Abstract
Objectives
To evaluate the impact of a drug interactions elective course on student knowledge and skills.
Design
A drug interactions elective which focused on assessment and application of drug interaction information and identification and management of commonly encountered drug interactions by therapeutic category was offered to third-year PharmD students. Students were expected to (1) determine whether a given interaction was clinically significant or required pharmacist intervention, and (2) make rational, scientifically sound, practical recommendations for management of drug interactions.
Evaluation and Assessment
Assessment included course evaluations, student self-assessments, and knowledge and skills assessments. Students who completed the course were more confident in their abilities relating to drug interactions than students who did not complete the course. Students who completed the course scored significantly better in all areas of the assessment compared to students who did not complete the course. Course evaluation results were also positive.
Conclusion
A course devoted to the identification and management of drug interactions improved PharmD students' knowledge and skills and could potentially improve the patient care they provide in the future.
Keywords: drug interactions, assessment, elective course
INTRODUCTION
More drugs and combinations of drugs are being used than ever before, with prescription drug sales increasing by 11.5% between 2005 and 2007.1 In 2007, 3.52 billion prescriptions were filled, averaging about 11 prescriptions per person in the United States.1 The medical management of many common diseases such as heart failure and diabetes continues to evolve to include increasing numbers of drugs as the standard of care.2,3 In addition, 64% of patient visits to a physician result in a prescription.4 With this explosion of medication use in the United States, individual patient medication regimens are becoming increasingly complex, with higher probabilities of adverse drug reactions (ADRs) and drug-drug interactions (DDIs).
Several studies from the 1990s demonstrated that ADRs were a leading cause of morbidity and mortality in the United States.5-9 Although there are few recent data on the epidemiology of ADRs in the United States, a 2004 United Kingdom analysis of over 18,000 hospital admissions, concluded that 6.5% of hospital admissions were related to ADRs which accounted for 4% of hospital bed capacity.10 In addition, the rate of ADRs increases exponentially after a patient is on 4 or more medications.11 DDIs represent approximately 3% to 5% of all in-hospital medication errors and are also an important cause of patient visits to emergency departments.12,13
While over 100,000 types of potential DDIs have been documented, most do not actually lead to adverse effects.14 Over the last decades, our understanding of the mechanisms of DDIs has vastly increased; however, a lack of definitive epidemiologic studies makes it impossible to predict which DDIs pose the most risk to our patients.15 The true frequency of clinically significant DDIs remains unknown.16 Therefore, the identification, assessment, and management of potential or actual DDIs are critical functions of pharmacists, with potentially the most challenging function being the ability to distinguish clinically significant interactions from those that are not.
While technologic advances such as computerized screening and alert systems may aid a pharmacist in identifying drug interactions, they often fall short. Screening programs that generate a large number of alerts with low clinical significance often lead pharmacists to override the computer system, forcing it to process the prescription, without adequately reviewing the interaction. One study evaluating how community pharmacists respond to DDI alerts found that most DDI alerts were routinely deemed insignificant by pharmacists and the majority of time reacting to DDIs was spent overriding them in the pharmacy's computer system.17 Another study assessing general practitioners' views on computerized drug interaction alerts revealed that 22% would frequently or very frequently override DDI alerts without properly checking them.18 In other words, too many alerts often desensitize the user and lead to “alert fatigue.”
In addition, computer alert programs may offer little or no help in terms of evaluating and managing DDIs in individualized patients, making them insufficient at preventing adverse drug reactions caused by DDIs. These findings should reinforce the need for pharmacists to develop their own systematic approach to identifying, evaluating, preventing, and managing drug interactions. Pharmacists must have the fundamental knowledge and understanding of mechanisms of drug interactions as well as the ability to evaluate a given DDI, synthesize drug information regarding the interaction, determine the clinical significance of the interaction, and recommend appropriate management that is evidence-based and patient-specific.19
The knowledge and abilities described above should be considered core components of pharmacy education and training; parts of which are likely already incorporated into PharmD curricula at multiple levels. For example, basic DDI mechanisms may be introduced in a pharmacology course, drug interaction screening programs may be reviewed in a drug information course, and individual DDIs may be reviewed in a therapeutics course. However, the teaching and learning should not end at memorizing or recalling specific drug interactions. Students must be challenged with case-based activities that require application of previous knowledge, assessment of patient specific variables, and interpretation of primary and tertiary references, which ultimately leads to their ability to make rational, practical, and individualized recommendations. This view is supported by the Accreditation Council for Pharmacy Education (ACPE) competency statements and Center for the Advancement of Pharmaceutical Education (CAPE) outcome statements discussing the provision of patient care and the use of practical teaching and learning methods that develop critical-thinking and problem-solving skills.20,21
The Northeastern University School of Pharmacy introduces students to basic concepts regarding drug interactions early in the curriculum and discusses disease-specific interactions throughout several core didactic courses such as therapeutics. However, a systematic, clinical, and practical approach to the assessment and management of drug interactions seemed to be missing. A drug interactions elective course was developed in an attempt to improve students' knowledge and skills regarding drug interactions. The objective of this study was to evaluate the impact of this elective course on student knowledge and skills regarding drug interactions.
DESIGN
Expected Outcomes
A 2-semester hour elective course entitled Drug Interactions was offered to third-year PharmD students in 2005. The course focused on the assessment and application of drug interaction information and identification and management of commonly encountered drug interactions. The primary goal of the course was to improve students' knowledge and skills related to drug interactions and enable students to prevent, identify, evaluate, and manage drug interactions in an evidence-based, patient-specific manner. Given real-world patient case scenarios, students were expected to (1) determine whether a given interaction was clinically significant or required pharmacist intervention, and (2) make rational, scientifically sound, practical recommendations for the management of drug interactions. The course objectives were to increase the students' ability to:
differentiate between pharmacokinetic and pharmacodynamic drug interactions
describe common mechanisms of drug interactions and provide examples
compare and contrast drug information references on drug interactions
describe commonly used drug-drug interaction classification systems
identify strengths and limitations of computer-based screening programs that alert practitioners of drug-drug interactions
evaluate a patient's medication list to identify potential drug interactions
retrieve, analyze, and interpret scientific literature regarding a given drug interaction
determine the clinical significance of a given drug interaction based on scientific literature and patient specific information
recommend appropriate management of a given drug interaction for a specific patient.
Educational Environment
The curriculum at Northeastern University School of Pharmacy provides the opportunity for students to complete up to 8 semester hours of elective course work in the third year. Elective courses can be professional pharmacy electives, interprofessional courses within the College of Health Sciences, or general electives through other University departments.
The Drug Interactions elective was offered in the spring semester so that students had completed the majority of the therapeutics-based curriculum prior to the course. To enroll in the course, students had to have successfully completed prerequisite coursework in pharmacology, drug information, literature evaluation, and introductory therapeutics. The course was developed and administered by 1 clinical faculty member of the Department of Pharmacy Practice. The faculty member was a board-certified pharmacotherapy specialist and practiced in the area of adult internal medicine. A teaching assistant was available for 10 hours per week. The class met once a week for 100 minutes. Enrollment in the course was limited to 25 students, which was approximately one quarter of the class. The course was held in a pharmacy practice laboratory equipped with 25 computer stations. Students had access to online reference guides including Clinical Pharmacology (www.clinicalpharmacology.com)22 and Micromedex (www.thomsonhc.com),23 as well as several core pharmacy textbooks, standard drug information, and pharmacy practice references. Students were required to purchase The Top 100 Drug Interactions: A Guide to Patient Management.15 Students were also required to check the Blackboard Web site (Blackboard, Inc; Washington, DC) periodically for class announcements, documents, and discussions. The course was offered for 3 years; however, the assessment activities reported in this paper are limited to the second year.
Pedagogy/Andragogy
The course used a variety of teaching and learning methods, including informal, interactive didactic teaching, and active learning. The students were broken into 6 groups of 4 students. These groups worked together on both in-class activities and homework. Each class period typically started with an active learning activity. Student groups were given an assignment, questions, or case scenario with specific instructions. All case scenarios were based on “real-world” patients from the instructor's clinical practice. Groups were brought back together for a large group discussion followed by didactic teaching when appropriate. Groups were encouraged to use a variety of drug information resources and gained experience with several online resources as well as texts and reference materials. Students were also encouraged to engage in debate and practice their oral communication skills during large group discussions.
Content
The first several weeks of the course set the foundation for the subsequent case-based application lectures. Early lectures were devoted to the scientific review of drug interactions, including common mechanism and genetic determinants of drug interactions. Subsequent classes targeted drug information skills including utilization of common online references to create drug interaction reports. Specific attention was paid to evaluating scientific literature commonly encountered when investigating drug interactions (eg, case studies, pharmacokinetic studies in healthy individuals). Computer-based screening and alert programs were reviewed and discussed as well. Once the foundation coursework was completed, lecture 6, “Clinical Approach to Drug Interactions,” was presented. Students were given a complex patient case with multiple drug interactions. Students worked either independently or in small groups to identify and evaluate the patient's drug interactions. The instructor and teaching assistant served as facilitators. The purpose of the exercise was to have students recognize their competence in identifying drug interactions and start moving their efforts towards practical and patient specific management of drug interactions. The remaining classes were devoted to reviewing commonly encountered drug interactions and managing patient case scenarios through a combination of short lectures, group discussion, and independent work. Multiple literature searches were performed and relevant primary, secondary, and tertiary literature totaling over 100 references was reviewed by the instructor to prepare for each week's activities.
EVALUATION AND ASSESSMENT
Curriculum Evaluation
Standard Course Evaluation.
At the end of the spring semester, 24 students (96%) completed the standard University course evaluation through the Center for Effective University Teaching. Evaluations consisted of 14 questions using a 5-point scale and open-ended questions regarding positive aspects of the course and suggestions for improvement. Results of the evaluation provide a comparison to other pharmacy courses taught that term.
Course evaluation results were positive. The mean overall rating of the course was 4.3 ± 0.7 based on a scale on which 5 = one of the best and 1 = one of the worst. This score compared favorably to a mean score of 3.6 ± 0.9 for all pharmacy courses taught that term. Using a scale in which 5 = almost always and 1 = almost never, the usefulness of in-class activities scored 4.8 ± 0.4 compared to 4.1 ± 1.0 for all pharmacy courses taught that term. Usefulness of outside assignments also scored favorably compared to other pharmacy courses (4.2 ± 1.1 vs. 3.5 ± 1.1, respectively). Students were asked “how much have you learned in this course” using a scale in which 5 = an exceptional amount and 1 = almost nothing. Student responses resulted in a mean score of 4.3 ± 0.6 compared to 3.6 ± 0.9 for other pharmacy courses. The most common comment on the evaluations was that the course should be a required part of the curriculum. This comment was also reiterated several times in student exit survey instruments the following year. Other students commented that a strength of the course was its focus on the application of knowledge and skills to real-world case scenarios.
Instructor's Course Evaluation.
The instructor completed a self-assessment which included a review of the course objectives, activities, student assessments, and course evaluations, as well as a reflection on all informal reflective comments received from students and fellow faculty members. Overall, the course was viewed as effective and successful at meeting its objectives. The course instructor held 3 focus group discussions with students the following year during advanced pharmacy practice experiences (APPEs). Several students commented on the usefulness of the course, particularly during the APPEs. Students noted that other members of their interprofessional teams most commonly relied on the pharmacist or pharmacy student to manage clinical issues surrounding 2 things: adverse effects and drug-drug interactions. Some students considered this to be more valuable than aiding in the decision making regarding an initial therapeutic plan. Students who completed the course felt comfortable and prepared for those situations while students who did not complete the course did not feel as well prepared.
Learner Evaluation
Class Participation and Examinations.
Several methods were used to evaluate student learning. Classroom participation counted for 20% of the student's grade. A classroom performance grading rubric was used to assess student's ability to make meaningful contributions to scientific classroom discussion. A formative midpoint evaluation was completed by the instructor and a self-evaluation was completed by the student. Five homework assignments counted for 25% of the student's grade (5% each). The homework assignments varied in nature and included creating a pocket reference highlighting different drug interaction references, responding to a hospital formulary request, and evaluating and managing a variety of patient case scenarios involving drug interactions. A strong emphasis was placed on accuracy and concise written communication skills. Two quizzes counted for 30% of the grade (15% each). Each quiz included a closed-reference section of multiple-choice questions and an open-reference case for which the students completed a SOAP format recommendation. The cumulative final examination was similar in format to each quiz and counted for 25% of the course grade. The open-reference section of the quizzes and final examination were graded by both the instructor and teaching assistant using a rubric. Major grading discrepancies were reviewed to reach consensus. Multiple evaluation strategies were used in the course to assess each course objective, ranging from retaining basic knowledge of drug interaction mechanisms, to effectively utilizing drug information resources, to synthesizing scientific literature, and finally recommending appropriate management of a given drug interaction for a specific patient.
Survey of Student Self-confidence.
A survey instrument was distributed to all students completing the third year of the PharmD program to evaluate the impact of the Drug Interactions course on student confidence levels. Students were given the objectives of the course and asked to rate their confidence levels regarding these skills on a 4-point scale on which 1 was very unconfident and 4 was very confident. Responses of students who completed the course were compared to responses of students who did not complete the course using the Mann-Whitney test.
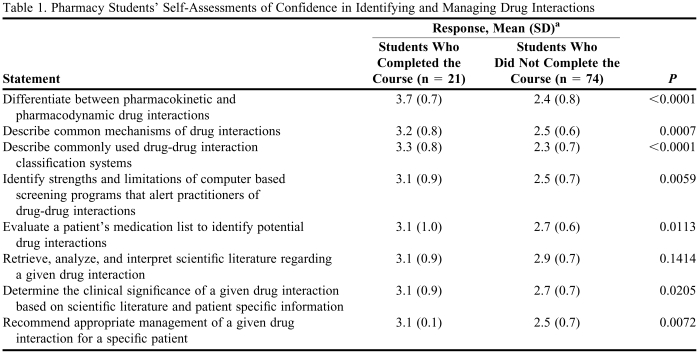
Students who completed the course were more confident in their abilities relating to drug interactions than students who did not complete the course (Table 1). Importantly, students who completed the class were more confident in their ability to determine the clinical significance of a given drug interaction based on the scientific literature and patient-specific information compared to students who did not complete the course (3.1 ± 0.9 vs. 2.7 ± 0.7, p = 0.02). They were also more confident in their ability to recommend appropriate management of a given drug interaction for a specific patient (3.1 ± 0.9 vs. 2.5 ± 0.7, p = 0.007). Responses to the statement, “retrieve, analyze, and interpret scientific literature regarding a given drug interaction,” were not significantly different between the 2 groups (3.1 ± 0.9 vs. 2.9 ± 0.7, p = 0.14).
Table 1.
Pharmacy Students' Self-Assessments of Confidence in Identifying and Managing Drug Interactions
a Responses were based on a 4-point scale on which 1 = very unconfident, 2 = unconfident, 3 = confident, and 4 = very confident.
Knowledge and Skills Evaluation.
To determine the performance of the learner (eg, gained knowledge and skills), students were asked to participate in an additional, nongraded assessment to evaluate their knowledge and skills related to drug interactions. The assessment consisted of a closed-reference multiple-choice section and an open-reference essay section. The closed reference section included 10 multiple-choice questions focused primarily on knowledge and recall of drug interaction information. Five of the multiple-choice questions listed 2 medications (eg, warfarin plus digoxin) and asked students the following question: “Which of the following phrases best describes the interaction potential of the drug combination: (a) there is no drug interaction between these 2 drugs, (b) there is an interaction but it is not clinically significant, and (c) there is a significant interaction between these 2 drugs.” The other 5 multiple-choice questions targeted knowledge related to mechanisms of interactions. Students had 15 minutes to complete the section.
The open-reference section of the assessment focused on critical thinking and application skills and included a patient case scenario in which the student was playing the role of an ambulatory care pharmacist. The case scenario involved a 65-year-old man with heart failure and atrial fibrillation. His medications included lisinopril, 20 mg, once daily; metoprolol XL, 50 mg, once daily; digoxin, 0.25 mg, once daily; and warfarin, 5 mg, once daily. He presented to the clinic with complaints of intermittent palpitations, dizziness, and fatigue, and his physician was planning to start amiodarone 400 mg, 3 times daily. The students were instructed to evaluate the patient's medication list for any potential drug-drug interactions, determine the clinical significance of each drug interaction, recommend appropriate management, and document the intervention using the SOAP format. Students had 45 minutes to complete the section.
The open reference section was scored using a rubric which was written by the instructor and reviewed by an additional clinical faculty member (available from the author). The rubric evaluated the student's assessment and plan (including identification of each DDI, description of mechanism and potential consequence of each DDI, assessment of clinical significance, and appropriate recommendation) and overall organization using a scale of 1 = poorest anticipated performance level, 2 = average performance level, and 3 = better than expected performance. Each student's assessment was scored by the blinded primary investigator using the rubric. Scores of students who completed the class were compared to students who did not.
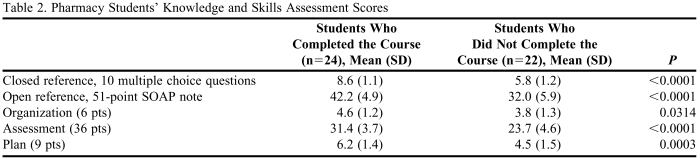
Results of the knowledge and skills assessment are presented in Table 2. Students who completed the course scored significantly better in all areas of the assessment than students who did not complete the course. In the closed reference, multiple-choice section, students who completed the course answered approximately 3 more questions correctly out of 10 compared to students who did not complete the course; a 33% difference between groups. In the open reference, SOAP format section, students who completed the course scored higher in all 3 graded components. A notable difference was in the assessment, where the average score for students who completed the course was 31.4 out of 36 (87.2%) compared to 23.7 out of 36 (65.8%) for students who did not complete the course (p < 0.0001). The majority of students in both groups appropriately identified the existing drug-drug interactions in the assessment section, but students who completed the course were more likely to describe the mechanism and potential consequence and assess the clinical significance of each interaction. Another notable difference was in the plan, where the average score for students who completed the course was 6.2 out of 9 (69%) compared to 4.5 out of 9 (49.4%) for students who did not complete the course (p = 0.0003). When looking specifically at the interaction between amiodarone and warfarin, students who completed the course were more likely to successfully recommend changing the dose of warfarin and monitoring more frequently compared to students who did not complete the course (92% vs. 41%, p = 0.0004). Seven students who did not complete the course (32%) recommended discontinuing amiodarone, and 3 (14%) recommended discontinuing warfarin. There were no significant differences in confidence levels regarding students' abilities to analyze and interpret scientific literature for a given drug interaction; however, the results of the knowledge and skills assessment indicated that students who completed the course were more successful at analyzing and interpreting scientific literature regarding the warfarin and amiodarone interaction, as evident by their management recommendations.
Table 2.
Pharmacy Students' Knowledge and Skills Assessment Scores
DISCUSSION
The logistics and resources required to implement a course in drug interactions are modest. Although several reference books related to drug interactions are readily available, a textbook that focuses on the evaluation and management of drug interactions is not. The instructor and teaching assistant spent a significant amount of time during the first year of the course performing literature searches and retrieving primary literature to fully prepare for the course. In addition, this course was conducted in the pharmacy practice laboratory so that each student would have access to a computer terminal and online resources. This author believes that conducting the course in such a simulated real-world environment is crucial to the overall effectiveness of the experience. Thus, the course was limited to the capacity of our pharmacy practice laboratory. If a school requires students to have personal laptop computers and their campuses have wireless connection options, the size limitation may not be an issue from a technology standpoint. However, small class sizes certainly lend themselves better to group discussions, interaction, and opportunities for students to improve their communication skills on a routine basis.
One strength of the course was the consistency obtained by having the same instructor each week. The group was able to build upon and expand on topics discussed in previous weeks and reinforce key principles. Also, the teaching assistant was able to help with literature retrieval, grading, and course maintenance issues. Grading assignments, quizzes, and examinations was time consuming. This time commitment could be decreased by allowing students to submit group work as opposed to individual work, and doing so also could improve interpersonal skills.
There are several limitations to consider with this study. First is the potential for bias. The confidence survey was developed by the course instructor using the course objectives and was not validated. In addition, the instructor created the knowledge and skills assessment tool and evaluation rubric. The author attempted to minimize bias by having an external clinical professor evaluate the assessment tool and rubric for external validity. In addition, all evaluations were blinded and reviewed by an external evaluator. There is the possibility that students who completed the course scored better on the assessments because they were more familiar with the format of the assessment as opposed to having improved knowledge and skills. There is also the possibility that students who enrolled in the course were better-performing students than students who did not enroll in the course. Although all students were required to complete an elective course during this semester, students who elected to complete this particular course could have been more motivated students, or students with higher grade point averages. Data regarding class rank and grade point averages were not obtained so comparisons between groups could not be made in this regard
The most noteworthy discovery was the overwhelming view that the content and skills of this course should be part of the core curriculum as opposed to an elective course. Understanding drug interaction information and applying that knowledge in patient case scenarios would seem to be a basic core content area within a pharmacy curriciulum. The reflective process, thus, focused heavily on considerations of how the course content could be integrated into existing core courses such as Therapeutics and delivered in an effective manner to all students. Discussions with students and fellow faculty members revealed that, although drug interactions were included in the core curriculum, the focus was primarily on (1) introductory content related to basic mechanisms early in the curriculum, and (2) the identification or memorization of key drug interactions. The curriculum offered little to no opportunities in the evaluation and management of drug interactions using a case-based approach. The results of this assessment illustrated this gap in the curriculum. Students who did not take the drug interactions elective course scored much lower on the assessment and often times made management decisions that were ineffective, unpractical, and unnecessary. For example, students who did not take the drug interactions course were more likely to recommend changing amiodarone to a less effective antiarrythmic agent instead of adjusting the dose of warfarin to manage the interaction between amiodarone and warfarin. This course was successful at increasing the faculty member's awareness that more often than not, the clinical issues surrounding drug interactions were getting lost in the last 5 minutes of every therapeutics lecture. Ultimately, this awareness translated into action, with several faculty members working to effectively incorporate the content and activities into current courses.
CONCLUSION
Identifying, evaluating, and managing significant drug interactions are essential roles of pharmacists. The purpose of this study was to evaluate the impact of an elective course on drug interactions on student knowledge and skills. Specific goals of the course were to improve students' ability to (1) determine whether a given interaction was clinically significant or required pharmacist intervention, and (2) make rational, scientifically sound, practical recommendations for the management of drug interactions. Based on the course evaluations, student self-assessments, and knowledge and skills assessments, the course was successful at meeting its goals and objectives. Offering a course devoted to the identification and management of drug interactions can improve knowledge and skills and potentially improve pharmaceutical care. However, integrating such content into the core curriculum may be more appropriate than offering it as an elective course. Based on the overall positive response to this elective course, the coordinators of our patient care and therapeutics based courses have been working on redesigning courses to longitudinally incorporate the content and activities from this elective course into the core PharmD curriculum.
REFERENCES
- 1. National Association of Chain Drug Stores, Inc. Alexandria, VA. 2007 Community Pharmacy Results. http://www.nacds.org/user-assets/pdfs/Pharmacy/2007CommunityPharmacyResults.pdf. Accessed February 27, 2009.
- 2.Masoudi FA, Baillie CA, Wang Y, et al. The complexity and cost of drug regimens of older patients hospitalized with heart failure in the United States, 1998-2001. Arch Intern Med. 2005;165(18):2069–76. doi: 10.1001/archinte.165.18.2069. [DOI] [PubMed] [Google Scholar]
- 3.Ibrahim IA, Kang E, Dansky KH. Polypharmacy and possible drug-drug interactions among diabetic patients receiving home health care services. Home Health Care Services Q. 2005;24:87–99. doi: 10.1300/J027v24n01_07. [DOI] [PubMed] [Google Scholar]
- 4.Schappert SM. Ambulatory care visits to physician offices, hospital outpatient departments, and emergency departments: United States, 1997. National Center for Health Statistics. Vital Health Stat. 1999;13(143) [PubMed] [Google Scholar]
- 5.Leape LL, Brennan TA, Laird N, et al. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Practice Study II. N Engl J Med. 1991;324(6):377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 6.Lazarou J, Pomeranz B, Corey PN. Incidence of adverse drug reactions in hospitalized patients: A meta-analysis of prospective studies. JAMA. 1998;279(15):1200–5. doi: 10.1001/jama.279.15.1200. [DOI] [PubMed] [Google Scholar]
- 7.Gurwitz JH, Field TS, Avorn J, et al. Incidence and preventability of adverse drug events in nursing homes. Am J Med. 2000;109(2):87–94. doi: 10.1016/s0002-9343(00)00451-4. [DOI] [PubMed] [Google Scholar]
- 8.Classen DC, Pestotnik SL, Evans RS, et al. Adverse drug events in hospitalized patients. Excess length of stay, extra costs, and attributable mortality. JAMA. 1997;277(4):301–6. [PubMed] [Google Scholar]
- 9.Johnson JA, Bootman JL. Drug-related morbidity and mortality. A cost-of-illness model. Arch Intern Med. 1995;155(18):1949–56. [PubMed] [Google Scholar]
- 10.Pirmohamed M, James S, Meakin S, et al. Adverse drug reactions as cause of admission to hospital: prospective analysis of 18,820 patients. Br Med J. 2004;329(7456):15–19. doi: 10.1136/bmj.329.7456.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jacubeit T, Drisch D, Weber E. Risk factors as reflected by an intensive drug monitoring system. Agents Actions. 1990;29:117–25. doi: 10.1007/978-3-0348-7292-8_13. [DOI] [PubMed] [Google Scholar]
- 12.Leape LL, Bates DW, Cullen DJ, et al. Systems analysis of adverse drug events. ADE Prevention Study Group. JAMA. 1995;274(1):35–43. [PubMed] [Google Scholar]
- 13.Raschetti R, Morgutti M, Menniti-Ippolito F, et al. Suspected adverse drug events requiring emergency department visits or hospital admissions. Eur J Clin Pharmacol. 1999;54(12):959–63. doi: 10.1007/s002280050582. [DOI] [PubMed] [Google Scholar]
- 14.Peterson JF, Bates DW. Preventable medication errors: identifying and eliminating serious drug interactions. J Am Pharm Assoc. 2001;41:159–60. doi: 10.1016/s1086-5802(16)31243-8. [DOI] [PubMed] [Google Scholar]
- 15.Hansten PD, Horn JR. The Top 100 Drug Interactions: A guide to patient management. 2007 Edition. Freeland, WA: H&H Publications, LLP; 2007. [Google Scholar]
- 16.Brunton LL, Lazo JS, Parker KL. 11th edition. New York: The McGraw-Hill Companies, Inc; 2006. Goodman and Gilman's The Pharmacological Basis of Therapeutics. [Google Scholar]
- 17.Murphy JE, Forrey RA, Desiraju U. Community pharmacists' responses to drug-drug interaction alerts. Am J Health-Syst Pharm. 2004;61:1484–7. doi: 10.1093/ajhp/61.14.1484. [DOI] [PubMed] [Google Scholar]
- 18.Magnus D, Rodgers S, Avery AJ. GP's views on computerized drug interaction alerts: questionnaire survey. J Clin Pharm Ther. 2002;27(5):377–82. doi: 10.1046/j.1365-2710.2002.00434.x. [DOI] [PubMed] [Google Scholar]
- 19. Center for Drug Evaluation and Research. Preventable adverse drug reactions: a focus on drug interactions. Available at http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/DrugInteractionsLabeling/ucm110632.htm. Accessed Jan 12, 2008.
- 20. Accreditation Council for Pharmacy Education: Accreditation Standards and Guidelines for the Professional Program in Pharmacy Leading to the Doctor of Pharmacy Degree. Available at: http://www.acpe-accredit.org/pdf/ACPE_Revised_PharmD_Standards_Adopted_Jan152006.pdf. Accessed February 27, 2009.
- 21. American Association of Colleges of Pharmacy Center for the Advancement of Pharmaceutical Education: Educational Outcomes 2004. Available at: http://www.aacp.org/resources/education/Documents/CAPE2004.pdf. Accessed June 13, 2005.
- 22. Clinical Pharmacology [Internet database]. Tampa, FL: Gold Standard, Inc.; 2008. http://www.clinicalpharmacology.com. Accessed February 27, 2009.
- 23. Micromedex Healthcare Series [Internet database]. Greenwood Village, CO: Thomson Healthcare; 2008. http://www.thomsonhc.com. Accessed February 27. 2009.