Abstract
Reactivity of lamina propria (LP) T cells to commensal bacteria has been demonstrated in animal models of inflammatory bowel disease (IBD) and in humans with IBD, but few studies have evaluated the function of such cells in normal individuals. LP mononuclear cells (LPMC) were disaggregated from healthy human intestinal tissue and cultured with heat-killed commensal and pathogenic bacteria. CD3+CD4+ IFN-γ-producing (Th1) cells reactive to commensal bacteria were demonstrated at frequencies ranging from 0.05 to 2.28% in LPMC. Bacteria-specific Th1 responses were inhibited by anti-HLA-DR antibodies and chloroquine exposure, were enriched in LP relative to peripheral blood, and expressed effector memory cell markers. Bacteria-specific CD4+ T cell proliferation in vitro was dependent on the presence of LP dendritic cells (DCs), which produced proinflammatory cytokines upon bacterial exposure. These results suggest that bacteria-reactive DCs and CD4+ T cells in normal LP have substantial pro-inflammatory potential that is revealed upon disaggregation in vitro.
Introduction
Understanding mechanisms of mucosal immunity and tolerance to bacteria is vital for improved mucosal vaccine design, as well as therapy for disorders with chronic intestinal inflammation. The immune system encounters both pathogenic and commensal bacteria, but commensals do not normally provoke inflammation. Rather they play key roles in normal gut homeostasis, including in nutrition as well as in stimulating the generation and population of the mucosal tissues with lymphocytes [1-5]. In part, the “friendly” aspect of commensal bacteria likely reflects their evolution into forms less invasive and inflammatory. However, their lack of pathogenicity also likely reflects multiple mechanisms by the host to both contain these bacteria, as well as to suppress undesired inflammation. Indeed, outside the context of the lumen, these bacteria are far from harmless, as they are common causes of severe life-threatening bacterial infections, particularly among hospitalized and debilitated patients. This indicates that mechanisms must normally exist to limit their penetration across mucosal defenses.
Studies in mice have implicated multiple mechanisms limiting bacterial translocation, including IgA-secreting B cells [6, 7], T lymphocytes [8, 9], and more importantly epithelial integrity [10] and mast cell, granulocyte and macrophage microbicidal mechanisms [11, 12]. The latter, in particular, if defective lead to rapidly lethal infections by commensal bacteria in mouse models [11]. On the other hand, animal models of inflammatory bowel disease (IBD), as well as in vitro studies of human IBD, suggest that T cell reactivity to commensal bacteria underlies chronic colitis [13-15]. These studies suggest that colitis results from a breakdown of regulatory T cells and other suppressive mechanisms which normally keep such bacteria-reactive T cells in check [15-18]. Importantly, these results imply the co-existence of cells with pathogenic potential and those that tightly regulate them in the normal intestine [15]. Given their hazardous potential, this observation hints that such cells must normally serve an important function in immune defense. The commensal bacteria-reactivity of these colitogenic T cells has been primarily inferred by whether or not colitis can be induced in germ (commensal) free mice as opposed to conventionally reared animals [17, 19], but remarkably little detail is known of the functional properties and recognition mechanisms employed by such cells. The few studies which have explored T cell reactivity in vitro have demonstrated substantial T cell proliferation to crude commensal antigen preparations in humans with IBD or in mice with IBD-like colitis, with only sporadic T cell reactivity seen in normal subjects or in mice [16, 20-22].
In the present study, using highly sensitive flow cytometric techniques, we sought to determine whether CD4+ T cells isolated from healthy human lamina propria (LP) of jejunal and colonic tissue would react by cytokine production or proliferation to whole heat killed commensal bacteria. We assessed intracellular IFN-γ and IL-2, produced by Th1 proinflammatory cells, the Th2 cytokine IL-5, as well as IL-17, the signature cytokine of the recently described Th17 T cell subset which has been implicated in reactivity against extracellular bacteria and may also play a role in autoimmune disease [23]. In contrast to previous in vitro studies, we observed significant frequencies of commensal bacteria-reactive, IFN-γ-producing CD4+ Th1 cells with proliferative potential in normal intestinal tissue. These responses were sensitive to inhibition by anti-MHC Class II antibodies as well as the antigen processing inhibitor chloroquine, and were significantly enriched in LP relative to peripheral blood. Only low frequencies of Th17 cells and no IL5-producing cells were observed in response to bacteria. Interestingly, the in vitro proliferative response of CD4+ T cells to commensal bacteria was dependent on the presence of intestinal dendritic cells (DCs), which themselves exhibited a proinflammatory phenotype through the production of commensal bacteria-induced tumor necrosis factor (TNF). Possible functions of commensal bacteria-reactive CD4+ T cells are discussed.
Materials and methods
Study subjects
Jejunum (n=6) and colon (n=3) tissue samples were obtained from patients undergoing elective surgery for intestinal neoplasms (n=5), pancreatic cancer (n=3), and other structural anomalies (n=1). The tissue samples represented otherwise discarded tissue from surgical anastomotic junctions and were macroscopically healthy and normal in appearance (tumors were at least 5cm away from histologically normal tissue). Tissue was stored at 4°C in saline until use. The cohort consisted of 3 males and 6 females with a median age of 43 years (range 21-66 years). Peripheral blood samples were obtained from healthy adults (4 males, 3 females) with a median age of 28 years (range 20-53 years) from the University of Colorado Denver who voluntarily consented. Collection of peripheral blood samples was approved by the Colorado Multiple Institutional Review Board (COMIRB) at the University of Colorado Denver.
Isolation of mononuclear cells from intestinal lamina propria and peripheral blood
LP cells were extracted from intestinal tissue using previously described techniques [24] with slight modifications. Tissue was rinsed with Hanks’ Balanced Salt Solution (HBSS, Cellgro, Manassas, VA), and fat, muscularis, and submucosa dissected from mucosal tissue. The mucosal tissue was then treated with 1.6 mM dithiothreitol (DTT, Sigma-Aldrich, St. Louis, MO) for 40 min at 37°C with end-over-end rotation, rinsed with HBSS, and the epithelium was removed with two 60 minute treatments of 1mM EDTA in HBSS and 0.1% bovine serum albumin (BSA, Sigma-Aldrich) at 37°C. Tissue was then minced into 1-2 mm square portions and digested with 1-2mg/ml of collagenase D (Roche, Nutley, NJ) in RPMI containing 1% penicillin, 1% streptomycin, and 1% glutamine (complete RPMI) supplemented with 10% fetal bovine serum (FBS, Sigma-Aldrich) or 0.1% BSA for two to four 60 minute treatments. Released LP mononuclear cells (LPMC) from each treatment were passed through a cell strainer, stored at 4°C, and all digested cells ultimately pooled, frozen in multiple aliquots in 10% DMSO in RPMI with 10% FBS, and stored in liquid nitrogen. In one instance, the sample was held overnight at 4°C prior to digestion. Previous experiments had shown that this had limited effect on T cell function compared to samples that were processed immediately. Peripheral blood mononuclear cells (PBMC) were isolated from heparinized blood by standard Ficoll-Hypaque (GE Healthcare Bioscience, Piscataway, NJ) density centrifugation and cryopreserved as for LPMC.
Whole bacterial preparations
Escherichia coli and Salmonella typhimurium stocks were obtained from American Type Culture Collection (ATCC, Manassas, VA, catalog #25922 and #35986, respectively). Enterobacter cloacae and Enterococcus species (sp.) were isolated from EDTA extracts from two of the surgical intestinal samples, following overnight incubation in RPMI + 10% FBS without antibiotics. Isolates were typed by the Clinical Microbiology Laboratory at University of Colorado Hospital. Bacteria were recovered and expanded with overnight incubation in LB medium. Heat-killed bacteria were prepared by washing the organisms with phosphate buffered saline (PBS, Invitrogen, Carlsbad, CA), treating for 2 hours at 56°C, and repeat washing and resuspension in PBS. Bacteria were frozen at stock concentrations of 3 × 109/ml in PBS and aliquots thawed prior to use. For intracytoplasmic flow cytometry, organisms were used at a concentration of 3 × 106/well, and for proliferation assays at 7.5 × 105/well.
Cell cultures for intracellular cytokine analysis
For intracellular flow cytometry assays, LPMC and PBMC were thawed in complete RPMI + 10% FBS + 100μg/ml DNase I (Sigma-Aldrich) and cultured at 500,000 cells/well in 100μl of complete RPMI + 10% human serum (HS, Gemini Bioproducts, Woodland, CA) in 96 well microtiter plates (Costar, Lowell, MA) with either heat-killed bacteria (3 × 106/well) or with E.coli GroEL (10μg/ml; StressGen, Ann Arbor, MI) or S. typhimurium flagellin (10μg/ml; InvivoGen, San Diego, CA). Control stimuli included Staphylococcal enterotoxin B (SEB, 1μg/ml; Toxin Technologies, Sarasota, FL), phytohemagglutinin (PHA) (1μg/ml; Sigma-Aldrich), or PMA (250 ng/ml, Sigma-Aldrich) plus ionomycin (1 ug/ml, Sigma-Aldrich). In some experiments, not all bacterial stimuli were tested due to insufficient sample cell numbers. For all stimulatory conditions except PMA plus Ionomycin, brefeldin A (Golgi Plug; BD Biosciences, San Jose, CA) was added after 4h to a concentration of 0.1% and the cells incubated for an additional 8 hours. For PMA plus Ionomycin stimulated cultures, these stimulants were added together with brefeldin for the final 8 hours. Cells were subsequently harvested and washed with PBS + 0.1% BSA + 2mM EDTA (staining buffer) and underwent intracellular flow cytometry staining as detailed below. In some experiments the following inhibitors were added: either normal mouse IgG (30 μg/ml; Invitrogen), L243 anti-HLA-DR (15 μg/ml: gift of Dr. A. Fontenot, University of Colorado Denver, Denver), W6/32 anti HLA-A,B,C (10% culture supernatant; ATCC) or cholorquine (100μM, Sigma-Aldrich).
T cell proliferation and activation assays
Thawed LPMC were pre-labeled with 0.5 μM 5,6-carboxylfluorescein diacetate succinimidyl ester (CFSE; Molecular Probes, Eugene, OR) in HBSS for 10 minutes at 37°C. The reaction was stopped with an excess of FBS, and the cells were washed, resuspended in complete medium and 100,000 cells added to 96 well microtiter plate wells in a volume of 100μl with heat-killed bacteria (7.5 × 105/well) or with E.coli GroEL (10μg/ml) or S. typhimurium flagellin (10μg/ml). After 4 days, cells were harvested, washed in staining buffer and T cell proliferation assessed by flow cytometry as described below. In some experiments, not all bacterial stimuli were included due to insufficient cell numbers.
For assays utilizing cell preparations after DC or B cell depletion, DCs and B cells were isolated from total cell preparations using BDCA-1 and CD19 magnetic bead cell isolation kits according to the manufacturer’s instructions (Miltenyi Biotec, Auburn, CA). Cell clumping was minimized by additional pretreatment with 100μg/ml DNase as well as a brief 5 min incubation at 37°C with PBS + 2mM EDTA + 0.5% BSA followed by up and down pipeting with a micropipet and re-cooling to 4°C immediately prior to application to magnetized columns. Results from multiple preparations (n = 5) indicated that BDCA-1 isolation resulted in depletion of BDCA-1+ cells by 98±1% from the starting cell preparation while CD19 isolation removed 93±5% of B cells. DC- and B cell-depleted preparations were cultured as described above for proliferation assays.
Flow cytometry
Standard intracellular flow cytometry staining techniques were followed. Briefly, cells were stained with appropriate combinations of PE anti-human CD3, PE-Cy7 anti-human CD8, APC anti-human CD4, PE-Cy7 anti-human CD19, PE-Cy5 anti-human CD45RA, (all BD Biosciences) and APC BDCA-1 (Miltenyi). Expression of CCR7 was determined by using purified anti-human CCR7 (BD Biosciences) followed by biotinylated mouse IgM (BD Biosciences) and Streptavidin-PE (Rockland, Gilbertsville, PA). At the completion of surface staining, all cells were fixed in formaldehyde (Medium A, Invitrogen) and then permeabilized with Medium B (Invitrogen). The following antibodies were used for intracellular cytokine detection: FITC anti-human IFN-γ, PE anti-human IL-2, FITC anti-human TNF, APC anti-human IL-5, (all BD Biosciences) and FITC IL-17A (eBioscience, San Diego, CA).
For assessment of T cell proliferation, cells were collected after 4 days in culture and stained with PE anti-human CD3, PE-Cy7 anti-human CD8 and APC anti-human CD4. T cell proliferation was assessed by determining levels of CFSE within gated T cell populations. For assays in which DC or B cells were depleted, T cell activation was assessed by staining cells with PE anti-human CD3, PE-Cy7 anti-human CD8, APC anti-human CD4 and FITC anti-human CD38 [25].
All cells were fixed with 2% formaldehyde prior to flow cytometry analysis. Cells were acquired on a FACScalibur flow cytometer (BD Biosciences) using CellQuest software (BD Biosciences), with 100,000-200,000 events obtained per condition.
Data analysis
Analysis was performed with FloJo Analysis Software (Treestar, Ashland, OR), and tabular data analyzed with Excel (Microsoft Office, Redmond, Washington) or JMP 6 software (SAS, Cary NC). For intracellular cytokine analysis, frequencies of responding cells were calculated as the number of cytokine-producing cells for a given subset divided by the total number of events within that population. Bacteria-specific (net) frequencies were calculated by subtracting the frequency of responding cells within control cultures without antigens from those with bacterial antigens, and these net frequencies were used as one criteria for comparative purposes. As an additional criteria, positive responses were defined as those with specific frequencies greater than 0.05% as described in our earlier studies of HIV specific CD4 responses [26]. The effect of anti-MHC antibodies or chloroquine on IFN-γ specific responses was expressed as percent inhibition, and calculated as 100 times the stimulus specific frequency in absence of inhibitor less that in the presence of inhibitor, divided by the stimulus specific frequency in the absence of inhibitor. All analysis was performed on gated small cells defined by forward and side scatter.
For T cell proliferation analysis, proliferating blasts were defined as large cells expressing high forward and side scatter and low expression of CFSE. Additionally, small cells were gated separately from blasts due to differences in background autofluorescence. Frequency of responding cells for a given T cell subset was defined as the number of proliferating blasts within that subset divided by the total number of cells in combined small and large cell gates. Bacteria-specific proliferation was calculated by subtracting the percentage of proliferating blasts within control cultures from that of bacteria-stimulated cultures. Stimulation indices were calculated by dividing the frequency of proliferating blasts within stimulated cultures by that within control cultures, and positive responses defined as those with indices greater than 3.
Frequency of activated T cells within cultures depleted of DCs or B cells were defined as the total CD38+ large cells (defined by light scatter) divided by the total number of cells within small and large cell gates and bacteria-specific cells calculated as above by subtracting frequencies within control cultures from that of bacteria-stimulated cultures.
Pairwise comparisons of median frequencies were evaluated with the Wilcoxon non-parametric test. Categorical data representing positive cytokine or proliferative resonses among different T cell subset were evaluated with the chi-square statistical test. Paired student-t tests were utilized to compare mean responses where indicated.
Results
Identification of bacteria-reactive, cytokine-producing T cells within LPMC
Intracytoplasmic flow cytometry was utilized to assess the potential of bacteria-reactive T cells within LPMC to produce cytokines. Previous work with polyclonal stimulation had suggested that normal LPMC produced abundant IFN-γ and IL-2 and much less but detectable IL-5 [27], and more recent data suggested capacity to produce IL-17 [28]. In initial experiments, we evaluated the cytokine profiles of T cell subsets in LPMC in response to the oligoclonal stimulus, SEB, as well as to the common commensal bacteria, E.coli. A representative example of the gating strategy used to evaluate IFN-γ cytokine production by LP CD4+, CD8+ and CD4-CD8- (double negative, DN) T cells in response to E. coli is shown in Fig. 1. E. coli-and SEB-specific frequencies of CD4+ LP T cells producing IFN-γ, IL-17, IL-2 and IL-5 from six intestinal samples, three jejunums and three colons, are depicted in Fig. 2. E.coli-specific IFN-γ-and IL-2-producing cells were readily detectable in all six samples, with frequencies ranging from 0.1% to 2.2% for IFN-γ (median 0.53%), and 0.07 to 0.6% for IL-2 (median 0.3%). IL-17-producing cells were also detectable but at lower frequencies (median 0.07%, range 0.01-0.3%), and frequencies of IL-5-producing cells were at or below the limit of detection in all samples (Fig. 2A). A similar hierachy of cytokine responsiveness was observed for SEB-reactive CD4+ cells in LP, with highest frequencies for IFN-γ (median 19.55%, range 3-22.4%) and IL-2 (median 27.25% (1.9-28.75%) production, intermediate for IL-17 production (median 0.56%, range 0.2-2.94%), and IL-5-producing cells at lowest frequencies (median 0.11%, range 0.02-1.16%) (Fig. 2B). Additional experiments with seven intestinal samples were performed with PMA and ionomycin as a polyclonal stimulus to evaluate the relative expression of IFN-γ and IL-17. These results indicated that most stimulated CD4+ T cells produced IFN-γ without IL-17 (median 74.8%, range 62.9-86.2%), a small proportion expressed IL-17 without IFN-γ (median 4.7%, range 0.8-7.5%), and a smaller fraction still expressed both cytokines (median 2.3%, range 0.9-8.0%). Given the high frequency of IFN-γ– relative to IL17-producing cells and lack of demonstrable IL5 production, further characterization of bacteria-specific LP T cells focused on those producing the Th1 signature cytokine, IFN-γ.
Figure 1.

Example of bacteria-reactive IFN-γ production by LP T cell subsets. LPMC were isolated and stimulated with heat-killed bacteria and T cell-specific cytokine production assessed using intracellular flow cytometry techniques. Gated CD8+CD4-, CD4-CD8- (double negative, DN) and CD8-CD4+ small lymphocyte populations were further defined for CD3 and IFN-γ expression. Virtually all CD8+ and CD4+ cells were CD3+, whereas CD3+ and CD3- subsets of DN cells could be visualized. IFN-γ-producing cells in response to E.coli were primarily observed among gated CD4+ T cells.
Figure 2.
Cytokine production by E.coli– and SEB-reactive LP CD4+ T cells. LPMC (n=6) were isolated and stimulated with heat-killed E.coli (panel A) or SEB (panel B) and cytoplasmic IFN-γ, IL-17, IL-2 and IL-5 production assessed within the CD4+ cell population. The net frequencies of E.coli- or SEB-specific cytokine-producing cells among CD4+ cells are shown (log scale), with median values indicated with a bar.
Analysis of the maturation phenotype of E.coli-reactive, IFN-γ-producing CD4+ T cells in a subset of intestinal samples (n=4) indicated that a median of 84% (range 70-95%) were CCR7-CD45RA- (effector memory, EM), a median of 12% (range 2-29%) were CCR7+CD45RA- (central memory, CM), with less than 2% of reactive cells (range 1-5%) exhibiting naïve (CCR7+CD45RA+) or terminally differentiated effector memory (CCR7-CD45RA+;) T cell phenotypes.
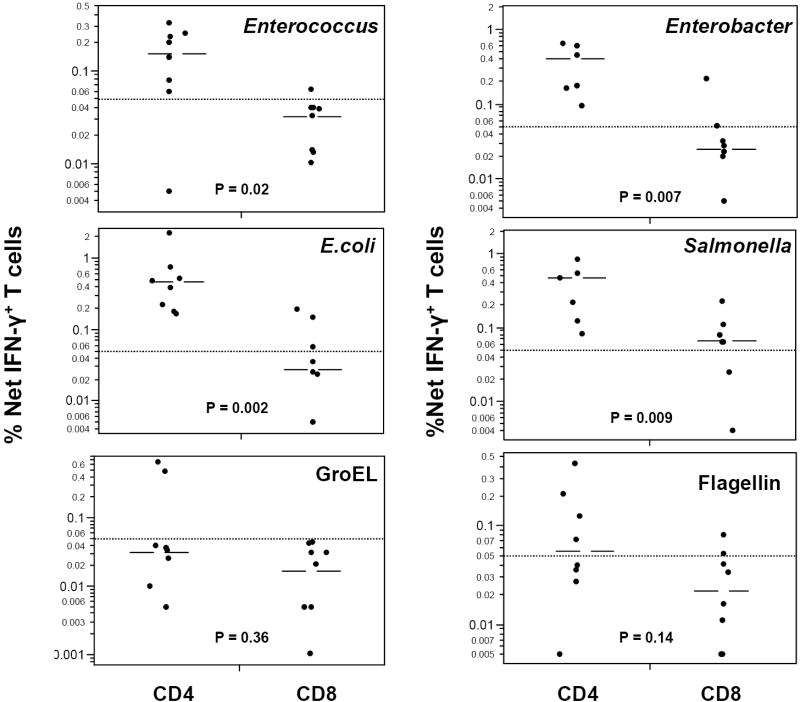
IFN-γ responses of CD4+ and CD8+ T cells in LPMC (n=8) to a number of whole, heat-killed commensal bacterial species, including E.coli, Enterobacter cloacae, and Enterococcus sp, and to the gastroenteritis-inducing pathogen, Salmonella typhimurium were next evaluated (Fig. 3). All four bacterial species induced significant IFN-γ T cell responses. For each bacterial preparation, the median frequency of CD4+- IFN-γ-producing T cells was significantly higher than that of CD8+ T cells (p≤0.02 for each bacteria). As an additional criterion for comparative purposes, “positive” bacteria-specific responses were arbitrarily defined as having net frequencies of IFN-γ producing T cells of at least 0.05% (see Materials and Methods). Out of a potential maximum of 32 responses (8 samples times 4 bacteria), 28 of 30 responses (93%) were positive among CD4 T cells (with two missing values due to insufficient cells at culture initiation). In contrast, only 11 of 30 responses (37%) were positive among CD8 T cells. The differences between CD4+ and CD8+ T cell responses were highly significant (p < 0.0001, chi-square).
Figure 3.
IFN-γ production by LP CD4+ and CD8+ T cells in response to multiple bacteria and bacterial antigens. LPMC (n=7-8) were stimulated with heat-killed preparations of the indicated bacterial species, or with E.Coli GroEL and Salmonella flagellin antigens as described in Materials and Methods. Net frequencies of IFN-γ-producing cells among gated CD3+CD4+CD8- or CD3+CD4-CD8+ populations are illustrated (log scale), with median values for each bacterial preparation depicted by a bar. Median responses of CD4+ and CD8+ T cells were compared for each stimulus, and p values, determined by the Wilcoxon non-parametric test are illustrated. Positive bacteria-specific responses were defined as those with net frequencies of IFN-γ producing T cells of at least 0.05% (level depicted with dotted line).
Moreover, in addition to using whole heat killed bacteria as stimulants, responses were also assessed to purified bacterial proteins, GroEL (isolated from E.coli) and flagellin (isolated from S. typhimurium). Both proteins have been shown to elicit MHC I-and II-restricted T cell responses [29-33]. T-dependent anti-flagellin antibody responses may exhibit cross-reactivity between different enteric bacterial species [34]. GroEL is a widely conserved heat shock protein (the homologue of human HSP 60) and is a common antigen targeted by CD4+ and CD8+ T cells [12, 30-33]. Both proteins also exhibit toll-like receptor (TLR) agonist activity, with GroEL stimulating through both TLR2 and TLR4 [35, 36] and flagellin through TLR5 [12]. Among CD4 T cells, cytokine responses to these purified proteins were consistently lower in frequency than responses to whole bacteria, and although negative in some individuals, were clearly robust in others (Fig. 3). Positive CD4+IFN-γ+ responses (based on net frequencies greater than 0.05% as detailed above) to GroEL were detected in 25% of samples whereas positive responses to flagellin were detected in half of the samples. In contrast, none of the LPMC samples exhibited positive CD8+IFN-γ+ responses to GroEL and only 25% had positive responses to flagellin. However, statistically significant differences between IFN-γ+ CD4 and CD8 T cell frequencies in response to GroEL or flagellin were not observed.
Proliferative responses of LP T cells to bacterial preparations
To ascertain whether the bacterial preparations that elicited cytokine responses were also capable of inducing T cell proliferation, LPMC were labeled with CFSE, stimulated for four days with heat killed bacteria or purified proteins, and proliferation was assessed by loss of CFSE fluorescence using flow cytometry. Fig. 4 illustrates the gating strategy employed, which allows simultaneous assessment of CD4, CD3 and CD8 expression on proliferating cells from a representative LPMC sample. The LPMC proliferative responses from up to six subjects for each of the bacterial preparations were determined. When percentages of proliferating bacterial antigen-specific blasts were determined, bacteria-specific CD4+ T cell proliferation frequencies exceeded those of CD8+ T cells for each bacterial antigen preparation and achieved statistical significance at the 0.05 level (Fig. 5). When positive responses were defined as those with stimulation indices greater than 3 (see Materials and Methods), positive proliferative responses were observed to virtually all bacterial antigen preparations by both CD4+ (96% response rate) and CD8+ (89% response rate) T cells.
Figure 4.
Flow cytometry gating strategy to assess proliferation of lymphocyte subsets in response to E.coli bacteria. LPMC were pre-stained with CFSE, cultured with media alone or heat-killed E. coli for 4 days, and levels of proliferation assessed by flow cytometry. Flow plots from unstimulated (upper plots) or E.coli-stimulated (lower plots) cultures are illustrated. Large cells (blasts) were gated based on forward and side scatter characteristics, and further defined for CD4 and CD8 expression. Gated CD4+CD8-, CD4-CD8+ and CD4-CD8- cells were analyzed for CD3 and CFSE expression. CFSElowCD4+CD8- and CFSElowCD4-CD8+ regions, which define proliferating cells within these subsets, are shown. CFSElow cells among CD3+ and CD3- DN cells were similarly enumerated.
Figure 5.
Proliferative responses of LP T cells to different bacteria and bacterial antigens. LPMC (n=3-6 samples) were pre-stained with CFSE, stimulated with heat-killed bacteria or bacterial proteins for 4 days, and analyzed for loss of CFSE dye, indicative of proliferation, as illustrated in Figure 4. Frequencies of bacteria-specific CFSElow cells within the CD4+ or CD8+ T cell subsets are shown with medians indicated with a bar. Statistical analysis was performed as described in Figure 3.
Comparisons of median IFN-γ-producing cell frequencies and proliferative responses amongst CD4+, CD8+ and DN T cells, and among CD3-CD4-CD8-LP cells in response to whole bacteria and bacterial antigens indicated that most bacteria-reactive IFN-γ-producing (Fig. 6A) and proliferating (Fig. 6B) cells were CD4+ T cells, with moderate frequencies of CD3- cells reactive to some bacteria and with very low frequencies of bacteria-reactive CD8+ T cells or DN T cells.
Figure 6.
Comparison of IFN-γ production and proliferation among CD4+, CD8+, DN T cell subsets, and non-T cells among LPMC. Data obtained in experiments depicted in Figures 3 and 5, were re-analyzed and expressed to illustrate the relative contribution of CD3+CD4+CD8- (CD4+ T cells), CD3+CD4-CD8+ (CD8+ T cells), CD3+CD4-CD8- (DN) T cells), and CD3-CD4-CD8- subsets for IFN-γ production (A) and proliferation (B). Frequencies of a given subset were defined as number of positive events divided by the total events within the live cell gate which consisted of only small cells for IFN-γ assays, and both small and large cells for proliferation assays.
Comparison of IFN-γ responses to bacteria between T cells in LPMC and PBMC
In order to determine whether the IFN-γ-predominant T cell responses observed to the bacterial preparations were enriched in LPMC relative to peripheral blood (PB), T cell cytokine responses to four bacterial preparations in LPMC were compared to those in unrelated donor PBMC. As shown in Figure 7, frequencies of IFN-γ-producing, bacteria-reactive CD4+ T cells were significantly lower in PBMC when compared with LPMC for a given bacteria (p≤ 0.03 for all comparisons). Although this comparison was made with a limited number of subjects, and intestinal and blood samples were not autologous, the magnitude of the median differences to each of the bacterial preparations in the two compartments ranged from 8.5-19.5 fold. If responses to each of the four bacteria were pooled, the median frequency of IFN-γ-producing LP CD4+ T cells was 0.24% compared to only 0.02% in PBMC, a difference which was highly significant (p<0.0001). In contrast, CD8 responses to whole, heat-killed bacteria were low in both LPMC and PBMC (median frequencies of 0.034% and 0.069%, respectively, among pooled data, p=0.39). Collectively, these results suggest that frequencies of bacteria-reactive CD4+ T cells are substantially enriched in lamina propria compared with peripheral blood.
Figure 7.
Comparison of bacteria-specific IFN-γ-producing T cell frequencies in LPMC versus PBMC. LPMC (n=7-8) and PBMC (n=4-7) were prepared and cultured with or without the indicated heat-killed bacteria, and analyzed as described in Figure 1. Frequencies of IFN-γ-producing cells among CD4+ T cells (upper plots) or CD8+ T cells (lower plots) are depicted. Statistical analysis was performed as described in Figure 3.
Inhibition of bacteria-reactive CD4 T cell responses by anti-MHC class II antibodies and chloroquine
In order to probe the mechanism of T cell reactivity to bacteria further, bacteria-specific IFN-γ production and proliferation of intestinal CD4+ T cells was evaluated in the presence of antibodies to MHC class II molecules. As shown in Figure 8, anti-HLA-DR antibody inhibited the CD4+ T cell IFN-γ response to E. coli by ~ 80% compared with control mouse IgG, whereas the same anti-HLA-DR antibodies only marginally inhibited a polyclonal PHA CD4+ T cell response. In additional experiments (n=5), anti-HLA-A,B,C antibodies only modestly inhibited E.coli-specific IFN-γ production by CD4+ T cells (mean=21±39%) relative to control, whereas anti-HLA-DR antibodies inhibited a significantly greater fraction of cells (mean=73.7±7.1%, p=0.01 comparing anti-HLA-A,B,C with anti-HLA-DR inhibition, student’s t-test). Similarly, comparisons of E.coli-specific CD4+ T cell proliferative responses using the CFSE dye dilution assay revealed that anti-HLA-DR antibodies inhibited such responses by 78±22% compared with control cultures without antibodies, whereas anti-HLA-A,B,C antibodies exhibited no inhibition (-7.2±19.5%, p=0.01 comparing anti-HLA-DR with anti-HLA-A,B,C inhibition). Inhibition of the weak IFN-γ and proliferative responses of CD8+ T cells to E.coli by anti-HLA-A,B,C antibodies was not consistently observed (data not shown), although control proliferative responses of magnetic bead-purified alloreactive CD8+ T cells to LP cells were inhibited by 58% by the anti-HLA-A,B,C antibodies. These results suggest that bacteria-specific IFN-γ and proliferative responses by CD4+ T cells appear to be, at least partially, dependent on HLA-DR molecules.
Figure 8.

Sensitivity of E.coli-reactive CD4+ T cell responses to anti-HLA-DR antibodies or chloroquine. In one set of experiments, LPMC were thawed, and control, E.coli-or PHA-stimulated cultures initiated and supplemented with either control mouse IgG or L243 anti-HLA-DR antibody. E.coli-and PHA-specific IFN-γ frequencies among CD4+ T cells were determined, and percent inhibition calculated as described in Materials and Methods. The mean (+ standard deviation) % inhibition by the indicated antibody supplements for 3 LPMC samples are shown. In a second set of experiments, LPMC were cultured alone or with E.coli or SEB. Parallel cultures were supplemented with 100μM chloroquine, and intracytoplasmic IFN-γ among stimulus-specific CD4+ T cells determined as above. Percentage inhibition by chloroquine was calculated, and mean (+ standard deviation) of 3 samples shown.
Since CD4+ T cell responses to conventional antigens are known to be susceptible to antigen-processing inhibitors such as chloroquine [37], and because T cell responses to unconventional bacterial antigens such as superantigens are MHC class II-dependent but chloroquine-resistant [38, 39], LP CD4+ T cell IFN-γ responses to bacteria were assessed in the presence of chloroquine. Results from three samples are depicted in Figure 8. Chloroquine at a dose of 100 μM nearly completely inhibited E.coli-specific CD4+ IFN-γ responses (mean inhibition 85± 8%), but had no effect on the response to the superantigen SEB (mean inhibition 5± 15%, p = 0.002 comparing inhibition of E.coli and SEB responses, paired t-test). In a fourth sample, we observed that chloroquine inhibited both SEB-specific (72%) and E.coli-specific (100%) IFN-γ responses. However, at a lower dose of 20 μM, chloroquine affected SEB responses minimally (14%), whereas E.coli responses were reduced by 62%. Collectively, these results are consistent with a recognition mechanism by bacteria-reactive CD4+ T cells which depends on antigen processing, but is unlikely to be related to recognition of superantigens.
Dendritic cells produce the pro-inflammatory cytokine TNF-α upon exposure to bacteria and are necessary for the proliferative response of bacteria-reactive CD4+ T cells
The finding that CD4+ T cell bacterial reactivity was dependent upon MHC class II molecules as well as lysozomal proteolysis implies the involvement of antigen presenting cells. DCs have been implicated in the sampling of commensal bacteria from the intestinal lumen [7, 40, 41]. Although classically defined as pivotal antigen-presenting cells for naive T cells, recent data in murine models suggest that dendritic cells may also play a key role in induction of proliferative responses of memory T cells in peripheral tissues [42, 43]. Therefore, we were interested in determining whether DCs were necessary for responses of LP CD4+ T cells to bacteria.
In order to avoid the in vitro activation of DCs inherent during positive selection, we utilized a negative selection approach to assess the T cell stimulatory capacity of LP DCs. In studies to be reported elsewhere (Dillon S et al., manuscript in preparation), we determined that the majority of total LP DCs could be specifically identified by flow cytometry based on expression of BDCA-1 (CD1c antigen), a myeloid DC marker [44], and that co-staining with the B cell marker CD19 excluded a small population of BDCA-1-expressing B cells (data not shown). Furthermore, viable DCs were readily preserved following tissue digestion and cryopreservation, with the median CD19-BDCA1+ DC frequency being 0.71% of all viable cells (range 0.41-1.13%, n = 8). In addition, we used an alternative approach to the CFSE assay to assess both T cell activation and proliferation that involved enumeration of large blasts expressing the activation marker CD38. We have previously observed this assay to be correlated with proliferation assays [25], and this approach minimized the stress on cells and improved cell recoveries after stimulation.
Initial experiments compared total LPMC with anti-BDCA-1 magnetic bead-depleted LPMC. Results from two preliminary experiments showed that depletion of BDCA-1+ cells from LPMC preparations reduced E.coli-specific CD38+ CD4+ T cell blasts after 4 days of culture by 69%-77%, and of Enterococcus-specific CD38+CD4+ T cell blasts by 95% (data not shown). However, because a portion of B cells also express BDCA-1, it was possible that the observed decrease in bacteria-specific T cell blastogenesis was due to a T cell-stimulating B cell subset. We therefore next compared LPMC depleted of cells bearing the B cell marker CD19 with those depleted of BDCA-1+ cells. BDCA-1 depletion substantially reduced E.coli- and Enterococcus -specific T cell blastogenesis relative to B cell depletion by anti-CD19 magnetic beads (Fig. 9A). The results from three independent experiments indicated that BDCA-1 depletion reduced E.coli-specific blastogenesis by 69.8±9.9% and Enterococcus-specific blastogenesis by 84.4±13.3% relative to CD19 depletion. Collectively, these results indicated that BDCA-1+ DCs were necessary for CD4+ T cell expansion and activation in response to bacteria. A separate experiment comparing the effects of CD19-and BDCA-1-depletion using both the E.coli-specific CD38 blastogenesis assay and the E.coli-specific CFSE dye dilution assay for proliferation gave identical results (data not shown).
Figure 9.
Activation and proliferation of bacteria-reactive LP CD4+ T cells is dependent on LP DCs. A) LPMC, depleted of either CD19+ B cells or BDCA-1+ DCs were cultured with media alone, E.coli or Enterococcus for 4 days, and the frequency of bacteria-specific CD38+CD3+CD4+ large cells among total small and large cells determined among CD19-depleted or BDCA-1-depleted LPMC as described in Materials and Methods. The results of three independent experiments are shown. B) LPMC from 4 independent gut samples were cultured with (solid histogram) or without (open histogram) Enterococcus under identical conditions as described in Figure 1, and net percentage of TNF-producing cells among gated BDCA-1+CD19- DCs was determined by subtracting values of control stimulated cultures from that of Enterococcus- stimulated cultures. DC TNFα responses among paired unstimulated and stimulated cultures for each sample are shown, with gates for positive responses within each histogram indicated along with net percentages.
Finally, because we observed a requirement for DCs in inducing CD4+ T cell proliferation in response to bacteria, we wished to determine whether this DC-dependence could be correlated with bacteria-induced proinflammatory cytokine production by DCs. Accordingly, LPMC were stimulated with whole, heat-killed bacteria, and intracytoplasmic TNF-α was assessed among gated BDCA-1+CD19- DCs. A consistent induction of TNF-α among Enterococcus-stimulated cultures was observed, as shown in Fig. 9B. Of four LPMC samples tested, we observed a mean frequency of 12 ± 8% TNF-α+ DCs in unstimulated cultures and 40±11 % TNF-α+ DCs in the presence of Enterococcus (p = 0.007).
DISCUSSION
In the present study, we have shown that human CD4+ T cells from the LP of healthy jejunem and colon tissue exhibit reactivity to commensal bacteria (Enterobacter, E. coli, Enterococcus species) as well as to the pathogen, Salmonella typhimurium. Functional analysis showed that reactive cells had primarily a Th1-like phenotype, with a minor fraction exhibiting a Th-17-like phenotype. This pro-inflammatory phenotype was associated with proliferation, and both IFN-γ and proliferative responses were inhibited by anti-MHC class II antibodies, suggesting a recognition mechanism dependent, at least in part, on MHC class II molecules. Finally, depletion studies suggested that bacteria-specific CD4+ T cell activation and proliferation were dependent on intestinal DCs, which themselves exhibited a pro-inflammatory cytokine profile upon bacterial stimulation.
Since there have been few studies directly addressing reactivity of T cells to bacteria using ex vivo or in vitro approaches as we have done here, much of the previous work on T cell responses to commensal bacteria have been inferred from observations of mice raised in germ-free, as opposed to conventional pathogen-free, conditions. Reactivity to commensals has in particular been revealed in animal models of IBD, and these have typically involved immunodeficient or knockout mice. However, studies have also identified memory T cells, which exist in normal mice under tonic suppression by IL-10-dependent regulatory cells, but with colitogenic potential and inferred commensal reactivity [15]. Moreover, whereas some studies have shown the generation and activation of IgA-producing B cells specific for commensal bacteria in a T-independent fashion [6], others have shown optimal IgA production to be dependent on T cells with presumed reactivity to commensal bacteria [45]. Collectively, these observations predict that T cells with commensal reactivity should be present in normal individuals, and that some activity may be pro-inflammatory and under tonic suppression by T regulatory cells or other mechanisms, whereas other activity may be involved in regulating B cell IgA production.
The few studies in rodent and man that have addressed the issue of T cell reactivity to commensal bacteria in vitro have been focused on their role in colitis or IBD. Two groups studied murine responses to crude fecal extracts and found evidence of high reactivity among T cells isolated from mice with colitis, but no or limited reactivity among T cells from normal mice [14, 21]. Similar results were observed looking at proliferative and IFN-γ responses, with no [46] or some detectable activity [47, 48] observed in normal individuals, but with much higher levels found in IBD patients [46, 48]. Khoo et al. demonstrated that human gut T cells proliferated poorly to crude extracts of E.coli, but some proliferation was apparent to partially purified preparations [22]. Our results here demonstrate that bacterial reactivity can be consistently identified in intestinal LP T cells of normal individuals using flow cytometric-based approaches. Whether the responses we observed reveal differences in sensitivity of techniques employed (flow cytometry vs. cytokine release in culture supernatants), preparation or dose of antigen (crude fecal extracts vs. heat killed whole bacteria), cell preparation or other technicalities is not clear. In any case, our results are compatible with the aforementioned murine in vivo studies which predict the presence of commensal bacteria-reactive T cells in normal individuals.
Lamina propria CD4+ T cells were primarily Th1 cells with a much lower fraction of Th17 cells. This was true whether we stimulated cytokine production with whole heat-killed E.coli, SEB, or the powerful mitogen combination of PMA and Ionomycin. This preponderance of Th1 over Th17 cells among LPMC mirrors the findings in previous studies of human PBMC [49, 50] and lamina propria cells [49]. Although recent studies have emphasized the reactivity of Th17 towards bacterial antigens [51], our current study serves as a reminder that a large fraction of Th1 cells exist as well in the gut with potential bacterial reactivity, and that these populations may act together in bacterial defense, a possibility recently suggested for fungal infections in human [50] and murine models of tuberculosis [52].
Although the recognition mechanism(s) involved remains to be clarified in detail, the finding that such responses were sensitive to inhibition by anti-MHC class II antibodies as well as by chloroquine suggest that these responses, at least in part, resemble those of conventional MHC class II-restricted CD4+ T cells to processed antigens. It seems unlikely that these responses are mediated by bacterial superantigens because such responses, although MHC class II-dependent, typically stimulate both CD4+ and CD8+ T cells, are more commonly seen with gram positive organisms than gram negative organisms such as E.coli, Salmonella or Enterobacter, and, importantly, are not sensitive to inhibitors of antigen-processing such as chloroquine [38, 53]. Zwitterionic carbohydrate antigens, which have best been characterized from Bacteroides species, are powerful CD4+ T cell mitogens that are both MHC class II-dependent and require processing [53, 54]. However, the vast majority of bacterial carbohydrates are not zwitterionic, and zwitterionic carbohydrates have not been described among the capsular carbohydrates for the organisms utilized in this study [55, 56]. Yet, it is conceivable that other non-conventional, processing-dependent molecules are involved in the observed stimulation. Finally, in that not all IFN-γ or proliferation could be inhibited by either chloroquine or anti-HLA-DR, it is possible that additional innate mechanisms exist which contribute to these responses. Previous studies have suggested that cytokines such as IL-12 are particularly potent in inducing IFN-γ from intra-epithelial human CD4+ T cells [57]. Our finding that bacteria also induce production of pro-inflammatory cytokines such as TNF-α, from LP myeloid DCs suggests a mechanism in which cytokines from DCs may be additive or synergistic with HLA-DR-dependent T cell stimulation in response to bacteria. In any case, it is clear that our understanding of the mechanisms of commensal bacteria recognition by T cells is limited. CD4+ T cells from some intestinal samples responded to the bacterial proteins GroEL and flagellin from E.coli and Salmonella, respectively. These well-defined molecules are known to stimulate both adaptive T cell responses as well as innate responses via interaction with TLR2 or TLR4, and TLR5, respectively [29, 30, 35, 36, 58]. Detailed characterization of T cell responses, particularly at the clonal level, to these molecules will assist in the approach to deciphering the mechanism of CD4+ T cell induction and in turn the role of DCs in this process.
The requirement for DCs in our T cell responses was observed in assays requiring T cell proliferation [25]. Traditionally, dendritic cells have been viewed as critical for the antigen-specific proliferation of naive rather than memory T cells. However, these views have recently been challenged with the findings in murine models that peripheral memory CD8 responses to viral antigens are largely DC-dependent [42, 43]. Although the details of the recognition mechanism(s) involved in our bacterial responses remain to be clarified in detail, our finding of dendritic cell- and class II-dependent bacteria-reactive proliferation among a population of largely effector memory CD4 T cells appears at face value to be analogous to the recent findings of CD8 T cells in mouse models [43]. These results imply that dendritic cells may have the unique potential to induce proliferation of effector memory T cells within non-lymphoid peripheral tissue compartments, and as such would provide a local mechanism to amplify the immune response.
It is important to note that we have not yet assessed whether DCs are necessary for bacteria-induced T cell cytokine responses, largely because such assays required larger numbers of cells than were available. Previous studies have indicated that macrophages [59] and myofibroblasts [60] comprise a substantial portion of the cells within lamina propria, and owing to their expression of costimulatory molecules and HLA-DR necessary for optimal antigen presentation [60], these cells could be contributing to the short term cytokine response—in particular IFNγ-- by bacteria-reactive CD4 T cells. On the other hand, recent evidence has pinpointed the critical role of dendritic cells in the production of IL-17 from Th17 cells [23]. The relative contribution of individual lamina propria accesory cells in the production of CD4 T cell derived cytokines thus remains to be clarified.
The finding of significant pro-inflammatory reactivity of intestinal T cells and DCs to commensal bacteria in normal individuals requires consideration in the context of normal gut homeostasis. As alluded to above, several previous reports in humans have found that ex-vivo analyzed intestinal T cells do in fact exhibit such Th1-like or Th17-like proinflammatory cytokine profiles in response to polyclonal stimulation [23, 27, 49], so the cytokine profiles we see are compatible with these studies. In addition, while some studies of lamina propria have emphasized inflammatory anergy of cells such as macrophages [59], others have indicated that human and mouse lamina propria dendritic cells can be induced to produce proinflammatory cytokines [61, 62]. Our finding here that mDCs can be induced to express TNF-α is compatible with the latter studies, and supports our contention that both lamina propria DC as well as T cells have proinflammatory potential. However, conventional wisdom would predict that commensal, bacteria-reactive, pro-inflammatory cells should not be tolerated under normal conditions. We thus cannot rule out -- in fact we favor-- the notion that by analyzing these cells outside the context of normal intestinal tissue we are releasing T cells from the influence of other cells or factors that would otherwise minimize such reactivity in vivo. Several inhibitory mechanisms can be considered. First, bacteria, antigen-presenting cells such as DCs, and T cells are likely to be compartmentalized in vivo, either physically or functionally, in such a way to prevent or, at least reduce, the interactions required for ultimate T cell stimulation. The epithelial barrier restricts entry of most commensal bacteria, so the numbers of bacteria present in our cultures are well in excess of the few that might normally be present in the intact LP. In addition, although it is known that DCs sample the intestinal lumen and shuttle commensal bacteria to inductive sites within intestinal mucosa [7, 40, 41], it is unclear whether or how LP DCs interact with effector memory T cells within the LP under normal steady state conditions. By analogy with other lymphoid tissues [63], such interactions are presumably regulated by chemokines, but it is conceivable that the chemokine milieu of the LP may normally not encourage DC-T cell interactions. Thus, the T cell and DC responses we observed in vitro might more likely resemble an in vivo situation after substantial bacterial breach has already occurred, rather than the steady state scenario. Finally, a number of mechanisms may actively down-regulate commensal-reactive T cell activity, including IL-10-dependent T regulatory cells [15, 64] and mediators produced by epithelial cells [65-67], as well other cells within the intestinal stroma [59, 68]. Thus, although our in vitro system reveals the pro-inflammatory potential of T cells and DCs within intestinal tissue, it likely underestimates the suppressive or compartmentalization mechanisms which normally operate in situ. Still, the convenience and manipulability of this in vitro model justifies its use to identify cells and soluble mediators involved in regulation of proinflammatory responses.
The potential hazard of pro-inflammatory, commensal bacteria-reactive T cells--even if under tight regulation-- makes little sense unless these T cells serve a very important function. We suggest that such cells would provide one of many levels of defense in the setting of mucosal breach, all of which can be easily justified considering the extraordinary proliferative potential of extracellular bacteria which would otherwise overwhelm the host immune system. Th1-like cells could function by cytokine-driven enhancement of macrophage or neutrophil [69] microbicidal mechanisms or by direct cytolytic mechanims, and Th17 cells by enhanced epithelial defensin production or neutrophil recruitment [23]. There is also evidence that Th1-like cells contribute to B cell responses in mice [70, 71], and recent findings suggest that human B cell IgA class switching and production can be induced by DCs activated with a number of mediators including IFN-γ [72].
Such T cells might contribute to the containment of commensal bacteria to a greater extent than is currently appreciated. Results from animal studies have suggested that while T cells reduce commensal bacterial translocation, they do not impact potential mortality from commensal bacterial infections [8, 9]. However, such models may underestimate the frequency with which mucosal disruption occurs in many human populations. Of particular relevance is the very high prevalence of intestinal helminth, protozoal and pathogenic bacterial infections in developing countries, many of which involve mucosal epithelial disruption. For example, it is well known that stronglyoides helminth invasion in humans is strongly associated with E.coli bacteremia [73]. Thus, relatively high level exposure to and hence need for defense against commensal bacteria within the lamina propria may be a continuous process in many humans.
Alternatively, commensal bacteria-induced T cells may function primarily in defense against cross reactive pathogenic bacteria. Insight into how such a mechanism might evolve can be gleaned from extensive sero-epidemiological studies of neisseria meningitidis in human subjects nearly 40 years ago [74], in which it was established that natural immunity to meningococcal disease develops in response to intermittent asymptomatic nasal carriage of different strains of meningococci [74, 75]. It was further suggested that in early childhood such a process begins with generation of immunity to non-pathogenic colonizing strains of neisseria, many of which could be shown to cross-react serologically with pathogenic meningococci [75]. Our observation here that frequencies of cells reactive to commensal enteric gram negative bacilli were similar to that of the pathogen Salmonella is consistent with extensive bacterial antigen cross reactivity at the CD4+ T cell level, although this obviously requires further investigation. It is tempting to speculate that the reactivity we see in adult human lamina propria, the well established finding that commensal bacteria induce generation of lamina propria T cells in young mice [1-5], and the detection of proinflammatory CD4+ T cells with presumed commensal reactivity in adult normal mice [15], reflect one and the same process, and that this may be conceptually analogous to the previously described evolution of humoral immunity to neisseria in humans [74, 75].
In sum, we have demonstrated substantial and readily detectable proinflammatory reactivity of both LP CD4+ T cells and DCs to commensal bacteria in vitro. Given the hazards of such reactivity for epithelial integrity, we propose that this potential 1) is normally under an actively maintained but readily reversible suppression mechanism in vivo, and 2) is primarily intended for defense against either cross-reactive pathogens or commensal bacteria which have breached the epithelium.
Acknowledgments
We wish to thank the study subjects for their generous participation. We also wish to acknowledge the Colorado Center for AIDS Research (CFAR) Immunology Core for assistance with flow cytometry.
Sources of support: This work was supported by NIH grants R01 AI065275 (C.W.), K24 AI074343 (C.W.), and was facilitated by the infrastructure and resources provided by the Colorado Center for AIDS Research (AI054907).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Macpherson AJ, Geuking MB, McCoy KD. Immune responses that adapt the intestinal mucosa to commensal intestinal bacteria. Immunology. 2005;115:153–162. doi: 10.1111/j.1365-2567.2005.02159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berg RD. The indigenous gastrointestinal microflora. Trends Microbiol. 1996;4:430–435. doi: 10.1016/0966-842x(96)10057-3. [DOI] [PubMed] [Google Scholar]
- 3.Benveniste J, Lespinats G, Adam C, Salomon JC. Immunoglobulins in intact, immunized, and contaminated axenic mice: study of serum IgA. J Immunol. 1971;107:1647–1655. [PubMed] [Google Scholar]
- 4.Backhed F, Ley RE, Sonnenburg JL, Peterson DA, Gordon JI. Host-bacterial mutualism in the human intestine. Science. 2005;307:1915–1920. doi: 10.1126/science.1104816. [DOI] [PubMed] [Google Scholar]
- 5.Rakoff-Nahoum S, Paglino J, Eslami-Varzaneh F, Edberg S, Medzhitov R. Recognition of commensal microflora by toll-like receptors is required for intestinal homeostasis. Cell. 2004;118:229–241. doi: 10.1016/j.cell.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 6.Macpherson AJ, Gatto D, Sainsbury E, Harriman GR, Hengartner H, Zinkernagel RM. A primitive T cell-independent mechanism of intestinal mucosal IgA responses to commensal bacteria. Science. 2000;288:2222–2226. doi: 10.1126/science.288.5474.2222. [DOI] [PubMed] [Google Scholar]
- 7.Macpherson AJ, Uhr T. Induction of protective IgA by intestinal dendritic cells carrying commensal bacteria. Science. 2004;303:1662–1665. doi: 10.1126/science.1091334. [DOI] [PubMed] [Google Scholar]
- 8.Gautreaux MD, Deitch EA, Berg RD. T lymphocytes in host defense against bacterial translocation from the gastrointestinal tract. Infect Immun. 1994;62:2874–2884. doi: 10.1128/iai.62.7.2874-2884.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Macpherson AJ, Martinic MM, Harris N. The functions of mucosal T cells in containing the indigenous commensal flora of the intestine. Cell Mol Life Sci. 2002;59:2088–2096. doi: 10.1007/s000180200009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nenci A, Becker C, Wullaert A, et al. Epithelial NEMO links innate immunity to chronic intestinal inflammation. Nature. 2007;446:557–561. doi: 10.1038/nature05698. [DOI] [PubMed] [Google Scholar]
- 11.Shiloh MU, MacMicking JD, Nicholson S, et al. Phenotype of mice and macrophages deficient in both phagocyte oxidase and inducible nitric oxide synthase. Immunity. 1999;10:29–38. doi: 10.1016/s1074-7613(00)80004-7. [DOI] [PubMed] [Google Scholar]
- 12.Rumbo M, Nempont C, Kraehenbuhl JP, Sirard JC. Mucosal interplay among commensal and pathogenic bacteria: lessons from flagellin and Toll-like receptor 5. FEBS Lett. 2006;580:2976–2984. doi: 10.1016/j.febslet.2006.04.036. [DOI] [PubMed] [Google Scholar]
- 13.Lodes MJ, Cong Y, Elson CO, et al. Bacterial flagellin is a dominant antigen in Crohn disease. J Clin Invest. 2004;113:1296–1306. doi: 10.1172/JCI20295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cong Y, Brandwein SL, McCabe RP, et al. CD4+ T cells reactive to enteric bacterial antigens in spontaneously colitic C3H/HeJBir mice: increased T helper cell type 1 response and ability to transfer disease. J Exp Med. 1998;187:855–864. doi: 10.1084/jem.187.6.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asseman C, Read S, Powrie F. Colitogenic Th1 cells are present in the antigen-experienced T cell pool in normal mice: control by CD4+ regulatory T cells and IL-10. J Immunol. 2003;171:971–978. doi: 10.4049/jimmunol.171.2.971. [DOI] [PubMed] [Google Scholar]
- 16.Cong Y, Weaver CT, Lazenby A, Elson CO. Bacterial-reactive T regulatory cells inhibit pathogenic immune responses to the enteric flora. J Immunol. 2002;169:6112–6119. doi: 10.4049/jimmunol.169.11.6112. [DOI] [PubMed] [Google Scholar]
- 17.Powrie F. Immune regulation in the intestine: a balancing act between effector and regulatory T cell responses. Ann N Y Acad Sci. 2004;1029:132–141. doi: 10.1196/annals.1309.030. [DOI] [PubMed] [Google Scholar]
- 18.Powrie F, Leach MW, Mauze S, Menon S, Caddle LB, Coffman RL. Inhibition of Th1 responses prevents inflammatory bowel disease in scid mice reconstituted with CD45RBhi CD4+ T cells. Immunity. 1994;1:553–562. doi: 10.1016/1074-7613(94)90045-0. [DOI] [PubMed] [Google Scholar]
- 19.Aranda R, Sydora BC, McAllister PL, et al. Analysis of intestinal lymphocytes in mouse colitis mediated by transfer of CD4+, CD45RBhigh T cells to SCID recipients. J Immunol. 1997;158:3464–3473. [PubMed] [Google Scholar]
- 20.Duchmann R, Schmitt E, Knolle P, Meyer zum Buschenfelde KH, Neurath M. Tolerance towards resident intestinal flora in mice is abrogated in experimental colitis and restored by treatment with interleukin-10 or antibodies to interleukin-12. Eur J Immunol. 1996;26:934–938. doi: 10.1002/eji.1830260432. [DOI] [PubMed] [Google Scholar]
- 21.Brimnes J, Reimann J, Nissen M, Claesson M. Enteric bacterial antigens activate CD4(+) T cells from scid mice with inflammatory bowel disease. Eur J Immunol. 2001;31:23–31. doi: 10.1002/1521-4141(200101)31:1<23::aid-immu23>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 22.Khoo UY, Proctor IE, Macpherson AJ. CD4+ T cell down-regulation in human intestinal mucosa: evidence for intestinal tolerance to luminal bacterial antigens. J Immunol. 1997;158:3626–3634. [PubMed] [Google Scholar]
- 23.Chen Z, O’Shea JJ. Regulation of IL-17 production in human lymphocytes. Cytokine. 2008;41:71–78. doi: 10.1016/j.cyto.2007.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hart AL, Al-Hassi HO, Rigby RJ, et al. Characteristics of intestinal dendritic cells in inflammatory bowel diseases. Gastroenterology. 2005;129:50–65. doi: 10.1053/j.gastro.2005.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Howe RC, Dhiman N, Ovsyannikova IG, Poland GA. Induction of CD4 T cell proliferation and in vitro Th1-like cytokine responses to measles virus. Clin Exp Immunol. 2005;140:333–342. doi: 10.1111/j.1365-2249.2005.02766.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Palmer BE, Boritz E, Blyveis N, Wilson CC. Discordance between frequency of human immunodeficiency virus type 1 (HIV-1)-specific gamma interferon-producing CD4(+) T cells and HIV-1-specific lymphoproliferation in HIV-1-infected subjects with active viral replication. J Virol. 2002;76:5925–5936. doi: 10.1128/JVI.76.12.5925-5936.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Fuss IJ, Neurath M, Boirivant M, et al. Disparate CD4+ lamina propria (LP) lymphokine secretion profiles in inflammatory bowel disease. Crohn’s disease LP cells manifest increased secretion of IFN-gamma, whereas ulcerative colitis LP cells manifest increased secretion of IL-5. J Immunol. 1996;157:1261–1270. [PubMed] [Google Scholar]
- 28.Saruta M, Yu QT, Avanesyan A, Fleshner PR, Targan SR, Papadakis KA. Phenotype and effector function of CC chemokine receptor 9-expressing lymphocytes in small intestinal Crohn’s disease. J Immunol. 2007;178:3293–3300. doi: 10.4049/jimmunol.178.5.3293. [DOI] [PubMed] [Google Scholar]
- 29.Cookson BT, Bevan MJ. Identification of a natural T cell epitope presented by Salmonella-infected macrophages and recognized by T cells from orally immunized mice. J Immunol. 1997;158:4310–4319. [PubMed] [Google Scholar]
- 30.Busch DH, Jassoy C, Brinckmann U, Girschick H, Huppertz HI. Detection of Borrelia burgdorferi-specific CD8+ cytotoxic T cells in patients with Lyme arthritis. J Immunol. 1996;157:3534–3541. [PubMed] [Google Scholar]
- 31.Salerno-Goncalves R, Fernandez-Vina M, Lewinsohn DM, Sztein MB. Identification of a human HLA-E-restricted CD8+ T cell subset in volunteers immunized with Salmonella enterica serovar Typhi strain Ty21a typhoid vaccine. J Immunol. 2004;173:5852–5862. doi: 10.4049/jimmunol.173.9.5852. [DOI] [PubMed] [Google Scholar]
- 32.Noll A, Autenrieth IB. Yersinia-hsp60-reactive T cells are efficiently stimulated by peptides of 12 and 13 amino acid residues in a MHC class II (I-Ab)-restricted manner. Clin Exp Immunol. 1996;105:231–237. doi: 10.1046/j.1365-2249.1996.d01-758.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Hermann E, Sucke B, Droste U, Meyer zum Buschenfelde KH. Klebsiella pneumoniae-reactive T cells in blood and synovial fluid of patients with ankylosing spondylitis. Comparison with HLA-B27+ healthy control subjects in a limiting dilution study and determination of the specificity of synovial fluid T cell clones. Arthritis Rheum. 1995;38:1277–1282. doi: 10.1002/art.1780380916. [DOI] [PubMed] [Google Scholar]
- 34.Sanders CJ, Yu Y, Moore DA, 3rd, Williams IR, Gewirtz AT. Humoral immune response to flagellin requires T cells and activation of innate immunity. J Immunol. 2006;177:2810–2818. doi: 10.4049/jimmunol.177.5.2810. [DOI] [PubMed] [Google Scholar]
- 35.Argueta JG, Shiota S, Yamaguchi N, Masuhiro Y, Hanazawa S. Induction of Porphyromonas gingivalis GroEL signaling via binding to Toll-like receptors 2 and 4. Oral Microbiol Immunol. 2006;21:245–251. doi: 10.1111/j.1399-302X.2006.00286.x. [DOI] [PubMed] [Google Scholar]
- 36.Habich C, Burkart V. Heat shock protein 60: regulatory role on innate immune cells. Cell Mol Life Sci. 2007;64:742–751. doi: 10.1007/s00018-007-6413-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Finnegan A, Needleman BW, Hodes RJ. Antigen processing requirements for T cell activation: differential requirements for presentation of soluble conventional antigen vs cell surface MHC determinants. J Immunol. 1985;134:2960–2965. [PubMed] [Google Scholar]
- 38.Janeway CA, Jr, Yagi J, Conrad PJ, et al. T-cell responses to Mls and to bacterial proteins that mimic its behavior. Immunol Rev. 1989;107:61–88. doi: 10.1111/j.1600-065x.1989.tb00003.x. [DOI] [PubMed] [Google Scholar]
- 39.Norton SD, Schlievert PM, Novick RP, Jenkins MK. Molecular requirements for T cell activation by the staphylococcal toxic shock syndrome toxin-1. J Immunol. 1990;144:2089–2095. [PubMed] [Google Scholar]
- 40.Rescigno M, Urbano M, Valzasina B, et al. Dendritic cells express tight junction proteins and penetrate gut epithelial monolayers to sample bacteria. Nat Immunol. 2001;2:361–367. doi: 10.1038/86373. [DOI] [PubMed] [Google Scholar]
- 41.Niess JH, Brand S, Gu X, et al. CX3CR1-mediated dendritic cell access to the intestinal lumen and bacterial clearance. Science. 2005;307:254–258. doi: 10.1126/science.1102901. [DOI] [PubMed] [Google Scholar]
- 42.Zammit DJ, Cauley LS, Pham QM, Lefrancois L. Dendritic cells maximize the memory CD8 T cell response to infection. Immunity. 2005;22:561–570. doi: 10.1016/j.immuni.2005.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wakim LM, Waithman J, van Rooijen N, Heath WR, Carbone FR. Dendritic cell-induced memory T cell activation in nonlymphoid tissues. Science. 2008;319:198–202. doi: 10.1126/science.1151869. [DOI] [PubMed] [Google Scholar]
- 44.Dzionek A, Fuchs A, Schmidt P, et al. BDCA-2, BDCA-3, and BDCA-4: three markers for distinct subsets of dendritic cells in human peripheral blood. J Immunol. 2000;165:6037–6046. doi: 10.4049/jimmunol.165.11.6037. [DOI] [PubMed] [Google Scholar]
- 45.Jiang HQ, Thurnheer MC, Zuercher AW, Boiko NV, Bos NA, Cebra JJ. Interactions of commensal gut microbes with subsets of B- and T-cells in the murine host. Vaccine. 2004;22:805–811. doi: 10.1016/j.vaccine.2003.11.022. [DOI] [PubMed] [Google Scholar]
- 46.Duchmann R, Kaiser I, Hermann E, Mayet W, Ewe K, Meyer zum Buschenfelde KH. Tolerance exists towards resident intestinal flora but is broken in active inflammatory bowel disease (IBD) Clin Exp Immunol. 1995;102:448–455. doi: 10.1111/j.1365-2249.1995.tb03836.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Duchmann R, May E, Heike M, Knolle P, Neurath M, Meyer zum Buschenfelde KH. T cell specificity and cross reactivity towards enterobacteria, bacteroides, bifidobacterium, and antigens from resident intestinal flora in humans. Gut. 1999;44:812–818. doi: 10.1136/gut.44.6.812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kamada N, Hisamatsu T, Okamoto S, et al. Unique CD14 intestinal macrophages contribute to the pathogenesis of Crohn disease via IL-23/IFN-gamma axis. J Clin Invest. 2008;118:2269–2280. doi: 10.1172/JCI34610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Annunziato F, Cosmi L, Santarlasci V, et al. Phenotypic and functional features of human Th17 cells. J Exp Med. 2007;204:1849–1861. doi: 10.1084/jem.20070663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Acosta-Rodriguez EV, Rivino L, Geginat J, et al. Surface phenotype and antigenic specificity of human interleukin 17-producing T helper memory cells. Nat Immunol. 2007;8:639–646. doi: 10.1038/ni1467. [DOI] [PubMed] [Google Scholar]
- 51.Brenchley JM, Paiardini M, Knox KS, et al. Differential Th17 CD4 T-cell depletion in pathogenic and nonpathogenic lentiviral infections. Blood. 2008;112:2826–2835. doi: 10.1182/blood-2008-05-159301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Khader SA, Cooper AM. IL-23 and IL-17 in tuberculosis. Cytokine. 2008;41:79–83. doi: 10.1016/j.cyto.2007.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cobb BA, Kasper DL. Zwitterionic capsular polysaccharides: the new MHCII-dependent antigens. Cell Microbiol. 2005;7:1398–1403. doi: 10.1111/j.1462-5822.2005.00591.x. [DOI] [PubMed] [Google Scholar]
- 54.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122:107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 55.Gallorini S, Berti F, Parente P, et al. Introduction of zwitterionic motifs into bacterial polysaccharides generates TLR2 agonists able to activate APCs. J Immunol. 2007;179:8208–8215. doi: 10.4049/jimmunol.179.12.8208. [DOI] [PubMed] [Google Scholar]
- 56.Tzianabos AO, Onderdonk AB, Rosner B, Cisneros RL, Kasper DL. Structural features of polysaccharides that induce intra-abdominal abscesses. Science. 1993;262:416–419. doi: 10.1126/science.8211161. [DOI] [PubMed] [Google Scholar]
- 57.Ebert EC, Jabri B. Massive interleukin-12-induced interferon-gamma production by interleukin-15-stimulated lamina propria lymphocytes followed by down-regulation of the interleukin-12 receptor. Immunology. 2008 doi: 10.1111/j.1365-2567.2007.02796.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Feige U, van Eden W. Infection, autoimmunity and autoimmune disease. Exs. 1996;77:359–373. doi: 10.1007/978-3-0348-9088-5_24. [DOI] [PubMed] [Google Scholar]
- 59.Smythies LE, Sellers M, Clements RH, et al. Human intestinal macrophages display profound inflammatory anergy despite avid phagocytic and bacteriocidal activity. J Clin Invest. 2005;115:66–75. doi: 10.1172/JCI19229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Saada JI, Pinchuk IV, Barrera CA, et al. Subepithelial myofibroblasts are novel nonprofessional APCs in the human colonic mucosa. J Immunol. 2006;177:5968–5979. doi: 10.4049/jimmunol.177.9.5968. [DOI] [PubMed] [Google Scholar]
- 61.Hall JA, Bouladoux N, Sun CM, et al. Commensal DNA limits regulatory T cell conversion and is a natural adjuvant of intestinal immune responses. Immunity. 2008;29:637–649. doi: 10.1016/j.immuni.2008.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hart AL, Lammers K, Brigidi P, et al. Modulation of human dendritic cell phenotype and function by probiotic bacteria. Gut. 2004;53:1602–1609. doi: 10.1136/gut.2003.037325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kim CH. The greater chemotactic network for lymphocyte trafficking: chemokines and beyond. Curr Opin Hematol. 2005;12:298–304. doi: 10.1097/01.moh.0000166496.18773.e3. [DOI] [PubMed] [Google Scholar]
- 64.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 65.Zaph C, Troy AE, Taylor BC, et al. Epithelial-cell-intrinsic IKK-beta expression regulates intestinal immune homeostasis. Nature. 2007;446:552–556. doi: 10.1038/nature05590. [DOI] [PubMed] [Google Scholar]
- 66.Jarry A, Bossard C, Bou-Hanna C, et al. Mucosal IL-10 and TGF-beta play crucial roles in preventing LPS-driven, IFN-gamma-mediated epithelial damage in human colon explants. J Clin Invest. 2008;118:1132–1142. doi: 10.1172/JCI32140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rimoldi M, Chieppa M, Salucci V, et al. Intestinal immune homeostasis is regulated by the crosstalk between epithelial cells and dendritic cells. Nat Immunol. 2005;6:507–514. doi: 10.1038/ni1192. [DOI] [PubMed] [Google Scholar]
- 68.Smith PD, Ochsenbauer-Jambor C, Smythies LE. Intestinal macrophages: unique effector cells of the innate immune system. Immunol Rev. 2005;206:149–159. doi: 10.1111/j.0105-2896.2005.00288.x. [DOI] [PubMed] [Google Scholar]
- 69.Moreno SE, Alves-Filho JC, Alfaya TM, da Silva JS, Ferreira SH, Liew FY. IL-12, but not IL-18, is critical to neutrophil activation and resistance to polymicrobial sepsis induced by cecal ligation and puncture. J Immunol. 2006;177:3218–3224. doi: 10.4049/jimmunol.177.5.3218. [DOI] [PubMed] [Google Scholar]
- 70.Coffman RL, Seymour BW, Lebman DA, et al. The role of helper T cell products in mouse B cell differentiation and isotype regulation. Immunol Rev. 1988;102:5–28. doi: 10.1111/j.1600-065x.1988.tb00739.x. [DOI] [PubMed] [Google Scholar]
- 71.Smith KM, Brewer JM, Rush CM, Riley J, Garside P. In vivo generated Th1 cells can migrate to B cell follicles to support B cell responses. J Immunol. 2004;173:1640–1646. doi: 10.4049/jimmunol.173.3.1640. [DOI] [PubMed] [Google Scholar]
- 72.Litinskiy MB, Nardelli B, Hilbert DM, et al. DCs induce CD40-independent immunoglobulin class switching through BLyS and APRIL. Nat Immunol. 2002;3:822–829. doi: 10.1038/ni829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Newberry AM, Williams DN, Stauffer WM, Boulware DR, Hendel-Paterson BR, Walker PF. Strongyloides hyperinfection presenting as acute respiratory failure and gram-negative sepsis. Chest. 2005;128:3681–3684. doi: 10.1378/chest.128.5.3681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. I. The role of humoral antibodies. J Exp Med. 1969;129:1307–1326. doi: 10.1084/jem.129.6.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Goldschneider I, Gotschlich EC, Artenstein MS. Human immunity to the meningococcus. II. Development of natural immunity. J Exp Med. 1969;129:1327–1348. doi: 10.1084/jem.129.6.1327. [DOI] [PMC free article] [PubMed] [Google Scholar]