Abstract
Background Depression has been associated with chronic changes in the serum concentration of C-reactive protein (CRP) in observational studies, but it is unclear if this association is causal or is due to confounding and bias. Genetic studies are less subject to this type of error and offer an opportunity to investigate if CRP is causally linked to depression, particularly because known polymorphisms of the CRP gene have been associated with high- and low-basal serum concentrations of CRP [single nucleotide polymorphisms (SNPs) rs1130864 and rs1205, respectively]. The aim of this study is to determine if polymorphisms of SNPs rs1130864 and rs1205 are associated with prevalent depression.
Methods We completed a cross-sectional study of a community sample of 3700 men aged ≥70 years, and used the 15-item Geriatric Depression Scale (GDS-15) to assess depressive symptoms. A GDS-15 score 7 or more indicates the presence of clinically significant depressive symptoms. Physical morbidity was assessed with the physical component summary score (PCS) of the SF-36 Health Survey. We collected fasting blood samples to measure high sensitivity CRP and to extract DNA for the genotyping of SNPs rs1130864 and rs1205 of the CRP gene.
Results One hundred and eighty-two men were depressed (4.9%). The odds of depression increased by 2% (95% CI = 1–4%) for every unit (mg/l) increase of CRP and nearly doubled for men with CRP ≥ 3 mg/l vs <1 mg/l [odds ratio (OR) = 1.95, 95% confidence interval (CI) = 1.27–2.98]. However, the association between high CRP (≥3 mg/l) and depression was no longer significant after the analyses were adjusted for smoking, age, body mass index (BMI) and PCS. Men with the CT and TT genotypes of rs1130864 had 1.36 (95% CI = 1.13–1.63) and 2.31 (95% CI = 1.65–3.24) greater odds of CRP ≥3 mg/l than CC carriers, but there was no association between this polymorphism and the presence of prevalent depression. The G > A polymorphism of SNP rs1205 was associated with 24% (95% CI = 16–32%) lower concentration of CRP compared with other genotypes. Men with the rs1205 AA genotype had 1.66 (95% CI = 1.07–2.57) and 1.67 (95% CI = 1.08–2.58) greater odds of having clinically significant depression than participants with the GA and GG genotypes, respectively.
Conclusion Our study shows that clinically significant depressive symptoms in later life are unlikely to be caused by an increase in the serum concentration of CRP. Instead, we found that the risk of depression was greater amongst people who carry the rs1205 G > A genetic polymorphism of the CRP gene, which was associated with ∼20% lower serum concentration of CRP compared with other genotypes. This suggests that CRP may be a compensatory response to external insults that predispose to depression, and that an increase in the concentration of CRP might be adaptive.
Keywords: Depression, depressive disorder, affective disorder, mood disorder, inflammation, allostasis, C-reactive protein, genetic polymorphism, aged, elderly
Introduction
C-reactive protein (CRP) is a ubiquitous protein amongst vertebrate and invertebrate animals that phylogenetically spans 400 million years of evolution.1 It reacts strongly with the C polysaccharide of pneumococcus (hence the name) and was initially found in the plasma of humans with acute bacterial infections.2 This acute phase reactant is produced in large quantities by the liver,1 and its concentration rises as much as 2000-fold during the first 24–48 h after the onset of tissue injury or inflammation.3 The precise physiological functions of CRP remain uncertain, although currently available evidence suggests it plays a key role in the recognition of foreign pathogens and of damaged cells of the host, and contributes to triggering the humoral and cellular responses that ultimately lead to their clearance.1,4 CRP has a calcium-dependent binding specificity for phosphocholine (PCh), which is present in the outer layer of most biological membranes, and for several nuclear constituents such as histones and ribonucleoprotein particles.4,5 Bound CRP is recognized by C1q and initiates cleavage and activation of C3 and C4 through the classical complement pathway.4,6,7 In addition, CRP has high affinity for phagocytic receptors8 and seems to promote phagocytosis.4
There is evidence from observational studies that high plasma concentration of CRP increases the risk of cardiovascular events in humans.9,10 Results from a nested case–control study of 28 263 post-menopausal women showed that those with CRP concentrations in the highest quartile were four times more likely to experience a cardiovascular event (death from coronary heart disease, non-fatal myocardial infarction, stroke or coronary revascularization) than women with CRP in the lowest quartile.9 Likewise, Danesh and colleagues10 found that participants in the Reykjavik study who had a fatal or non-fatal myocardial infarction were 45% more likely than controls to have had CRP in the highest tertile (>2.0 mg/l) 4–18 years earlier. A meta-analysis of 22 prospective studies published after 2003 confirmed that adults with CRP plasma concentrations in the highest tertile have increased odds of coronary heart disease compared with those in the lowest tertile,10 but the results of other investigations have raised doubts as to whether CRP is causally related to cardiovascular events.11 Higher basal CRP concentrations have also been associated with numerous other health outcomes,12–15 including depressed mood.16
Kop et al.17 examined 4260 adults aged ≥65 years enroled in the Cardiovascular Health Study, of whom 510 showed clinically significant symptoms of depression. Older adults with depression had higher plasma CRP concentrations than non-depressed participants, although differences between the groups became non-significant once the analyses were adjusted for age, gender, ethnic background, height, weight, smoking status, systolic blood pressure and diabetes (P = 0.056). We have recently confirmed that older men with depression are 56% [95% confidence interval (CI): 20–111%] more likely than non-depressed men to have high CRP concentrations (>3 mg/l) but, like Kop et al.,17 we found that the association between high CRP and depression weakened once measures of physical morbidity were taken into account.18 We suggested that the association between high CRP and depression was probably due to the poor physical health of participants, but were unable to dismiss the possibility that CRP mediates the link between poor physical health and low mood via mechanisms such as cerebrovascular disease and apoptosis.19
Recently, genetic epidemiologists have proposed that Mendelian randomization can be used to infer causality between an exposure (such as raised CRP) and a clinical outcome (such as depression).20 The rationale in this case is that the concentration of CRP in the plasma is influenced by certain polymorphisms of the CRP gene.21–24 For example, the rs1130864 C > T variant increases basal and stimulated CRP levels,21 whereas the rs1205 G > A variant has the opposite effect.22 Consequently, these polymorphisms produce a natural (Mendelian) randomization, with individuals allocated to higher or lower plasma CRP according to a random assortment of heritable units during gamete production and fertilization.25 This approach presupposes that these genetic polymorphisms have no other biologically relevant effect than to modulate the serum concentration CRP. It also assumes that serum CRP lies on the causal pathway to depression.
The aim of the present study was to determine if high CRP concentration is causally related to depression in later life. We hypothesized that older men with depression would have higher plasma CRP and would be more likely to carry the rs1130864 C > T homozygote variant of the CRP gene. We also hypothesized that men with the rs1205 AA genotype would have lower plasma CRP concentration and lower probability of clinically significant symptoms of depression.
Methods
Recruitment of participants
Our analyses are based on a community-derived sample of older men living in Perth, Western Australia, who collectively constitute the Health In Men Study (HIMS) cohort. Details regarding enrolment and assessment procedures have been described elsewhere.26 Briefly, 12 203 men aged ≥65 years were recruited via random sampling from the Australian electoral roll between 1996 and 1998, enrolment to vote being compulsory for all adult Australian citizens. During the years 2001–04 those men who were still alive were contacted and invited to complete a follow-up assessment and donate a fasting blood sample (n = 9718). This report refers to 3700 subjects who consented to follow-up and agreed to donate a blood sample for biochemical and genetic analysis. The Human Research Ethics Committee of the University of Western Australia approved the study protocol.
Procedures and clinical assessment
Consenting men were asked to complete a self-report questionnaire that included items assessing demographic and clinical information. Participants were asked which language they first learned as a child, and those who reported a language other than English were considered to have come from a non-English speaking background (NESB). Age was calculated as the difference in years between the date of the follow-up assessment and the subject's date of birth. Education was rated as the highest level of education attained: no schooling, primary school, some high school, completed high school, completed university or other tertiary degree. Participants were asked how often they smoked now, and men who answered ‘every day’ or ‘not every day’ were considered current smokers (the third possible answer was ‘not at all’). A trained research assistant used standard procedures to measure each participant's height (to 0.5 cm) and weight (to 0.2 kg). The body mass index (BMI) was calculated as weight (kg)/height2 (m2).
Participants were asked to complete the 15-item Geriatric Depression Scale (GDS-15) and, a priori, those with a total score of seven or more were considered to display clinically significant depressive symptoms at the time of assessment (primary outcome of interest). This relatively high cut-point was chosen to ensure high specificity for the diagnosis of depression in this sample.27
In addition, participating men completed the SF-36 Health Survey.28 For the purposes of this study, the analyses were limited to the physical component summary measure (PCS). The mean PCS for the Australian population is 50, with a standard deviation of 10.29 We used the PCS score as a proxy measure of physical health.
Biochemical and genetic analyses
Blood samples were collected between 08:00 and 10:30 a.m. following overnight fasting. Plasma was prepared immediately following phlebotomy, stored on ice and assayed within 3 h. Biochemical assays and genetic analyses were performed in the Department of Biochemistry, PathWest, Royal Perth Hospital, Western Australia. We measured the serum concentration of CRP with a high-sensitivity particle-enhanced immunonephelometry system on a BNII analyser (Dade Behring, Minnesota, Minneapolis, USA). The interassay coefficient of variation (CV) for this test ranges from 4 to 7%, and a serum concentration of ≥3 mg/l is associated with tissue damage or inflammation.30
We extracted DNA from the buffy coat fraction of centrifuged blood. The primary CRP genotyping technique was Taqman fluorescent single nucleotide peptide (SNP) allelic discrimination by means of an ABI 7900HT (Applied Biosystems, Foster City, CA, USA) with the primers and probes designed by Applied Biosystems. Two SNPs were assayed: 1444 C > T (rs1130864) and 1846 G > A (rs1205). The distribution of these SNPs within the CRP gene region has been described by others.25,31
Analysis of the data
Data were managed and analysed with the statistical package Stata release 10.0 (StataCorp, College Station, TX, USA). Participants were grouped according to whether they showed evidence of clinically significant depressive symptoms and the odds ratios (ORs) (plus 95% CIs) were calculated for measured exposures: age, NESB, education, BMI, smoking and physical health comorbidity. We used t-tests to estimate the difference between groups for age and serum concentration of log-transformed CRP (transformation required due to skewness of the data; Shapiro–Wilk test z = 18.59, P < 0.001). We also divided men into three groups according to CRP concentration: low (<1 mg/l), intermediate (1–2.9 mg/l) and high (≥3 mg/l) and calculated the odds of depression for the intermediate and high concentration groups. The correlation between CRP concentration and PCS scores was measured with Spearman's rho. We then investigated if the distribution of alleles at CRP SNPs rs1130864 and rs1205 were in equilibrium according to the Hardy–Weinberg test (exact method) and calculated the OR of the associations between the heterozygote and homozygote minor allele carriers with the geometric concentration of CRP (i.e. natural logarithmic transformed concentration of serum CRP), CRP group, depression status and other measured sociodemographic, clinical and biochemical factors.
Sample size calculation
Based on a preliminary analysis of available data for genotyping, we established that the study would have 182 and 3518 men with and without depression, respectively. The results of our previous study had shown that a CRP concentration of ≥3 mg/l was associated with a 2-fold increase in the odds of depression18 and published data have demonstrated that carriers of the rs1130864 C > T polymorphism have more than double the serum concentration of CRP.21 We estimated that with a sample size of 3700 men, the study would have 80% power to declare as significant a difference in the prevalence of depression between carriers of the major (3.5%) and minor alleles (8.5%), with two-sided alpha set at 5%.
Results
One hundred and eighty-two (4.9%) of 3700 participants had clinically significant symptoms of depression at assessment. The age of participants ranged from 70.8 to 88.6 years, and the mean age [± standard deviation (SD)] of men with and without depression was 77.6 ± 3.8 and 77.0 ± 3.6 years, respectively (t = –2.24, P = 0.025). Table 1 summarizes the frequency distribution of demographic, lifestyle, clinical and biochemical characteristics of men with and without depression. The odds of depression were increased among men with BMI ≥ 30, or with a history of current of smoking, and poor self-perceived physical health as measured by the PCS score (Table 1). Men with depression had higher serum concentrations of CRP than non-depressed men (geometric mean ± SD = 2.7 ± 3.2 vs 2.0 ± 2.8 mg/l, t = 3.89, P < 0.001). The odds of depression increased 2% (95% CI = 1–4%) for every unit (mg/l) increase of CRP, and men with CRP ≥ 3 mg/l were nearly twice as likely to be depressed as men with CRP < 1 mg/l (OR = 1.95, 95% CI = 1.27–2.98). The confidence limits of the odds of depression associated with high CRP included one after the model was adjusted for smoking, age, BMI and PCS groups (OR = 1.30, 95% CI = 0.83–2.03). The serum concentration of CRP had a weak inverse correlation with PCS scores (Spearman's rho = –0.13, P < 0.001), and men with PCS < 30 were twice as likely to have serum concentration of CRP ≥ 3 mg/l (OR = 2.00, 95% CI = 1.60–2.51) as men with PCS ≥ 50.
Table 1.
Sociodemographic, lifestyle, health and biochemical characteristics of older men with and without depression
| No depression n = 3518 | Depression n = 182 | Crude OR (95% CI) | |
|---|---|---|---|
| Age (years), n (%) | |||
| 70–74 | 1308 (37.2) | 56 (30.8) | 1 (Reference) |
| 75–79 | 1522 (43.3) | 75 (41.2) | 1.16 (0.81–1.64) |
| 80–84 | 556 (15.8) | 43 (23.6) | 1.81 (1.20–2.72) |
| 85+ | 132 (3.7) | 8 (4.4) | 1.42 (0.66–3.03) |
| NESBa | 450 (12.9) | 31 (17.1) | 1.40 (0.94–2.09) |
| Education: completed high schoolb | 1709 (48.6) | 74 (40.7) | 0.72 (0.53–0.98) |
| BMIc | |||
| <25 | 1192 (33.9) | 48 (27.6) | 1 (Reference) |
| 25–29.9 | 1785 (50.8) | 84 (48.3) | 1.17 (0.81–1.68) |
| 30+ | 534 (15.2) | 42 (24.1) | 1.95 (1.27–2.99) |
| Smoker (current) | 172 (4.9) | 19 (10.4) | 2.27 (1.38–3.74) |
| PCSd | |||
| 50+ | 861 (24.7) | 12 (6.7) | 1 (Reference) |
| 30–49 | 2128 (60.9) | 94 (52.5) | 3.17 (1.73–5.81) |
| <30 | 503 (14.4) | 73 (40.8) | 10.41 (5.60–19.36) |
| CRP concentration (mg/l) | |||
| <1 | 871 (24.8) | 31 (17.0) | 1 (Reference) |
| 1–2.9 | 1536 (43.7) | 74 (40.7) | 1.35 (0.88–2.08)e |
| ≥3 | 1111 (31.6) | 77 (42.3) | 1.95 (1.27–2.98)f |
aInformation missing for 21 participants.
bInformation missing for 4 participants.
cInformation missing for 15 participants.
dInformation missing for 29 participants.
eOR = 1.09 (95% CI = 0.70–1.70), after adjustment for smoking, age, PCS and BMI.
ffOR = 1.30 (95% CI = 0.83–2.03), after adjustment for smoking, age, PCS and BMI.
Table 2 shows that the genotypic distribution of CRP SNPs rs1130864 and rs1205 in our sample did not deviate significantly from Hardy–Weinberg equilibrium and was similar to Euro-centric populations (dbSNP database, http://www.ncbi.nlm.nih.gov/projects/SNP/). As expected, the mean serum concentration of CRP was higher in the presence of the rs1130864 C > T polymorphism: 1.8 mg/l amongst men with the CC genotype to 2.1 and 2.5 mg/l for those with the CT and TT genotypes, respectively (Cuzick's test for trend: z = 5.09, P < 0.001). Men with the CT and TT genotypes had 1.36 (95% CI = 1.13–1.63) and 2.31 (1.65–3.24) greater odds of having high concentration of CRP (≥3 mg/l compared with <1 mg/l) at the time of assessment than CC carriers. As Table 3 shows, the rs1130864 C > T polymorphism was not associated with any other socio- demographic background or measures of health (including depression).
Table 2.
Allelic frequency for two common CRP SNPs
| Major homozygote (%) | Heterozygote (%) | Minor homozygote (%) | Minor allele frequency (SE) | HWET P | |
|---|---|---|---|---|---|
| CRP1444-rs1130864 C>T | 1808 (48.9) | 1548 (41.8) | 344 (9.3) | 0.30 (0.005) | 0.640 |
| CRP1846-rs1205 G>A | 1630 (44.0) | 1667 (45.0) | 403 (10.9) | 0.33 (0.005) | 0.460 |
HWET = Hardy–Weinberg Equilibrium Test, p-value.
Table 3.
Socio-demographic, lifestyle, health and biochemical measures associated with the allelic distribution of rs1130864 SNP
| CRP1444-rs1130864 |
OR |
OR |
|||
|---|---|---|---|---|---|
| CC (n = 1808) | CT (n = 1548) | TT (n = 344) | CT/CC (95% CI) | TT/CC (95% CI) | |
| Age (years), n (%) | |||||
| 70–74 | 651 (36.0) | 573 (37.0) | 140 (40.7) | 1 (Reference) | 1 (Reference) |
| 75–79 | 792 (43.8) | 672 (43.4) | 133 (38.7) | 0.96 (0.83–1.12) | 0.78 (0.60–1.01) |
| 80–84 | 294 (16.3) | 246 (15.9) | 59 (17.1) | 0.95 (0.78–1.16) | 0.93 (0.67–1.30) |
| ≥85 | 71 (3.9) | 57 (3.7) | 12 (3.5) | 0.91 (0.63–1.32) | 0.79 (0.41–1.49) |
| NESB, n (%)a | 231 (12.9) | 200 (13.0) | 50 (14.6) | 1.01 (0.82–1.24) | 1.16 (0.83–1.61) |
| Education: completed high school, n (%)b | 884 (49.0) | 740 (47.8) | 159 (46.2) | 0.96 (0.83–1.09) | 0.90 (0.71–1.13) |
| BMI, n (%)c | |||||
| <25 | 614 (34.1) | 515 (33.4) | 111 (32.5) | 1 (Reference) | 1 (Reference) |
| 25–29.9 | 909 (50.4) | 786 (51.0) | 174 (50.9) | 1.03 (0.89–1.20) | 1.06 (0.82–1.37) |
| ≥30 | 279 (15.5) | 240 (15.6) | 57 (16.7) | 1.03 (0.83–1.26) | 1.13 (0.80–1.60) |
| Smoker (current), n (%) | 103 (5.7) | 70 (4.5) | 18 (5.2) | 0.78 (0.57–1.07) | 0.91 (0.55–1.53) |
| Depression, n (%) | 101 (5.6) | 65 (4.2) | 16 (4.6) | 0.74 (0.54–1.02) | 0.82 (0.48–1.42) |
| GDS score, median (IQR) | 2 (1, 3) | 2 (1, 3) | 1 (1, 3) | 0.97 (0.94–1.00) | 0.97 (0.92–1.03) |
| PCS, n (%)d | |||||
| ≥50 | 437 (24.4) | 354 (23.0) | 82 (23.9) | 1 (Reference) | 1 (Reference) |
| 30–49.9 | 1063 (59.4) | 947 (61.6) | 212 (61.8) | 1.10 (0.93–1.30) | 1.06 (0.80–1.40) |
| <30 | 290 (16.2) | 237 (15.4) | 49 (14.3) | 1.01 (0.81–1.26) | 0.90 (0.61–1.32) |
| CRP, geometric mean (SD), (mg/l) | 1.8 (2.8) | 2.1 (2.9) | 2.5 (2.8) | 1.12 (1.05–1.19)e | 1.31 (1.17–1.46)f |
| CRP concentration (mg/l), n (%) | |||||
| <1 | 498 (27.5) | 350 (22.6) | 54 (15.7) | 1 (Reference) | 1 (Reference) |
| 1–2.9 | 771 (42.6) | 684 (44.2) | 155 (45.1) | 1.26 (1.06–1.50)g | 1.85 (1.33–2.58)h |
| ≥3 | 539 (29.8) | 514 (33.2) | 135 (39.2) | 1.36 (1.13–1.63)i | 2.31 (1.65–3.24)j |
aInformation missing for 21 participants.
bInformation missing for 4 participants.
cInformation missing for 15 participants.
dInformation missing for 29 participants.
ez-statistic = 3.31, P = 0.001.
fz-statistic = 4.77, P < 0.001.
gz-statistic = 2.67, P = 0.008.
hz-statistic = 3.67, P < 0.001.
iz-statistic = 3.28, P = 0.001.
jz-statistic = 4.85, P < 0.001.
IQR = interquartile range.
The G > A polymorphism of the CRP SNP rs1205 was associated with lower mean concentrations of serum CRP, from 2.3 mg/l for men with the GG genotype to 1.8 and 1.6 mg/l for GA and AA carriers, respectively (Cuzik's test for trend: z = –8.20, P < 0.001) (Table 4). Similarly, men with the AA genotype were less likely to have CRP concentration ≥3 mg/l than those with GA (OR = 0.77, 95% CI = 0.60–0.99) or GG carriers (OR = 0.52, 95% CI = 0.41–0.67). They also had a serum concentration of CRP 24% lower than men with the other genotypes grouped together (OR of the natural logarithmically transformed data, OR = 0.76, 95% CI = 0.68–0.84). In addition, GDS-15 scores increased from the GG to the AA genotype (Cuzik's test for trend: z = 2.11, P = 0.035), and men with the AA genotype had 66% (OR = 1.66, 95% CI = 1.07–2.57) and 67% (OR = 1.67, 95% CI = 1.08–2.58) greater odds for clinically significant depression than GA and GG carriers, respectively (see also Table 4). The population attributable fraction of depression associated with the AA genotype at SNP rs1205 was 11%. The G > A polymorphism of SNP rs1205 was not associated with any other socio-demographic or clinical measures (Table 4).
Table 4.
Socio-demographic, lifestyle, health and biochemical measures associated with the allelic distribution of rs1205 SNP
| CRP1846-rs1205 |
OR |
OR |
|||
|---|---|---|---|---|---|
| GG (n = 1630) | GA (n = 1667) | AA (n = 403) | GA/GG (95% CI) | AA/GG (95% CI) | |
| Age (years), n (%) | |||||
| 70–74 | 602 (36.9) | 622 (37.3) | 140 (34.7) | 1 (Reference) | 1 (Reference) |
| 75–79 | 716 (43.9) | 706 (42.3) | 175 (43.4) | 0.95 (0.82–1.11) | 1.05 (0.82–1.35) |
| 80–84 | 260 (15.9) | 265 (15.9) | 74 (18.4) | 0.99 (0.80–1.21) | 1.22 (0.89–1.68) |
| ≥85 | 52 (3.2) | 74 (4.4) | 14 (3.5) | 1.38 (0.95–2.00) | 1.16 (0.62–2.15) |
| NESB, n (%)a | 210 (12.9) | 212 (12.8) | 59 (14.7) | 0.99 (0.81–1.22) | 1.16 (0.85–1.58) |
| Education: completed high school, n (%)b | 795 (48.8) | 788 (47.4) | 200 (49.6) | 0.94 (0.82–1.08) | 1.03 (0.83–1.29) |
| BMI, n (%)c | |||||
| <25 | 534 (32.9) | 566 (34.1) | 140 (34.8) | 1 (Reference) | 1 (Reference) |
| 25–29.9 | 830 (51.1) | 834 (50.3) | 205 (51.0) | 0.95 (0.81–1.10) | 0.94 (0.74–1.20) |
| ≥30 | 261 (16.1) | 258 (15.6) | 57 (14.2) | 0.93 (0.76–1.15) | 0.83 (0.59–1.17) |
| Smoker (current), n (%) | 76 (4.7) | 91 (5.5) | 24 (6.0) | 1.18 (0.86–1.61) | 1.29 (0.81–2.08) |
| Depression, n (%) | 75 (4.6) | 77 (4.6) | 30 (7.4) | 1.00 (0.72–1.39) | 1.67 (1.08–2.58)e |
| GDS score, median (IQR) | 2 (1, 3) | 2 (1, 3) | 2 (1, 3) | 1.01 (0.98–1.04) | 1.05 (1.01–1.10)f |
| PCS, n (%)d | |||||
| ≥50 | 404 (24.9) | 377 (22.8) | 92 (23.0) | 1 (Reference) | 1 (Reference) |
| 30–49.9 | 972 (60.0) | 1009 (61.1) | 241 (60.2) | 1.11 (0.94–1.31) | 1.09 (0.83–1.42) |
| <30 | 244 (15.1) | 265 (16.0) | 67 (16.7) | 1.16 (0.93–1.46) | 1.21 (0.85–1.72) |
| CRP, geometric mean (SD), (mg/l) | 2.3 (2.8) | 1.8 (2.9) | 1.6 (2.6) | 0.81 (0.75–0.86)g | 0.66 (0.59–0.74)h |
| CRP concentration (mg/l), n (%) | |||||
| <1 | 306 (18.8) | 465 (27.9) | 131 (32.5) | 1 (Reference) | 1 (Reference) |
| 1–2.9 | 713 (43.7) | 721 (43.2) | 176 (43.7) | 0.67 (0.56–0.79)i | 0.58 (0.44–0.75)j |
| ≥3 | 611 (37.5) | 481 (28.8) | 96 (23.8) | 0.52 (0.43–0.62)k | 0.37 (0.27–0.49)l |
aInformation missing for 21 participants.
bInformation missing for 4 participants.
cInformation missing for 15 participants.
dInformation missing for 29 participants.
ez-statistic = 2.29, P = 0.022.
fz-statistic = 2.18, P = 0.029.
gz-statistic = 4.11, P < 0.001.
hz-statistic = 7.02, P < 0.001.
iz-statistic = 4.50, P < 0.001.
jz-statistic = 6.62, P < 0.001.
kz-statistic = 4.85, P < 0.001.
lz-statistic = 6.62, P < 0.001.
IQR = interquartile range.
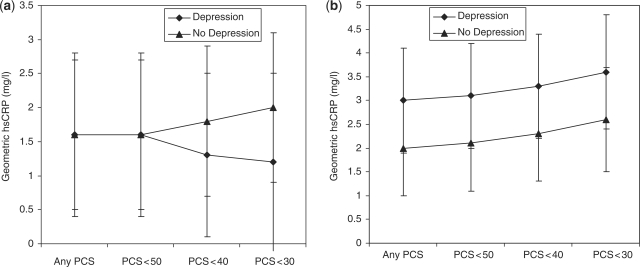
We also conducted a series of post hoc analyses to investigate how depression, poor physical health and the G > A polymorphism at SNP1205 of the CRP gene interacted to modulate the serum concentration of CRP. The geometric mean levels of CRP for AA carriers with PCS scores <50 were 1.6 mg/l (SD = 2.5) and 1.6 mg/l (SD = 2.6) for men with and without depression. Once the analysis was restricted to participants with PCS scores <40, the mean geometric mean concentration of CRP fell to 1.3 mg/l (SD = 2.3) for men with depression and increased to 1.8 mg/l (SD = 2.7) amongst men without clinically significant depressive symptoms. A further drop in the serum concentration of CRP was observed amongst AA carriers who had a PCS score <30 and were depressed (geometric mean: 1.2 mg/l, SD = 2.3), but increased to 2.0 mg/l (SD = 2.5) amongst those who did not display clinically significant symptoms of depression. Figure 1a and b shows the geometric mean concentration of CRP according to depression and PCS groups for men with AA or GA/GG genotypes at SNPrs1205.
Figure 1.
Geometric mean concentration of hsCRP according to the PCS of men with and without depression (the short lines represent the standard error of the geometric mean). (a) Results for AA carriers only and (b) results for GA or GG carriers at SNP rs1205 of the CRP gene
Table 5 shows the distribution of depression and CRP concentrations according to the combined genotypes of SNPs rs1130864 and rs1205 (two locus genotypes). None of the participants had the genotype TTAA, and genotypes CTAA and TTGA were rare. The reported effect on mood of SNP rs1205 was, therefore, not influenced by the genotype at SNP rs1130864.
Table 5.
Depression and CRP concentration according to genotypic combinations of the CRP gene at rs1130864 and rs1205 SNPs
| CCGG (n = 518) | CCGA (n = 889) | CCAA (n = 401) | CTGG (n = 771) | CTGA (n = 775) | CTAA (n = 2) | TTGG (n = 341) | TTGA (n = 3) | TTAA (n = 0) | |
|---|---|---|---|---|---|---|---|---|---|
| Depression, n (%) | 26 (5.0) | 45 (5.1) | 30 (7.5) | 33 (4.3) | 32 (4.1) | 0 | 16 (4.7) | 0 | – |
| CRP, geometric mean (SD), (mg/l) | 2.2 (2.8) | 1.8 (2.8) | 1.6 (2.6) | 2.4 (2.8) | 1.9 (2.9) | 2.7 (1.5) | 2.5 (2.8) | 4.1 (2.7) | – |
| CRP concentration, n (%) (mg/l) | |||||||||
| <1 | 108 (20.8) | 259 (29.1) | 131 (32.7) | 144 (18.7) | 206 (26.6) | 0 | 54 (15.8) | 0 | – |
| 1–2.9 | 220 (42.5) | 376 (42.3) | 175 (43.6) | 340 (44.1) | 343 (44.3) | 1 (50.0) | 153 (44.9) | 2 (66.7) | – |
| ≥3 | 190 (36.7) | 254 (28.6) | 95 (23.7) | 287 (37.2) | 226 (29.2) | 1 (50.0) | 134 (39.3) | 1 (33.3) | – |
The haplotype carrying both minor alleles is not observed in our sample. In other words, the haplotypes that exist are carrying one major (or common) allele and both combinations of haplotypes carrying one major and one minor allele. It is possible that this implies the T–A haplotype decreases the probability of survival into old age or that the SNPs are so close together that there have been almost no recombination events occurring between them over time (these SNPs are located 858 bp apart). This is reflected in the calculated linkage disequilibrium (LD) parameter D′ (D-prime) of 98.9% given these allele frequencies. Consequently, there is an exceedingly high LD between the common allele of one SNP and the rare allele of the other (and vice versa). If the common alleles at one locus had the same effect as the rare alleles in a locus in tight LD (e.g. difference of 90 vs 95%), then our study would most likely have been underpowered to demonstrate such a difference.
Discussion
This study failed to demonstrate a significant association between increasing serum CRP concentration and prevalent depression. In addition, the results confirmed that the minor allele of the CRP gene at SNP rs1130864 is associated with an increase in the serum concentration of CRP but not an increase in odds of depression. These data suggest that the association between CRP and depression is confounded by other factors, such as age and medical comorbidities, and is unlikely to be causal. Moreover, our finding that the rs1205 G > A polymorphism is associated with an increase in the risk of depression of 70% and a decrease in the serum concentration of CRP of 30% may offer further insights into the physiological pathway linking CRP to depression.
Before discussing our findings, we need to acknowledge the limitations of our study. This survey has the merit of having used a large and well-established community-representative sample of older men for whom relevant clinical, genetic and biochemical information was available.26 We accept, however, that we cannot infer causality between the factors under investigation because of the cross-sectional nature of the survey. There is also evidence that our older men with depression were less likely to agree to a blood test, and that those who did not have a blood test had poorer physical health.32 This would have biased our results towards a healthier sample and diminished our ability to investigate the associations between depression, physical morbidity, serum concentration of CRP and CRP polymorphisms. This type of bias is associated with decreased power but not with type I error. In addition, we used a validated scale and a demanding cut-point to establish the presence of depression in this sample. We recognize, however, that our definition of depression was not based on a formal assessment of mental state leading to the diagnosis of a depressive episode. As a result, we cannot be certain that our findings would be directly transferable to patients meeting DSM-IV criteria for major depression or ICD-10 criteria for a depressive episode. Our study included men only and the findings may not necessarily apply to women. Moreover, we limited our analyses to two SNPs of the CRP gene and this does not provide a complete picture of the possible haplotypes that control its expression. However, our focus was on two previously well-described SNPs associated with high- and low-serum concentration of CRP21 with the specific aim of teasing out the effects of CRP on depressive symptoms. We also acknowledge that the polymorphisms that we investigated in the two SNPs of the CRP gene are related to CRP (one increasing and the other decreasing its serum concentration) but not to depression (i.e. the C > T polymorphism at SNP rs1130864 increases the serum concentration of CRP but does not reduce the odds of depression). This may indicate that the association between the rs1205 G > A polymorphism and depression is either not causal or is affected by pleiotrophy. Finally, as we did not systematically collect information about the ethnic background of participants, we were unable to investigate population stratification in our dataset, although, as previously noted, the genotypic distribution of the relevant SNPs in our sample was similar to that in Euro-centric populations.
Our results suggest that it may be failure to raise the serum concentration of CRP as a response to injury that increases the risk of depression. Recent evidence implicates a relative deficiency of CRP in the pathogenesis of autoimmune diseases such as systemic lupus erythematosus (SLE). Russell and colleagues33 reported that adults with SLE show an inefficient and dampened CRP response during acute flares of disease, a finding that is not dissimilar from what we observed amongst our men with depression. They also found that the G > A polymorphism at SNP1205 was associated with the diagnosis of SLE and nuclear autoantibody production, and concluded that defective disposal of immunogenic material contributed to the pathogenesis of SLE.33
Curiously, common behavioural and psychological symptoms associated with SLE have been attributed to autoimmunity,34 and there is evidence that autoimmune antibodies trigger depressive symptoms in animals models35 as well as in humans with SLE.36 A recent review of the association between depression and immunity reported a consistent link between impaired lymphocyte activation and depression, as well as between reduced natural killer cell activity and depression.37 There is also growing evidence that immune and inflammatory reactions affect brain function through the modulation of afferent peripheral nerve responses to cytokines and peripherally produced pathogen-associated molecular patterns (PAMPs), as well as via a direct humoral pathway.38 Dantzer and colleagues38 argued that sickness behaviour—which is often characterized by nausea, loss of appetite, diminished interest in the surrounding environment, tiredness, disturbed sleep and poor concentration—is an integral part of the brain's response to injury. In addition, they suggested that when the activation of the peripheral immune system continues unabated for a prolonged period of time—such as in autoimmune diseases, cancer or systemic infections—depression may ensue in predisposed individuals.38
Our results are consistent with the possibility that carriers of the SNP rs1205 AA genotype produce a deficient CRP response to injury, which in turn may perpetuate immune and inflammatory reactions that ultimately lead to the expression of clinically significant depressive symptoms. In this model, the rs1205 G > A polymorphism would contribute to the development of depression by diminishing the individual's ability to respond efficiently to stress and injury. Ten years ago, McEwen39 suggested that organisms react to real or perceived challenges in two ways: (i) they mount an allostatic response that initiates a complex adaptive pathway (for example, to combat an infection), and (ii) they turn off the allostatic response when the threat is no longer present. Whilst the acute response is commonly adaptive, chronic allostatic load may result in damage to the organism because of repeated hits, lack of adaptation (i.e. decreased ability to turn off the allostatic response), prolonged (i.e. no recovery) or inadequate responses.39 McEwen suggested that a flattened or inadequate acute allostatic response to insult leads to compensatory hyperactivity of other stress-related mediators (such as cytokines),40 and this could contribute to the development of sickness behaviour and depression. However, such an explanation can only be considered speculative at this stage, as our study lacked supportive data to test the various steps involved in the allostatic model directly.
Our findings have potential implications for the management of depression. The data are consistent with the possibility that carriers of the AA genotype at CRP SNP rs1205 have a dampened CRP response to increasingly poor physical health, a relative fall in the serum concentration of CRP of ∼30%, and an increase in the prevalence of depression of 70%. If this relationship is truly causal, then raising the serum concentration of CRP by 30% could potentially lead to the remission of depressive symptoms amongst older AA carriers at CRP SNP rs1205. This possibility remains to be tested by randomized trials, although CRP is not readily available for consumption and potential safety issues associated with its infusion would need to be considered.41,42
In summary, the results of our study show that clinically significant depressive symptoms in later life are unlikely to be caused by an increase in the serum concentration of CRP, as previously suggested.43 Instead, we found that the odds of depression are greater amongst people who carry the rs1205 G > A genetic polymorphism of the CRP gene, which reduces the serum concentration of CRP. This suggests that CRP may be a compensatory response to external insults that predispose to depression, and that an increase in the concentration of CRP might be adaptive and potentially protective. In this case, the association between CRP and depression could be analogous to indication bias, where treatment for a disease state is associated with that disease. These findings should now be replicated in independent samples and, if the observed associations are confirmed, future studies will need to clarify if an increase in the serum concentration of CRP can reverse or avert depression amongst people at risk.
Funding
National Health and Medical Research Council of Australia (project grant numbers 279408, 379600 and 403963).
Acknowledgements
The investigators thank HIMS participants and research staff.
Conflict of interest: None declared.
KEY MESSAGES.
Older men with depression have high serum concentration of CRP, but the lack of association between prevalent depression and polymorphisms of the CRP gene that either increase or decrease the basal serum concentration of CRP suggests that this relationship is not causal.
The G > A polymorphism at SNP rs1205 is associated with lower serum concentration of CRP, with AA carriers showing <24% CRP than men with other genotypes.
Men with the AA genotype at SNP rs1205 had 1.66 (95% CI: 1.07–2.57) and 1.67 (95% CI: 1.08–2.58) greater odds of displaying clinically significant symptoms of depression than men with the GA and GG genotypes, respectively.
The results of this study suggest that changes in the serum concentration of CRP may represent a compensatory response to external insults that predispose to depression; failure to raise CRP in those circumstances may increase the odds of depression.
References
- 1.Kilpatrick JM, Volanakis JE. Molecular genetics, structure, and function of C-reactive protein. Immunol Res. 1991;10:43–53. doi: 10.1007/BF02918166. [DOI] [PubMed] [Google Scholar]
- 2.Tillett WS, Francis T., Jr Serological reactions in pneumonia with a nonprotein somatic fraction of pneumococcus. J Exp Med. 1930;52:561–85. doi: 10.1084/jem.52.4.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Morley JJ, Kushner I. Serum C-reactive protein levels in disease. Ann N Y Acad Sci. 1982;389:406–18. doi: 10.1111/j.1749-6632.1982.tb22153.x. [DOI] [PubMed] [Google Scholar]
- 4.Volanakis JE. Human C-reactive protein: expression, structure, and function. Mol Immunol. 2001;38:189–97. doi: 10.1016/s0161-5890(01)00042-6. [DOI] [PubMed] [Google Scholar]
- 5.Robey FA, Jones KD, Steinberg AD. C-reactive protein mediates the solubilization of nuclear DNA by complement in vitro. J Exp Med. 1985;161:1344–56. doi: 10.1084/jem.161.6.1344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kaplan MH, Volanakis JE. Interaction of C-reactive protein complexes with the complement system. I. Consumption of human complement associated with the reaction of C-reactive protein with pneumococcal C-polysaccharide and with the choline phosphatides, lecithin and sphingomyelin. J Immunol. 1974;112:2135–47. [PubMed] [Google Scholar]
- 7.Volanakis JE, Kaplan MH. Interaction of C-reactive protein complexes with the complement system. II. Consumption of guinea pig complement by CRP complexes: requirement for human C1q. J Immunol. 1974;113:9–17. [PubMed] [Google Scholar]
- 8.Marnell LL, Mold C, Volzer MA, Burlingame RW, Du Clos TW. C-reactive protein binds to Fc gamma RI in transfected COS cells. J Immunol. 1995;155:2185–93. [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–43. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Danesh J, Wheeler JG, Hirschfield GM, et al. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–97. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 11.Lawlor DA, Harbord RM, Timpson NJ, et al. The association of C-reactive protein and CRP genotype with coronary heart disease: findings from five studies with 4,610 cases amongst 18,637 participants. PLoS ONE. 2008;3:e3011. doi: 10.1371/journal.pone.0003011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Marsik C, Kazemi-Shirazi L, Schickbauer T, et al. C-reactive protein and all-cause mortality in a large hospital-based cohort. Clin Chem. 2008;54:343–49. doi: 10.1373/clinchem.2007.091959. [DOI] [PubMed] [Google Scholar]
- 13.Vainas T, Lubbers T, Stassen FR, et al. Serum C-reactive protein level is associated with abdominal aortic aneurysm size and may be produced by aneurysmal tissue. Circulation. 2003;107:1103–1105. doi: 10.1161/01.cir.0000059938.95404.92. [DOI] [PubMed] [Google Scholar]
- 14.Kravitz MS, Pitashny M, Shoenfeld Y. Protective molecules–C-reactive protein (CRP), serum amyloid P (SAP), pentraxin3 (PTX3), mannose-binding lectin (MBL), and apolipoprotein A1 (Apo A1), and their autoantibodies: prevalence and clinical significance in autoimmunity. J Clin Immunol. 2005;25:582–91. doi: 10.1007/s10875-005-7828-2. [DOI] [PubMed] [Google Scholar]
- 15.Gruenewald TL, Seeman TE, Ryff CD, Karlamangla AS, Singer BH. Combinations of biomarkers predictive of later life mortality. Proc Natl Acad Sci USA. 2006;103:14158–63. doi: 10.1073/pnas.0606215103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ford DE, Erlinger TP. Depression and C-reactive protein in US adults: data from the Third National Health and Nutrition Examination Survey. Arch Intern Med. 2004;164:1010–14. doi: 10.1001/archinte.164.9.1010. [DOI] [PubMed] [Google Scholar]
- 17.Kop WJ, Gottdiener JS, Tangen CM, et al. Inflammation and coagulation factors in persons >65 years of age with symptoms of depression but without evidence of myocardial ischemia. Am J Cardiol. 2002;89:419–24. doi: 10.1016/s0002-9149(01)02264-0. [DOI] [PubMed] [Google Scholar]
- 18.Almeida OP, Norman P, Hankey GJ, Jamrozik K, Flicker L. The association between C-reactive protein concentration and depression in later life is due to poor physical health: results from the Health in Men Study (HIMS) Psychol Med. 2007;37:1775–86. doi: 10.1017/S0033291707000827. [DOI] [PubMed] [Google Scholar]
- 19.Fujii H, Li SH, Szmitko PE, Fedak PW, Verma S. C-reactive protein alters antioxidant defenses and promotes apoptosis in endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2006;26:2476–82. doi: 10.1161/01.ATV.0000242794.65541.02. [DOI] [PubMed] [Google Scholar]
- 20.Davey Smith G, Ebrahim S, Lewis S, Hansell AL, Palmer LJ, Burton PR. Genetic epidemiology and public health: hope, hype, and future prospects. Lancet. 2005;366:1484–98. doi: 10.1016/S0140-6736(05)67601-5. [DOI] [PubMed] [Google Scholar]
- 21.Brull DJ, Serrano N, Zito F, et al. Human CRP gene polymorphism influences CRP levels: implications for the prediction and pathogenesis of coronary heart disease. Arterioscler Thromb Vasc Biol. 2003;23:2063–69. doi: 10.1161/01.ATV.0000084640.21712.9C. [DOI] [PubMed] [Google Scholar]
- 22.Crawford DC, Sanders CL, Qin X, et al. Genetic variation is associated with C-reactive protein levels in the Third National Health and Nutrition Examination Survey. Circulation. 2006;114:2458–65. doi: 10.1161/CIRCULATIONAHA.106.615740. [DOI] [PubMed] [Google Scholar]
- 23.Lange LA, Carlson CS, Hindorff LA, et al. Association of polymorphisms in the CRP gene with circulating C-reactive protein levels and cardiovascular events. JAMA. 2006;296:2703–11. doi: 10.1001/jama.296.22.2703. [DOI] [PubMed] [Google Scholar]
- 24.Verzilli C, Shah T, Casas JP, et al. Bayesian meta-analysis of genetic association studies with different sets of markers. Am J Hum Genet. 2008;82:859–72. doi: 10.1016/j.ajhg.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Carlson CS, Aldred SF, Lee PK, et al. Polymorphisms within the C-reactive protein (CRP) promoter region are associated with plasma CRP levels. Am J Hum Genet. 2005;77:64–77. doi: 10.1086/431366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Norman PE, Flicker L, Almeida OP, Hankey GJ, Hyde Z, Jamrozik K. Cohort profile: The Health In Men Study (HIMS) Int J Epidemiol. 2009;38:48–52. doi: 10.1093/ije/dyn041. [DOI] [PubMed] [Google Scholar]
- 27.Almeida OP, Almeida SA. Short versions of the geriatric depression scale: a study of their validity for the diagnosis of a major depressive episode according to ICD-10 and DSM-IV. Int J Geriatr Psychiatry. 1999;14:858–65. doi: 10.1002/(sici)1099-1166(199910)14:10<858::aid-gps35>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- 28.Ware JE, Kosinski M, Gandek B. SF-36 Health Survey: Manual & Interpretation Guide. Lincoln, RI: QualityMetric Incorporated; 2000. [Google Scholar]
- 29.ABS. National Health Survey: SF-36 Population Norms. Australia: Australian Bureau of Statistics, Canberra; 1995. [Google Scholar]
- 30.Pearson TA, Mensah GA, Alexander RW, et al. Markers of inflammation and cardiovascular disease: application to clinical and public health practice: a statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- 31.Timpson NJ, Lawlor DA, Harbord RM, et al. C-reactive protein and its role in metabolic syndrome: mendelian randomisation study. Lancet. 2005;366:1954–59. doi: 10.1016/S0140-6736(05)67786-0. [DOI] [PubMed] [Google Scholar]
- 32.Almeida OP, Yeap BB, Hankey GJ, Jamrozik K, Flicker L. Low free testosterone concentration as a potentially treatable cause of depressive symptoms in older men. Arch Gen Psychiatry. 2008;65:283–89. doi: 10.1001/archgenpsychiatry.2007.33. [DOI] [PubMed] [Google Scholar]
- 33.Russell AI, Cunninghame Graham DS, Shepherd C, et al. Polymorphism at the C-reactive protein locus influences gene expression and predisposes to systemic lupus erythematosus. Hum Mol Genet. 2004;13:137–47. doi: 10.1093/hmg/ddh021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Strous RD, Shoenfeld Y. Behavioral changes in systemic lupus erythematosus are of an autoimmune nature. Nat Clin Pract Rheumatol. 2007;3:592–93. doi: 10.1038/ncprheum0622. [DOI] [PubMed] [Google Scholar]
- 35.Katzav A, Solodeev I, Brodsky O, et al. Induction of autoimmune depression in mice by anti-ribosomal P antibodies via the limbic system. Arthritis Rheum. 2007;56:938–48. doi: 10.1002/art.22419. [DOI] [PubMed] [Google Scholar]
- 36.Lapteva L, Nowak M, Yarboro CH, et al. Anti-N-methyl-D-aspartate receptor antibodies, cognitive dysfunction, and depression in systemic lupus erythematosus. Arthritis Rheum. 2006;54:2505–14. doi: 10.1002/art.22031. [DOI] [PubMed] [Google Scholar]
- 37.Irwin MR, Miller AH. Depressive disorders and immunity: 20 years of progress and discovery. Brain Behav Immun. 2007;21:374–83. doi: 10.1016/j.bbi.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 38.Dantzer R, O’Connor JC, Freund GG, Johnson RW, Kelley KW. From inflammation to sickness and depression: when the immune system subjugates the brain. Nat Rev Neurosci. 2008;9:46–56. doi: 10.1038/nrn2297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.McEwen BS. Protective and damaging effects of stress mediators. N Engl J Med. 1998;338:171–79. doi: 10.1056/NEJM199801153380307. [DOI] [PubMed] [Google Scholar]
- 40.McEwen BS. Protective and damaging effects of stress mediators: central role of the brain. Dialogues Clin Neurosci. 2006;8:367–81. doi: 10.31887/DCNS.2006.8.4/bmcewen. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bisoendial RJ, Kastelein JJ, Peters SL, et al. Effects of CRP infusion on endothelial function and coagulation in normocholesterolemic and hypercholesterolemic subjects. J Lipid Res. 2007;48:952–60. doi: 10.1194/jlr.P600014-JLR200. [DOI] [PubMed] [Google Scholar]
- 42.Birjmohun RS, Bisoendial RJ, van Leuven SI, et al. A single bolus infusion of C-reactive protein increases gluconeogenesis and plasma glucose concentration in humans. Metabolism. 2007;56:1576–82. doi: 10.1016/j.metabol.2007.06.026. [DOI] [PubMed] [Google Scholar]
- 43.Penninx BW, Kritchevsky SB, Yaffe K, et al. Inflammatory markers and depressed mood in older persons: results from the Health, Aging and Body Composition study. Biol Psychiatry. 2003;54:566–72. doi: 10.1016/s0006-3223(02)01811-5. [DOI] [PubMed] [Google Scholar]