness, and impaired coronary flow velocity reserve are present even in young patients with ADPKD who have normal blood pressure and well-preserved renal function. These findings suggest that cardiovascular involvement starts very early in the course of ADPKD. Intracranial and extracranial aneurysms and cardiac valvular defects are other potential cardiovascular problems in patients with ADPKD. Early diagnosis and treatment of hypertension, with drugs that block the renin-angiotensin-aldosterone system, has the potential to decrease the cardiovascular complications and slow the progression of renal disease in ADPKD.
Introduction
Autosomal-dominant polycystic kidney disease (ADPKD) is the most common inherited renal disease, and occurs in 1 of 400–1,000 individuals.1 Its prevalence is higher than that of Huntington disease, hemophilia, sickle cell disease, cystic fibrosis, myotonic dystrophy and Down syndrome combined.2 ADPKD is genetically heterogeneous; two genes—PKD1 and PKD2—are implicated in its development.1 The disease is characterized by renal and extrarenal involvement with cystic and noncystic manifestations. With the availability of renal replacement therapies for patients with end-stage renal disease (ESRD), cardiovascular complications have emerged as a major cause of death in patients with ADPKD.3,4 Hypertension is a common early finding and can be the symptom that leads to the diagnosis of ADPKD.5,6 Hypertension is also associated with a rapid progression to ESRD and increased cardiovascular complications.7 Left ventricular hypertrophy (LVH), which is an important risk factor for premature cardiovascular death, occurs frequently in patients with ADPKD.8 Aneurysms and cardiac valvular abnormalities are other cardiovascular manifestations of this disease. This Review focuses on the cardiovascular problems of patients with ADPKD.
Hypertension
Hypertension is very common in ADPKD, and occurs in 50–70% of patients before any substantial reduction in glomerular filtration rate is observed.5,6 Furthermore, hypertension occurs at a much earlier age in patients with ADPKD than in the general population.9 The median age at diagnosis of hypertension in ADPKD was 32 years for males and 34 years for females,10 compared with a median age of 45–55 years in patients with essential hypertension. Studies have reported that hypertension occurs in 20–30% of children with ADPKD.11–14 Moreover, Schrier et al.10 demonstrated that the likelihood of hypertension in both men and women with ADPKD was significantly greater when the affected parent was hypertensive. Thus, the presence of hypertension in the ADPKD-affected parent of a patient with ADPKD should alert the clinician to the need for early detection and treatment of hypertension.
Role of the renin-angiotensin-aldosterone system
Renal structural changes have an important role in the pathogenesis of hypertension in patients with ADPKD. Gabow et al.15 reported that adult patients with ADPKD and hypertension had significantly greater renal volume than patients with normal blood pressure. This relationship between hypertension and cystic involvement also exists in children with ADPKD.12 These findings suggest that activation of the renin-angiotensin-aldosterone system (RAAS) as a result of cyst expansion and local renal ischemia has an important role in the development of hypertension in this disease. Immunohistochemical studies of nephrectomy specimens from patients with ADPKD have shown hyperplasia of renin-secreting cells of the juxtaglomerular apparatus, which suggests chronic stimulation of the RAAS is present.16 In addition, high levels of renin were found in cyst fluid obtained from patients with ADPKD.17 Loghman-Adham et al.18 showed that other components of the RAAS, including angiotensinogen, angiotensin-converting enzyme (ACE), angiotensin II receptor and angiotensin II peptide were also present in the cysts and dilated tubules of ADPKD kidneys.
Key points.
Cardiovascular problems are a major cause of morbidity and mortality in patients with autosomal-dominant polycystic kidney disease (ADPKD)
Hypertension, a common symptom of ADPKD, is associated with rapid progression to end-stage renal disease; the renin-angiotensin-aldosterone system (RAAS) is important in the development of hypertension in this setting
Early vascular changes have been reported even in young patients with ADPKD and normal blood pressure
Left ventricular hypertrophy is a common finding in patients with ADPKD
Patients with ADPKD have a higher prevalence of aneurysms and cardiac valvular abnormalities than the general population
Early and effective treatment of hypertension is very important to decrease the morbidity and mortality of patients with ADPKD, and drugs that inhibit the RAAS might be beneficial in this context
Activation of the RAAS in ADPKD has been confirmed by the demonstration that plasma renin activity and aldosterone concentrations in the supine and upright positions, as well as after Captopril administration, are greater in patients with ADPKD, hypertension and normal renal function than in patients with essential hypertension who are of the same age, sex, body surface area, sodium excretion, renal function and mean arterial pressure.19 Studies have also shown that administration of ACE inhibitors decreases mean arterial pressure, renal vascular resistance and filtration fraction significantly more in hypertensive patients with ADPKD than in healthy controls with normal blood pressure.19–21 Doulton et al.22 reported that blood pressure and hormonal responses of the RAAS after high and low sodium intake and after the administration of the ACE inhibitor enalapril were not different in hypertensive patients with ADPKD and in controls with essential hypertension. These results suggest that the intrarenal RAAS might be more important than the circulating RAAS in the development of hypertension in patients with ADPKD.
To investigate the early hemodynamic abnormalities of ADPKD, Harrap et al.23 studied adult patients with normal blood pressure and well-preserved renal function. Total exchangeable sodium, plasma renin activity and plasma aldosterone levels were significantly higher in these patients than in family members of the same age and sex. Similarly, Barrett et al.24 reported that during chronically high sodium intake, plasma renin activity was higher in patients with ADPKD who had normal blood pressure and creatinine clearance greater than 70ml/min/1.73m2 than in unaffected controls from the same families. These findings suggest that the RAAS is activated at an early stage of ADPKD, and this activation precedes hypertension and the major clinical manifestations of the disease.
Torres et al.25 investigated the natriuretic response to plasma volume expansion in patients with ADPKD who had normal renal function. These investigators found that the blood pressure-natriuresis regression line was significantly shifted to the right compared with that of unaffected control individuals, which indicated that the sodium balance was maintained at the cost of increased blood pressure. In this study, plasma renin activity was not suppressed in response to plasma volume expansion, again suggesting that RAAS activity was increased.
Roles of other factors
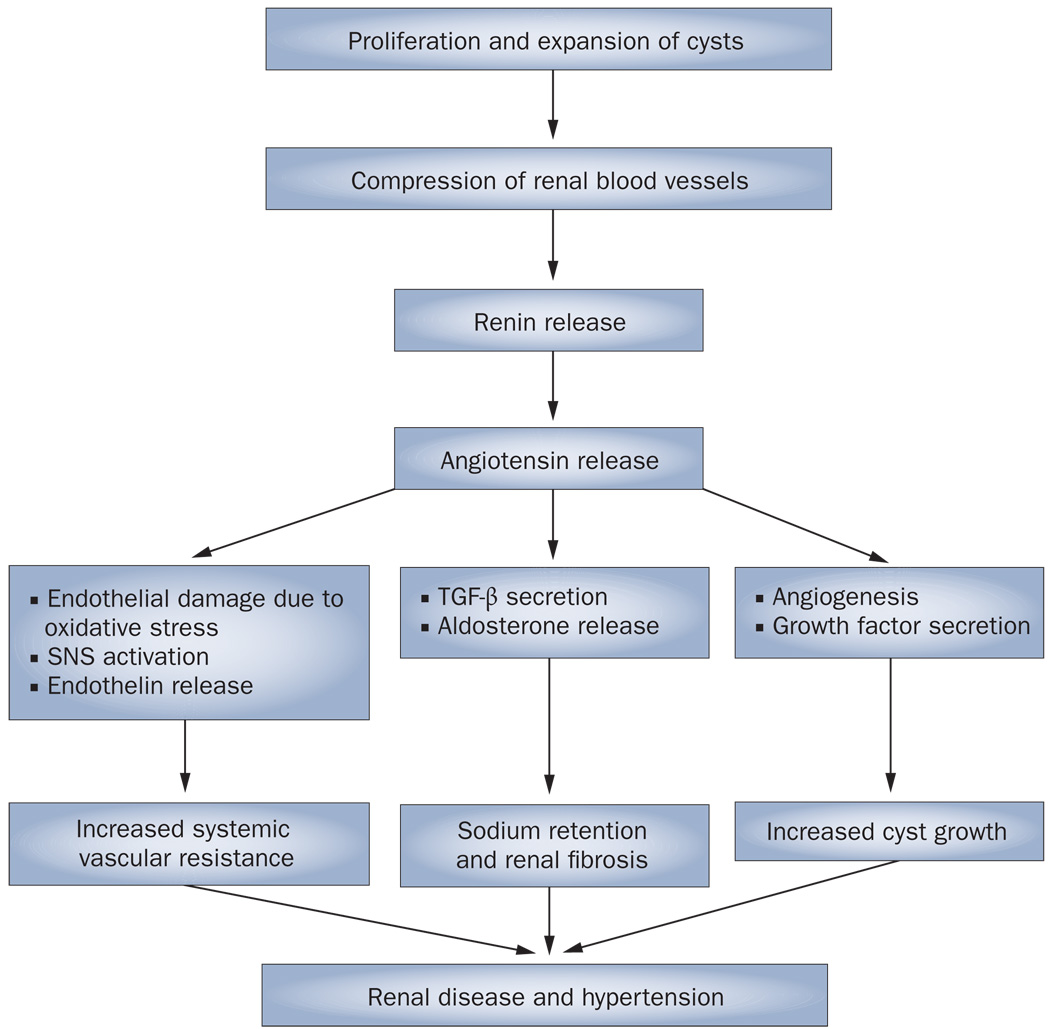
Although activation of the RAAS seems to have a major role in the pathogenesis of hypertension in ADPKD, other factors might also be involved (Figure 1). Klein et al.26 demonstrated that muscle sympathetic nerve activity was increased in hypertensive patients with ADPKD regardless of renal function, which suggests that sympathetic hyperactivity could contribute to the pathogenesis of hypertension in this disease. Of note, the RAAS is stimulated by increased sympathetic activity, and angiotensin stimulates the sympathetic nervous system.
Figure 1.
Potential pathogenetic mechanisms of hypertension in autosomal-dominant polycystic kidney disease.97 Abbreviations: SNS, sympathetic nervous system; TGF-β, transforming growth factor β.
Plasma vasopressin levels correlate with blood pressure levels in salt-sensitive forms of human and experimental hypertension.27–29 Since plasma vasopressin concentrations are increased in patients with ADPKD, vasopressin could also contribute to the development of hypertension in this disease;29,30 however, this theory remains to be proven.
The cystic epithelium of kidney sections from patients with ADPKD exhibits increased expression of endothelin 1.31 Endothelin 1 is also found in the cyst fluid.32 Patients with ADPKD have higher plasma levels of endothelin 1 than do healthy controls and patients with essential hypertension.33 Moreover, endothelium-dependent relaxation is impaired and endothelial nitric oxide synthase activity is decreased in patients with ADPKD.34,35 These findings suggest that endothelial dysfunction secondary to impaired release of nitric oxide exists in these patients. An imbalance in endothelium-derived vasoactive mediators (that is, endothelin and nitric oxide) might, therefore, contribute to the pathogenesis of hypertension in ADPKD.36,37
In a 2008 study, Wang et al.38 investigated asymmetric dimethylarginine (ADMA) as a marker of nitric oxide synthase inhibition and the lipid peroxidation product 13-hydroxyoctadecadienoic acid as a marker of oxidative stress in patients with early ADPKD. The investigators found that these patients had significantly increased plasma ADMA and plasma 13-hydroxyoctadecadienoic acid levels and significantly decreased urinary clearance of ADMA compared with healthy controls. Demonstration of endothelial dysfunction and increased carotid intima-media thickness in both hypertensive and normotensive patients with ADPKD and well-preserved renal function shows that atherosclerosis starts very early in the course of this disease.39
The findings of early-onset endothelial dysfunction mentioned above were confirmed by a study that reported decreased coronary flow velocity reserve—which represents the capacity of the coronary circulation to dilate after an increase in myocardial oxygen demand—in both hypertensive and normotensive patients with ADPKD.40 Furthermore, Borresen et al.41 investigated arterial stiffness in early ADPKD by pulse-wave analysis and measurement of pulse-wave velocity. They found that pulse-wave reflection was amplified even in young patients with ADPKD who have normal blood pressure and renal function, which demonstrates that pathological changes in the arterial system occur early in the course of disease.
The protein products of PKD1 and PKD2, polycystin 1 and polycystin 2, are expressed in vascular smooth muscle and endothelium.42,43 Thus, primary disruption of polycystin function in blood vessels could also be responsible for the early development of hypertension and renal vascular remodeling in patients with ADPKD.
Left ventricular hypertrophy
LVH is a powerful independent risk factor for cardiovascular morbidity and mortality.44 Chapman et al.8 reported that 48% of hypertensive patients with ADPKD had this condition. Increased left ventricular mass index (LVMI) is associated with poor renal and overall outcomes in these patients.7 A significant correlation between hypertension and LVMI has been demonstrated in both children and adults with ADPKD.13,44–47 A 2008 study of 85 children with ADPKD revealed that even children whose blood pressure was within the upper quartile of the normal range had a significantly greater LVMI than those with lower blood pressure.47
Other studies have also reported increased LVMI in normotensive patients with ADPKD.8,48 Bardaji et al.49 reported that young ADPKD patients with normal blood pressure and preserved renal function had increased LVMIs and Doppler abnormalities consistent with early diastolic dysfunction. Moreover, Oflaz et al.50 showed that biventricular diastolic dysfunction was present in both hypertensive and normotensive patients with ADPKD and well-preserved renal function.
Patients with hypertension whose blood pressure remains elevated at night (‘non-dippers’) show higher LVMI than those whose blood pressure falls (‘dippers’).51 Thus, ambulatory blood pressure measurement is important to evaluate the cardiovascular risk of these patients. Li Kam Wa et al.52 demonstrated that patients with ADPKD, hypertension and normal renal function or mild renal impairment exhibited a significantly lower nocturnal fall in blood pressure than patients with essential hypertension. Moreover, Valero et al.53 reported that the nocturnal fall in blood pressure was attenuated even in young patients with ADPKD who did not have hypertension. In these patients, LVMI was elevated in proportion to the ambulatory systolic blood pressure. Several other studies also showed a significant association between ambulatory blood pressure and LVMI in young, normotensive patients with ADPKD.54,55 Such patients show an exaggerated diastolic blood pressure response during exercise, which suggests that they have an impaired capacity for exercise-induced vasodilatation. This exaggerated response might indicate an increased risk of developing hypertension.54
An association between insulin resistance and LVH has been reported.56,57 Insulin resistance has been found in patients with ADPKD58 and, therefore, Lumiaho et al.59 investigated whether this symptom, determined using the homeostasis model assessment method, was associated with LVMI in 106 patients with ADPKD type 1 (PKD1) and their 70 healthy relatives. The investigators found that insulin resistance was significantly associated with LVMI in both groups independent of other factors known to increase LVMI, such as age, weight, systolic blood pressure and albuminuria. Thus, the stimulation of angiotensin II60 and the sympathetic nervous system61 secondary to hyperinsulinemia might contribute to increased LVMI in patients with PKD1.
An association between the deletion/deletion polymorphism of the ACE gene (D/Dgenotype) and increased LVMI has been reported in patients with essential hypertension62 or type 2 diabetes mellitus.63 The effects of ACE genetic polymorphisms on progression to ESRD in patients with ADPKD are controversial.64–67 However, in a cross-sectional study that included 407 white patients with ADPKD, no significant association was found between ACE gene polymorphisms and the prevalence of LVH.68
Aneurysms
Patients with ADPKD have a greater prevalence of intracranial aneurysms than the general population (4.0–11.7% versus 1.0%).69,70 Ruptured intracranial aneurysms account for 4–7% of deaths in patients with ADPKD and such deaths occur at a younger age than in the general population.71 Aneurysmal involvement of extracranial arteries, such as the coronary arteries, abdominal aorta, renal artery and splenic artery has also been reported in patients with ADPKD.72–74 Other reported vascular manifestations of ADPKD are dolichoectasias (elongations and distentions of the arteries caused by weakening of the vessel walls) and dissections.71 Since polycystin 1 and polycystin 2 are both expressed in vascular smooth muscle cells, interactions of these proteins with a single pathway might have a role in the pathogenesis of aneurysms in ADPKD.
Rupture of an intracranial aneurysm seems to cluster in certain families with ADPKD.75,76 A family history of ruptured intracranial aneurysm, therefore, is an indication for screening imaging. Patients with a previous sub-arachnoid hemorrhage should also be screened. Magnetic resonance angiography is currently the preferred screening technique.77 If magnetic resonance angiography findings show intracranial aneurysm, follow-up imaging is indicated.76 However, if the magnetic resonance angiography findings are negative, follow-up imaging is probably only necessary in patients who have a family history of a ruptured intracranial aneurysm.77,78
Cardiac valvular abnormalities
Cardiac valvular abnormalities are common in patients with ADPKD. In a combined retrospective and prospective study of 62 patients with this disease, Leier et al.79 reported that 18% of the patients overall and 27% of the patients for whom autopsy findings were available had one or more cardiovascular abnormalities. Defects of the aortic root, annulus, and valve and the mitral valve were the predominant abnormalities. In a study of 163 patients with ADPKD, 26% had mitral valve prolapse, 31% had mitral incompetence, 8% had aortic incompetence, 15% had tricuspid incompetence and 6% had tricuspid valve prolapse.80 Likewise, in a study of 228 patients with ADPKD, 25% had mitral valve prolapse, 30% had mitral incompetence, 5% had tricuspid valve prolapse and 19% had aortic regurgitation.81 In an echocardiographic study, the prevalence of mitral valve prolapse among children with ADPKD was 12%, compared with 3% among unaffected children from the same families.13 In another study, mitral valve prolapse was found in 28 of 109 patients (26%) with PKD1.82 Moreover, 13% of this cohort had hemodynamically significant (grade 2 or 3) mitral regurgitation. However, prevalences of other valve abnormalities in the ADPKD group were not significantly different from their prevalences in the healthy control group. Although mitral valve prolapse seems to be a characteristic finding in patients with PKD1 mutations, mitral regurgitation can be secondary to elevated blood pressure. Screening echocardiography is not indicated for patients with ADPKD unless a murmur is detected on examination.
Effects of antihypertensive treatment
Early and effective treatment of hypertension is very important in patients with ADPKD to slow the progression of renal failure and prevent cardiovascular complications.
Renal effects
In the Modification of Diet in Renal Disease (MDRD) study, which included 200 patients with ADPKD, aggressive blood pressure control was not associated with a reduced rate of progressive glomerular filtration rate impairment compared with standard blood pressure control.83 However, the difference in mean arterial pressure between the aggressive and standard blood pressure control groups was only 4.7mmHg, rather than the treatment goal of 15 mmHg (92 mmHg versus 107 mmHg). Moreover, the patients with ADPKD had advanced renal disease (glomerular filtration rate <55ml/min/1.73 m2), the average duration of follow-up was only 2.2 years, the type of antihypertensive therapy was not controlled, the structural progression of the kidney and heart abnormalities was not assessed and cardiovascular events were not reported.
Since the RAAS is activated in ADPKD and drugs that block the RAAS slow the progression of diabetic and non-diabetic kidney diseases, several studies have investigated the renoprotective properties of these drugs in patients with ADPKD. In the Angiotensin-converting-enzyme Inhibition in Progressive Renal Insufficiency (AIPRI) study, 64 patients with ADPKD were treated with the ACE inhibitor benazepril.84 The investigators found that patients with glomerular diseases or diabetes mellitus benefited most from this treatment, and those with ADPKD benefited least. However, similar to the MDRD trial, the patients with ADPKD already had substantial renal impairment (mean baseline creatinine clearance was 43 ml/min) and the duration of the study was only 3 years. In a randomized, prospective, 5-year study of 24 patients with ADPKD who had well-preserved renal function, use of a long-acting calcium-channel blocker (amlodipine) to maintain blood pressure below 140/90 mmHg was associated with a similar decline in creatinine clearance to that achieved with an ACE inhibitor (enalapril).85 However, only enalapril showed a sustained antialbuminuric effect. Since increased urinary protein excretion has been shown to correlate with an increased rate of renal progression in ADPKD,86 ACE inhibitors might have kidney-protective effects in the long term.
In a study by van Dijk et al.87 in patients with ADPKD who had well-preserved renal function, those with normal blood pressure were randomly assigned an ACE inhibitor (enalapril) or placebo and those with hypertension were randomly assigned enalapril or a β-blocker (atenolol); both groups were followed-up for 3 years. No difference in losses of renal function was detected between the cohorts. Similarly, in a prospective, randomized, double-blind study by Zeltner et al. in hypertensive patients with ADPKD,88 no differences in glomerular filtration rate, urinary albumin excretion or LVMI were observed between individuals who received the ACE inhibitor ramipril and those who received the β-blocker metoprolol, after 3 years of follow-up. However, a post hoc analysis showed that rigorous blood pressure control (target mean arterial pressure ≤97 mmHg) reduced urinary albumin excretion and prevented an increase in LVMI compared with standard blood pressure control (target mean arterial pressure >97mmHg), which suggested that blood pressure control has a crucial role in slowing the progression of renal and cardiac damage in ADPKD.
An epidemiological study compared blood pressure control and renal survival over two separate periods, 1985–1992 and 1992–2001, in 513 patients with ADPKD.89 The patients followed-up in the later period had significantly lower blood pressure, longer renal survival and greater use of ACE inhibitors than those followed during the initial period. Another study showed that the proportion of hypertensive patients with ADPKD who achieve blood pressure control of less than 140/90 mmHg has increased from 38% to 64% over a 15-year period.90 Education programs for patients with ADPKD and their primary-care physicians could have contributed to this improvement.
As performed in the studies by van Dijk and Zeltner, the comparison of an ACE inhibitor with a β-blocker, both of which inhibit the RAAS, might obscure the specific beneficial effects of ACE inhibition. On the other hand, a comparison of effects of using an ACE inhibitor (which inhibits the RAAS) and a diuretic (which stimulates the RAAS) to achieve blood pressure control could reveal a role of the RAAS apart from its effect on blood pressure. In a nonrandomized, prospective study in patients with ADPKD who had hypertension,91 the annual loss of renal function during a mean follow-up period of 5 years was greater in patients who were receiving diuretics without any ACE inhibitors than in patients who were taking ACE inhibitors without any diuretics (5.3ml/min/l.73 m2 versus 2.7ml/min/1.73m2, P<0.05), despite similar blood pressure control. Moreover, a significant increase in urinary protein excretion occurred in the diuretic group but not in the ACE-inhibitor group. These observations suggest that the RAAS contributes to the progression of renal disease in ADPKD in a manner that is independent of its effect on blood pressure.
In a multicenter, prospective, randomized clinical trial, both urinary albumin excretion and the decrease in creatinine clearance were significantly lower after 3 years of follow-up in patients with ADPKD who received the angiotensin-receptor blocker candesartan than in those who received the calcium-channel blocker amlodipine.92 These results were independent of blood pressure control.
Finally a meta-analysis of eight randomized trials that included 142 patients with ADPKD reported that regimens that included ACE inhibitors were more effective in lowering urinary protein excretion than were regimens without ACE inhibitors, and that this benefit was greatest in patients with high baseline levels of proteinuria.93 However, the overall rate of kidney disease progression was not significantly slower with ACE inhibitors than with other agents.
Cardiovascular effects
The early and effective treatment of hypertension is also important for the prevention of cardiovascular complications in ADPKD. Antihypertensive treatment with an ACE inhibitor reversed LVH over a 7-year follow-up period, which decreased an important risk factor for cardiovascular death in patients with ADPKD.94 A 7-year prospective, randomized study in 75 hypertensive patients with ADPKD and LVH compared the effects of rigorous and standard blood pressure control (<120/80 mmHg versus 135–140/85–90 mmHg) on LVH and renal function.95 Both strategies decreased LVH significantly; however, rigorous blood pressure control was significantly more effective in decreasing LVMI than was standard blood pressure control. In addition, significantly more patients in the rigorous-control group (71%) than in the standard-group (44%) achieved normal LVMI. A subgroup analysis showed that patients who received the ACE inhibitor enalapril experienced a significantly greater decrease in LVH than patients who received the calcium-channel blocker amlodipine, despite similar blood pressure control. A blood pressure goal of less than 120/80 mmHg and the use of an ACE inhibitor is, therefore, recommended for patients with ADPKD who have hypertension and LVH.
Optimal therapy
The ongoing HALT PKD (Halt Progression of Polycystic Kidney Disease) study is designed to determine whether combined therapy with an ACE inhibitor (lisinopril) and an angiotensin-receptor blocker (telmisartan) is superior to an ACE inhibitor alone in delaying the progression of renal disease, independent of blood pressure control, in patients with ADPKD.96 The HALT PKD study will also determine whether a low blood pressure target (95–110/65–75 mmHg) is superior to a standard blood pressure target (120–130/70–80 mmHg) in patients with preserved renal function.
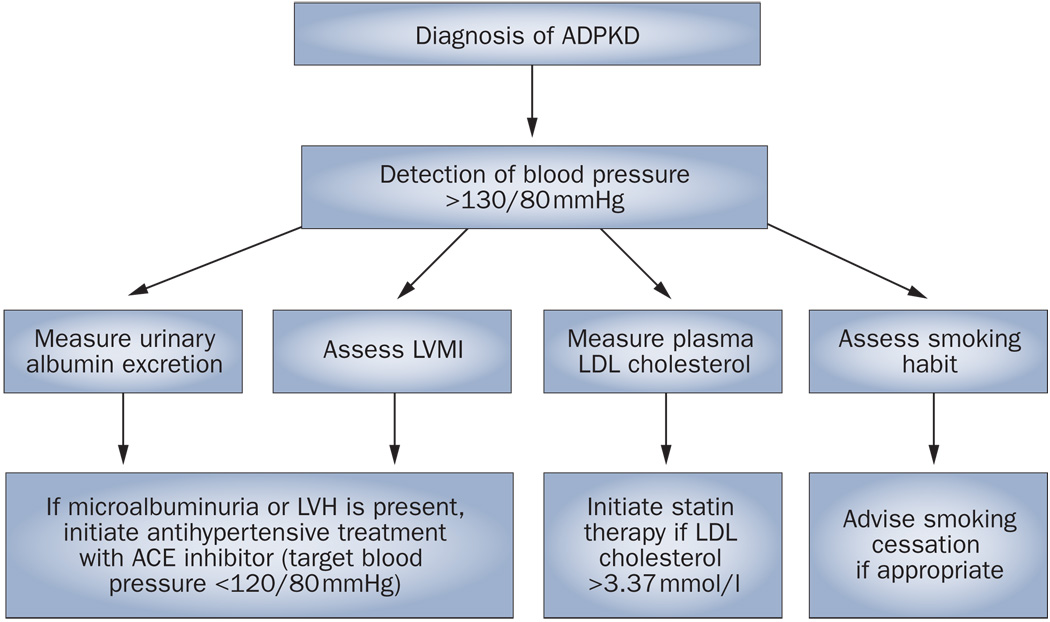
Until the results of this study are available, the optimal treatment of patients with ADPKD should include a blood pressure goal of 120/80 mmHg and the initiation of early RAAS inhibition if microalbuminuria or LVH is present97 (Figure 2). Furthermore, management of other cardiovascular risk factors, including smoking and dyslipidemia, is extremely important.
Figure 2.
An approach to assessment of cardiovascular risk factors and management in patients with autosomal-dominant polycystic kidney disease. If blood pressure is above 130/80 mmHg, urinary albumin excretion and plasma LDL cholesterol should be measured, LVMI should be calculated and smoking habit should be assessed. If microalbuminuria or LVH is present, blood pressure should be maintained below 120/80 mmHg with a regimen that includes an ACE inhibitor. Cessation of smoking and maintenance of plasma LDL cholesterol below 3.4mmol/l by use of statins is recommended. Abbreviations: ACE, angiotensin-converting enzyme; ADPKD, autosomal-dominant polycystic kidney disease, LVH, left ventricular hypertrophy; LVMI, left ventricular mass index.
Conclusions
Cardiovascular abnormalities are common in patients with ADPKD. Hypertension is a frequent finding and is associated with compromised cardiovascular and renal outcome. LVH, aneurysms and cardiac valvular defects are other cardiovascular manifestations of ADPKD. Early and effective treatment of hypertension, preferably with drugs that block the RAAS, is necessary to improve the cardiovascular and renal outcome of patients with ADPKD. The demonstration of vascular changes such as endothelial dysfunction and increased carotid intima-media thickness even in young patients with normal blood pressure and well-preserved renal function suggests that atherosclerosis starts very early during the course of this disease. Thus, aggressive control of other cardiovascular risk factors is also very important to improve the cardiovascular prognosis of affected patients.
Review criteria.
The PubMed database was searched with the terms “autosomal-dominant polycystic kidney disease”, “polycystic kidney disease”, “hypertension”, “cardiovascular”, “left ventricular hypertrophy” and “aneurysms”. No date or language restriction was placed on the search.
Footnotes
Competing interests
RW Schrier has declared associations with the following companies: Amgen, Astellas and Otsuka. See the article online for full details of the relationships. T Ecder declared no competing interests.
References
- 1.Ecder T. In: Diseases of the Kidney and Urinary Tract. Schriei RW, et al., editors. Philadelphia: Lippincott Williams & Wilkins; 2007. pp. 502–539. [Google Scholar]
- 2.PKD Foundation. Polycystic Kidney Disease: The Most Common Life-Threatening Genetic Disease. Kansas City: Polycystic Kidney Research Foundation; 2000. [Google Scholar]
- 3.Fick GM, et al. Causes of death In autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1995;5:2048–2056. doi: 10.1681/ASN.V5122048. [DOI] [PubMed] [Google Scholar]
- 4.Perrone RD, et al. Survival after end-stage renal disease In autosomal dominant polycystic kidney disease: contribution of extrarenal complications to mortality. Am. J. Kidney Dis. 2001;38:777–784. doi: 10.1053/ajkd.2001.27720. [DOI] [PubMed] [Google Scholar]
- 5.Chapman AB, Schrier RW. Pathogenesis of hypertension in autosomal dominant polycystic kidney disease. Semin. Nephrol. 1991;11:653–660. [PubMed] [Google Scholar]
- 6.Ecder T, Schrier RW. Hypertension in autosomal-dominant polycystic kidney disease: early occurrence and unique aspects. J. Am. Soc. Nephrol. 2001;12:194–200. doi: 10.1681/ASN.V121194. [DOI] [PubMed] [Google Scholar]
- 7.Gabow PA, et al. Factors affecting the progression of renal disease In autosomal-dominant polycystic kidney disease. Kidney Int. 1992;41:1311–1319. doi: 10.1038/ki.1992.195. [DOI] [PubMed] [Google Scholar]
- 8.Chapman AA et al. Left ventricular hypertrophy In autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1997;8:1292–1297. doi: 10.1681/ASN.V881292. [DOI] [PubMed] [Google Scholar]
- 9.Kelleher CL, et al. Characteristics of hypertension in young adults with autosomal dominant polycystic kidney disease compared with the general U.S. population. Am. J. Hypertens. 2004;17:1029–1034. doi: 10.1016/j.amjhyper.2004.06.020. [DOI] [PubMed] [Google Scholar]
- 10.Schrier RW, et al. The role of parental hypertension in the frequency and age of diagnosis of hypertension in offspring with autosomal-dominant polycystic kidney disease. Kidney Int. 2003;64:1792–1799. doi: 10.1046/j.1523-1755.2003.00264.x. [DOI] [PubMed] [Google Scholar]
- 11.Sedman A, et al. Autosomal dominant polycystic kidney disease In childhood: a longitudinal study. Kidney Int. 1987;31:1000–1005. doi: 10.1038/ki.1987.98. [DOI] [PubMed] [Google Scholar]
- 12.Fick GM, et al. The spectrum of autosomal dominant polycystic kidney disease In children. J. Am. Soc. Nephrol. 1994;4:1654–1660. doi: 10.1681/ASN.V491654. [DOI] [PubMed] [Google Scholar]
- 13.Ivy DD, et al. Cardiovascular abnormalities in children with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1995;5:2032–2036. doi: 10.1681/ASN.V5122032. [DOI] [PubMed] [Google Scholar]
- 14.Shamshirsaz A, et al. Autosomal-dominant polycystic kidney disease In infancy and childhood: progression and outcome. Kidney Int. 2005;68:2218–2224. doi: 10.1111/j.1523-1755.2005.00678.x. [DOI] [PubMed] [Google Scholar]
- 15.Gabow RA, et al. Renal structure and hypertension in autosomal dominant polycystic kidney disease. Kidney Int. 1990;38:1177–1180. doi: 10.1038/ki.1990.330. [DOI] [PubMed] [Google Scholar]
- 16.Graham RC, Lindop GBM. The anatomy of the renin-secreting cell In adult polycystic kidney disease. Kidney Int. 1988;33:1084–1090. doi: 10.1038/ki.1988.115. [DOI] [PubMed] [Google Scholar]
- 17.Torres VE, et al. Synthesis of renin by tubulocystic epithelium In autosomal-dominant polycystic kidney disease. Kidney Int. 1992;42:364–373. doi: 10.1038/ki.1992.297. [DOI] [PubMed] [Google Scholar]
- 18.Loghman-Adham M, et al. The intrarenal renin-angiotensin system in autosomal dominant polycystic kidney disease. Am. J. Physiol. Renal Physiol. 2004;287:F775–F788. doi: 10.1152/ajprenal.00370.2003. [DOI] [PubMed] [Google Scholar]
- 19.Chapman AB, et al. The renin-angiotensin-aldosterone system and autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1990;323:1091–1096. doi: 10.1056/NEJM199010183231602. [DOI] [PubMed] [Google Scholar]
- 20.Torres VE, et al. Effect of Inhibition of converting enzyme on renal hemodynamics and sodium management In polycystic kidney disease. Mayo Clin. Proc. 1991;66:1010–1017. doi: 10.1016/s0025-6196(12)61724-8. [DOI] [PubMed] [Google Scholar]
- 21.Watson ML, et al. Effects of angiotensin converting enzyme inhibition in adult polycystic kidney disease. Kidney Int. 1991;41:206–210. doi: 10.1038/ki.1992.28. [DOI] [PubMed] [Google Scholar]
- 22.Doulton TW, et al. The effect of sodium and angiotensin-converting enzyme inhibition on the classic circulating renin-angiotensin system in autosomal-dominant polycystic kidney disease patients. J. Hypertens. 2006;24:939–945. doi: 10.1097/01.hjh.0000222765.30348.0d. [DOI] [PubMed] [Google Scholar]
- 23.Harrap SB, et al. Renal, cardiovascular and hormonal characteristics of young adults with autosomal dominant polycystic kidney disease. Kidney Int. 1991;40:501–508. doi: 10.1038/ki.1991.238. [DOI] [PubMed] [Google Scholar]
- 24.Barrett BJ, et al. Differences in hormonal and renal vascular responses between normotensive patients with autosomal dominant polycystic kidney disease and unaffected family members. Kidney Int. 1994;46:1118–1123. doi: 10.1038/ki.1994.374. [DOI] [PubMed] [Google Scholar]
- 25.Torres VE, et al. Natriuretic response to volume expansion in polycystic kidney disease. Mayo Clin. Proc. 1989;64:509–515. doi: 10.1016/s0025-6196(12)65554-2. [DOI] [PubMed] [Google Scholar]
- 26.Klein IH, et al. Sympathetic activity is increased in polycystic kidney disease and is associated with hypertension. J. Am. Soc. Nephrol. 2001;12:2427–2433. doi: 10.1681/ASN.V12112427. [DOI] [PubMed] [Google Scholar]
- 27.Bakris G, et al. Role of vasopressin In essential hypertension: racial differences. J. Hypertens. 1997;15:545–550. doi: 10.1097/00004872-199715050-00011. [DOI] [PubMed] [Google Scholar]
- 28.Fernandes S, et al. Chronic V2 vasopressin receptor stimulation Increases basal blood pressure and exacerbates deoxycorticosterone acetate-salt hypertension. Endocrinology. 2002;143:2759–2766. doi: 10.1210/endo.143.7.8918. [DOI] [PubMed] [Google Scholar]
- 29.Torres VE. Vasopressin antagonists In polycystic kidney disease. Kidney Int. 2005;68:2405–2418. doi: 10.1111/j.1523-1755.2005.00703.x. [DOI] [PubMed] [Google Scholar]
- 30.Danielsen H, et al. Expansion of extracellular volume in early polycystic kidney disease. Acta Med. Scand. 1986;219:399–405. doi: 10.1111/j.0954-6820.1986.tb03330.x. [DOI] [PubMed] [Google Scholar]
- 31.Hocher B, et al. Renal endothelin system in polycystic kidney disease. J. Am. Soc. Nephrol. 1998;9:1169–1177. doi: 10.1681/ASN.V971169. [DOI] [PubMed] [Google Scholar]
- 32.Munemura C, et al. Epidermal growth factor and endothelin In cyst fluid from autosomal dominant polycystic kidney disease cases: Possible evidence of heterogeneity in cystogenesis. Am. J. Kidney Dis. 1994;24:561–568. doi: 10.1016/s0272-6386(12)80212-5. [DOI] [PubMed] [Google Scholar]
- 33.Giusti R, et al. Plasma concentration of endothelin and arterial pressure In patients with ADPKD. Contrib. Nephrol. 1995;115:118–121. doi: 10.1159/000424407. [DOI] [PubMed] [Google Scholar]
- 34.Wang D, et al. Endothelium-dependent relaxation of small resistance vessels Is impaired In patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 2000;11:1371–1376. doi: 10.1681/ASN.V1181371. [DOI] [PubMed] [Google Scholar]
- 35.Wang D, et al. Endothelial dysfunction and reduced nitric oxide in resistance arteries In autosomal dominant polycystic kidney disease. Kidney Int. 2003;64:1381–1388. doi: 10.1046/j.1523-1755.2003.00236.x. [DOI] [PubMed] [Google Scholar]
- 36.Al-Nimri MA, et al. Endothelium-derived vasoactive mediators in polycystic kidney disease. Kidney Int. 2003;63:1776–1784. doi: 10.1046/j.1523-1755.2003.00913.x. [DOI] [PubMed] [Google Scholar]
- 37.Merta M, et al. Role of endothelin and nitric oxide in the pathogenesis of arterial hypertension in autosomal dominant polycystic kidney disease. Physiol. Res. 2003;52:433–437. [PubMed] [Google Scholar]
- 38.Wang D, et al. Asymmetric dimethylarginine and lipid peroxidation products In early autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2008;51:184–191. doi: 10.1053/j.ajkd.2007.09.020. [DOI] [PubMed] [Google Scholar]
- 39.Kocaman O, et al. Endothelial dysfunction and increased carotid intlma-media thickness in patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2004;43:854–860. doi: 10.1053/j.ajkd.2004.01.011. [DOI] [PubMed] [Google Scholar]
- 40.Turkmen K, et al. Coronary flow velocity reserve and carotid intima media thickness in patients with autosomal dominant polycystic kidney disease: from impaired tubules to impaired carotid and coronary arteries. Clin. J. Am. Soc. Nephrol. 2008;3:986–991. doi: 10.2215/CJN.02330607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Borresen ML, et al. Pulse wave reflection Is amplified in normotensive patients with autosomal-dominant polycystic kidney disease and normal renal function. Am. J. Nephrol. 2007;27:240–246. doi: 10.1159/000101369. [DOI] [PubMed] [Google Scholar]
- 42.Griffin MD, et al. Vascular expression of polycystin. J. Am. Soc. Nephrol. 1997;8:616–626. doi: 10.1681/ASN.V84616. [DOI] [PubMed] [Google Scholar]
- 43.Torres VE, et al. Vascular expression of polycystin-2. J. Am. Soc. Nephrol. 2001;12:1–9. doi: 10.1681/ASN.V1211. [DOI] [PubMed] [Google Scholar]
- 44.Koren MJ, et al. Relationship of left ventricular mass and geometry to morbidity and mortality In uncomplicated essential hypertension. Ann. Intern. Med. 1991;114:345–352. doi: 10.7326/0003-4819-114-5-345. [DOI] [PubMed] [Google Scholar]
- 45.Zeier M, et al. Elevated blood pressure profile and left ventricular mass in children and young adults with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1993;3:1451–1457. doi: 10.1681/ASN.V381451. [DOI] [PubMed] [Google Scholar]
- 46.Bardaji A, et al. Cardiac involvement in autosomal dominant polycystic kidney disease: a hypertensive heart disease. Clin. Nephrol. 2002;56:211–220. [PubMed] [Google Scholar]
- 47.Cadnapaphornchai MA, et al. Increased left ventricular mass In children with autosomal dominant polycystic kidney disease and borderline hypertension. Kidney Int. 2008;74:1192–1196. doi: 10.1038/ki.2008.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Saggar-Malik A, et al. Left ventricular mass In normotensive subjects with autosomal dominant polycystic kidney disease. BMJ. 1994;309:1617–1618. doi: 10.1136/bmj.309.6969.1617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Bardaji A, et al. Left ventricular mass and diastolic function in normotensive young adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 1998;32:970–975. doi: 10.1016/s0272-6386(98)70071-x. [DOI] [PubMed] [Google Scholar]
- 50.Oflaz H, et al. Biventricular diastolic dysfunction in patients with autosomal dominant polycystic kidney disease. Kidney Int. 2005;68:2244–2249. doi: 10.1111/j.1523-1755.2005.00682.x. [DOI] [PubMed] [Google Scholar]
- 51.Verdecchia P, et al. Circadian blood pressure changes and left ventricular hypertrophy in essential hypertension. Circulation. 1990;81:528–536. doi: 10.1161/01.cir.81.2.528. [DOI] [PubMed] [Google Scholar]
- 52.Li Kam Wa TC, et al. Ambulatory blood pressure in hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 1997;12:2075–2080. doi: 10.1093/ndt/12.10.2075. [DOI] [PubMed] [Google Scholar]
- 53.Valero FA, et al. Ambulatory blood pressure and left ventricular mass in normotensive patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1999;10:1020–1026. doi: 10.1681/ASN.V1051020. [DOI] [PubMed] [Google Scholar]
- 54.Martinez-Vea A, et al. Exercise blood pressure, cardiac structure, and diastolic function in young normotensive patients with polycystic kidney disease: a prehypertensive state. Am. J. Kidney Dis. 2004;44:216–223. doi: 10.1053/j.ajkd.2004.04.026. [DOI] [PubMed] [Google Scholar]
- 55.Almeida EA, et al. Tissue Doppler Imaging In the evaluation of left ventricular function In young adults with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2006;47:587–592. doi: 10.1053/j.ajkd.2005.12.023. [DOI] [PubMed] [Google Scholar]
- 56.Phillips RA, et al. Relation among left ventricular mass, insulin resistance, and blood pressure in nonobese subjects. J. Clin. Endocrinol. Metab. 1998;83:4284–4288. doi: 10.1210/jcem.83.12.5331. [DOI] [PubMed] [Google Scholar]
- 57.Ohya Y, et al. Hyperinsulinemia and left ventricular geometry in a work-site population In Japan. Hypertension. 1996;27:729–734. doi: 10.1161/01.hyp.27.3.729. [DOI] [PubMed] [Google Scholar]
- 58.Vareesangthip K, et al. Insulin resistance in adult polycystic kidney disease. Kidney Int. 1997;52:503–508. doi: 10.1038/ki.1997.360. [DOI] [PubMed] [Google Scholar]
- 59.Lumiaho A, et al. Insulin resistance is related to left ventricular hypertrophy in patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 2003;41:1219–1224. doi: 10.1016/s0272-6386(03)00354-8. [DOI] [PubMed] [Google Scholar]
- 60.Rocchini AP, et al. Hyperinsulinemia and the aldosterone and pressor responses to angiotensin II. Hypertension. 1990;15:861–866. doi: 10.1161/01.hyp.15.6.861. [DOI] [PubMed] [Google Scholar]
- 61.Lembo G, et al. Abnormal sympathetic overactivity evoked by Insulin In the skeletal muscle of patients with essential hypertension. J. Clin. Invest. 1992;90:24–29. doi: 10.1172/JCI115842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Gharavi AG, et al. Deletion polymorphism of the angiotensin-converting enzyme gene is independently associated with left ventricular mass and geometric remodeling In systemic hypertension. Am. J. Cardiol. 1996;77:1315–1319. doi: 10.1016/s0002-9149(96)00198-1. [DOI] [PubMed] [Google Scholar]
- 63.Estado RO, et al. Deletion polymorphism of the angiotensin-converting enzyme gene is associated with an Increase in left ventricular mass in men with type 2 diabetes mellitus. Am. J. Hypertens. 1999;12:637–643. doi: 10.1016/s0895-7061(99)00013-8. [DOI] [PubMed] [Google Scholar]
- 64.Baboolal K, et al. Association of the angiotensin I converting enzyme gene deletion polymorphism with early onset of ESRF in PKD1 adult polycystic kidney disease. Kidney Int. 1997;52:607–613. doi: 10.1038/ki.1997.373. [DOI] [PubMed] [Google Scholar]
- 65.Perez-Oiler L, et al. Influence of the ACE gene polymorphism In the progression of renal failure in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 1999;34:273–278. doi: 10.1016/s0272-6386(99)70355-0. [DOI] [PubMed] [Google Scholar]
- 66.Van Dijk MA, et al. The ACE Insertion/deletion polymorphism has no Influence on progression of renal function loss In autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2000;15:836–839. doi: 10.1093/ndt/15.6.836. [DOI] [PubMed] [Google Scholar]
- 67.Schlavello T, et al. Angiotensin-Converting enzyme activity and the ACE Alu polymorphism in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2001;16:2323–2327. doi: 10.1093/ndt/16.12.2323. [DOI] [PubMed] [Google Scholar]
- 68.Ecder T, et al. No effect of angiotensin-converting enzyme gene polymorphism on disease progression and left ventricular hypertrophy in autosomal dominant polycystic kidney disease. Am. J. Nephrol. 2003;23:466–470. doi: 10.1159/000074653. [DOI] [PubMed] [Google Scholar]
- 69.Chapman AB, et al. Intracranial aneurysms in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1992;327:916–920. doi: 10.1056/NEJM199209243271303. [DOI] [PubMed] [Google Scholar]
- 70.Ruggleri PM, et al. Occult intracranial aneurysms in polycystic kidney disease: screening with MR angiography. Radiology. 1994;191:33–39. doi: 10.1148/radiology.191.1.8134594. [DOI] [PubMed] [Google Scholar]
- 71.Graf S, et al. Intracranial aneurysms and dolichoectasia in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2002;17:819–823. doi: 10.1093/ndt/17.5.819. [DOI] [PubMed] [Google Scholar]
- 72.Hadimeri H, et al. Coronary aneurysms In patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1998;9:837–841. doi: 10.1681/ASN.V95837. [DOI] [PubMed] [Google Scholar]
- 73.Kanagasundaram NS, et al. Aneurysm of the splenic artery in a patient with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 1999;14:183–184. doi: 10.1093/ndt/14.1.183. [DOI] [PubMed] [Google Scholar]
- 74.Torra R, et al. Abdominal aortic aneurysms and autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1996;7:2483–2486. doi: 10.1681/ASN.V7112483. [DOI] [PubMed] [Google Scholar]
- 75.Belz MM, et al. Familial clustering of ruptured intracranial aneurysms in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2001;38:770–776. doi: 10.1053/ajkd.2001.27694. [DOI] [PubMed] [Google Scholar]
- 76.Belz MM, et al. Recurrence of intracranial aneurysms in autosomal-dominant polycystic kidney disease. Kidney Int. 2003;63:1824–1830. doi: 10.1046/j.1523-1755.2003.00918.x. [DOI] [PubMed] [Google Scholar]
- 77.Schrier RW. Optimal care of autosomal dominant polycystic kidney disease patients. Nephrology. 2006;11:124–130. doi: 10.1111/j.1440-1797.2006.00535.x. [DOI] [PubMed] [Google Scholar]
- 78.Schrier RW, et al. Repeat Imaging for intracranial aneurysms in patients with autosomal dominant polycystic kidney disease with initially negative studies: a prospective ten-year follow-up. J. Am. Soc. Nephrol. 2004;15:1023–1028. doi: 10.1097/01.asn.0000118527.74850.66. [DOI] [PubMed] [Google Scholar]
- 79.Leier CV, et al. Cardiovascular abnormalities associated with adult polycystic kidney disease. Ann. Intern. Med. 1984;100:683–688. doi: 10.7326/0003-4819-100-5-683. [DOI] [PubMed] [Google Scholar]
- 80.Hossack KF, et al. Echocardiographic findings in autosomal dominant polycystic kidney disease. N. Engl. J. Med. 1988;319:907–912. doi: 10.1056/NEJM198810063191404. [DOI] [PubMed] [Google Scholar]
- 81.Timio M, et al. The spectrum of cardiovascular abnormalities in autosomal dominant polycystic kidney disease: a 10-year follow-up in a five-generation kindred. Clin. Nephrol. 1992;37:245–251. [PubMed] [Google Scholar]
- 82.Lumiaho A, et al. Mitral valve prolapse and mitral regurgitation are common In patients with polycystic kidney disease type 1. Am. J. Kidney Dis. 2001;38:1208–1216. doi: 10.1053/ajkd.2001.29216. [DOI] [PubMed] [Google Scholar]
- 83.Klahr S, et al. Dietary protein restriction, blood pressure control, and the progression of polycystic kidney disease. J. Am. Soc. Nephrol. 1995;5:2037–2047. doi: 10.1681/ASN.V5122037. [DOI] [PubMed] [Google Scholar]
- 84.Maschio G, et al. Effect of the angiotensin-converting-enzyme inhibitor benazepril on the progression of chronic renal insufficiency. N. Engl. J. Med. 1996;334:939–945. doi: 10.1056/NEJM199604113341502. [DOI] [PubMed] [Google Scholar]
- 85.Ecder T, et al. Effect of antihypertensive therapy on renal function and urinary albumin excretion in hypertensive patients with autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2000;35:427–432. doi: 10.1016/s0272-6386(00)70195-8. [DOI] [PubMed] [Google Scholar]
- 86.Chapman AB, et al. Overt proteinuria and microalbuminuria In autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. 1994;5:1349–1354. doi: 10.1681/ASN.V561349. [DOI] [PubMed] [Google Scholar]
- 87.Van Dijk MA, et al. No effect of enalapril on progression in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2003;18:2314–2320. doi: 10.1093/ndt/gfg417. [DOI] [PubMed] [Google Scholar]
- 88.Zeltner R, et al. Renal and cardiac effects of antihypertensive treatment with ramipril vs metoprolol in autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 2008;23:573–579. doi: 10.1093/ndt/gfm731. [DOI] [PubMed] [Google Scholar]
- 89.Schrier RW, et al. Epidemiological study of kidney survival in autosomal dominant polycystic kidney disease. Kidney Int. 2003;63:678–685. doi: 10.1046/j.1523-1755.2003.00776.x. [DOI] [PubMed] [Google Scholar]
- 90.Ecder T, et al. Progress in the blood pressure control in autosomal dominant polycystic kidney disease. Am. J. Kidney Dis. 2000;36:266–271. doi: 10.1053/ajkd.2000.8970. [DOI] [PubMed] [Google Scholar]
- 91.Ecder T, et al. Diuretics versus angiotensin-converting enzyme inhibitors In autosomal dominant polycystic kidney disease. Am. J. Nephrol. 2001;21:98–103. doi: 10.1159/000046231. [DOI] [PubMed] [Google Scholar]
- 92.Nutahara K, et al. Calcium channel blocker versus angiotensin II receptor blocker in autosomal dominant polycystic kidney disease. Nephron Clin. Pract. 2005;99:c18–c23. doi: 10.1159/000081790. [DOI] [PubMed] [Google Scholar]
- 93.Jafar TH, et al. The effect of angiotensin-converting enzyme Inhibitors on progression of advanced polycystic kidney disease. Kidney Int. 2005;67:265–271. doi: 10.1111/j.1523-1755.2005.00077.x. [DOI] [PubMed] [Google Scholar]
- 94.Ecder T, et al. Reversal of left ventricular hypertrophy with angiotensin converting enzyme Inhibition In hypertensive patients with autosomal dominant polycystic kidney disease. Nephrol. Dial. Transplant. 1999;14:1113–1116. doi: 10.1093/ndt/14.5.1113. [DOI] [PubMed] [Google Scholar]
- 95.Schrier R, et al. Cardiac and renal effects of standard versus rigorous blood pressure control in autosomal-dominant polycystic kidney disease: results of a seven-year prospective randomized study. J. Am. Soc. Nephrol. 2002;13:1733–1739. doi: 10.1097/01.asn.0000018407.60002.b9. [DOI] [PubMed] [Google Scholar]
- 96.Chapman AB. Approaches to testing new treatments In autosomal dominant polycystic kidney disease: Insights from the CRISP and HALT-PKD studies. Clin. J. Am. Soc. Nephrol. 2008;3:1197–1204. doi: 10.2215/CJN.00060108. [DOI] [PubMed] [Google Scholar]
- 97.Schrier RW. Renal volume, renin-angiotensin-aldosterone system, hypertension and left ventricular hypertrophy in patients with autosomal dominant polycystic kidney disease. J. Am. Soc. Nephrol. doi: 10.1681/ASN.2008080882. (In press). [DOI] [PubMed] [Google Scholar]