Abstract
Background Cancer registries in the 1970s showed that parts of Golestan Province in Iran had the highest rate of oesophageal squamous cell carcinoma (OSCC) in the world. More recent studies have shown that while rates are still high, they are approximately half of what they were before, which might be attributable to improved socio-economic status (SES) and living conditions in this area. We examined a wide range of SES indicators to investigate the association between different SES components and risk of OSCC in the region.
Methods Data were obtained from a population-based case–control study conducted between 2003 and 2007 with 300 histologically proven OSCC cases and 571 matched neighbourhood controls. We used conditional logistic regression to compare cases and controls for individual SES indicators, for a composite wealth score constructed using multiple correspondence analysis, and for factors obtained from factors analysis.
Results We found that various dimensions of SES, such as education, wealth and being married were all inversely related to OSCC. The strongest inverse association was found with education. Compared with no education, the adjusted odds ratios (95% confidence intervals) for primary education and high school or beyond were 0.52 (0.27–0.98) and 0.20 (0.06–0.65), respectively.
Conclusions The strong association of SES with OSCC after adjustment for known risk factors implies the presence of yet unidentified risk factors that are correlated with our SES measures; identification of these factors could be the target of future studies. Our results also emphasize the importance of using multiple SES measures in epidemiological studies.
Keywords: Oesophageal cancer, socio-economic status, case–control, epidemiology, Iran, factor analysis, correspondence analysis
Introduction
Studies conducted in the late 1960s and 1970s reported very high rates of oesophageal squamous cell carcinoma (OSCC) in Golestan Province, in the eastern part of the Caspian Littoral of Iran, with age-adjusted incidence rates of more than 100 per 100 000 (World Standard Population) in both sexes.1 Although the incidence rates of OSCC are still very high in both sexes in this region, recent studies have shown a decrease.2
A change in the distribution of risk factors may be responsible for these decreasing rates. Suggested risk factors for OSCC in this area are tobacco and opium consumption,3–6 low intake of fresh fruits and vegetables,7 drinking very hot beverages7,8 and low socio-economic status (SES).7 While many of these have changed over time, perhaps the most striking change during the past few decades has been an improvement in living conditions, with greater availability of schools, electricity, safe drinking water, telephone communication and roads.2 This has resulted in improved SES. For example, the number of people aged 7–29 years in the study area who had no formal education decreased from 75% in 1966 to 7% in 1996, and the percentage of households with access to electricity increased from 11% in 1966 to 95% in 1996.9,10 This improvement in SES could be one of the main reasons for the observed decrease in the incidence of OSCC.2,11
In relation to health status, SES may be defined as a measure of access to the basic resources required to achieve and maintain good health.12 These resources may be grouped into three areas: material and monetary goods (material capital), skills and capabilities (human capital) and strength of social relationships (social capital).13 Although SES is not itself a direct biological causal factor, it can influence health via behaviour and lifestyle, environmental exposure and access to health care.14 A priori selection of one or more specific indicators to study the relationship of SES to health outcomes can be very difficult, because some SES markers may have different meanings in different populations or different time periods,12 and some SES indicators may not precisely convey the purpose of their collection. For example, living in suburban areas may be a sign of wealth in some countries or regions, but it may be a sign of poverty in others; or reported income may only partially capture economic status, because such a measure may not include assets such as inherited wealth, savings, or income earned from informal economy.15 SES indicators tend to be correlated, so the results found for one variable may be confounded by others; on the other hand, the correlation may not be strong enough to justify the use of the correlated SES indicators (e.g. education and income) as proxies for each other.15,16 Several papers have provided detailed discussions of the general views on selecting SES indicators in health studies.12–18 To overcome some of the above problems, it is generally recommended to use several SES indicators.17,18
Only one case–control study, conducted in the 1970s, has examined the association between SES and OSCC in Golestan, and it suggested an inverse association.7 Therefore, we sought to re-evaluate and corroborate this association using data collected from a recent case–control study, to identify which of the factors that contribute to SES influence the incidence of OSCC. We collected data on a large number of SES indicators in a population-based case–control study in Golestan Province, and we analysed the data in several ways.
Materials and methods
Study population
Details of subject selection have been reported earlier.6 Briefly, the case subjects included all patients who presented to Atrak Clinic, the only specialized clinic for upper gastrointestinal tract cancers in eastern Golestan, from December 2003 to June 2007 and who received a histopathological diagnosis of OSCC and agreed to participate in the study. Other eligibility criteria included being at least 18 years of age, residing in eastern Golestan at the time of enrolment, and having no history of any other cancer. Physicians in the study catchment area were asked to refer their patients suspected of having upper gastrointestinal tract cancers to Atrak Clinic, and results of the Golestan Cancer Registry show that ∼70% of all incident cases in this area during this time period were in fact referred to Atrak Clinic (unpublished results). For each case subject, we attempted to select two population-based control subjects, individually matched to the case for neighbourhood of residence, age (±2 years) and gender, using the family health census that is conducted annually by the Iranian Primary Health Care System. For rural patients, our interview group identified all of the potentially eligible controls in each village and randomly selected one to interview. If the first person could not be interviewed for any reason, the second person on the list was invited and so forth. In urban areas, a list of the eligible controls was ordered by geographic proximity to the case's residence, with the list starting from the eligible person living closest to the case's residence. If the first person was not available for interview, then the second closest person was invited and so forth. Seventy-seven percent of the controls enrolled in this study were the first randomly selected individuals, and 11 and 3%, respectively, were the second and third choices. The reason for not participating in the study in nearly all instances was the absence of the eligible control at the time of invitation.
The study was reviewed and approved by the Institutional Review Boards of the Digestive Disease Research Center of Tehran University of Medical Sciences and the US National Cancer Institute.
Case ascertainment method
A detailed description of the diagnostic procedure, including endoscopy, is available elsewhere.19 The biopsy samples were sent to the Digestive Disease Research Center of Tehran University of Medical Sciences, where they were examined by experienced pathologists and confirmed to be OSCC. Therefore, 100% of the cases had histological diagnosis.
Data collection
After obtaining written informed consent, participants were interviewed by trained interviewers who collected detailed information on demographic characteristics, SES indicators and potential confounders of interest, such as life-long history of tobacco, opium or alcohol use, using a structured questionnaire. Dietary data were collected by a nutritionist, using a food frequency questionnaire specifically developed for this population.20
The potential SES indicators that we investigated in this study were education level (highest level attained), head of the household's education level, marital status, number of first degree relatives, house ownership, house structure, house size in square metres (m2), number of people living together in the current house, ownership of household appliances, including bath in the residence, personal car, motorbike, black and white TV (B/W TV), colour TV, refrigerator, freezer, vacuum and washing machine, and the duration of owning these appliances. Questions on relatives and family structure were included because these factors could be indicators of social contacts and therefore social capital in Golestan, where there are few formal social activities, especially in rural areas. We also asked questions about the current job or the most recent occupation for retired or disabled subjects. Occupations were categorized as: (i) farmers, people whose main occupation was cultivating agricultural products in their own land or raising their own livestock; (ii) unskilled manual workers, which included construction, industry and agriculture labourers; (iii) skilled manual workers; (iv) service (‘white-collar’) workers, which included clerks and people working in financial, educational or other services; or (v) supported by aid organizations, which usually included very poor, unemployed people. When subjects had more than one occupation, the occupation with the most stable situation and earnings (e.g. non-seasonal) was used. Since few women in this study had an occupation, we asked women about the current or latest job of the household head. The participants were not questioned about their income, which was shown to be unreliable in a pilot study we conducted in Golestan (unpublished data).
Statistical analysis
Numbers and percentages were calculated and presented for categorical variables. To compare the distribution of the variables of interest between cases and matched controls, we used the chi-square test for categorical variables and the Wilcoxon matched pairs test for continuous variables.
We aimed to create a wealth score for each individual. One approach to building such a score is summing up the number of household appliances owned by each subject, but this method has the problem of giving an equal weight to each appliance regardless of its value or its ability to distinguish wealthier from poorer people.21 Also, it is difficult to incorporate information from job or other qualitative variables in such a score. Therefore, to build a composite score for wealth based on appliances and other variables, we utilized multiple correspondence analysis (MCA) on personal car, motorbike, B/W TV, colour TV, refrigerator, freezer, vacuum and washing machine ownership variables, as well as house ownership, house structure, house size (tertiles), having a bath in the residence and occupation. MCA may be used as an exploratory tool and is appropriate for qualitative variables.22–26 We have briefly described the principals of this analysis in Appendix 1 (available as supplementary data at IJE online). The scores were calculated and categorized as quintiles according to the observed coordinates among control subjects.
In accord with the matched design of the study, conditional logistic regression was used to calculate unadjusted and adjusted odds ratios (ORs) and corresponding 95% confidence intervals (CIs) for each SES variable. By design, case and control subjects were matched for age, sex and place of residence. Conditional logistic regression models were adjusted for potential confounders, including ethnicity, daily vegetable intake, alcohol drinking, consumption of opium and/or tobacco (including cigarette, pipe and hookah smoking and nass chewing), duration of residence in rural areas, education level, marital status and the wealth score. We calculated population attributable fractions (PAFs) for selected variables that showed associations with risk of OSCC.
Finally, we used an exploratory factor analysis, principal component factor method,27 as an additional analysis to examine the effect of derived uncorrelated factor variables on risk of OSCC, and to compare the results with those of our main analyses, which were described above. We selected factors with eigenvalue >1.0 and rotated them using the varimax rotation method. Variables with factor loadings of 0.4 or greater (–0.4 or less) within a particular factor are conventionally considered to be its major components.28 The rotated factors were labelled according to their high-loading variables. We calculated ORs (95% CIs) for each factor in conditional logistic regression models with adjustments for the other derived factors and for the above mentioned potential confounders. All statistical analyses were performed using STATA software, version 10.0 (StataCorp., College Station, TX, USA). Since there were no major differences between men and women in patterns of association of the studied variables with risk of OSCC in the multivariate models, we reported our results for both sexes combined.
Results
A total of 300 cases and 571 controls were recruited into the study. All cases had at least one matched control and the large majority (∼90%) had two matched controls. Table 1 shows the distribution of demographic variables in case and control subjects. Among both cases and controls, ∼50% were male; 73% were residing in rural areas, and mean [standard deviation (SD)] age was 64 (10) years. There was no difference in the distribution of ethnicity in the cases compared with the controls (chi-square test P = 0.64).
Table 1.
Characteristics of 300 OSCC cases and 571 controls, Golestan Province, Northern Iran, 2003–07a
| Characteristic | Case | Control | P-valueb |
|---|---|---|---|
| Age, mean (SD), years | 64.5 (10.1) | 64.3 (10.4) | 0.81 |
| Gender | 0.70 | ||
| Male | 150 (50.0%) | 278 (48.6%) | |
| Female | 150 (50.0%) | 293 (51.4%) | |
| Place of residence | 0.77 | ||
| Urban | 82 (27.3%) | 150 (26.3%) | |
| Rural | 218 (72.7%) | 421 (73.7%) | |
| Ethnicity | 0.64 | ||
| Turkmen | 171 (57.0%) | 312 (54.6%) | |
| Non-Turkmen | 129 (43.0%) | 259 (45.4%) | |
| Alcohol ever use | 7 (2.3%) | 15 (2.6%) | 0.86 |
| Tobaccoc or opium use | 0.0002 | ||
| Used neither tobacco nor opium | 166 (55.5%) | 398 (69.8%) | |
| Used tobacco but not opium | 43 (14.4%) | 66 (11.6%) | |
| Used opium but not tobacco | 30 (10.0%) | 34 (6.0%) | |
| Used both tobacco and opium | 60 (20.1%) | 72 (12.6%) |
aAlthough cases and controls were individually matched, the percentages of cases and controls are not necessarily equal in each age, gender or place of residence category, because some cases have one and the others have two matched controls.
bP-values come from chi-square tests for categorical variables (chi-square for trend in variables with more than two categories) and Wilcoxon Rank Sum tests for continuous variables.
cAny kind of tobacco smoking or chewing.
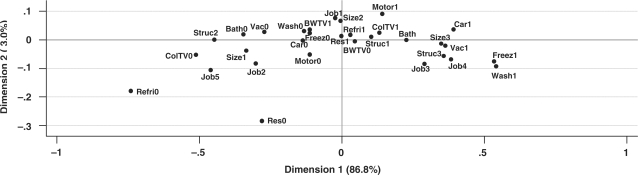
Figure 1 shows the results for the first two dimensions (axes) of the MCA analysis among control subjects. The first dimension explained the majority (86.8%) of the total chi-square variation in the data; therefore we used only the first dimension for further analyses. In this figure, indicators of higher wealth are found in the right side of the graph. For example, having a washing machine is in the extreme right side of the graph, and it means that only the wealthiest people in that area own a washing machine. In contrast, not having a refrigerator is a sign of extreme poverty. Service and skilled manual jobs were associated with higher wealth. In contrast, as expected, people supported by aid organizations had the lowest wealth status among the job categories. A wealth score was calculated by weighing each variable by weights reported in the first dimension and then summing these weights for each subject (see the footnote of Figure 1).
Figure 1.
Visualization of the coordinates of wealth variables in the MCA among 571 controls, Golestan Province, Northern Iran, 2003–07. Appliance ownership: Bath = bath in the residence; Car = automobile; Motor = motorbike; BW = B/W TV; ColTV = colour TV; Refri = refrigerator; Freez = freezer; Vac = vacuum; Wash = washing machine; suffix ‘0’ is the indicator of not owning the appliance; suffix ‘1’ is the indicator of owning the appliance. Housing: Res0 = not owned a residence; Res1 = owned a residence; Struc1 = house made of burned brick; Struc2 = house made of mud brick/clay; Struc3 = other house structures; Size1 = first tertile of house size (m2); Size2 = second tertile of house size; Size3 = third tertile of house size. Occupation: Job1 = farmers; Job2 = non-skilled manual worker; Job3 = skilled manual occupations; Job4 = service (white-collar); Job5 = supported by aid organizations. The indicators of higher wealth are located on the right side of the plot. The first dimension explains the majority (86.8%) of the chi-square variation of the data. The wealth score was calculated by weighing each variable by weights reported in the first dimension and then summing. For example, the weights for owning or not owning a refrigerator in the first dimension were 0.038 and –0.733, and for owning or not owning a freezer were 0.542 and –0.105, respectively. If a subject owned a refrigerator but did not have a freezer, the corresponding weights (0.038 and –0.105) were summed up; this procedure was continued until the weights of all MCA variables were included in this calculation.
Among controls, residence in urban areas and school attendance were associated with higher wealth scores (Appendix Table 1 available as supplementary data at IJE online). There was no trend in wealth score by ethnicity or marital status. Among controls, both in urban and in rural areas, younger subjects were more educated and had higher wealth than older subjects (Appendix Tables 2 and 3 available as supplementary data at IJE online). The percentages of controls with formal education in each group was higher in urban than in rural subjects.
Table 2 shows the ORs (95% CIs) for the association of each of the SES indicators with OSCC. There was a suggestion that residence in rural areas for >20 years was associated with increased risk (OR = 2.79, 95% CI 0.97–8.03). Compared with no formal education, attending primary school (OR = 0.52, 95% CI 0.27–0.98) or middle school or higher levels (OR = 0.20, 95% CI 0.06–0.65) was inversely associated with risk of OSCC. Education level of the head of household was not associated with risk. All subjects had a history of marriage, and only two cases were divorced. Compared with being widowed/divorced, being married was inversely associated with risk of OSCC (OR = 0.41, 95% CI 0.27–0.63). The second tertile of the number of children, compared with the fist tertile, showed an inverse association with risk of OSCC in non-adjusted analyses. No association between the number of children, the number of siblings or the number of people in the household and risk of OSCC was observed in the multivariate analyses.
Table 2.
ORs for oesophageal squamous cell carcinoma according to various potential measures of SES among 300 cases and 571 controls, Golestan Province, Northern Iran, 2003–07
| Case (%)/Control (%) | Crude OR (95% CI) | Adjusted ORa (95% CI) | |
|---|---|---|---|
| Duration of residence in rural areas | |||
| Nil | 9 (3.0)/29 (5.1) | 1.00 | 1.00 |
| 1–20 years | 26 (8.7)/51 (8.9) | 1.88 (0.71–4.97) | 1.74 (0.52–5.84) |
| >20 years | 264 (88.3)/491 (86.0) | 2.63 (1.04–6.64) | 2.79 (0.97–8.03) |
| Formal education | |||
| No school | 267 (89.0)/474 (83.0) | 1.00 | 1.00 |
| Primary school | 25 (8.3)/64 (11.2) | 0.53 (0.30–0.92) | 0.52 (0.27–0.98) |
| Middle school or higher | 8 (2.7)/33 (5.8) | 0.19 (0.07–0.57) | 0.20 (0.06–0.65) |
| Head of household education | |||
| No school | 190 (64.0)/367 (64.8) | 1.00 | 1.00 |
| Primary school | 60 (20.2)/104 (18.4) | 1.12 (0.75–1.67) | 1.41 (0.79–2.54) |
| Middle school or higher | 47 (15.8)/95 (16.8) | 0.88 (0.56–1.39) | 0.99 (0.53–1.85) |
| Marital status | |||
| Widowed/divorced | 103 (34.3)/125 (21.9) | 1.00 | 1.00 |
| Married | 197 (65.7)/446 (78.1) | 0.40 (0.27–0.60) | 0.41 (0.27–0.63) |
| Number of siblings | |||
| First tertile (0–3) | 107 (35.8)/180 (31.6) | 1.00 | 1.00 |
| Second tertile (4–5) | 87 (29.1)/171 (30.0) | 0.84 (0.59–1.19) | 1.00 (0.67–1.49) |
| Third tertile (6+) | 105 (35.1)/219 (38.4) | 0.75 (0.52–1.08) | 0.89 (0.59–1.33) |
| Number of children | |||
| First tertile (0–6) | 145 (48.7)/241 (42.3) | 1.00 | 1.00 |
| Second tertile (7–8) | 65 (21.8)/167 (29.3) | 0.65 (0.45–0.93) | 0.79 (0.53–1.20) |
| Third tertile (9+) | 88 (29.5)/162 (28.4) | 0.90 (0.63–1.26) | 1.06 (0.71–1.59) |
| Number of people in household | |||
| First tertile (1–4) | 106 (35.8)/197 (34.7) | 1.00 | 1.00 |
| Second tertile (5–6) | 83 (28.0)/155 (27.3) | 0.96 (0.67–1.37) | 0.92 (0.60–1.40) |
| Third tertile (7+) | 107 (36.2)/215 (37.9) | 0.89 (0.62–1.28) | 0.84 (0.54–1.32) |
| House ownership | |||
| No | 21 (7.0)/11 (1.9) | 1.00 | 1.00 |
| Yes | 278 (93.0)/558 (98.1) | 0.30 (0.14–0.63) | 0.31 (0.13–0.74) |
| House structure | |||
| Burned brick | 236 (78.9)/447 (78.4) | 1.00 | 1.00 |
| Mud brick–clay | 61 (20.4)/118 (20.7) | 0.99 (0.66–1.50) | 0.79 (0.47–1.33) |
| Other | 2 (0.7)/5 (0.9) | 0.71 (0.14–3.74) | 0.54 (0.08–3.64) |
| House area (m2) | |||
| First tertile (<80) | 99 (33.2)/188 (33.0) | 1.00 | 1.00 |
| Second tertile (80–100) | 102 (34.2)/210 (36.8) | 0.90 (0.64–1.27) | 0.89 (0.58–1.36) |
| Third tertile (>100) | 97 (32.6)/172 (30.2) | 1.05 (0.73–1.53) | 1.56 (0.92–2.64) |
| House area (m2) per person | |||
| First tertile (≤12.5) | 91 (30.7)/198 (34.9) | 1.00 | 1.00 |
| Second tertile (12.6–20) | 99 (33.5)/188 (33.2) | 1.16 (0.81–1.65) | 1.29 (0.85–1.97) |
| Third tertile (>20) | 106 (35.8)/181 (31.9) | 1.36 (0.93–1.97) | 1.63 (1.06–2.50) |
| Appliance ownershipb | |||
| Bath in residence | 179 (60.0)/335 (58.9) | 1.01 (0.73–1.39) | 1.23 (0.84–1.78) |
| Car | 47 (15.7)/139 (24.4) | 0.54 (0.37–0.79) | 0.53 (0.35–0.81) |
| Motorbike | 130 (43.5)/233 (40.9) | 1.13 (0.82–1.57) | 1.25 (0.88–1.80) |
| B/W TV | 124 (41.5)/192 (33.7) | 1.41 (1.04–1.91) | 1.40 (0.99–1.99) |
| Colour TV | 222 (74.3)/446 (78.4) | 0.76 (0.53–1.08) | 0.91 (0.61–1.34) |
| Refrigerator | 282 (94.3)/540 (94.9) | 0.83 (0.42–1.62) | 1.57 (0.72–3.47) |
| Freezer | 43 (14.4)/92 (16.2) | 0.83 (0.54–1.28) | 0.94 (0.57–1.52) |
| Vacuum | 118 (39.5)/236 (41.5) | 0.85 (0.61–1.18) | 1.03 (0.71–1.49) |
| Washing machine | 44 (14.7)/105 (18.5) | 0.64 (0.40–1.00) | 0.60 (0.36–0.99) |
| Wealth score | |||
| First quintile | 65 (21.7)/116 (20.3) | 1.00 | 1.00 |
| Second quintile | 57 (19.1)/105 (18.4) | 0.92 (0.58–1.47) | 0.96 (0.57–1.63) |
| Third quintile | 74 (24.7)/121 (21.2) | 1.03 (0.66–1.63) | 1.32 (0.80–2.17) |
| Fourth quintile | 60 (20.1)/111 (19.4) | 0.88 (0.54–1.43) | 1.24 (0.72–2.14) |
| Fifth quintile | 43 (14.4)/118 (20.7) | 0.49 (0.28–0.86) | 0.64 (0.35–1.19) |
| Occupation | |||
| Farmer | 172 (57.5)/320 (56.1) | 1.00 | 1.00 |
| Manual (unskilled) | 55 (18.4)/94 (16.5) | 1.05 (0.70–1.58) | 0.85 (0.52–1.39) |
| Manual (skilled) | 17 (5.7)/34 (6.0) | 0.88 (0.45–1.72) | 0.74 (0.35–1.56) |
| Service (white-collar) | 39 (13.0)/92 (16.1) | 0.72 (0.44–1.15) | 0.77 (0.44–1.35) |
| Aid organizations support | 16 (5.4)/30 (5.3) | 1.06 (0.51–2.18) | 0.79 (0.33–1.84) |
aAdjusted for ethnicity (0 = non-Turkmen; 1 = Turkmen), daily vegetable intake (in logarithmic scale), alcohol consumption (0 = never; 1 = ever), tobacco or opium ever use (0 = none; 1 = only tobacco; 2 = only opium; 3 = both), duration of residence in rural areas (0 = nil; 1 = 1–20 years; 2 = >20 years), education level (0 = no school; 1 = primary school; 2 = middle school or higher), marital status (0 = widowed/divorced; 1 = married) and wealth score (quintiles). When one of these possible confounders was the variable of interest, the adjustments were made for the other possible confounders.
bThe reference group for each appliance was the subjects that did not own that appliance. In the multivariate analysis, these appliance ownership variables were not adjusted for the wealth score.
Owning a house was inversely associated with OSCC risk (OR = 0.31, 95% CI 0.13–0.74); however, only 7.0% of cases and 1.9% of controls did not own a residence. Neither house structure nor total house area was associated with risk of OSCC, but the third tertile of residence area per person, compared with the first tertile, showed an association with risk (OR = 1.63, 95% CI 1.06–2.50). Owning an automobile (OR = 0.53, 95% CI 0.35–0.81) or a washing machine (OR = 0.60, 95% CI 0.36–0.99) was inversely associated with risk of OSCC in the multivariate models that did not include the wealth score. After adding the wealth score to the models, the association remained only for owning an automobile (data not shown). None of the other appliance ownership variables had an association with risk of OSCC, although owning a B/W TV showed a marginal association (OR = 1.40, 95% CI 0.99–1.99). The fifth quintile of the composite wealth score (compared with the first quintile) showed an inverse association with risk of OSCC in the non-adjusted analyses. This association was not present in the multivariate model, mainly as a result of adjustment for vegetable intake. There was no difference between cases and controls in duration of owning appliances (data not shown). Since cases and controls were matched for age, longer duration of having appliances was equivalent to having them at younger ages.
The PAFs for having no formal education, not being in the highest quintile of the wealth score and being widowed/divorced were 35, 30 and 16%, respectively (Table 3). The overall PAF for these variables was 69%.
Table 3.
Population attributable fraction (PAF) for selected variables in Golestan Case–Control Study, among 300 OSCC cases and 571 controls, Golestan Province, Northern Iran, 2003–07
| Risk associated characteristic | PAF (%) |
|---|---|
| No formal education vs some formal education | 35 |
| Wealth score quintiles 1–4 vs quintile 5 | 30 |
| Being widowed/divorced vs being married | 16 |
| All three of the above characteristics | 69 |
In the factor analysis, three factors were identified (Table 4). The first factor, for which the main components were education level and wealth score (OR = 0.73, 95% CI 0.58–0.92); the second factor, for which the main components were the number of children, the number of people in the household and the wealth score (OR = 0.83, 95% CI 0.70–0.99); and the third factor, for which the main component was marital status (OR = 0.66, 95% CI 0.54–0.80), had inverse associations with risk of OSCC.
Table 4.
Summary of the factor analysis, principal component factor method with varimax rotations, and adjusted ORs (95% CIs) for the derived factors in conditional logistic regression models, for 300 OSCC cases and 571 controls, Golestan Province, Northern Iran, 2003–07
| SES measure | Factor 1: education/wealth | Factor 2: family size/wealth | Factor 3: marital status |
|---|---|---|---|
| Education levela | 0.83 | –0.13 | 0.29 |
| Head of household education levela | 0.83 | –0.03 | –0.32 |
| Marital statusb | 0.00 | 0.11 | 0.94 |
| Number of sibling | 0.22 | 0.35 | –0.18 |
| Number of children | –0.25 | 0.68 | 0.03 |
| Number of people in household | 0.14 | 0.75 | 0.23 |
| Wealth scorec | 0.58 | 0.53 | 0.04 |
| Total variance (%) | 27 | 22 | 15 |
| Adjusted OR (95% CI)d | 0.73 (0.58–0.92) | 0.83 (0.70–0.99) | 0.66 (0.54–0.80) |
aCategorized as 0 = no formal education; 1 = primary school; 2 = middle school or higher.
bCategorized as 0 = widowed/divorced; 1 = married.
cContinues variable.
dAdjusted for ethnicity (0 = non-Turkmen; 1 = Turkmen), daily vegetable intake (in logarithmic scale), alcohol consumption (0 = never; 1 = ever), tobacco or opium ever use (0 = none; 1 = only tobacco; 2 = only opium; 3 = both), duration of residence in rural areas (in year, contentious) and other derived factors. Since the factors are standardized, one unit change in the factor means a change equaled to one standard deviation of the factor.
Discussion
Our study confirms a strong association between low SES and risk of OSCC in eastern Golestan. Several indicators of SES, including formal education, being married and high-wealth status were inversely associated with risk of OSCC. Education level showed a strong dose–response inverse association that was unaffected by adjustment for several potential confounders. Marital status also showed a strong inverse association that was largely unaffected by adjustment. The protective effect of high wealth score, however, was only seen for the highest quintile and disappeared after adjustment for other variables (mainly vegetable intake).
Using education as a marker of SES has several advantages and disadvantages. Questioning about education usually produces reliable results and is not affected by recall bias. Another advantage is that it is not altered by health status in old age. Education level is unlikely to change after early adulthood and it has been shown to be the main indicator of childhood SES with respect to some future health outcomes, such as overall mortality, as reported in a major review of prospective studies.29 One disadvantage of using education as a marker of SES in our study population was the usually limited years of schooling among most older people.15 The inverse association between education level and risk of OSCC found in our study is in agreement with the previous literature.7,30 While this association is strong and consistent across studies, and is therefore most probably real, it is difficult to know exactly how education affects risk of OSCC. Higher education may reflect a higher SES status of the family during childhood, which may have an effect on future health. People with higher levels of education may get jobs with higher income.15 In addition to years of schooling, early childhood education itself seems to have health benefits.14 One of the non-economic social effects of education is acquiring general and health-related knowledge, which can affect health outcomes.17
In our study, widowed/divorced (mainly widowed) people were at a higher risk of OSCC. This is consistent with a few other studies that have reported an association between being widowed or divorced and higher mortality from oesophageal cancer, or an inverse association between duration of living with a partner and risk of OSCC.31,32 We can only speculate on the reasons for these observations. Being widowed or divorced would result in loss of emotional support by the former spouse, and could result in less income, fewer social relationships, and less balanced nutrition. Nonetheless, adjustments for vegetable intake did not change our results.
We evaluated several variables related to living in more or less crowded environments, including number of siblings (related to one's environment during childhood), number of children, number of people living in the household, total house area and house area per person living in the household as potential indicators of SES. People with larger families and more crowded accommodations may have less adequate diets and higher exposure to infections.33,34 On the other hand, in more traditional societies with closer family relationships, people with larger families may be better supported by family members and may have closer social relationships.35,36 In our study, the number of siblings, the number of children and the number of people living in the household did not have independent associations with risk of OSCC, even though these variables and the wealth score had high loading factors in the second factor of our factor analysis (Table 4), which indicates that they were correlated. The house area per household member showed a positive association with risk of OSCC, which probably reflects the importance of living closely with family members in this society.
Collecting data on income and savings can be a sensitive issue in epidemiological studies.15,17 In addition, income may not reflect the true economic status of some individuals, especially older people, who usually do not work.15 Therefore, other measures of wealth may be more useful economic status indicators for these people.18 Collecting information on non-monetary indicators of wealth, such as owning appliances, can be relatively simple and a less sensitive issue,37 particularly in non-Western countries.38 In studies conducted in Golestan in the 1970s, only the number of cows owned at the age of 25 years showed an inverse association with risk of oesophageal cancer; other indicators of cattle or farm ownership were not associated with the risk.7 We asked about ownership of household appliances as a measure of wealth. Owning an automobile, which is more expensive than most other appliances and could be considered as an indicator of high income, was inversely associated with risk of OSCC. The MCA-based wealth score and owning an automobile were correlated (Spearman's rank correlation coefficient = 0.53; Appendix Table 1 available as supplementary data at IJE online). However, the protective association of owning a car persisted after adjustment for the wealth score, so owning a car may also be a proxy for some other more direct factors. For example, it may provide convenient access to food shopping or it may enhance social contacts.15 The protective effect of high wealth scores was observed only in the group with the highest wealth status; interestingly, there was no difference in the association between wealth score and risk of OSCC among the least wealthy 80% of the population.
In developed countries, occupation can be a good surrogate for income and social prestige, and it may also correlate with education level. Nonetheless, income inequalities can exist within one occupation, e.g. between ethnic groups or between men and women.15,39 In addition, it is not clear whether the husband's occupation is a good SES indicator for a housewife.15 In our study, there was limited variation in the occupation types; >25 and 65% of study subjects in urban and rural areas, respectively, were farmers. Although some occupations were associated with higher wealth score, none of the occupation categories was associated with risk of OSCC.
Duration of residence in a rural area showed a positive association with risk of OSCC, despite the fact that this association might have been attenuated due to matching cases and controls on current place of residence. A recent study has shown that the rural dwellers in Golestan have much lower vitamin intake than the urban dwellers,40 which might explain some of the increased risk of OSCC in this subgroup.
If improved SES is indeed an important factor in the observed recent decrease in OSCC incidence in Golestan, one might expect that further improvements in SES could lead to a further reduction in the incidence of this disease. Changing some factors that influence SES, such as the general economy, may not be achievable in the short term, and it may be beyond the reach of public health programs. However, if SES influences health outcomes via its effect on behaviour and lifestyle, one practical way to reduce OSCC incidence may be to conduct active educational programmes to increase general awareness of known and potentially important risk factors for this disease. Known risk factors of OSCC in Golestan include low fruit and vegetable intake and tobacco or opium consumption6,7 and other suggested risk factors may be poor oral hygiene,41 drinking hot tea,8 and high exposure to some environmental factors like polycyclic aromatic hydrocarbons42,43 or infections.44 The protective effect observed in our study for attending even primary school, which can help children acquire health-related knowledge, suggests the potential importance of increasing disease-related awareness in the general population.
This study has several strengths and limitations. The strengths include histological proof of OSCC, administration of pre-tested structured questionnaires by well-trained interviewers, and adjustments for several potential confounders. In addition, we utilized different methods to investigate associations between SES indicators and risk of OSCC, which all showed consistent results. One of the limitations of this study is the retrospective assessment of exposures. However, we mainly asked questions about easily recalled facts, such as educational level or ownership of residence or appliances, which reduces the possibility of recall bias. Although recruiting neighbourhood controls can attenuate some associations, it provides efficient control for some factors and thereby reduces the possibility of spurious associations. By selecting controls randomly from an updated family health census and having a good response rate, the possibility of selection bias was minimized.
In conclusion, the results of this study confirm the inverse association between SES and OSCC risk seen in most other studies,30 and they particularly confirm the results of the one previous case–control study conducted in Golestan. The presence of an SES effect on risk of OSCC after adjustment for known more direct risk factors may indicate that there are some yet unidentified risk factors for OSCC which are correlated with SES measures. Further studies are needed to identify these risk factors. The results of this study also suggest that conducting an active program to increase general awareness of known and probable OSCC risk factors may be helpful in further reducing the incidence of OSCC in Golestan. Finally, our results also demonstrate the importance of using multiple SES indicators in epidemiological studies.
Supplementary Data
Supplementary data are available at IJE online.
Funding
Digestive Disease Research Center of Tehran University of Medical Sciences (grant number 82-603); intramural funds of the National Cancer Institute, National Institutes of Health.
Acknowledgements
We sincerely thank the Atrak Clinic staff, including Dr Noushin Taghavi, Dr Haji-Amin Marjani, Dr Rabaeh Rajabzadeh, Monireh Badakhshan, Bita Mohammadi, Halimeh Eskandarnejhad, Safora Kor, Soleiman Kasalkheh and Ashor Yolmeh. We are deeply grateful to Dr Saman Fahimi, Dr Ramin Shakeri, Dr Noorli Radgohar, Dr Abdolazim Khozeini, Dr Rahmat Ghaziani, Dr Mohammad Hasan Brazandeh, Dr Abdolhakim Ebadati, Dr Naser Keramat and Dr Ahmad Nosrati for their valuable help. We especially thank and appreciate the local health networks and health workers (Behvarzes) in the study area for their assistance in the recruitment of controls. We thank the Iranian Social Security Organization for their strong local support.
Conflict of interest: None declared.
KEY MESSAGES.
We found a strong inverse association between SES and risk of OSCC in Golestan Province, northern Iran. This province has one of the highest risks of oesophageal cancer in the world.
Since this inverse association persisted after adjusting for known risk factors of OSCC, it implies the presence of yet unidentified, more-direct risk factors that are correlated with socio-economic measures.
Results of the study also emphasize the importance of using multiple socio-economic measures in epidemiological studies, especially in developing countries.
References
- 1.Mahboubi E, Kmet J, Cook PJ, Day NE, Ghadirian P, Salmasizadeh S. Oesophageal cancer studies in the Caspian Littoral of Iran: the Caspian cancer registry. Br J Cancer. 1973;28:197–214. doi: 10.1038/bjc.1973.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Semnani S, Sadjadi A, Fahimi S, et al. Declining incidence of esophageal cancer in the Turkmen Plain, eastern part of the Caspian Littoral of Iran: a retrospective cancer surveillance. Cancer Detect Prev. 2006;30:14–19. doi: 10.1016/j.cdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 3.Joint Iran-IARC group. Esophageal cancer studies in the Caspian littoral of Iran: results of population studies—a prodrome. Joint Iran-International Agency for Research on Cancer Study Group. J Natl Cancer Inst. 1977;59:1127–38. [PubMed] [Google Scholar]
- 4.Dowlatshahi K, Miller RJ. Role of opium in esophageal cancer: a hypothesis. Cancer Res. 1985;45:1906–7. [PubMed] [Google Scholar]
- 5.Ghadirian P, Stein GF, Gorodetzky C, et al. Oesophageal cancer studies in the Caspian littoral of Iran: some residual results, including opium use as a risk factor. Int J Cancer. 1985;35:593–97. doi: 10.1002/ijc.2910350505. [DOI] [PubMed] [Google Scholar]
- 6.Nasrollahzadeh D, Kamangar F, Aghcheli K, et al. Opium, tobacco, and alcohol use in relation to oesophageal squamous cell carcinoma in a high-risk area of Iran. Br J Cancer. 2008;98:1857–63. doi: 10.1038/sj.bjc.6604369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cook-Mozaffari PJ, Azordegan F, Day NE, Ressicaud A, Sabai C, Aramesh B. Oesophageal cancer studies in the Caspian Littoral of Iran: results of a case-control study. Br J Cancer. 1979;39:293–309. doi: 10.1038/bjc.1979.54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ghadirian P. Thermal irritation and esophageal cancer in northern Iran. Cancer. 1987;60:1909–14. doi: 10.1002/1097-0142(19871015)60:8<1909::aid-cncr2820600840>3.0.co;2-y. [DOI] [PubMed] [Google Scholar]
- 9.Iran Statistical Center. National Census of Population and Housing of Iran, November 1966. Tehran: Iran Statistical Center; 1968. [Google Scholar]
- 10.Iran Statistical Center. National Census of Population and Housing of Iran, November 1996. Tehran: Iran Statistical Center; 1997. [Google Scholar]
- 11.Kamangar F, Malekzadeh R, Dawsey SM, Saidi F. Esophageal cancer in northeastern iran: a review. Arch Iran Med. 2007;10:70–82. [PubMed] [Google Scholar]
- 12.Shavers VL. Measurement of socioeconomic status in health disparities research. J Natl Med Assoc. 2007;99:1013–23. [PMC free article] [PubMed] [Google Scholar]
- 13.Oakes JM, Rossi PH. The measurement of SES in health research: current practice and steps toward a new approach. Soc Sci Med. 2003;56:769–84. doi: 10.1016/s0277-9536(02)00073-4. [DOI] [PubMed] [Google Scholar]
- 14.Adler NE, Newman K. Socioeconomic disparities in health: pathways and policies. Health Aff. 2002;21:60–76. doi: 10.1377/hlthaff.21.2.60. [DOI] [PubMed] [Google Scholar]
- 15.Berkman LF, Macintyre S. The measurement of social class in health studies: old measures and new formulations. IARC Sci Publ. 1997;138:51–64. [PubMed] [Google Scholar]
- 16.Glaeser EL, Laibson D, Sacerdote B. An economic approach to social capital. Econ J. 2002;112:F437–58. [Google Scholar]
- 17.Braveman PA, Cubbin C, Egerter S, et al. Socioeconomic status in health research: one size does not fit all. JAMA. 2005;294:2879–88. doi: 10.1001/jama.294.22.2879. [DOI] [PubMed] [Google Scholar]
- 18.Galobardes B, Lynch J, Davey Smith G. Measuring socioeconomic position in health research. Br Med Bull. 2007;81–82:21–37. doi: 10.1093/bmb/ldm001. [DOI] [PubMed] [Google Scholar]
- 19.Islami F, Kamangar F, Aghcheli K, et al. Epidemiologic features of upper gastrointestinal tract cancers in Northeastern Iran. Br J Cancer. 2004;90:1402–1406. doi: 10.1038/sj.bjc.6601737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Malekshah AF, Kimiagar M, Saadatian-Elahi M, et al. Validity and reliability of a new food frequency questionnaire compared to 24 h recalls and biochemical measurements: pilot phase of Golestan cohort study of esophageal cancer. Eur J Clin Nutr. 2006;60:971–77. doi: 10.1038/sj.ejcn.1602407. [DOI] [PubMed] [Google Scholar]
- 21.Vyas S, Kumaranayake L. Constructing socio-economic status indices: how to use principal components analysis. Health Policy Plan. 2006;21:459–68. doi: 10.1093/heapol/czl029. [DOI] [PubMed] [Google Scholar]
- 22.Coste J, Bouyer J, Job-Spira N. Construction of composite scales for risk assessment in epidemiology: an application to ectopic pregnancy. Am J Epidemiol. 1997;145:278–89. doi: 10.1093/oxfordjournals.aje.a009101. [DOI] [PubMed] [Google Scholar]
- 23.Dohoo IR, Ducrot C, Fourichon C, Donald A, Hurnik D. An overview of techniques for dealing with large numbers of independent variables in epidemiologic studies. Prev Vet Med. 1997;29:221–39. doi: 10.1016/s0167-5877(96)01074-4. [DOI] [PubMed] [Google Scholar]
- 24.Howe LD, Hargreaves JR, Huttly SR. Issues in the construction of wealth indices for the measurement of socio-economic position in low-income countries. Emerg Themes Epidemiol. 2008;5:3. doi: 10.1186/1742-7622-5-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Greenacre M. Correspondence analysis of the Spanish National Health Survey. Gac Sanit. 2002;16:160–70. doi: 10.1016/s0213-9111(02)71648-8. [DOI] [PubMed] [Google Scholar]
- 26.Kroonenberg PM, Greenacre MJ. Correspondence analysis. In: Kotz S, Read CB, Balakrishnan N, et al., editors. Encyclopedia of Statistical Sciences. Hoboken: John Wiley & Sons; 2006. [Google Scholar]
- 27.Manly BFJ. Factor analysis. In: Manly BFJ, editor. Multivariate Statistical Methods: A Primer. Boca Raton, FL: CRC Press; 2004. pp. 91–104. [Google Scholar]
- 28.Lawlor DA, Ebrahim S, May M, Davey SG. (Mis)use of factor analysis in the study of insulin resistance syndrome. Am J Epidemiol. 2004;159:1013–18. doi: 10.1093/aje/kwh150. [DOI] [PubMed] [Google Scholar]
- 29.Galobardes B, Lynch JW, Davey Smith G. Is the association between childhood socioeconomic circumstances and cause-specific mortality established? Update of a systematic review. J Epidemiol Community Health. 2008;62:387–90. doi: 10.1136/jech.2007.065508. [DOI] [PubMed] [Google Scholar]
- 30.Blot WJ, McLaughlin JK, Fraumeni JF. Esophageal cancer. In: Schottenfeld D, Fraumeni JF, editors. Cancer Epidemiology and Prevention. New York: Oxford University Press; 2006. [Google Scholar]
- 31.Hemminki K, Li X. Lifestyle and cancer: effect of widowhood and divorce. Cancer Epidemiol Biomarkers Prev. 2003;12:899–904. [PubMed] [Google Scholar]
- 32.Jansson C, Johansson AL, Nyren O, Lagergren J. Socioeconomic factors and risk of esophageal adenocarcinoma: a nationwide Swedish case-control study. Cancer Epidemiol Biomarkers Prev. 2005;14:1754–61. doi: 10.1158/1055-9965.EPI-05-0140. [DOI] [PubMed] [Google Scholar]
- 33.Lundberg O. The impact of childhood living conditions on illness and mortality in adulthood. Soc Sci Med. 1993;36:1047–52. doi: 10.1016/0277-9536(93)90122-k. [DOI] [PubMed] [Google Scholar]
- 34.Hart CL, Davey Smith G. Relation between number of siblings and adult mortality and stroke risk: 25 year follow up of men in the Collaborative study. J Epidemiol Community Health. 2003;57:385–91. doi: 10.1136/jech.57.5.385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Liang J, Gu S, Krause N. Social support among the aged in Wuhan, China. Asia Pac Popul J. 1992;7:33–62. [PubMed] [Google Scholar]
- 36.Knodel J, Debavalya N. Social and economic support systems for the elderly in Asia: an introduction. Asia Pac Popul J. 1992;7:5–12. [PubMed] [Google Scholar]
- 37.Pollack CE, Chideya S, Cubbin C, Williams B, Dekker M, Braveman P. Should health studies measure wealth? A systematic review. Am J Prev Med. 2007;33:250–64. doi: 10.1016/j.amepre.2007.04.033. [DOI] [PubMed] [Google Scholar]
- 38.Zimmer Z, Amornsirisomboon P. Socioeconomic status and health among older adults in Thailand: an examination using multiple indicators. Soc Sci Med. 2001;52:1297–311. doi: 10.1016/s0277-9536(00)00232-x. [DOI] [PubMed] [Google Scholar]
- 39.Lynch J. Social position and health. Ann Epidemiol. 1996;6:21–23. [Google Scholar]
- 40.Islami F, Malekshah AF, Kimiagar M, et al. Patterns of food and nutrient consumption in northern Iran, a high-risk area for esophageal cancer. Nutr Cancer. doi: 10.1080/01635580902803735. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sepehr A, Kamangar F, Fahimi S, Saidi F, Abnet CC, Dawsey SM. Poor oral health as a risk factor for esophageal squamous dysplasia in northeastern Iran. Anticancer Res. 2005;25:543–46. [PubMed] [Google Scholar]
- 42.Kamangar F, Strickland PT, Pourshams A, et al. High exposure to polycyclic aromatic hydrocarbons may contribute to high risk of esophageal cancer in northeastern Iran. Anticancer Res. 2005;25:425–28. [PubMed] [Google Scholar]
- 43.Hakami R, Mohtadinia J, Etemadi A, et al. Dietary intake of benzo(a)pyrene and risk of esophageal cancer in North of Iran. Nutr Cancer. 2008;60:216–21. doi: 10.1080/01635580701684831. [DOI] [PubMed] [Google Scholar]
- 44.Farhadi M, Tahmasebi Z, Merat S, Kamangar F, Nasrollahzadeh D, Malekzadeh R. Human papillomavirus in squamous cell carcinoma of esophagus in a high-risk population. World J Gastroenterol. 2005;11:1200–3. doi: 10.3748/wjg.v11.i8.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.