Abstract
Objectives
To implement and evaluate the effectiveness of a laboratory component involving deoxyribonucleic acid (DNA) testing to a required pharmacogenomics course.
Design
Second-year doctor of pharmacy (PharmD) students extracted DNA from saliva samples, evaluated DNA quantity, and performed genotyping analysis of single nucleotide polymorphisms by fast-throughput technology. The students calculated the frequency of polymorphic alleles of the gene encoding arylamine N-acetyltransferase 2 (NAT2); performed stratification of the class into fast, slow, and intermediate acetylators; and discussed the clinical significance of genetic analysis in patients.
Assessment
An attitudinal survey tool with standardized scaled questions was developed and administered to evaluate whether the goals of the laboratory had been achieved. The student responses to the survey demonstrated that introduction of modern, fast-throughput genotyping technologies in the academic process facilitated comprehension of the potential that pharmacogenomics holds for pharmacy practice.
Conclusions
A laboratory session in pharmacogenomics helped students understand the relevance of pharmacogenomic analysis for use in planning/creating individualized medication regimens.
Keywords: pharmacogenomics, instructional design, DNA
INTRODUCTION
The Human Genome Project has catalyzed the rapid increase in the wealth of knowledge on how genetic background modulates the individual's response to drug therapy. In the decades to come, pharmacotherapy based on an individual's genetic profile will significantly change the manner in which drugs are prescribed and administered. The key to such personalized medicine is pharmacogenomics, a discipline spanning classical pharmacology and human genetics.1 While the ethical and social aspects of pharmacogenomics are vividly discussed in the biomedical community, the education of future medical professionals on the potential of pharmacogenomics and its implementation into clinical practice remains at a basic level. Knowledge about this emerging discipline will be essential for pharmacists to enhance therapeutic outcomes by maximizing efficacy and decreasing toxicity of drug therapy.
The 2002 Academic Affairs Committee of the American Association of Colleges of Pharmacy (AACP) published a report identifying the need to include curricular outcomes relating to pharmacogenetics and pharmacogenomics in the pharmacy curriculum.2 This Committee reviewed the Core Competencies in Genetics Essential for All Health Care Professionals developed by the National Coalition for Health Professional Education in Genetics3 and published a subset of competencies that relate specifically to pharmacists. The 2004 Center for Advancement of Pharmacy Education (CAPE) educational outcomes for graduating pharmacists lists the ability to utilize knowledge of the biomedical sciences and emerging technologies to provide pharmaceutical care as a member of the health care team as a competency. In the 2007 Standards, the Accreditation Council for Pharmacy Education (ACPE) has identified pharmacogenomics/pharmacogenetics as a subject that should be included in the science foundation for pharmacy students.4
Despite a consensus among healthcare providers and academicians regarding the need to educate all healthcare students about the potential impact of pharmacogenomics on patient care, the coverage of this material in colleges of pharmacy is evolving slowly. Brock and colleagues surveyed curriculum committee chairpersons in 2001, of the 50 schools responding, 70% indicated that their curriculum dedicated 5 hours or less to the science of pharmacogenomics.5 Sixty percent indicated that 2 hours or less were dedicated to practical applications, and 90% indicated a similar level of time devoted to the discussion of ethical considerations. Latif et al surveyed pharmacy school deans in 2004 and found that of the colleges responding, 78% taught pharmacogenetics in their curriculum.6 In students' evaluations of a required 2-credit course on human genomics, pharmacogenomics, and bioinformatics offered at the University of Buffalo, their major criticism was that the topic lacked relevance to the current practice of pharmacy.7
To stimulate PharmD students' interest in the practical application of pharmacogenomics, a laboratory component was added to the didactic material covering drug metabolism and pharmacogenomics to the second-year Pharmaceutics III course at Temple University School of Pharmacy. The objectives of the laboratory, which was entitled “Pharmacogenetic Diversity of the Class of 2009” were: (1) to introduce students to the concepts and technologies of pharmacogenomics; (2) to demonstrate to the students the universal character of genetic variability in genes coding for drug-metabolizing enzymes, and (3) to demonstrate the importance of genetic analysis for practical pharmacotherapy.
DESIGN
In their second-year of the PharmD curriculum, students in the Pharmaceutics III course had already completed a course in biochemistry and had a strong foundation in medicinal chemistry and pharmacology. The students were well aware of the basic concepts of pharmacogenomics and prepared for the practical laboratory component added to this course. The laboratory was added to the section of the course covering didactic material on drug metabolism and pharmacogenomics. This string of lectures consisted of 3 sections structured to introduce students to: (1) concepts of drug metabolism; (2) basics of human genetics and genetic variability; and (3) pharmacologically relevant examples of polymorphic drug-metabolizing enzymes.
The primary goal of the laboratory was to demonstrate to the students the universal character of genetic variability in genes coding for drug-metabolizing enzymes and the importance of genetic analysis for practical pharmacotherapy. To this end, we established a laboratory where students performed single nucleotide polymorphism (SNP) analysis of an important NAT2 gene coding for arylamine N-acetyltransferase 2 (NAT2). Genotyping analysis was performed using a TaqMan SNP Genotyping assay designed to detect SNP rs1801280. This non-synonymous SNP (341T>C) results in Ile114Thr mutation and is responsible for a significant decrease in NAT2 activity.8 The phenotypic manifestation of this SNP is isoniazid-induced neuritis, procainamide- and hydralazine-induced systemic lupus erythromatosus, and several other well-documented adverse events.9 The laboratory protocols employed a fast-throughput screening technique allowing the students to extract DNA, evaluate DNA quantity, and perform genotyping analysis of SNPs. Using the results generated during the 2 laboratory sessions, students calculated frequency of alleles responsible for polymorphism in the activity of NAT2, and performed a comparative bioinformatics analysis of the data.
The theoretical background of several genotyping methods, including TaqMan technology (basic concepts and protocols), were discussed during the regular lecture hours 1 week prior to the beginning of the laboratory exercise. The students were instructed to review the analysis protocol before each class. The protocols were available on the School's Web site, and students could access them from computer facilities on campus or using their own computer off-campus. Every laboratory session was preceded by a 30-minute presentation by the teaching assistant who provided detailed explanations of the protocol.
The class of 150 students was separated into 3 groups of approximately 50 students each. Each group participated in 2 laboratory sessions (3 hours each), and 1 discussion section (1 hour). Four teaching assistants were assigned to each laboratory session. Because the teaching assistants had limited experience with DNA isolation and characterization, they were given a special training session in major protocols (ie, DNA precipitation and re-dissolving) prior to the laboratory exercise. The teaching assistants provided instructions to the students prior to each laboratory, distributed and collected equipment and samples, answered questions, and supervised the laboratory work. During the first laboratory exercise, students collected saliva samples and extracted DNA. Teaching assistants helped students to use microcentrifuges and to identify and handle DNA precipitates. During the second exercise, students prepared DNA for quantification, and evaluated DNA quantity in their samples. Teaching assistants helped students with distributing the samples in 96-well plates, performed measurements of fluorescence, and provided help with evaluation of data. The results of analysis were available to each group through a computer network. Students were asked to finalize the quantitative evaluation of results using a predesigned electronic spreadsheet and report their results for evaluation by an instructor. A single discussion session followed the laboratory experiences where students discussed the results of the genotyping assay and received instructions on the preparation of a laboratory report. The laboratory report submitted at the end of the recitation summarized the experimental procedures and results of the genotyping experiment, and included class statistics.
The Temple University Internal Review Board (IRB) determined that this laboratory did not meet the definition of research. Therefore, no IRB approval for this exercise was required. However, defined procedures had to be followed for sample collection and processing, and samples could not be associated with a specific individual by the instructor, which limited data analysis.
Collection of Samples
Sample (saliva) collection was performed using Oragene DNA self-collection containers (DNA Genotek, Canada). Students were supplied with Oragene containers pre-labeled with identification (ID) numbers, which they used to track their samples throughout the laboratory. To maintain anonymity, instructors did not record ID numbers and did not match students' personal information with container labels.
Prior to collecting saliva, students were instructed to rinse their mouths with water. After at least 30 seconds, students collected their own saliva using the Oragene containers (about 2 ml) and proceeded with DNA extraction. The saliva samples mixed with the preservative solution were incubated for 1 hour at 50°C, and DNA was precipitated with 95% ethanol. The DNA precipitate was collected by centrifugation in a microcentrifuge. The DNA precipitate was dissolved in 100 μl of TE buffer (1 mM EDTA - 10 mM TrisHCl pH 7.5). The tubes with DNA were kept in a storage box at (+4°C) until completion of the laboratory. Used plastic ware (collection containers, pipettes, tubes, etc) and the DNA samples were disposed in orange biohazard bags and autoclaved after the laboratory session. DNA samples were destroyed by autoclaving after completion of the laboratory.
DNA Quantification
DNA quantification was performed using a Quant-iT PicoGreen dsDNA assay kit (Invitrogen, Carlsbad, CA) in 96-well plates, according to the manufacturer's instructions. Briefly, 2 μl of DNA sample was added to a microplate well containing 98 μl TE buffer; standard DNA solutions (25-800 ng/ml) were loaded in separate wells for calibration. Diluted PicoGreen reagent, 100 μl (1:200), was added to the wells with standard and sample DNA, and fluorescence (excitation 480 nm, emission 520 nm) was measured using an M2 microplate fluorometer (Molecular Devices, Sunnyvale, CA). Every plate contained 8 DNA standards and about 50 DNA samples.
Genotyping Assay
A TaqMan SNP Genotyping assay (Applied Biosystems, Foster City, CA) was used to detect SNP rs1801280 in the NAT2 locus of the human genome. The genotyping assay was performed using an ABI 7300 Real-Time Polymerase Chain Reaction (RT-PCR) instrument (Applied Biosystems, Foster City, CA) according to the manufacturer's instructions. Briefly, 5-20 ng (11.2 μl) DNA was added to each well of the 96-well reaction plate containing 13.8 μl reaction mix (12.5 ul 2xTaqMan Universal PCR Master Mix, No AmpErase UNG, and 1.25 ul 20XSNP Genotyping Assay Mix), along with negative (no template control) and positive (DNA samples with rs1801280) controls. The plate was sealed with an optical adhesive cover and briefly centrifuged. PCR amplification was performed using the following conditions: Step 1, incubation at 92°C for 10 minutes; Step 2: 40 cycles (incubation at 95°C for 15 seconds followed by incubation at 60°C for 1 minute). Data were collected in real time. Results of the genotyping experiment were presented as a table. A complete list of instrumentation and reagents used for DNA extraction, quantification, and genotyping analysis is available from the corresponding author upon request.
DNA collection, extraction, and quantification.
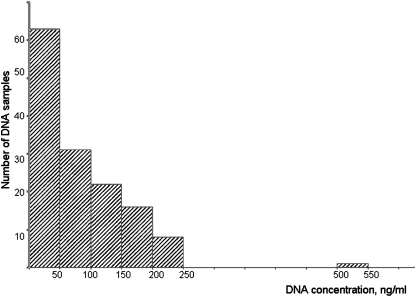
One hundred forty-one DNA preparations were received for quantification. Sixty-two samples with DNA concentrations below 50 ng/ml were excluded from further analysis. A histogram demonstrating the amount of DNA isolated from saliva using Oragene containers is shown in Figure 1. Quantification of DNA was performed using fluorescent analysis in a 96-well format. This technology allowed simultaneous quantification of 50 or more samples, plus the DNA standards for preparation of a calibration curve, in 1 plate. After dissolving DNA and dispensing samples into the assigned wells, students moved to the computer laboratory. The results of the analysis were distributed by e-mail, and students were instructed to perform calculations using the predesigned spreadsheet. The Pharmacy School computer laboratory was available during this session for calculations, and the teaching assistant's were available to assist students.
Figure 1.
Histogram of the amount of DNA isolated from saliva using Oragene containers. X axis, DNA concentration, ng/ml; Y axis, number of samples. DNA preparations with concentration less than 50 ng/ml (62 samples) were excluded from further analysis.
Genotyping analysis.
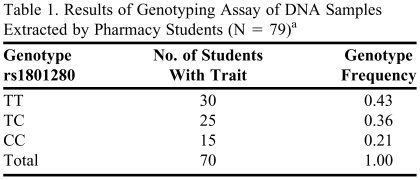
Genotyping of DNA samples was performed by the staff of the pharmacogenomics laboratory. Seventy-nine samples were distributed in a single 96-well plate, along with reagents for Allele Discrimination assay. The entire analysis took about 2 hours. Nine samples did not produce amplified product and were excluded from the genotyping analysis. The results of the genotyping assay are shown in Table 1. All 3 possible genotypes—TT, TC, and CC—were found in a group of 70 analyzed individuals. Students calculated the genotype frequencies, and allele frequencies for allele T and allele C, and compared results with known allele frequencies reported in the dbSNP database. The results were discussed in the recitation session.
Table 1.
Results of Genotyping Assay of DNA Samples Extracted by Pharmacy Students (N = 79)a
Nine samples (11%) did not produce amplified product and were excluded from the genotyping analysis.
EVALUATION AND ASSESSMENT
The grades for the laboratory were based on the reports the students submitted, which constituted 5% of the final grade for the course. All students successfully completed the laboratory and submitted the laboratory reports. Only 3 (2%) of the 144 students were unable to correctly quantify DNA using the calibration curve and spreadsheet. All students correctly calculated the genotype and allele frequencies from the genotyping data.
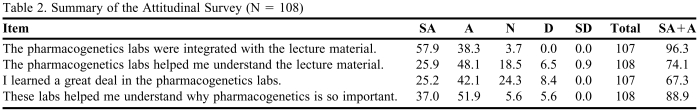
An attitudinal survey tool with standardized scaled questions was developed and administered to evaluate whether the goals of the laboratory had been achieved. Students were asked to complete the survey instrument at the conclusion of the laboratory/recitation sequence. The students were informed that participation in the survey was voluntary and anonymous.
One hundred eight (74%) of the 144 students completed a survey instrument. A summary of the participants' responses is listed in the Table 2. The majority of students indicated that the laboratory (1) helped them understand the concepts of pharmacogenomic analysis (74%), (2) was “appropriately” integrated with the lecture material (96%), and (3) highlighted the importance of this topic to their future practice (89%). About 67% of respondents indicated that they learned “a great deal” from the pharmacogenomic laboratory.
Table 2.
Summary of the Attitudinal Survey (N = 108)
Abbreviations: SA = strongly agree; A = agree; N = neutral; D = disagree; SD = strongly disagree
Students' perception of the laboratory were further expressed in their responses to open-ended survey questions; students expressed their interest in the topic of pharmacogenomics, particularly in the context of real data generated during the laboratory exercise: “…Use of real data in determining genotype and phenotype distribution was very beneficial”.
DISCUSSION
Knowledge of pharmacogenomics will be essential for pharmacists to prospectively individualize drug therapy using information about the patient's genetic profile, in addition to age, diseases states, and organ function. Evaluating a patient's genetic background prior to beginning pharmacotherapy is increasingly becoming a reality in pharmacy practice.10
Clinically important and well-characterized genetic polymorphisms have been identified as important predictors of adverse events; existing technology has made the detection and interpretation of these polymorphisms fast and easy enough to be routinely used in diagnostic centers. In the past several years, the US Food and Drug Administration has recommended genetic tests for several polymorphic enzymes including cytochrome P450 isoforms 2D6 and 2C9; thiopurine S-methyltransferase (TPMT); and vitamin K epoxide reductase complex, subunit 1.11
Nevertheless, the systematic use of pharmacogenomic data in clinical practice is limited; decisions regarding drug therapy are rarely based upon the genotype despite the availability of this technology. While a biochemical analysis of enzyme level in a patient's sample (eg, TPMT) is universally accepted as a guide for individualizing therapeutic regimens, genetic analysis continues to be perceived as a violation of privacy.
This limited use of pharmacogenomics in current clinical practice makes it difficult to convince students of the practical relevance of genetic information for pharmacists. This slow implementation of pharmacogenomic analysis also often costs unnecessary human morbidity and mortality.1 Teaching students entering the healthcare professions about the principles and potential of pharmacogenomics should close the gap between clinical studies and clinical practice.12
Pharmacogenetics is a required component of the curriculum during the fall semester of the second year of the PharmD program at Temple University School of Pharmacy. Pharmacy students are exposed to the “Introduction to Drug Metabolism and Pharmacogenomics” section during 20 academic hours as a part of Pharmaceutics III course. The lectures are logically separated into 3 parts: drug metabolism, flow of genetic information, and applied pharmacogenomics. The practical exercise was synchronized with the last part of the section, after the students had acquired the initial knowledge about the proteins involved in drug metabolism and drug distribution and learned about variability in human genome. The practical work, consisting of two 3-hour laboratories and a 1-hour recitation, was a key component of this course; it was incorporated with the goals of giving students a hands-on experience in using the analytical techniques of genetic analysis to reinforce concepts covered in lectures and to make this information more relevant. The practical exercise was complemented by the lecture “Genetic polymorphism of arylamine N-acetyltransferases,” in which biochemistry, genetics, and medical implications of genetic polymorphism in the NAT protein family were discussed.
To demonstrate the importance of genetic polymorphism for pharmacotherapy in a class population, we chose the NAT2 gene. Genetic polymorphism of NAT2 was the first reported example of inherited variation in drug metabolism,13 the molecular mechanism and clinical implications of which have been well studied.9 Importantly, the frequency of slow acetylators is high, and genetic variations in this locus usually are present even in a small group of individuals.
Frequency of the causative allele C is high in the Caucasian (43%-46%) and African American (28%-38%) populations, in contrast to individuals of Chinese and Japanese origins (0-3%).14 Therefore, significantly distinct allele frequencies in ethnic groups provided an opportunity to discuss ethnic differences in drug metabolism with the class. The student population participating in this laboratory was highly ethnically diverse, and all 3 possible genotypes were detected among students in the class. The importance of genotype as a risk factor for development of adverse events in slow acetylators was presented to the class based on real statistics (Table 1).
Traditional methods of genetic analysis (eg, Southern hybridization, PCR-RFLP, etc) are time consuming and labor intensive, thus precluding their use in classroom exercises. In contrast, the simplicity and practicality of the laboratory presented here allowed 150 students to receive hands-on experience in DNA extraction, genotyping, and interpretation of genetic data. With many schools of pharmacy having access to molecular analysis facilities, the laboratory design suggested in our manuscript can be easily reproduced.
In the experimental design, serious consideration was given to maintaining confidentiality of the genotyping results. First, the ethical aspects of collecting and handling genetic information were discussed with students during regular lectures prior to the laboratory. Next, the confidentiality of genetic information was assured by following specific procedures for collecting DNA samples and processing genotype information. The containers for sample collection were randomly labeled, and teaching assistants did not match samples with student ID numbers. Students were given an option to use an anonymous DNA sample in case they were unwilling to extract their own DNA. In these settings (de-identified samples and lack of records on individual samples), Institutional Review Board approval is not required. Students were not hesitant about collecting samples and none requested anonymous DNA for analysis.
To perform DNA isolation, quantification, and genotyping within a class of 150 students, we used recent developments in technology that allowed the students to process and analyze multiple samples in parallel. There are 3 key elements that made this possible. First, a new technique for collection and purification of DNA based on a saliva sample obviated the need for medical procedures as would be required for extracting DNA from blood. Second, quantification of isolated DNA was performed in a 96-well format using a microplate spectrofluorometer, reducing the time for analysis of 40-50 samples to 1-2 minutes. Finally, a fast-throughput technology was used for genotyping experiments that permitted the collection of genotyping data simultaneously from 79 samples in approximately 2 hours.
The robust protocol for DNA isolation allowed students with little or no experience to extract DNA in visible amounts (students clearly saw the DNA precipitate) and with sufficient purity for genotyping experiments. In 62 samples (43% of all collected samples), DNA concentration was below 50 ng/ml. We did not use samples with low DNA concentration for genotyping experiments because such preparations may have needed an additional purification/concentration step for accurate genotyping.
DNA extraction did not require precise pipetting; students accomplished DNA extraction using disposable polyethylene pipettes. Further, the use of multiple parallel reading instruments, such as an M2 spectrofluorometer and real-time PCR instrument ABI7300, permitted the analysis of all samples well within the laboratory hours (3 hours per session, including 30 minutes of preliminary instruction by teaching assistants). Additional resources (reagents and instrumentation) were required to implement this teaching innovation. The cost of reagents and consumables (saliva collection kits, plastic ware, etc) was about $30 per student. This estimate did not include the cost and operating time for the instruments (a microplate reader, and a real-time PCR machine) which were available for this work through the Jayne Haines Center for Pharmacogenomics and Drug Safety at Temple University School of Pharmacy.
During the final recitation, students discussed principles of genotyping technology, the results of genotyping analysis, calculated allele frequencies from genotype frequencies, and possible effects of slow acetylator phenotypes on drug metabolism. In the future classes, we plan to introduce students to the Hardy-Weinberg analysis. Comparison of expected genotypic frequencies with the observed genotypic frequencies using chi-square test could be a useful extension of the current laboratory exercise. Students were encouraged to use the Pharmacogenomics Knowledge Base Web site (www.PharmGKB.org) to extract information regarding the clinical implications of NAT2 polymorphism, and for preparing the final reports. Allele frequencies (T allele, 0.61; C allele, 0.39) were similar to those found in other epidemiological studies or published in Internet-accessible databases PharmGKB, dbSNP, and HapMap.15,16 The results of the pharmacogenomic analysis performed during the laboratory exercise were presented to students. About 20% of the class had genotype rs1801280:C/C (a causative polymorphism for the slow acetylator phenotype). Therefore, our students were exposed to real data indicative of increased risk in nearly a fifth of the class for adverse events to the antitubercular drug isoniazid and several other important medications. Students were excited about the laboratory and actively participated. The individuals who responded to the survey after the recitation session indicated that the laboratory increased their learning and helped them see the relevance of the course material. The results of our survey indicated that the new laboratory exercise directly demonstrated to the class that genetic variability is a risk factor even in a small group of individuals (150 students). Furthermore, the laboratory helped students to better understand the basic principles and technology behind pharmacogenomic analysis, and supplemented the didactic material of the lectures with a genuine example of a pharmacologically important pharmacogenetic polymorphism. We consider this practical exercise as an important element in maintaining professional competence of pharmacy students, which will add to their ability to identify and analyze emerging issues and services.
SUMMARY
Modern health care providers should be educated about the genetic risk factors that affect the outcomes of pharmacotherapy. We established a laboratory exercise designed to introduce pharmacy students to pharmacogenomic analysis and educate them about existing scientific and technological tools to identify individuals specifically at risk of developing adverse drug reactions due to their genetic background. About 150 students received a hands-on experience in DNA extraction, genotyping, and interpretation of genetic data. The student responses to the survey demonstrated that introduction of modern, fast-throughput genotyping technologies in the academic process did not represent a barrier for students; rather, it facilitated comprehension of the potential that pharmacogenomics holds for pharmacy practice.
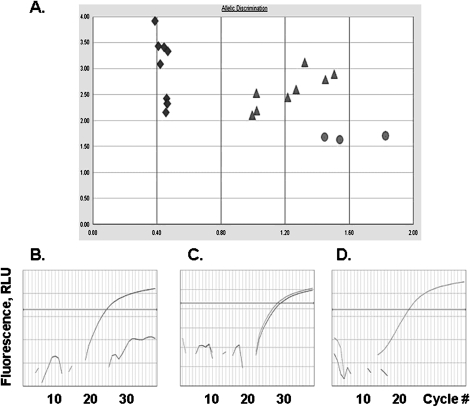
Figure 2.
Allele discrimination report and representative amplification plots of DNA samples isolated from saliva by students. (A) Allelic discrimination report of genotyping analysis in 19 DNA samples: Diamonds, rs1801280:T/T; Triangles, rs1801280:C/T; Circles, rs1801280:C/C Amplification plots of: (B) CC genotype (variant homozygous); (C) CT genotype (heterozygous); (D) TT genotype (wild type homozygous). Dye fluorescence is displayed as a function of cycle number in Polymerase Chain Reaction (PCR). Increase in fluorescence reveals allele-specific amplification of SNP rs1801280.
ACKNOWLEDGEMENT
We are grateful to Dean Peter Doukas for his encouragement and full support of this laboratory; Dr. Natalia Krynetskaia for her help in creating the concept and experimental design of the laboratory; and Dr. Anurag Mishra (Jayne Haines Center for Pharmacogenomics and Drug Safety at Temple University School of Pharmacy) and Barbara Grissani (Computer laboratory) for their help in performing genotyping and data evaluation. We wish to thank Dr. Michael Jacobs for his valuable advice in organizing the collection and processing of saliva samples.
REFERENCES
- 1.Gurwitz D, Lunshof JE, Dedoussis G, et al. Pharmacogenomics education: International Society of Pharmacogenomics recommendations for medical, pharmaceutical, and health schools deans of education. Pharmacogen J. 2005;5(4):221–5. doi: 10.1038/sj.tpj.6500312. [DOI] [PubMed] [Google Scholar]
- 2.Johnson JA, Bootman JL, Evans WE, et al. Pharmacogenomics: a scientific revolution in pharmaceutical sciences and pharmacy practice. Report of the 2001/02 Academic Affairs Committee. Am J Pharm Educ. 2002;66(4):12S–4S. [Google Scholar]
- 3. Core Competencies in Genetics Essential for all health-care professionals. January 2001. National Coalition for Health Professional Education in Genetics (NCHPEG) 2001. http://www.nchpeg.org/core/Core_Comps_English_2007.pdf.
- 4. Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. February 2006. Accreditation Council for Pharmacy Education; 2006. http://www.acpe-accredit.org/
- 5.Brock TP, Faulkner CM, Williams DM, Smith SR. Continuing-education programs in pharmacogenomics for pharmacists. Am J Health-Syst Pharm. 2002;59(8):722–5. doi: 10.1093/ajhp/59.8.722. [DOI] [PubMed] [Google Scholar]
- 6.Latif D, McKay A. Pharmacogenetics and pharmacogenomics instruction in colleges and schools of pharmacy in the United States. Am J Pharm Educ. 2005;69(2):152–6. [Google Scholar]
- 7.Brazeau D, Brazeau GA. A required course in human genomics, pharmacogenomics, and bioinformatics. Am J Pharm Educ. 2006;70(6):1–6. doi: 10.5688/aj7006125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hein DW, Doll MA, Rustan TD, Ferguson RJ. Metabolic activation of N-hydroxyarylamines and N-hydroxyarylamides by 16 recombinant human NAT2 allozymes: effects of 7 specific NAT2 nucleic acid substitutions. Cancer Res. 1995;55(16):3531–6. [PubMed] [Google Scholar]
- 9.Daly AK. Pharmacogenetics of the major polymorphic metabolizing enzymes. Fundamentals Clin Pharmacol. 2003;17(1):27–41. doi: 10.1046/j.1472-8206.2003.00119.x. [DOI] [PubMed] [Google Scholar]
- 10.Krynetskiy E, McDonnell P. Building individualized medicine: prevention of adverse reactions to warfarin therapy. J Pharmacol Exp Ther. 2007;322(2):427–34. doi: 10.1124/jpet.106.117952. [DOI] [PubMed] [Google Scholar]
- 11. Genomics at FDA. Center for Drug Evaluation and Research. U.S. Food and Drug Administration. Available at: http://www.fda.gov/Drugs/ScienceResearch/ResearchAreas/Pharmacogenetics/ucm085427.htm. Accessed June 6, 2008.
- 12.Krynetskiy EY, Evans WE. Closing the gap between science and clinical practice: the thiopurine S-methyltransferase polymorphism moves forward. Pharmacogenetics. 2004;14(7):395–6. doi: 10.1097/01.fpc.0000114753.08559.e9. [DOI] [PubMed] [Google Scholar]
- 13.Evans DAP, Manley KA, McKuisick VA. Genetic control of isoniazid acetylation in man. Br Med J. 1960;2(5197):485–91. doi: 10.1136/bmj.2.5197.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Patin E, Barreiro LB, Sabeti PC, et al. Deciphering the ancient and complex evolutionary history of human arylamine N-acetyltransferase genes. Am J Hum Gen. 2006;78(3):423–36. doi: 10.1086/500614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Cascorbi I, Brockmoller J, Mrozikiewicz PM, Muller A, Roots I. Arylamine N-acetyltransferase activity in man. Drug Metab Rev. 1999;31(2):489–502. doi: 10.1081/dmr-100101932. [DOI] [PubMed] [Google Scholar]
- 16.Hein DW, Doll MA, Fretland AJ, et al. Molecular genetics and epidemiology of the NAT1 and NAT2 acetylation polymorphisms. Cancer Epidemiol Biomarkers Prev. 2000;9(1):29–42. [PubMed] [Google Scholar]