Abstract
The cyclization reaction of an epoxyalcohol is catalyzed by a synthetic cavitand receptor with an inwardly-directed carboxylic acid function. The receptor features a hydrophobic pocket in which the substrate is bound and positioned to react in a regioselective manner. The nature of this substrate-catalyst complex and its dynamic properties were investigated by NMR methods and with the aid of a model compound lacking the epoxide function. The kinetic parameters of the cyclization reaction were also studied. A catalytic cycle is proposed and diverse inhibition mechanisms are identified that parallel those encounterd in enzymology.
Introduction
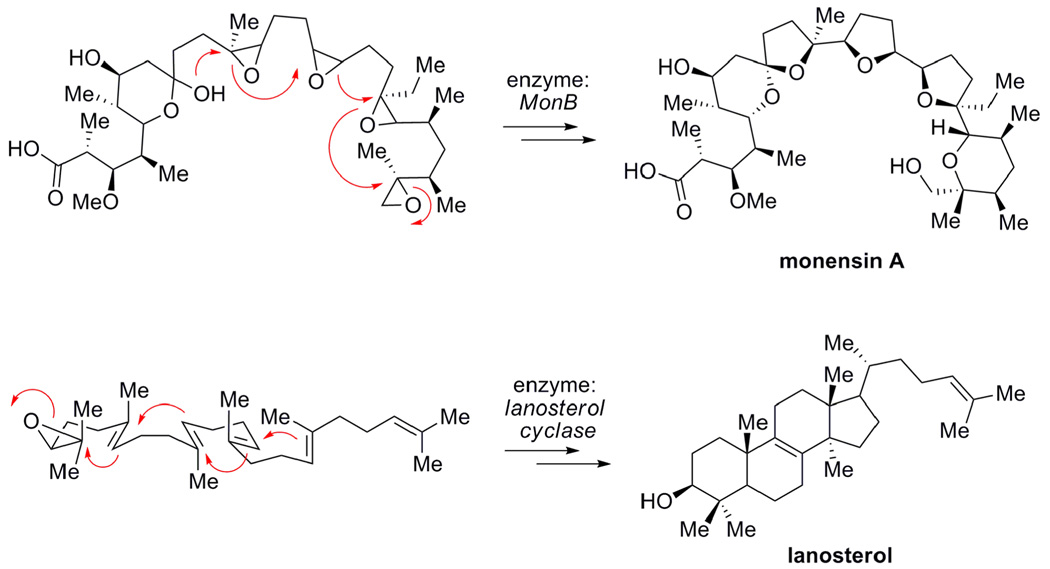
A recent perspective by Ringe and Petsko1 identifies the key features of catalysis that have emerged from the study of enzymes over the last decades. These comprise positioning of reactive species, isolation in a special microenvironment, distortion of the substrate to a reactive shape and stabilizing transition states and intermediates that channel the reaction along a specific path. Another proposal implicates the formation of covalent bonds between enzyme and substrate in cases where enormous rate effects are encountered.2 We describe here the application of cavitands to epoxide opening cyclization reactions. These systems show some of the features responsible for enzyme catalysis of these cascade transformations which are widespread in nature3 (Figure 1): (1) the binding of a substrate into conformations resembling the transition structures of the cyclization reactions, (2) initiating the epoxide ring-opening events by Brønsted acid catalysis, (3) accelerating the reaction rates by stabilizing cationic intermediates, and (4) shielding these reactive species from capture by external nucleophiles.4
Figure 1.
Epoxide ring-opening cyclizations in the biosynthesis of polyether and terpene natural products.
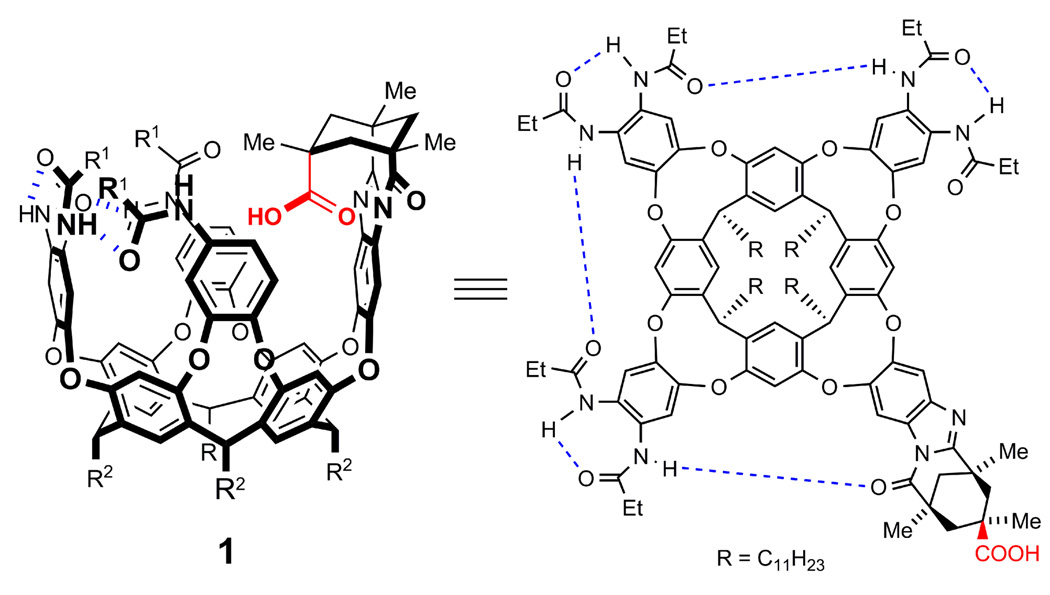
Cavitand 15 is a supramolecular receptor that is closed at one end and open to the bulk solution at the other (Figure 2). A resorcinarene base and four aromatic walls form a deep cylindrical π-surface that provides an isolated environment similar to the interiors of enzymes.6 An inwardly-directed Kemp’s triacid7 derivative can stabilize a host-guest complex through hydrogen bonding interactions,8 while also providing a source of Brønsted acid-base chemistry. Six secondary amides confer a cyclic array of hydrogen bonds around the polar rim of the cavitand and stabilize the receptor in its vase-like conformation.9
Figure 2.
Cavitand 1 depicted in its folded vase-like conformation (left, some amide groups were omitted for clarity) and as its detailed Lewis structure (right).
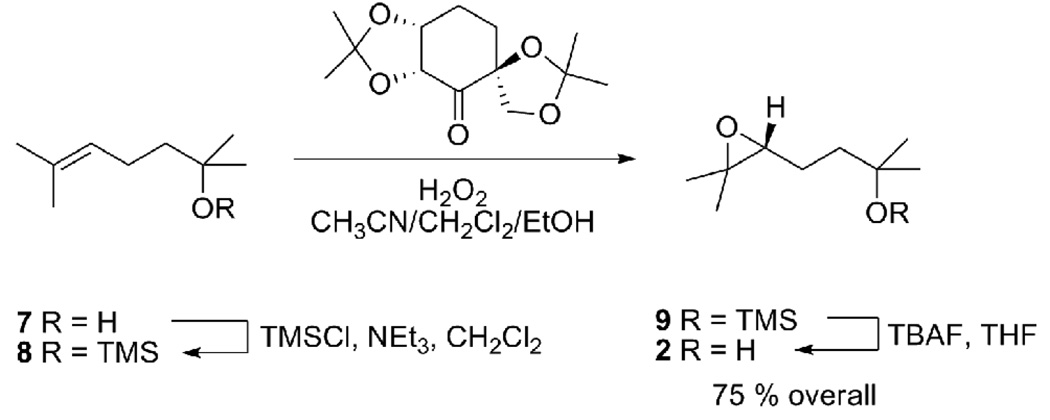
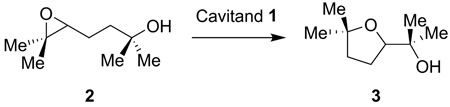
We recently demonstrated that cavitand 1 promotes highly regioselective epoxide ring-opening cyclization reactions.10 Upon binding the 1,5-epoxyalcohol 2, the receptor catalyzed the formation of the five-membered ring ether 3 as the exclusive product of the reaction (eq. 1). Here, we discuss the mechanistic details of this transformation and describe the scope and limitations of cavitand 1 as a functional mimic of enzymes. We chose 1,5-epoxy alcohol 2 as the substrate in this mechanistic study due to the high regioselectivity11 and slow reaction rate observed in its cyclization reaction within cavitand 1.
 |
(1) |
Results and Discussion
The binding of epoxyalcohol 2 within cavitand 1 involves molecular recognition. This event is driven in part by the desolvation of both the cavitand and epoxyalcohol molecule, and also the attractive non-covalent interactions between the host and the bound guest12, 6 (dispersion forces, CH-π and cation-π interactions, and hydrogen bonding to the inwardly-directed acid).
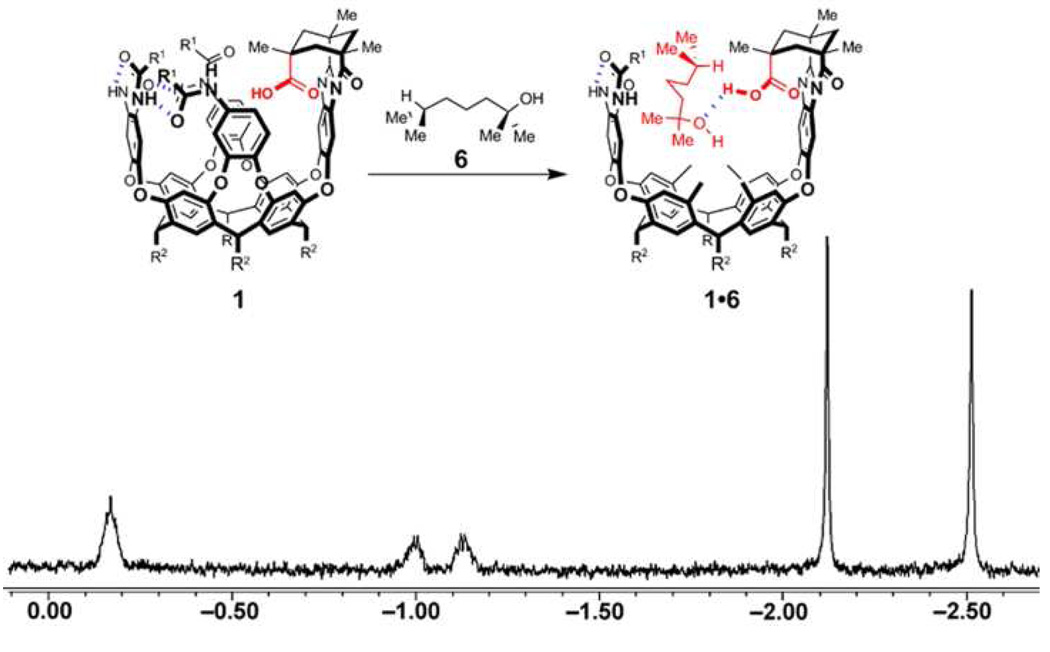
The thermodynamic parameters involved in the host-guest complexation of cavitand 1 and 1,5-epoxyalcohol 2 were investigated. Cavitand 4 is a receptor that bears eight secondary propanoyl amides around its rim and lacks an inwardly-directed carboxylic acid functionality.9 When a solution of substrate 2 in mesitylene-d12 (a solvent to large to compete for the space inside) was added to cavitand 4, no host-guest complexation was observed by 1H NMR spectroscopy (eq. 2). Neither was host-guest complexation observed when cavitand 1 was treated with a solution of the substrate analog 5, an alkyl epoxide lacking a hydroxyl functionality (eq. 3). When a solution of 6, an alcohol lacking an epoxide function was added to cavitand 1 in mesitylene-d12, we observed immediate host-guest complexation as indicated by the characteristic signals in the upfield region of the 1H NMR spectrum (Figure 3). These results indicated that entropic effects alone cannot account for the binding of 1,5-epoxyalcohol 2 by cavitand 1 and that this complexation is primarily driven by the enthalpic benefits of forming hydrogen bonding interactions between the inwardly-directed acid of the host and the hydroxyl group of the guests.13
Figure 3.
Upfield region of the 1H NMR spectrum of the host-guest complex formed between cavitand 1 and alcohol 6.
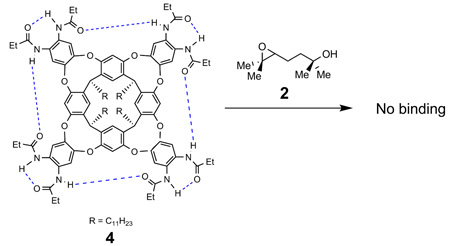 |
(2) |
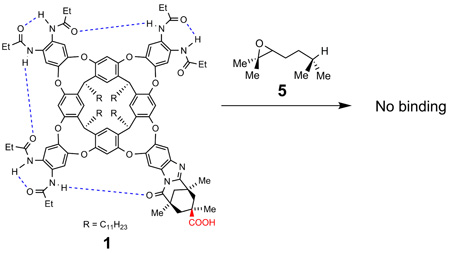 |
(3) |
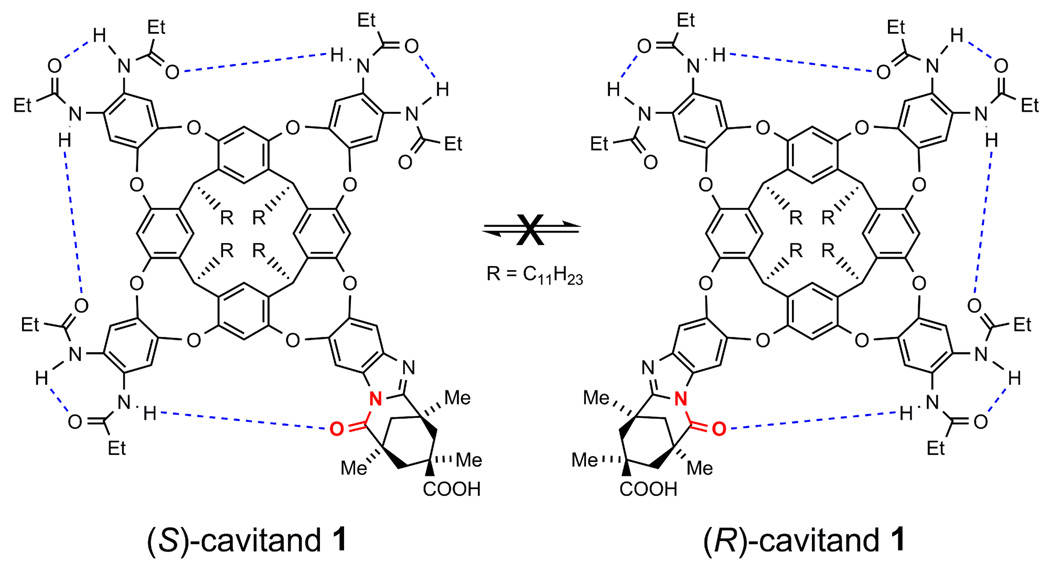
The stereochemical properties of this system are complex. As described above, cavitand 1 is stabilized by a seam hydrogen bonds conferred from six secondary amides positioned around the rim of the vaselike receptor. A clockwise and counterclockwise directionality in this hydrogen bond acceptor-to-donor network is established by the amide carbonyl of the Kemp’s triacid derivative (Scheme 1). This directionality renders cavitand 1 chiral, and any host-guest complexes it makes with chiral guests are accordingly diastereomeric. In practice, the inner space of the folded cavitand is not very asymmetric either in the steric sense (the cavitands surfaces are mostly flat π bonds) or magnetic sense.14 Except when otherwise stated, the studies represented in this article represent host-guest complexes between racemic 1 and racemic 1,5-epoxyalcohol 2.
Scheme 1.
Non-interconvertable Cycloenantiomers of Cavitand 1.
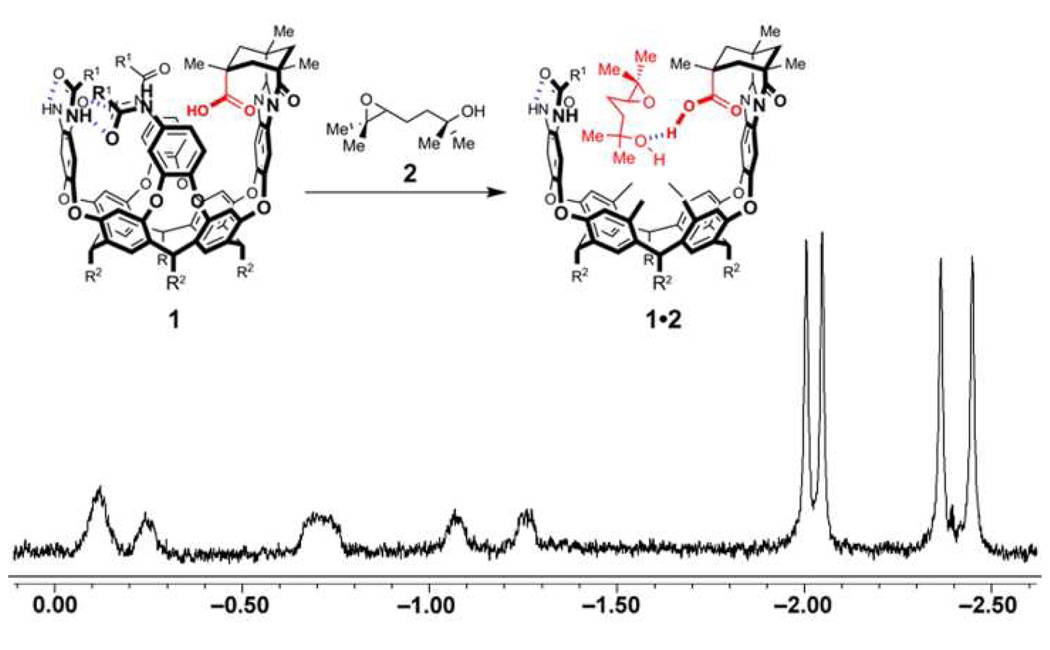
Upon treating cavitand 1 with a solution of 1,5-epoxyalcohol 2 in mesetylene-d12, the upfield region in the 1H NMR spectrum of the initial complex displays four prominent singlet resonances (δ = −1.9, −2.0, −2.3 and −2.4 ppm) with approximately the same integral values. These represent methyl substituents on substrate 2 (Figure 4). Since epoxyalcohol 2 contains two sets of geminal diasterotopic methyls (one set at the epoxide terminus and another at the alcohol terminus), it was unclear whether these four signals in the 1H NMR represented all four methyl substituents, or only one pair of geminal methyl groups from the two possible diastereomeric complexes formed upon binding a racemic 2 by racemic cavitand 1.
Figure 4.
Selected upfield region of the 1H NMR spectrum of host-guest complex 1•2.
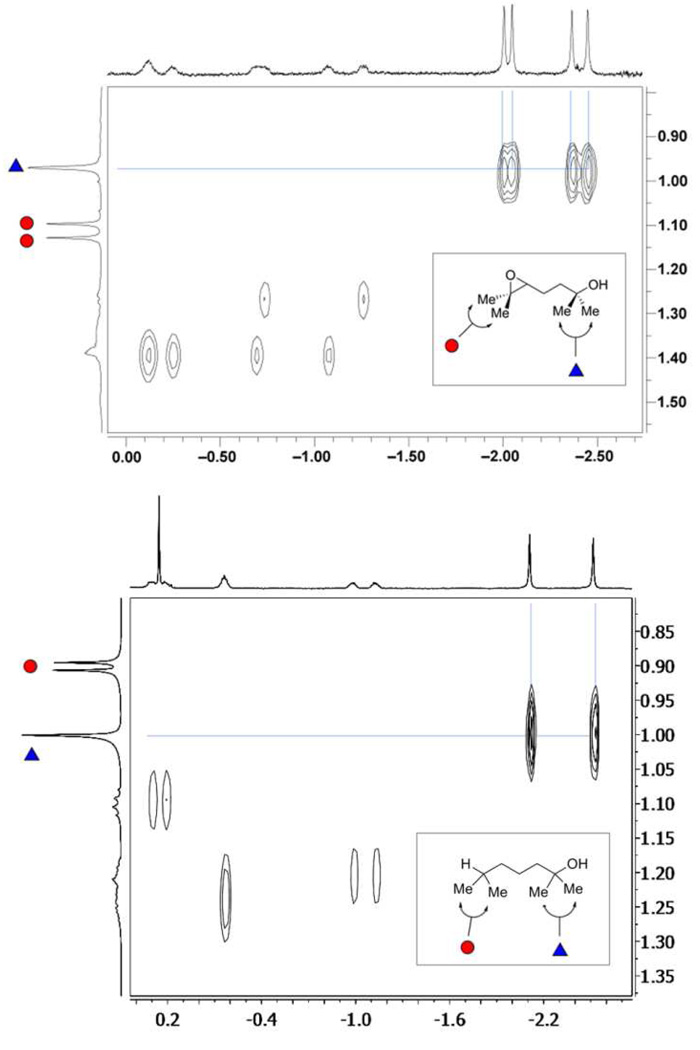
Analysis of complex 1•2 by 2D NOESY showed chemical exchange cross-peaks between all four upfield singlet signals from the bound substrate and a broad singlet resonance at δ = 0.99 ppm, which represented one pair of geminal methyl substituents on the unbound epoxide (Figure 5). HMBC and HMQC spectroscopy (see Supporting Information) of the 1,5-epoxyalcohol 2 in mesitylene-d12 allowed the assignment of the resonance at δ = 0.99 ppm to the geminal methyl substituents from the alcohol terminus of substrate 2. Similarly, the upfield sharp resonances observed in complex 1•6 showed exchange with the methyl groups at the alcohol terminus of 6 (δ = 1.00 ppm).
Figure 5.
Expansions of the 2D NOESY (600 MHz, mesitylene-d12) spectra of complexes 1•2 (top) and 1•6 (bottom) formed by treatment of cavitand 1 to a solution of either 2 or 6 at 300 K. This region shows chemical exchange cross-peaks between the singlet resonances from the methyl groups of the bound guest and one pair of geminal methyl groups of the unbound substrate (a spectrum of free guests 2 and 6 in mesitylene-d12 is shown in the vertical axis). Note that whereas the diastereotopic methyl groups at the epoxide terminus of 2 appear as two singlets at δ 1.13 and 1.15 ppm, the methyl substituents on the aliphatic region of 6 show up as a single doublet (J = 6.7 Hz) at δ0.90 ppm.
Taken together, these NMR experiments indicate that substrate 2 is bound with the hydroxyl function in contact with the acid and the methyls near the alcohol directed deep into the cavity of receptor 1. The spectrum of complex 1•2 bears a strong resemblance to the complex formed from racemic 1 and achiral alcohol 6, with the caveat that this spectrum is simplified by virtue of 1•6 existing as a mixture of indistinguishable enantiomers (compare Figure 3 and Figure 4).15
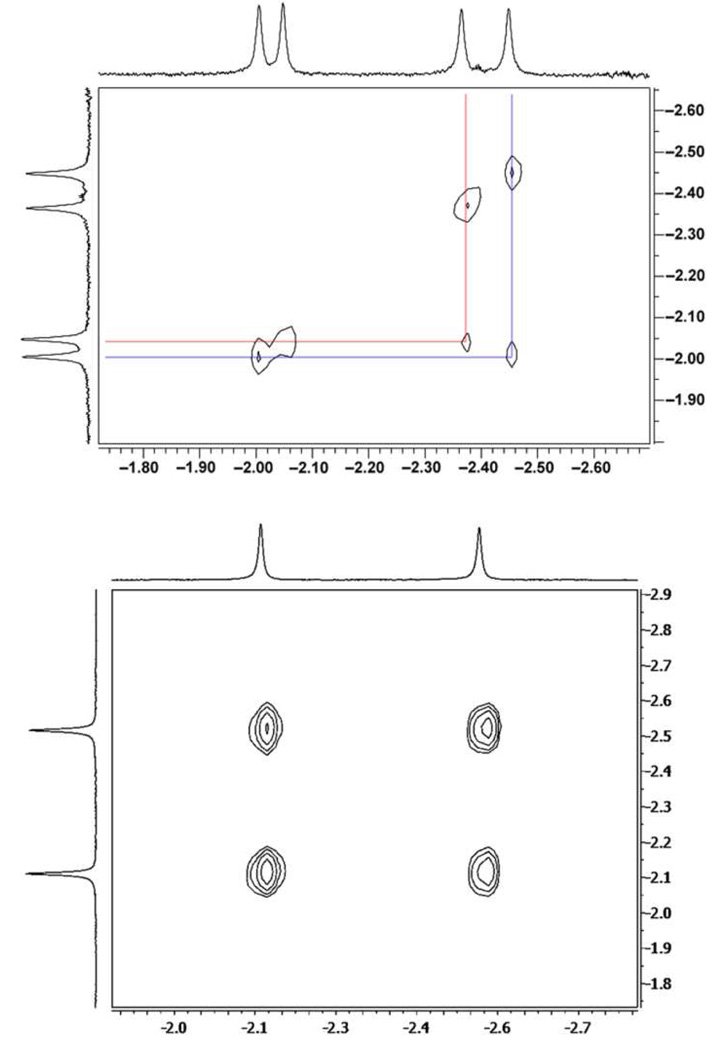
Further analysis of the NMR spectra revealed the existence of the two isomeric complexes on both systems (Figure 6). The 2D NOESY spectrum of complex 1•6 shows chemical exchange cross peaks between the two buried methyl groups (δ = −2.11, −2.52 ppm) which are rendered diastereotopic upon binding by virtue of their different stereochemical environments: one of them is deep in the resorcinarene cone and is further shifted upfield (Δδ = 0.41 ppm) than the other which is directed towards one of the walls and is less shielded (Figure 7).16 Since the two observed species only differ in the orientation of the guest within the host cavity we can consider them conformational isomers. The upfield methyl resonances are doubled in the case of complex 1•2 due to the formation of true diastereomeric complexes (configurational isomers). Since it is not possible to racemize either cavitand 1 or epoxyalcohol 2 only two chemical exchange cross peaks are observed (indicating that this process is occurring at a faster rate than the catalyzed reaction), one between the singlet resonances at δ = −1.98 and −2.46 ppm and another between the singlet resonances at δ = −2.04 and −2.38 ppm. Although these methyl groups are already diastereotopic in the free guest in a strict sense, they are isochronous in practice (Figure 5) and are only resolved when bound within 1 because of their different positioning in the aromatic cavity as is the case with 6. The species showing chemical exchange have again Δδ values around 0.4 ppm whereas the change in the configuration of 2 results in less than 0.1 ppm difference.
Figure 6.
Expansions of the 2D NOESY spectra (600 MHz, mesitylene-d12, 300 K) of complexes 1•2 (top) and 1•6 (bottom). This region shows chemical exchange cross-peaks between the methyl substituents at the alcohol terminus on guests 2 and 6 in two different stereoelectronic environments.
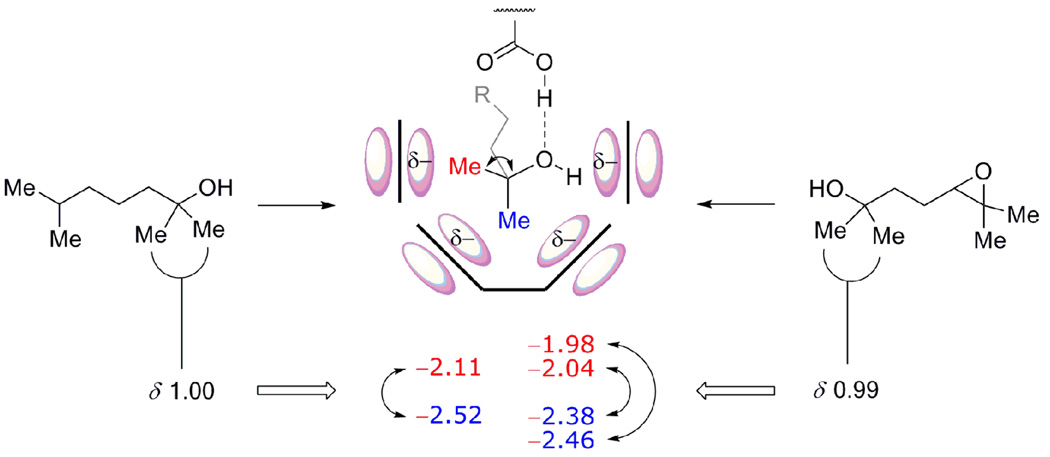
Figure 7.
Different stereoelectronic environments experienced by the buried methyl groups of 2 and 6 bound within 1. The methyl group directed towards the cavitand wall (equatorial, in red) only experiences the anisotropic shielding of a single aromatic ring at a given time whiles the one deep in the cavity (axial, blue) experiences the effects of the four aromatics of the resorcinarene cone (NMR shifts expressed in ppm).
We repeated the binding experiment with an enantioenriched sample of 2 (er 85:1517, Scheme 2) which displayed the same relative intensities for all the upfield resonances of 2, indicating that host 1 does not show any selectivity in the binding of different enantiomers of 2. This observation reinforces the idea that the chirality of this receptor might only be prominent in a magnetic sense near the amide rim but its inner cavity is almost symmetric in all senses, offering little chance for discrimination between enantiomeric guests or enantiotopic groups within the same guest.14
Scheme 2.
Synthesis of enantioenriched 2.
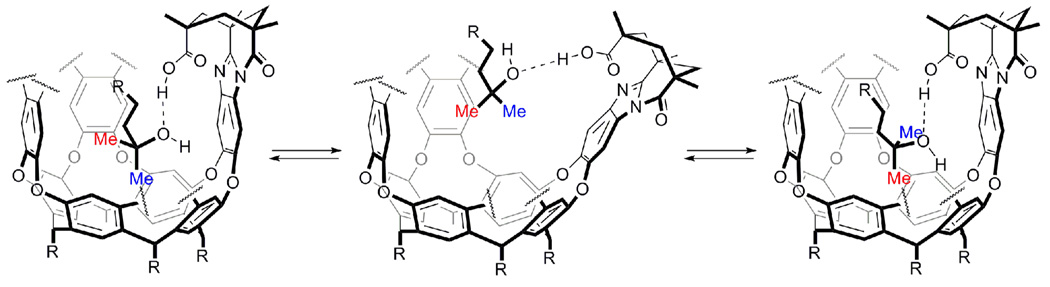
As a further test of the conformational equilibriums proposed, additional NMR experiments were performed using complex 1•6 as a model (Figure 5 and Figure 6), a system which is more convenient for an accurate analysis involving long acquisition experiments18. For this system the 2D NOESY spectrum shows chemical exchange not only between the upfield methyl resonances but also between the neighboring methylene groups (see Supporting Information). Application of the EXSY technique19 to this system provides an energy barrier for the exchange of the two guests inside the cavity of 17.0 kcal/mol.20 Although this might be regarded as a very high value for a process which involves rotation of a non-covalently bound guest, a similar value has been previously reported for the tumbling motion of 1,4-diazabicyclo[2.2.2]octane (DABCO) inside an analogous cavitand.8c A feasible mechanism is depicted in Figure 8. The methyl group buried deep in the cavity (axial) has little space to move in the narrow space defined by the resorcinarene concavity and is fixed at the same time by the hydrogen bond(s) to the carboxylic acid. The only way this methyl can move to the upper position (equatorial) and exchange environments is a partial opening of the cavitand’s wall bearing the acid function. This allows the guest to move up to the wider region of the cavity where σ bond rotation is possible. In this scenario a high-energy barrier seems feasible since some hydrogen bonds need to be broken and the cavitand’s wall has to bend to a disfavored half-open state.21
Figure 8.
Proposed mechanism for the interconversion of conformational isomers of 2 and 6 within 1.
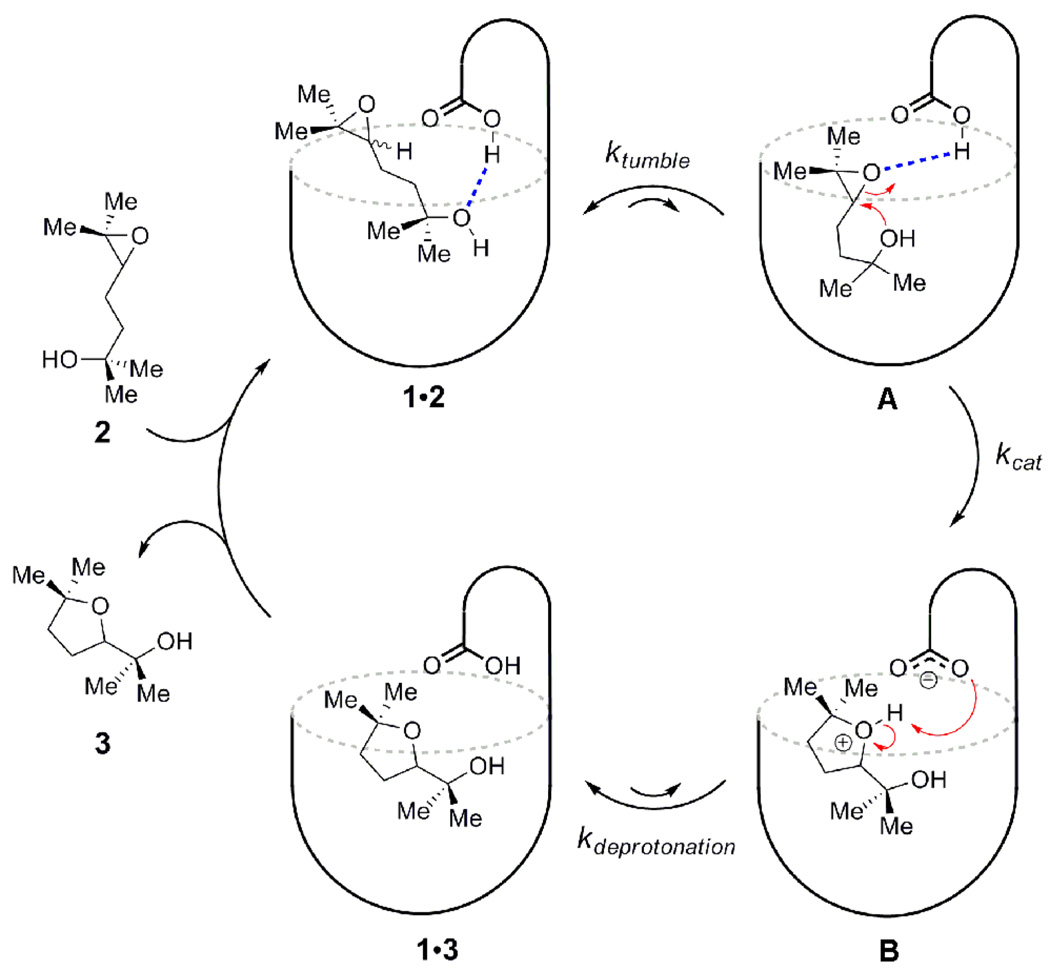
Based on the data obtained from our binding studies and NMR experiments, we proposed the following mechanism for the conversion of substrate 2 into product 3 using cavitand 1 as the catalyst (Scheme 3). Hydrogen bonding interactions between the hydroxyl functionality of the guest and the carboxylic functionality of the host position 1,5-epoxyalcohol 2 within cavitand 1. Regardless of the hydrogen bond pattern inside the cavitand the two forms of the 1•2 observed by 1H NMR spectroscopy are resting states of the catalytic cycle. While the guest can associate and dissociate from the cavitand, it has also some mobility within the receptor. This allows the epoxide terminus of the guest to occasionally contact the acid function of the host (Scheme 3, species A), leading to reaction. A first-order, rate-determining step follows, resulting in the epoxide ring-opening cyclization to form a protonated ether (Scheme 3, species B). The electron-rich π-surface of cavitand 1 is well-suited to stabilize the formation of such cationic species. The ether in intermediate B is readily deprotonated by the carboxylate functionality of the host, leading to a kinetically unstable host-product complex 1•3, which is quickly dissociates and is replaced by another molecule of 2, driving turnover. When the reaction has completely consumed the starting material, no host-guest complexation is observed by 1H NMR, indicating that the product ether 3 is not a good guest for cavitand 1. Apparently, the lack of product inhibition in this system is due to a mismatch in shape-complementarity between cavitand 1 and the cyclic ether product 3. Upon the completion of the reaction, addition of another portion of 1,5-epoxy alcohol 2 regenerates the host-guest complex 1•2, and the cyclization reaction proceeds with no loss of catalytic function.
Scheme 3.
Proposed Catalytic Cycle.
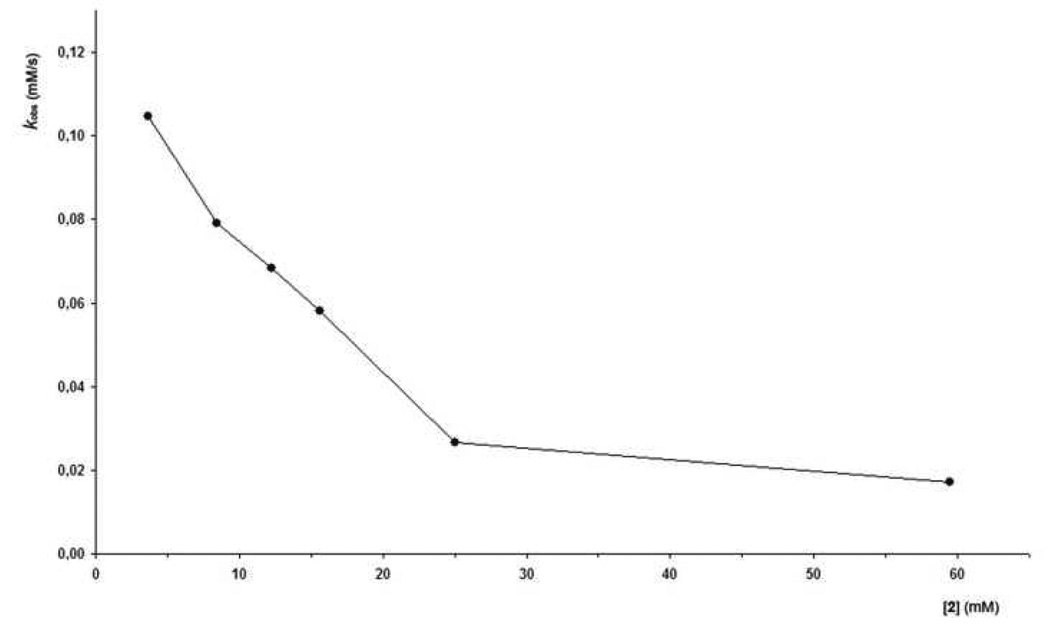
The catalytic cycle depicted in Scheme 3 suggests that this system parallels Michaelis-Menten kinetics of enzymatic function. For example, the folding of the cavitand around the guest epoxide to form complex 1•2 establishes a pre-equilibrium state akin to the formation of an enzyme-substrate complex. The following cyclization reaction, a first-order rate limiting step, is responsible for the conversion of the complex 1•2 into complex 1•3 and parallels the conversion of an enzyme-substrate complex into an enzyme-product complex. In the case of Michaelis-Menten kinetics, the rate of a reaction increases linearly with substrate concentration until reaching a maximum velocity Vmax. It is at this maximum velocity that saturation kinetics can be observed. In order to assess the function of cavitand 1 as an enzyme mimic, we measured the rate of the epoxide ring-opening cyclization reaction in cavitand 1 as a function of 1,5-epoxyalcohol concentration.22 Surprisingly, in our case, the rate of reaction diminished as a function of substrate concentration (Figure 9).
Figure 9.
Plot of kobs (mM/s) vs. [2] (mM) displays substrate inhibition.
The observed decrease in reaction rate as a function of increasing substrate concentration indicated that our system experiences substrate inhibition. Since the reaction is first order in the starting material 2 within a wide range of concentrations (see Supporting Info), the reaction could not be competitively inhibited by the substrate. Instead, the inhibition of receptor 1 should be occurring at a position other than the catalytic site. Such a situation is known commonly referred to as allosteric-inhibition in biological systems.23
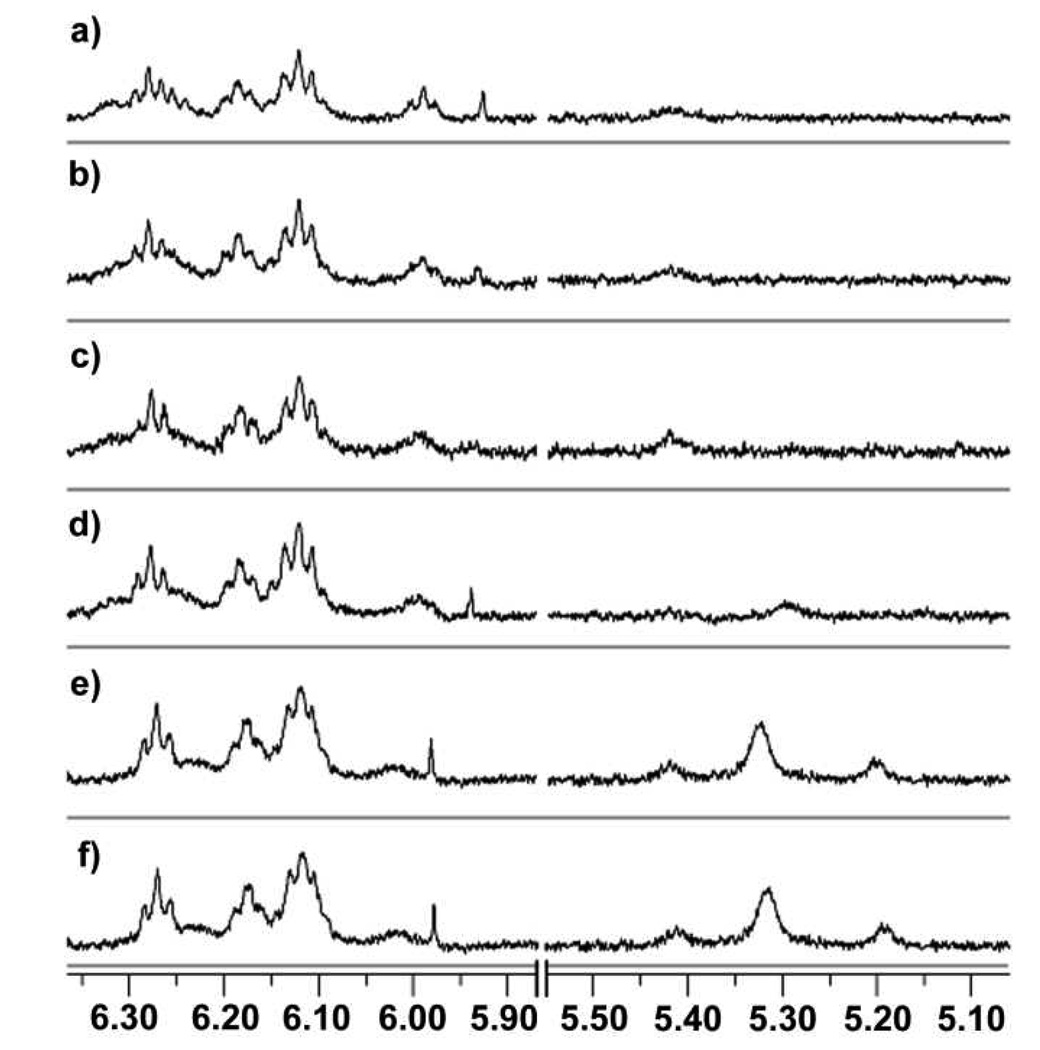
The nature of the present effect is believed to involve the hydrogen bond seam that stabilizes the cavitand in its vase-like conformation. Although the hydroxyl group is crucial for the binding of substrate 2 in cavitand 1, it can also serve to disrupt the cavitand’s hydrogen bond seam. Such a phenomenon is observed in related systems in which protic solvents can promote the dissociation of supramolecular assemblies held together by hydrogen bonding interactions,24 but contrasts with those cases where protic solvents are required to stabilize the assemblies.25 In the case of cavitand 1, the disruption of the cavitand’s hydrogen bond seam results in the unfolding (deactivation) of the catalyst. Such unfolding results in the appearance of new signals in the region of the 1H NMR between δ = 5.00 and 5.50 ppm. These resonances correspond to the four methine protons of the cavitand’s resorcinarene base. When folded into a vase-like conformation, these methine protons appear as sharp triplet resonances between δ = 6.00 and 6.50 ppm,26 because they are placed within the anisotropically deshielded zone of the four resorcinarene aromatic rings. As the cavitand begins to unfold and the resorcinarene adopts a more kite-like conformation, the methine protons shift into zones that experience more anisotropic shielding. The broadening of methine resonances at δ = 6.00 to 6.50 ppm and the appearance of new methine resonances at δ = 5.00 and 5.50 ppm indicate that such a phenomenon occurs at high substrate concentrations (Figure 10).
Figure 10.
Region of the 1H NMR spectrum of the host-guest complex 1•2 showing the broadening of triplet resonances at δ = 6.00–6.50 ppm, and appearances of new signals at δ = 5.00–5.50 ppm as a function [2]: (a) 2.5 equiv. 2, (b) 5 equiv. 2, (c) 7.5 equiv. 2, (d) 10.0 equiv 2, (e) 20.0 equiv. 2, (f) 40.0 equiv. 2.
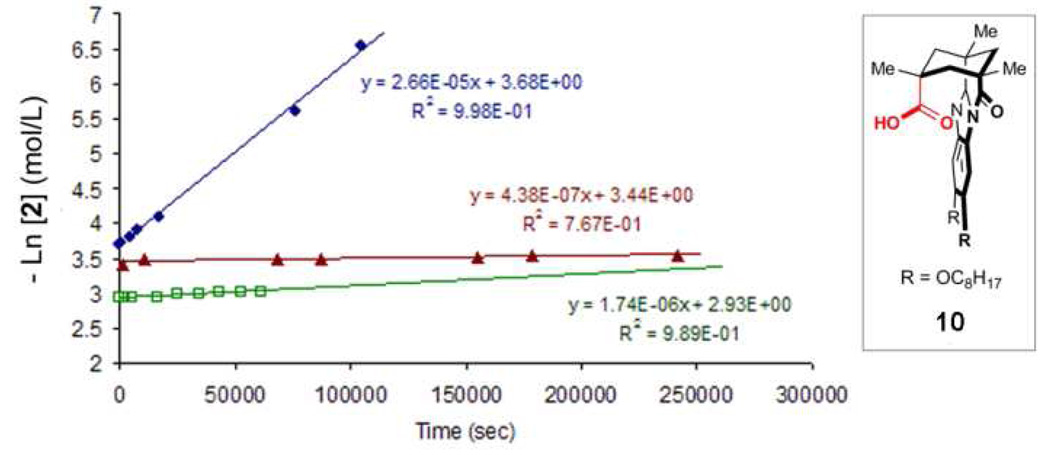
The catalytic function of cavitand 1 can also be diminished by the action of another suitable guest molecule that competes with substrate 2 for the binding site – competitive inhibition. The rate of cyclization in our system is decreased in the presence of methylene chloride, a solvent molecule that is strongly bound by cavitand 1. The rate of the cyclization reaction using 5% loading of the catalyst 1 in a solution of 1,5-epoxyalcohol 2 in mesitylene-d12 is 2.66 × 10−5 s−1 (Figure 11). Upon the addition of 10 mol % of methylenechloride to this solution, we observed a 15-fold diminution in reaction rate (1.74 × 10−6 M/s). When the reaction is performed in CD2Cl2 as the solvent, neither guest-binding nor the formation of cyclic ether 3 was observed, even after one week. Likewise, using control acid 10,27 the rate and regioselectivity of this reaction was diminished in the absence of cavitand 1. However, the rate of this background reaction is unchanged in the presence of 10% CH2Cl2. These data indicate that cavitand 1 is not simply serving as a Brønsted acid catalyst for the epoxide ring-opening cyclization reaction of 1,5-epoxyalcohol 2, but also that the binding of this substrate within the active site of cavitand is important for the cyclization reaction to take place.
Figure 11.
First-order rate law plots of the reaction of substrate 2 in cavitand 1 with (a) 5 mol % 1, 25 mM 2 in mesitylene-d12 (blue circles), (b) 5 mol % 1, 25 mM 2, 10 mol % CH2Cl2 in mesitylene-d12 (green squares) and (c) 5 mol % 10, 25 mM 2, 10 mol % CH2Cl2 in mesitylene-d12 (red triangles).
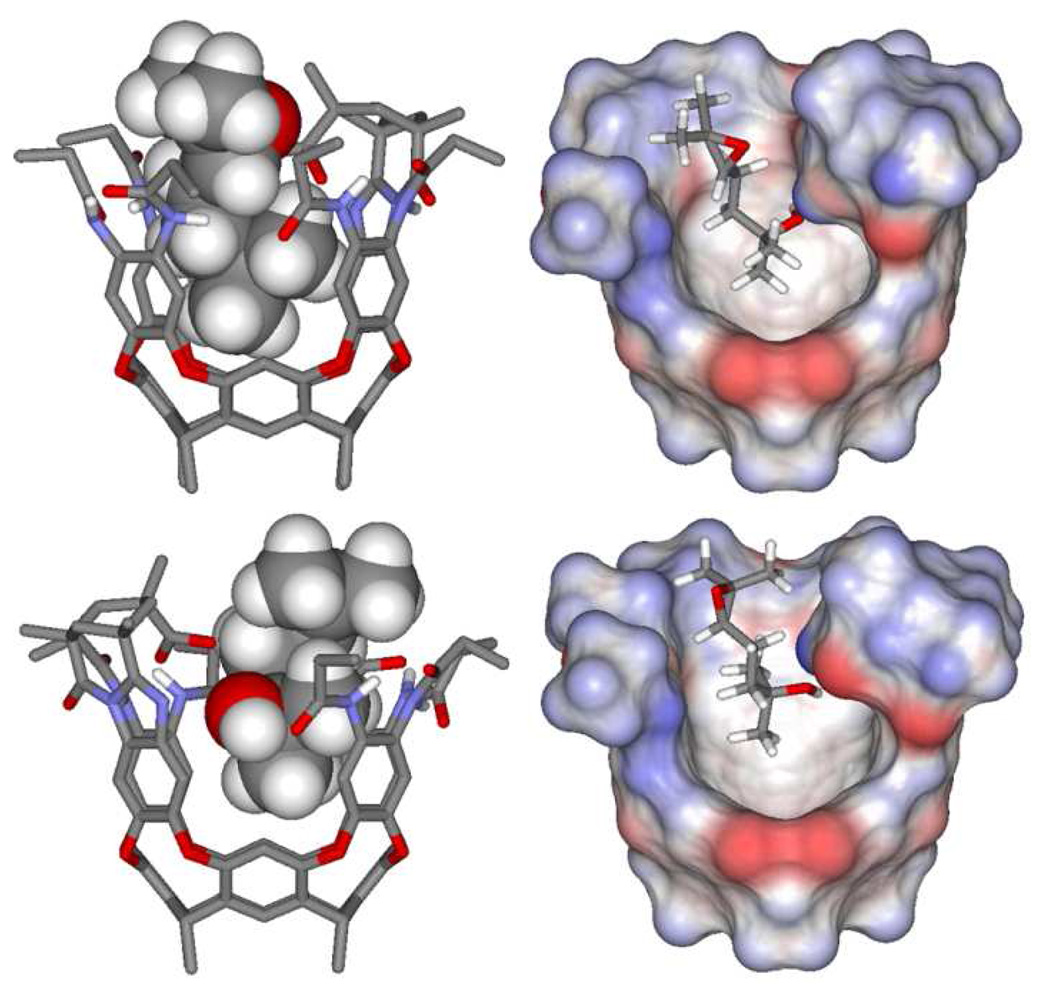
Molecular modeling experiments were used to predict a tentative structure for the initial host-guest complex 1•2. In accordance with our binding studies using substrate analog 6, this models show that the alcohol terminus of the substrate 2 is in close association with the inwardly-directed carboxylic functionality of our receptor. The remainder of the 1,5-epoxyalcohol 2 substrate extends out from the opening of the cavitand and is slightly coiled, minimizing steric repulsions with the neighboring amide groups (Figure 12). Two energy minima were located in which the same enantiomer of 2 has methyl groups that clearly adopt distinct axial and equatorial orientations in good agreement with what is observed by 1H NMR.
Figure 12.
Minimized representations of two conformers of one enantiomer of 1,5-epoxyalcohol 2 in a simplified model of cavitand 1 (Spartan ’04, PM3 level of theory). Left: CPK/tube models show the distinct axial/equatorial disposition of the methyl groups. Right: a different view displaying the solvent-accessible surface (1.4 Å probe) of the host (the front aromatic wall is omitted for clarity).
Conclusions
The cavitand bearing an inwardly-directed carboxylic acid group binds and situates 1,5-epoxyalcohol substrates within an electron-rich concave aromatic surface. The epoxide ring-opening cyclization reaction takes place inside. A hydroxyl group on the substrate was shown to be crucial for host-guest complexation, but can also play a deleterious role in disrupting the hydrogen bond seam stabilizing the active vase-like confirmation of the cavitand. The unfolding of the cavitand results in the deactivation of the catalyst - substrate inhibition. The structure of the initial host-guest complex, and its unique stereochemical properties were determined by NMR spectroscopy, and further corroborated by molecular modeling experiments.
Supplementary Material
General experimental, synthesis and spectroscopic assignment of enantioenriched 2, expanded 2D NOESY spectra for complexe 1•6, details for the EXSY calculations and kinetic measurements for the conversion of 2 into 3.
Acknowledgement
We are grateful to the Skaggs Institute for Research, and the National Institutes of Health (GM 27932) for financial support. S.R.S. thanks the San Diego Foundation Blasker Science and Technology Fellowship for financial support. A.L. thanks MICINN (Ministry of Science and Innovation, Spain) for a fellowship.
References
- 1.Ringe D, Petsko GA. Science. 2008;320:1428–1429. doi: 10.1126/science.1159747. [DOI] [PubMed] [Google Scholar]
- 2.Zhang X, Houk KN. Acc. Chem. Res. 2005;38:379–385. doi: 10.1021/ar040257s. [DOI] [PubMed] [Google Scholar]
- 3.a) Thoma R, Schulz-Gasch T, D'Arcy B, Benz J, Aebi J, Dehmlow H, Hennig M, Stihle M, Ruf A. Nature. 2004;432:118–122. doi: 10.1038/nature02993. [DOI] [PubMed] [Google Scholar]; b) Hoshino T, Sakai Y. Tetrahedron Lett. 2001;42:7319–7323. [Google Scholar]; c) Hayashi N, Fujiwara K, Murai A. Tetrahedron. 1997;53:12425–12468. [Google Scholar]; d) Koert U. Angew. Chem. Int. Ed. 1995;34:298–300. [Google Scholar]; e) Fish PV, Sudhakar AR, Johnson WS. Tetrahedron Lett. 1993;34:7849–7852. [Google Scholar]; f) Lee MS, Qin G-W, Nakanishi K, Zagorski MG. J. Am. Chem. Soc. 1989;111:6234–6241. [Google Scholar]; g) Chou HN, Shimizu Y. J. Am. Chem. Soc. 1987;109:2184–2185. [Google Scholar]; h) Cane DE, Celmer WD, Westley JW. J. Am. Chem. Soc. 1983;105:3594–3600. [Google Scholar]; i) Lin Y-Y, Risk M, Ray SM, Van Engen D, Clardy J, Golik J, James JC, Nakanishi K. J. Am. Chem. Soc. 1981;103:6773–6775. [Google Scholar]; j) Cane DE, Liang T-C, Hasler H. J. Am. Chem. Soc. 1981;103:5962–5965. [Google Scholar]; k) Schmid G, Fukuyama T, Akasaka K, Kishi Y. J. Am. Chem. Soc. 1979;101:259–260. [Google Scholar]
- 4.a) Wendt KU, Schulz GE, Corey EJ, Liu DR. Angew. Chem. Int. Ed. 2000;39:2812–2833. [PubMed] [Google Scholar]; b) Cane DE, Celmer WD, Westley JW. J. Am. Chem. Soc. 1983;105:3594–3600. [Google Scholar]
- 5.Renslo AR, Rebek JJ. Angew. Chem. Int. Ed. 2000;39:3281–3283. doi: 10.1002/1521-3773(20000915)39:18<3281::aid-anie3281>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 6.Meyer EA, Castellano RK, Diederich F. Angew. Chem. Int. Ed. 2003;42:1210–1250. doi: 10.1002/anie.200390319. [DOI] [PubMed] [Google Scholar]
- 7.Kemp DS, Petrakis KS. J. Org. Chem. 1981;46:5140–5143. [Google Scholar]
- 8.a) Paul L, Wash ARR, Julius Rebek., Jr Angew. Chem. Int. Ed. 2001;40:1221–1222. [PubMed] [Google Scholar]; b) Butterfield SM, Rebek J., Jr J. Am. Chem. Soc. 2006;128:15366–15367. doi: 10.1021/ja0663374. [DOI] [PubMed] [Google Scholar]; c) Purse BW, Butterfield SM, Ballester P, Shivanyuk A, Rebek J. J. Org. Chem. 2008;73:6480–6488. doi: 10.1021/jo8008534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.a) Rudkevich D, Hilmersson G, Rebek JJ. J. Am. Chem. Soc. 1998;120:12216–12225. [Google Scholar]; b) Rudkevich DM, Hilmersson G, Rebek J. J. Am. Chem. Soc. 1997;119:9911–9912. [Google Scholar]; c) Tucci FC, Rudkevich DM, Rebek J., Jr J. Org. Chem. 1999;64:4555–4559. [Google Scholar]
- 10.Shenoy SR, Pinacho Crisóstomo FR, Iwasawa T, Rebek J. J. Am. Chem. Soc. 2008;130:5658–5659. doi: 10.1021/ja801107r. [DOI] [PubMed] [Google Scholar]
- 11.a) Morimoto Y, Nishikawa Y, Ueba C, Tanaka T. Angew. Chem. Int. Ed. 2006;45:810–812. doi: 10.1002/anie.200503143. [DOI] [PubMed] [Google Scholar]; b) Baldwin JE, Thomas RC, Kruse LI, Silberman L. J. Org. Chem. 1977;42:3846–3852. [Google Scholar]; c) Baldwin JE, Reiss JA. J. Chem. Soc. Chem. Commun. 1977:77. [Google Scholar]; d) Baldwin JE, Cutting J, Dupont W, Kruse L, Silberman L, Thomas RC. J. Chem. Soc. Chem. Commun. 1976:736. [Google Scholar]; e) Baldwin JE. J. Chem. Soc. Chem. Commun. 1976:734. [Google Scholar]
- 12.Nishio M, Umezawa Y, Hirota M, Takeuchi Y. Tetrahedron. 1995;51:8665–8701. [Google Scholar]
- 13.The stabilization energy for this interaction has been found to be at least 6 Kcal/mol for the binding of amines, see ref. 8c.
- 14.Mann E, Rebek J., Jr Tetrahedron. 2008;64:8484–8487. [Google Scholar]
- 15.The same upfield signature is also observed for the methyl groups of bound isobutylamine within the analogous ethyl-footed introverted acid, see ref. 5.
- 16.Ajami D, Iwasawa T, Rebek J. Proc. Nat. Ac. Sci. 2006;103:8934–8936. doi: 10.1073/pnas.0602781103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Determined by 1H NMR using a lanthanide shift reagent, see Supporting Info for details.
- 18.For long acquisition time NMR experiments the use of excess 2 ensures that the substrate is not consumed, in part due to the system displaying product inhibition (see following paragraphs), although this is detrimental for the signal to noise ratio of the bound species and thus gives poor NMR data. When model compound 6 is used the system is static and less than 1 fold excess guest is required to obtain 75% binding which translates in a 2D NOESY with has much better signal to noise ratio on the relevant peaks.
- 19.Perrin CL, Dwyer TJ. Chem. Rev. 1990;90:935–967. [Google Scholar]
- 20.Obtained by integration of the methyl peaks in the 2D spectra (see Supporting Info). Integration of the weaker methylene resonances provides a similar value.
- 21.Previous studies on unfunctionalized cavitands establish a 10–12 kcal/mol barrier for the kite/vase conformational change, see: Cram DJ, Choi HJ, Bryant JA, Knobler CB. J. Am. Chem. Soc. 1992;114:7748–7765. Moran JR, Ericson JL, Dalcanale E, Bryant JA, Knobler CB, Cram DJ. J. Am. Chem. Soc. 1991;113:5707–5714.
- 22.The conversion of epoxide 2 into ether 3 was monitored by the disappearance of the starting material resonances at 2.43 ppm and the appearance of product resonances at 3.52 ppm.
- 23.Changeux J-P, Edelstein SJ. Science. 2005;308:1424–1428. doi: 10.1126/science.1108595. [DOI] [PubMed] [Google Scholar]
- 24.Körner SK, Tucci FC, Rudkevich DM, Heinz T, Rebek J., Jr Chem. Eur. J. 2000;6:187–195. doi: 10.1002/(sici)1521-3765(20000103)6:1<187::aid-chem187>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]; Shivanyuk A, Rebek J., Jr Chem. Comm. 2001:2374–2375. doi: 10.1039/b106793c. [DOI] [PubMed] [Google Scholar]
- 25.a) MacGillivray LR, Atwood JL. Nature. 1997;389:469–472. [Google Scholar]; b) Shivanyuk A, Rebek J., Jr Chem. Comm. 2001:2424–2425. doi: 10.1039/b109464p. [DOI] [PubMed] [Google Scholar]; c) Ugono O, Holman KT. Chem. Comm. 2006:2144. doi: 10.1039/b604148e. [DOI] [PubMed] [Google Scholar]
- 26.Moran JR, Karbach S, Cram DJ. J. Am. Chem. Soc. 1982;104:5826–5828. [Google Scholar]
- 27.Purse BW, Ballester P, Rebek J. J. Am. Chem. Soc. 2003;125:14682–14683. doi: 10.1021/ja036595q. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
General experimental, synthesis and spectroscopic assignment of enantioenriched 2, expanded 2D NOESY spectra for complexe 1•6, details for the EXSY calculations and kinetic measurements for the conversion of 2 into 3.