Abstract
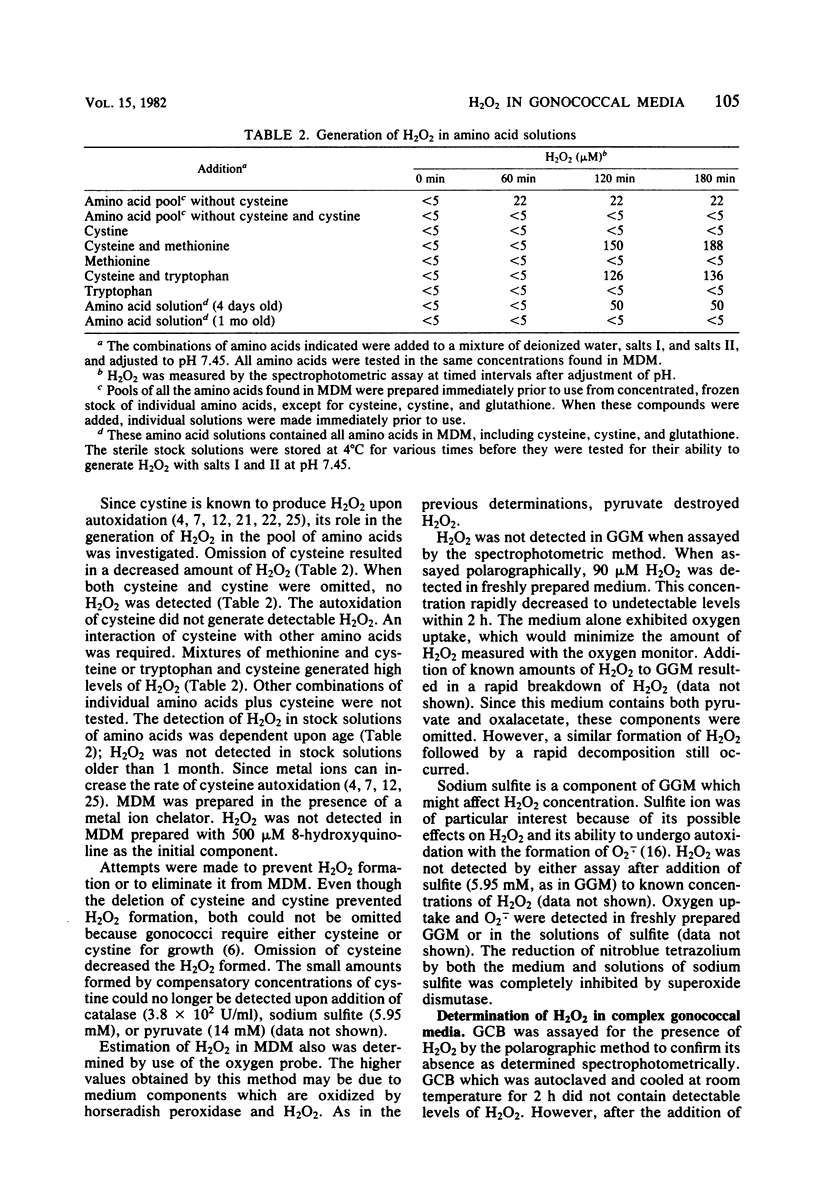
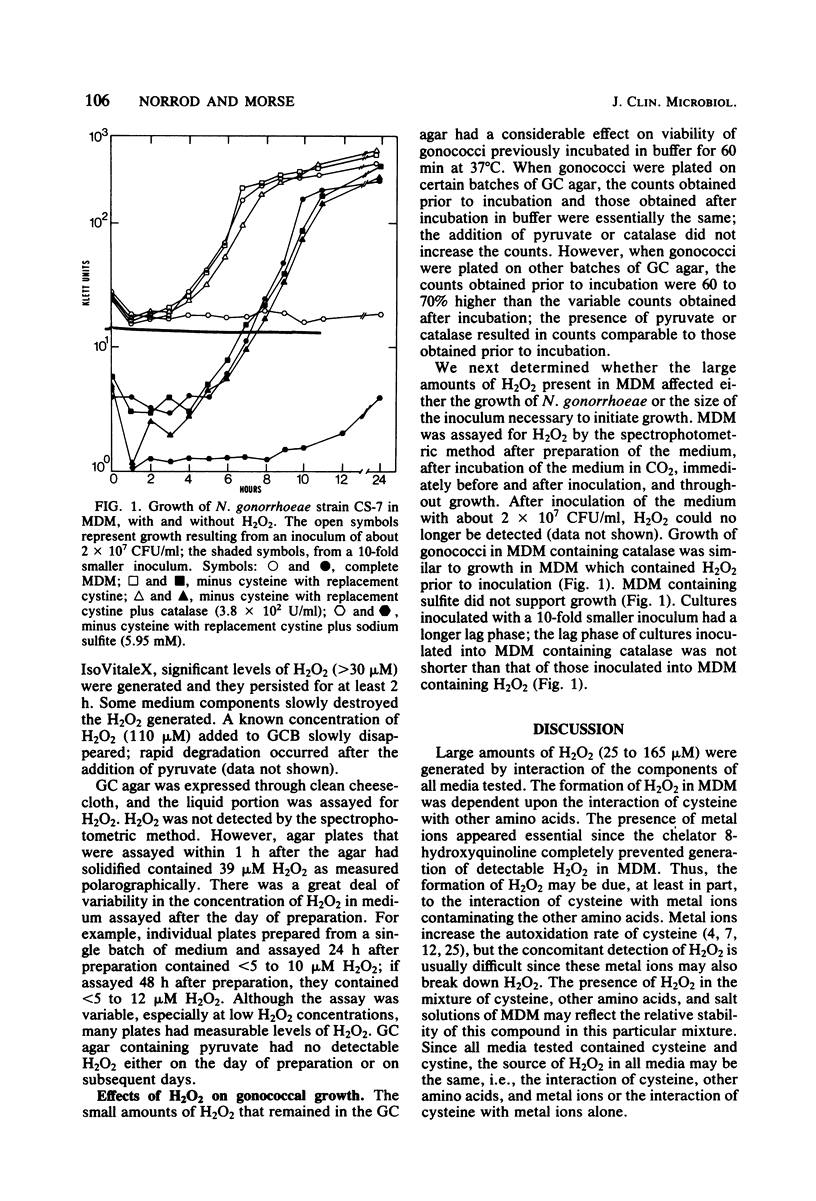
Defined complex media used for cultivation of Neisseria gonorrhoeae were tested for the presence of H2O2 by both a spectrophotometric and a polarographic assay. H2O2 (35 to 165 microM) was present in all media tested. In the defined media, H2O2 was generated by the interaction of cysteine with other amino acids. The addition of the chelator 8-hydroxyquinoline prevented formation of detectable H2O2, suggesting that metal ions were necessary. The persistence of H2O2 varied greatly among different media. Medium components which affected the presence of H2O2 were pyruvate, oxalacetate, and sodium sulfite. Sodium sulfite also generated superoxide radical. In liquid medium containing H2O2, the endogenous gonococcal catalase present in an inoculum of about 2 X 10(7) colony-forming units/ml destroyed detectable H2O2. The long lag phase which resulted from a 10-fold lower inoculum could not be shortened by the addition of exogenous catalase. Small amounts of residual H2O2 in agar plates of complex medium affected the viability of gonococci which had been suspended in buffer and incubated for 60 min at 37 degrees C. Addition of pyruvate or catalase increased viable counts in medium containing H2O2.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bairdparker A. C., Davenport E. The effect of recovery medium on the isolation of Staphylococcus aureus after heat treatment and after the storage of frozen or dried cells. J Appl Bacteriol. 1965 Dec;28(3):390–402. doi: 10.1111/j.1365-2672.1965.tb02169.x. [DOI] [PubMed] [Google Scholar]
- Beauchamp C., Fridovich I. Superoxide dismutase: improved assays and an assay applicable to acrylamide gels. Anal Biochem. 1971 Nov;44(1):276–287. doi: 10.1016/0003-2697(71)90370-8. [DOI] [PubMed] [Google Scholar]
- Brewer D. G., Martin S. E., Ordal Z. J. Beneficial effects of catalase or pyruvate in a most-probable-number technique for the detection of Staphylococcus aureus. Appl Environ Microbiol. 1977 Dec;34(6):797–800. doi: 10.1128/aem.34.6.797-800.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlsson J., Granberg G. P., Nyberg G. K., Edlund M. B. Bactericidal effect of cysteine exposed to atmospheric oxygen. Appl Environ Microbiol. 1979 Mar;37(3):383–390. doi: 10.1128/aem.37.3.383-390.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catlin B. W. Nutritional profiles of Neisseria gonorrhoeae, Neisseria meningitidis, and Neisseria lactamica in chemically defined media and the use of growth requirements for gonococcal typing. J Infect Dis. 1973 Aug;128(2):178–194. doi: 10.1093/infdis/128.2.178. [DOI] [PubMed] [Google Scholar]
- Cavallini D., De Marco C., Dupré S. Luminol chemiluminescence studies of the oxidation of cysteine and other thiols to disulfides. Arch Biochem Biophys. 1968 Mar 20;124(1):18–26. doi: 10.1016/0003-9861(68)90299-3. [DOI] [PubMed] [Google Scholar]
- Flowers R. S., Martin S. E., Brewer D. G., Ordal Z. J. Catalase and enumeration of stressed Staphylococcus aureus cells. Appl Environ Microbiol. 1977 May;33(5):1112–1117. doi: 10.1128/aem.33.5.1112-1117.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frölander F., Carlsson J. Bactericidal effect of anaerobic broth exposed to atmospheric oxygen tested on Peptostreptococcus anaerobius. J Clin Microbiol. 1977 Aug;6(2):117–123. doi: 10.1128/jcm.6.2.117-123.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon S. M., Kautter D. A. Beneficial effect of catalase treatment on growth of Clostridium perfringens. Appl Environ Microbiol. 1976 Sep;32(3):409–416. doi: 10.1128/aem.32.3.409-416.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail G., Sawyer W. D., Wegener W. S. Effect of hydrogen peroxidase and superoxide radical on viability of Neisseria gonorrhoeae and related bacteria. Proc Soc Exp Biol Med. 1977 Jun;155(2):264–269. doi: 10.3181/00379727-155-39786. [DOI] [PubMed] [Google Scholar]
- Kobashi K. Catalytic oxidation of sulfhydryl groups by o-phenanthroline copper complex. Biochim Biophys Acta. 1968 May;158(2):239–245. doi: 10.1016/0304-4165(68)90136-0. [DOI] [PubMed] [Google Scholar]
- La Scolea L. J., Jr, Young F. E. Development of a defined minimal medium for the growth of Neisseria gonorrhoeae. Appl Microbiol. 1974 Jul;28(1):70–76. doi: 10.1128/am.28.1.70-76.1974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin S. E., Flowers R. S., Ordal Z. J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976 Nov;32(5):731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. The utility of superoxide dismutase in studying free radical reactions. I. Radicals generated by the interaction of sulfite, dimethyl sulfoxide, and oxygen. J Biol Chem. 1969 Nov 25;244(22):6056–6063. [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Factors affecting autolysis of Neisseria gonorrhoeae. Proc Soc Exp Biol Med. 1974 Apr;145(4):1418–1421. doi: 10.3181/00379727-145-38025. [DOI] [PubMed] [Google Scholar]
- Morse S. A., Bartenstein L. Purine metabolism in Neisseria gonorrhoeae: the requirement for hypoxanthine. Can J Microbiol. 1980 Jan;26(1):13–20. doi: 10.1139/m80-003. [DOI] [PubMed] [Google Scholar]
- Norrod P., Morse S. A. Absence of superoxide dismutase in some strains of Neisseria gonorrhoeae. Biochem Biophys Res Commun. 1979 Oct 29;90(4):1287–1294. doi: 10.1016/0006-291x(79)91176-8. [DOI] [PubMed] [Google Scholar]
- Nyberg G. K., Granberg G. P., Carlsson J. Bovine superoxide dismutase and copper ions potentiate the bactericidal effect of autoxidizing cysteine. Appl Environ Microbiol. 1979 Jul;38(1):29–34. doi: 10.1128/aem.38.1.29-34.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsen J., Davis L. The oxidation of dithiothreitol by peroxidases and oxygen. Biochim Biophys Acta. 1976 Sep 14;445(2):324–329. doi: 10.1016/0005-2744(76)90086-3. [DOI] [PubMed] [Google Scholar]
- PROOM H., WOIWOD A. J., BARNES J. M., ORBELL W. G. A growth-inhibitory effect on Shigella dysenteriae which occurs with some batches of nutrient agar and is associated with the production of peroxide. J Gen Microbiol. 1950 May;4(2):270–276. doi: 10.1099/00221287-4-2-270. [DOI] [PubMed] [Google Scholar]
- Rest R. F. Killing of Neisseria gonorrhoeae by human polymorphonuclear neutrophil granule extracts. Infect Immun. 1979 Aug;25(2):574–579. doi: 10.1128/iai.25.2.574-579.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor J. E., Yan J. F., Wang J. L. The iron(3)-catalyzed oxidation of cysteine by molecular oxygen in the aqueous phase. An example of a two-thirds-order reaction. J Am Chem Soc. 1966 Apr 20;88(8):1663–1667. doi: 10.1021/ja00960a016. [DOI] [PubMed] [Google Scholar]



