Planar cell polarity (PCP) is a property of epithelial tissues where cellular structures coordinately orient along a two-dimensional plane lying orthogonal to the axis of apical-basal polarity[1]. PCP is particularly striking in tissues where multiciliate cells generate a directed fluid flow, as seen, for example, in the ciliated epithelia lining the respiratory airways or the ventricles of the brain. To produce directed flow, ciliated cells orient along a common planar axis, in a direction set by tissue patterning, but how this is achieved in any ciliated epithelium is unknown[2]. Here we show that the planar orientation of Xenopus multiciliate cells is disrupted when components in the PCP signaling pathway are altered non-cell autonomously. We also show that wild type ciliated cells, located at a mutant clone border, reorient toward cells with low Vangl2 or high Frizzled activity and away from those with high Vangl2 activity. These results indicate that the PCP pathway provides directional non-cell autonomous cues to orient ciliated cells as they differentiate, thus playing a critical role in establishing directed ciliary flow.
PCP has been extensively studied in Drosophila where it is evident in the ordered projection of hairs on the wing and abdomen, or in the orientation of ommatidia in the eye[3]. The genes required to orient these structures include those encoding the core components of the PCP signaling pathway, namely three intracellular proteins, Prickle, Dishevelled and Diego, and three transmembrane proteins, Flamingo, Frizzled and Van Gogh (also known as Strabismus) (reviewed in [4-6]). In genetic mosaics, two of these genes, Frizzled and Van Gogh, produce profound non-cell autonomous phenotypes in which the orientation of wild type cells adjacent to a mutant clone are redirected inwards or outward [6-8]. In addition, Frizzled and Van Gogh dynamically accumulate during PCP signaling at opposite sides of a polarized cell (reviewed in [4]). These and other observations suggest that Frizzled and Van Gogh, in combination with Flamingo, act as directional cues to align cells along a planar axis based on local cell-to-cell comparisons [9-11]. PCP signaling is also conserved in vertebrates where several PCP homologs localize asymmetrically within polarized cells such as cochlear hair cells, and disrupt PCP in several tissues when mutant (reviewed in [5, 12]).
The PCP pathway has been studied thus far in multiciliate cells, by targeting the cytoplasmic component, Dishevelled, or two downstream effectors of PCP called inturned and fuzzy in Xenopus skin cells using morpholinos [13, 14]. When all three Xenopus Dishevelled homologs are targeted by morpholinos (Dvl1-3), basal bodies (BBs) fail to dock at the apical surface and cilia are lost, a phenotype also observed in morphants of inturned and fuzzy. While this phenotype is not a defect in PCP per se, it does suggest that PCP components are required in a targeting mechanism that localizes and docks BBs at the apical membrane where cilia outgrowth occurs. However, Dishevelled function can also be disrupted in embryos by expressing a well-characterized, dominant-negative mutant of Dvl2, called Xdd1[14]. In these embryos, cilia now form and beat, but fail to polarize along a planar axis, suggesting that Dishevelled also functions downstream of BB docking in a mechanism that establishes their rotational orientation. Since the Dishevelled proteins have functions outside of the PCP pathway[15, 16], it remains unclear whether cell-cell interactions involving the PCP pathway is required to align ciliated cells along a planar axis.
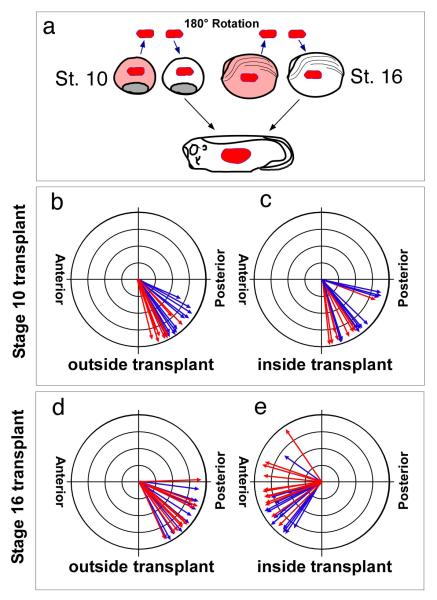
To address the role of cell-cell interactions in orienting ciliated cells, we exploited how these cells arise and are patterned during Xenopus skin development[17]. Classic grafting experiments in other amphibian species have shown that the direction of ciliary flow along the anterior to posterior axis (A-P) is set by a patterning event that occurs soon after gastrulation and prior to ciliated cell differentiation [18, 19]. At this stage, the developing skin in Xenopus embryos is not one epithelial layer as found in other amphibians, but is two layered, and the ciliated cells arise as precursors in the inner layer before intercalating into the outer epithelial layer. Thus, if the global axis of planar polarity is also fixed this early in the Xenopus skin, then ciliated cell precursors presumably acquire an orientation when they intercalate, based on cues established earlier in the outer epithelium. To confirm when the A-P polar axis is set in the skin of Xenopus laevis, we rotated a small patch of developing skin before and after gastrulation, allowed the embryos to develop, and scored the subsequent orientation of ciliated cells in the graft relative to the host (Fig. 1A). Cilia orientation was examined in these grafts functionally by flow measurements (Supplementary Movies 1 and 2) and by measuring the rotational orientation of basal bodies using a confocal assay [14]. The confocal assay measures basal body orientation by using two fusion proteins to label basal bodies with RFP and the rootlets with GFP (see Material and Methods and Fig. 2). The results show that the planar orientation of the skin is set in Xenopus soon after gastrulation (Fig. 1) and prior to ciliated cell differentiation, implying that the, ciliated cells only acquire their planar orientation when they later join the epithelium during intercalation.
Figure 1. Timing of planar axis determination.
(a) Diagram of a grafting experiment where both layers of the developing skin were isolated at the indicated stage, rotated 180° and transplanted homotopically onto host embryos. At stage 28, the orientation of ciliated cells both inside and outside the graft was determined by confocal microscopy (see Materials and Methods, Fig. 2). (b-e) Ciliated cell orientation following a stage 10 (b,c), or stage 16 (d,e) skin transplant, scored outside the transplant (b,d) and inside the transplant (c.e). Each arrow represents the mean orientation of BBs within a cell, while arrow length represents the compliment of circular variance around that mean. Colors represent data from separate embryos. Ciliated cells normally orient posteriorly with a ventral bias.
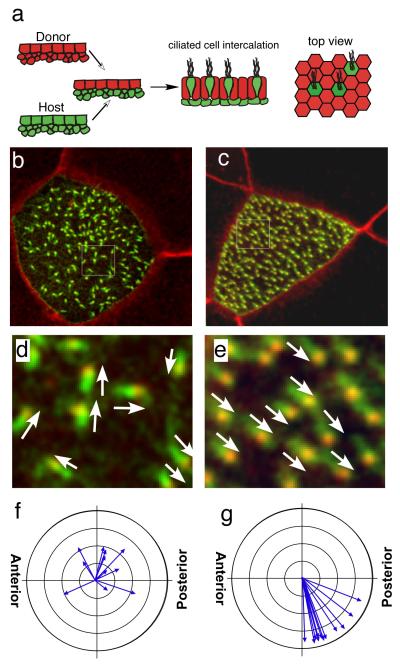
Figure 2. Xdd1 functions cell autonomously to disrupt cilia orientation.
(a) Diagram of the assay where outer cells are grafted from a donor onto a host embryo prior to gastrulation. (b,c) Confocal image of a Xdd1 expressing ciliated cell surrounded by a transplant of wild type outer cells (b), or of a wildtype ciliated cells surrounded by a transplant of Xdd1 expressing outer cells (c). Ciliated cells express a Centrin-RFP (red) fusion protein that labels BBs, and a CLAMP-GFP (green) fusion protein that labels the rootlet. (d,e) Area indicated in panels b and c are magnified 5X in d and e, respectively. Arrows indicate the direction of cilium orientation based on centrin and CLAMP staining (f,g) Circular graphs depicting mean cilia orientation of cells from a transplant of wild type outer cells onto Xdd1 injected ciliated cells (d) or Xdd1 injected outer cells transplanted onto wild type ciliated cells (e). Each arrow represents the mean direction of BBs within a cell, while the length represents one minus the circular variance around the mean. Cells with short arrows, therefore, vary more in BB orientation than those with long arrows. In wild type embryos, ciliated cells beat in a posterior direction with a ventral bias.
We next asked whether components of the PCP pathway are required for this secondary patterning event, by selectively disrupting the PCP pathway in ciliated cells or in the epithelia into which they intercalate, by separating the two layers apart before gastrulation and transplanting the layers between donor and host embryos (Fig. 2a). This assay was first used to determine whether the Dishevelled mutant, Xdd1, disrupts the rotational axis of BBs by acting solely in ciliated cells, or whether it also disrupts the ability of outer cells to orient ciliated cells non-cell autonomously[14]. When Xdd1-expressing ciliated cells intercalate into wild type outer cells, the polar orientation of the cilia is severely disrupted, as predicted for a cell autonomous phenotype (Fig. 2b,d). This disorientation was evident at two levels: BB orientation was severely disorganized within cells (Fig. 2b,d and short arrows in Fig. 2f) and the mean cilia orientation of ciliated cells failed to converge along the A-P axis (mean direction of arrows in Fig. 2f). By contrast, when wild-type ciliated cells intercalated into outer epithelium expressing Xdd1, their BBs orient normally within cells (Fig. 2c,e and long arrows in Fig. 2g) and ciliated cells were polarized in a posterior direction with a ventral bias as normal (mean direction of arrows in Fig. 2g compared to Fig. 1b,d). Thus, Xddl disrupts BB orientation cell autonomously, but cannot disrupt ciliated cell polar orientation in a non-cell autonomous fashion.
We next asked whether the transmembrane components of the PCP pathway are required to orient ciliated cells, initially by targeting a Xenopus homolog of Van Gogh, called Vangl2, using a morpholino designed to block the translation of Vangl2 RNA (Vangl2MO). The ability of the Vangl2MO to disrupt Vangl2 function was first tested in the mesoderm where PCP signaling in general, and Vangl2 in particular is required for the polarized cell movements that underlie axial elongation [20]. As predicted, injecting Vangl2MO but not a control MO into the marginal zone of two-cell embryos produced strong defects in axial elongation (Supplementary Fig. 1). We then asked whether disrupting Vangl2 function in the skin by injecting the Vangl2MO into animal pole of two-cell embryos causes defects in ciliogenesis, as reported previously for a knock down of Dvl1-3, Inturned or Fuzzy [13, 14]. Indeed, in Vangl2MO morphants, BBs failed to dock at the apical surface and cilia were dramatically reduced in number, and this phenotype was substantially rescued by co-injecting a syntheticVangl2 mRNA lacking sequences targeted by the morpholino (Supplementary Fig. 2). Although fewer in number, the extant cilia in Vangl2 morphants have a similar beat frequency to that of wild type cilia but are disorganized in orientation (Supplementary Fig. 3, Supplementary Movies 3 and 4). Thus, these results are consistent with a role for the PCP pathway and specifically Vangl2 in basal body apical localization and ciliogenesis but not cilia motility[13, 14].
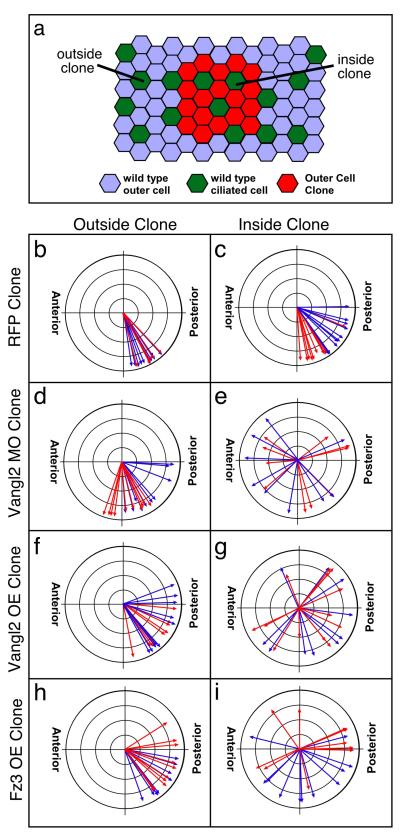
Since the deleterious effects of Vangl2 on ciliogenesis is likely to be cell autonomous as shown above for Xdd1, we next asked whether a loss of Vangl2 function in outer cells resulted in non-cell autonomous effects on the polarity of wildtype ciliated cells (Fig. 3a). As a control, ciliated cell orientation was found to be normal when a wild type outer layer (mRFP) was transplanted onto a wild type embryo (Fig. 3 compare panels b and c). By contrast, when outer layer cells were transplanted from Vangl2MO-injected donors onto wild-type hosts, the orientation of the intercalated wild-type ciliated cells within the clone was severely disorganized compared with cells outside the clone (Fig. 3, compare panels e and d). Significantly, the non-cell autonomous disruption of cilia orientation obtained in this experiment was distinct from that obtained cell autonomously with Xdd1 above. Specifically, the Vangl2 mutant outer cells did not disrupt the orientation of BBs within ciliated cells or the coordinated beating of cilia (longer arrows in Fig. 3e, Compare Supplementary Movie 2 to Movie 3), but rather the orientation of ciliated cells along the A-P axis. Indeed, ciliated cell orientation varied significantly more on average around a mean direction within a Vangl2MO injected clone compared to a control (p=0.000471). These results suggest that ciliated cells acquire their orientation via cell-cell interactions with the outer layer as they intercalate and suggest that the orientation cue requires Vangl2.
Figure 3. Ciliated cell orientation is disrupted non-cell autonomously by changes in Vangl2 and Fz3 activity.
(a) Diagram of an assay where outer cells from donor embryos injected with Vangl2MO, Vangl2, Fz3 or RFP RNA were grafted onto wildtype host embryos. Ciliated cell orientation was measured using confocal microscopy (see Materials and Methods, Fig. 2) either outside the clone (>30 cell diameters from the clone) or inside the clone (b-g) Circular graphs of ciliated cell orientation for wild type cells outside the clones (b, d, f, h) and for wild type cells surrounded by transplanted outer cells injected with mRFP (c), Vangl2MO (e), Vangl2 OE (g) or Fz3 OE (i)
In cases where PCP signaling has been shown to act, over-expression of components of the PCP pathway in gain-of-function experiments often cause similar polarity defects as those observed in loss-of-function experiments. Thus, to further assess the role of the PCP pathway in outer cells, we transplanted outer cells over-expressing Vangl2 RNA (Vangl2OE) onto wild type hosts. Ciliated cell orientation within these Vangl2OE clones was also disrupted (Fig.3, compare panels f and g), varying significantly more around a mean direction compared to controls (p=0.0075). Thus, both the loss and gain-of function experiments with Vangl2 support the idea that outer cells provide cues to orient ciliated cells, and that the proper levels of Vangl2 are required to generate this cue.
In Drosophila, changing the activity of Frizzled in mutant clones also has profound non-cell autonomous effects on neighboring wild type cells[1]. However vertebrates have a large number of Frizzled homologs, in contrast to Vangl2, and it is not clear which and how many of these might be required for PCP signaling in the skin [5, 12]. We therefore focused on the over-expression of Frizzled-3 (Fz3OE), since mice mutant for Fz3 when combined with those in Fz6 have defects in C-E and polar orientation defects in hair cells [21]. When wild type ciliated cells intercalate into outer cells that over-express Fz3, their orientation is also disrupted, showing more variation around a mean direction compared to control (p=0.00021) (Fig. 3, compare panel i and h). Thus, these results suggest that changes in the levels of PCP signaling alter ciliated cell orientation, non-cell autonomously.
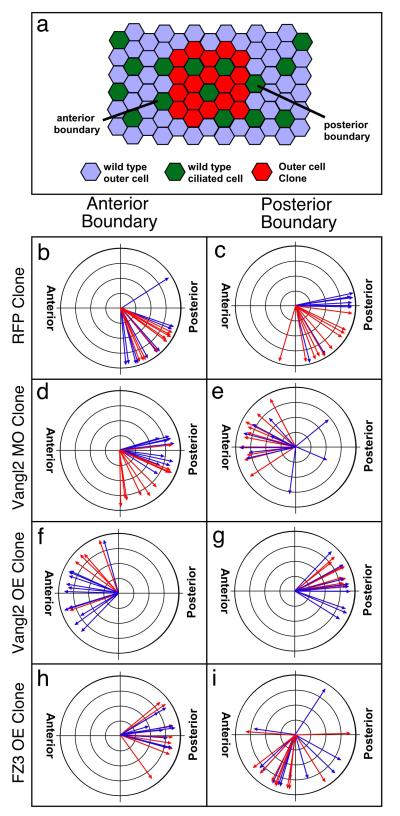
One interpretation of the results above is that outer cells require the proper levels of Vangl2 and Frizzled activity, perhaps indirectly, to generate an orientation cue for ciliated cells. Alternatively, Vangl2 and Frizzled may be acting as they do in PCP signaling in Drosophila, by instructively polarizing neighboring ciliated cells. The key observations that distinguish between these two possibilities in Drosophila are the different directional non-autonomous phenotypes that occur at clone boundaries mutant for Frizzled and Van Gogh[7, 8]. Thus, to determine whether Vangl2 is also acting instructively in outer cells to orient ciliated cells, we analyzed the orientation of ciliated cells lying at the anterior and posterior boundary of an outer cell transplant (Fig. 4a, Supplementary Fig. 4).
Figure 4. Vangl2 and Fz3 have directional, non-cell autonomous effects on ciliated cell orientation.
(a) Diagram of an assay where wildtype ciliated cells are located at the anterior and posterior border of an outer cell clone from donor embryos injected with Vangl2MO, Vangl2, Fz3 or RFP RNA. Ciliated cell orientation at the clone border was measured using confocal microscopy (Supplementary Fig. 4) (b-g) Circular graphs of ciliated cell orientation for cells located at the anterior border (b, d, f, h) or for those located at the posterior border (c, e, g, i) under the indicated experimental conditions. Different colors represent data from different experiments.
Ciliated cells located at the anterior and posterior borders of a clone of wild type cells (mRFP) orient their cilia in the normal posterior direction with an approximately 45° ventral bias (Fig. 4b-c, Supplementary Fig. 5a,b). Ciliated cells located at the anterior border of Vangl2MO clones are still oriented posteriorly but with a less pronounced ventral bias (Fig. 4d, Supplementary Fig. 5c). In stark contrast, ciliated cells at posterior border of Vangl2MO clones are significantly reversed on average 124° (p=7.57E-5) relative to control cells, and are 166° reversed relative to the cells at the anterior border (Fig. 4e, Supplementary Fig. 5d). We see a reciprocal effect on the orientation of ciliated cells at the border of Vangl2OE clones (Fig. 4f,g). Ciliated cells at the anterior border of Vangl2OE clones (Fig. 4f, Supplementary Fig. 5e) are reversed 154° relative to controls (p=1.81E-18) while those at the posterior border (Fig. 4g, Supplementary Fig. 5f) have lost their ventral bias, and thus shifted 48° relative to the control. In Drosophila, Van Gogh and Frizzled have reciprocal effects on orienting cells at clone boundaries[6-8]. While less striking than the Vangl2 results, the ciliated cells located at the anterior border of Fz3OE clones (Fig. 4h) are shifted 37° relative to controls (p=9.7E-7) but are still oriented in a posterior direction. Ciliated cells at the posterior borders, however, are reversed 71° relative to the controls in an anterior direction (p=6.03E-7) and are on average shifted 108° relative to the cells at the anterior border. These results provide strong evidence that Vangl2 and Frizzled levels in outer cells instructively orient the planar polarity of ciliated cells, but in opposite directions: ciliated cells orient the beating of their cilia towards outer cells with lower levels of Vangl2 activity and to those with higher levels of Frizzled.
These data support a model where ciliated cells are patterned along the A-P axis of the developing skin based on cues they encounter when they intercalate into the outer epithelium. Moreover, our data suggest that PCP signaling acts as a major cue in this patterning event whereby Vangl2 and Fz3 (or related Frizzled(s)) instruct the polar orientation of intercalating cells in a reciprocal manner. The instructive signaling cues we observe in our transplant assay, therefore, provide strong functional evidence that ciliated cells are oriented by local asymmetry in PCP activity and represents the first direct evidence for directional non-cell autonomy during PCP signaling in a vertebrate system. PCP signaling in Drosophila is accompanied by, and potentially attributed to, the asymmetric localization of Frizzled and Van Gogh along the planar axis to opposite sides of each cell (reviewed in [4]). Accordingly, our functional data would predict that Vangl2 and Frizzled activity is differentially restricted to the posterior and anterior sides of skin cells, respectively. Whether this differential activity involves asymmetrical protein localization as proposed in Drosophila, or another mechanism, remains to be determined.
In contrast to Vangl2 and Fz3, the Dvl2 mutant, Xdd1, did not cause a disorientation of ciliated cells when expressed in a clones of outer cells even though in the converse experiment, it disrupted the orientation of BBs in a cell-autonomous manner. This result is reminiscent of findings in Drosophila, suggesting that Dishevelled is not required to generate or propagate intercellular PCP signaling, at least over clone distances, but is only required intracellularly for cells to polarize [22, 23]. Disrupting Dishevelled activity in the outer cells using additional approaches will be required to fully address the role of Dishevelled in PCP signal propagation both intercellularly and intracellularly.
Our data also show that BBs fail to dock apically, and ciliogenesis fails in Vangl2 morphants as reported previously for Dvl1-3, inturned, and Fuzzy[13, 14]. Intriguingly, mammalian Vangl2 has been reported on vesicular structures that localize to the BB in human respiratory ciliated cells[24]. Our data, therefore, adds further support to the idea that BBs are positioned apically in ciliated cells by vesicular targeting events involving multiple components of the PCP pathway[14]. Due to the ciliogenesis defects, we cannot test whether Vangl2 also has a cell autonomous role in establishing the rotational orientation of individual BBs. Nonetheless, our observations suggest that at the same time PCP components position BBs apically, they are also used to orient ciliated cells along the planar axis via interactions with cells in the outer epithelium.
Materials and Methods
Transplant assays and explant cultures
Xenopus laevis embryos were obtained by in vitro fertilization using standard protocols [25].To mark transplanted tissue embryos were injected four times at the two- to four-cell stages with capped, synthetic mRNA [25]encoding membrane-localized form of RFP (mRFP). At stage 10, a fine needle or hair was used to peel off the outer layer from a region of the ectoderm from a donor embryo, which was transferred onto the host embryos after removing a similar patch of outer cells. While the transplanted tissue healed onto the host embryo, it was kept in place by pressing down with a small piece of a glass coverslip, held in place with silicone grease. In experimental transplants, host embryos were not only injected with mRFP but also with a Vangl2 MO (ACTGGGAATCGTTGTCCATGTTTC, Gene Tools), Control MO (CTAGCGCTGTAAGGAGCCATCCTGT), Vangl2[26] or Fz3 RNA [27]. Transplants were performed in Danilchik’s buffer + 0.1% BSA [28]. After healing of the transplanted tissue, embryos were returned to 0.1x Marc’s Modified Ringers (MMR) [25], until stage 28 when they fixed overnight on ice in 4.0% paraformaldehyde in phosphate buffered saline (PBS). After mounting, tadpoles were imaged using a BioRad Radiance 2100 confocal mounted to a Zeiss inverted microscope using a 63x objective. Grafted tissues were identified based on the RFP tracer and were analyzed when localized to the middle flank. Ciliated cells were imaged that were either within the grafted tissues, at least 30 cell diameters outside the grafted tissue, or located at the anterior or posterior border and touching both grafted and host cells.
Confocal assay for cilia orientation
To score ciliated cell orientation, cilia direction along the polar axis was determined by measuring BB orientation using confocal microscopy [14]. This assay involves expressing two fusion proteins in host embryos: one protein, called CLAMP, is fused to GFP and localizes to the striated rootlet, a structure that marks the rotational axis of the BB by projecting in the opposite direction of ciliary beating. The second protein, Centrin2, is fused to RFP and localizes to the basal body. When expressed in ciliated cells using RNA injection, the two fusion proteins decorate the BB and rootlet such that orientation can be easily scored by confocal microscopy. The orientation of approximately 100 BBs was scored per cell and used to calculate the mean orientation of cilia within a cell, as a measure of a cell’s overall planar polarity, where the mean direction of a cell is denoted as an arrow on a circular graph and the length of the arrow represents the variance around that mean [29].
Immunostaining and confocal microscopy
Ciliated cells were immunostained by fixing embryos with 4.0% paraformaldehyde in phosphate buffered saline (PBS) for one hour on ice. Tissue was stained by overnight incubation in rabbit anti-ZO-1 (Zymed 1:200) and mouse monoclonal anti-acetylated tubulin (Sigma 1:1000) or anti-γ tubulin GTU88 (Sigma 1: 500) primary antibodies, and a four to six hour incubation in anti-rabbit Cy3 and anti-mouse Cy2 secondary antibodies (Jackson Immunoresearch). Antibody incubations were performed using PBS containing 0.1% TritonX-100 and 10% heat-inactivated normal goat serum, and washed with PBS containing 0.1% TritonX-100. Basal bodies and rootlets were labeled by injecting synthetic messenger RNA encoding centrin2-RFP and Clamp-GFP fused at the carboxy terminus as previously described (Park2008). After mounting, embryos were imaged on a BioRad Radiance 2100 confocal mounted to a Zeiss inverted microscope using a 63x objective. Movies of cilia beating were taken at 6688 fps using a Vision Research Phantom 7.2 mounted to an Olympus BX51 microscope with a 100X objective.
Data Analysis
Basal Body / Rootlet orientation was scored using Matlab and statistical analysis and circular plotting was done using Oriana 2.0 (Kovach Computing Services) circular statistics software. Each arrow on the polar plot represents the orientation of a single ciliated cell based on scoring the orientation of on average 100 cilia (basal body / rootlets) per cell. Experimental values were compared to control values using a two-tailed student’s t-test.
Supplementary Material
Acknowledgements
The authors thank members of the Kintner lab for comments on the manuscript. This work was supported in part by the UCI Center for Complex Systems Biology under NIH P50GM076516, by the UCI Institute for Surface and Interface Science, by a NIH grant GM076507 to C.K, and by a Parker B Francis fellowship to B.M..
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Zallen JA. Planar polarity and tissue morphogenesis. Cell. 2007;129:1051–1063. doi: 10.1016/j.cell.2007.05.050. [DOI] [PubMed] [Google Scholar]
- 2.Marshall WF, Kintner C. Cilia orientation and the fluid mechanics of development. Curr Opin Cell Biol. 2008;20:48–52. doi: 10.1016/j.ceb.2007.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adler PN. Planar signaling and morphogenesis in Drosophila. Dev Cell. 2002;2:525–535. doi: 10.1016/s1534-5807(02)00176-4. [DOI] [PubMed] [Google Scholar]
- 4.Strutt D. The planar polarity pathway. Curr Biol. 2008;18:R898–902. doi: 10.1016/j.cub.2008.07.055. [DOI] [PubMed] [Google Scholar]
- 5.Seifert JR, Mlodzik M. Frizzled/PCP signalling: a conserved mechanism regulating cell polarity and directed motility. Nat Rev Genet. 2007;8:126–138. doi: 10.1038/nrg2042. [DOI] [PubMed] [Google Scholar]
- 6.Lawrence PA, Struhl G, Casal J. Planar cell polarity: one or two pathways? Nat Rev Genet. 2007;8:555–563. doi: 10.1038/nrg2125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Vinson CR, Adler PN. Directional non-cell autonomy and the transmission of polarity information by the frizzled gene of Drosophila. Nature. 1987;329:549–551. doi: 10.1038/329549a0. [DOI] [PubMed] [Google Scholar]
- 8.Taylor J, Abramova N, Charlton J, Adler PN. Van Gogh: a new Drosophila tissue polarity gene. Genetics. 1998;150:199–210. doi: 10.1093/genetics/150.1.199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Wu J, Mlodzik M. The frizzled extracellular domain is a ligand for Van Gogh/Stbm during nonautonomous planar cell polarity signaling. Dev Cell. 2008;15:462–469. doi: 10.1016/j.devcel.2008.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen WS, Antic D, Matis M, Logan CY, Povelones M, Anderson GA, Nusse R, Axelrod JD. Asymmetric homotypic interactions of the atypical cadherin flamingo mediate intercellular polarity signaling. Cell. 2008;133:1093–1105. doi: 10.1016/j.cell.2008.04.048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Strutt H, Strutt D. Differential stability of flamingo protein complexes underlies the establishment of planar polarity. Curr Biol. 2008;18:1555–1564. doi: 10.1016/j.cub.2008.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang Y, Nathans J. Tissue/planar cell polarity in vertebrates: new insights and new questions. Development. 2007;134:647–658. doi: 10.1242/dev.02772. [DOI] [PubMed] [Google Scholar]
- 13.Park TJ, Haigo SL, Wallingford JB. Ciliogenesis defects in embryos lacking inturned or fuzzy function are associated with failure of planar cell polarity and Hedgehog signaling. Nat Genet. 2006;38:303–311. doi: 10.1038/ng1753. [DOI] [PubMed] [Google Scholar]
- 14.Park TJ, Mitchell BJ, Abitua PB, Kintner C, Wallingford JB. Dishevelled controls apical docking and planar polarization of basal bodies in ciliated epithelial cells. Nat Genet. 2008;40:871–879. doi: 10.1038/ng.104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wallingford JB, Habas R. The developmental biology of Dishevelled: an enigmatic protein governing cell fate and cell polarity. Development. 2005;132:4421–4436. doi: 10.1242/dev.02068. [DOI] [PubMed] [Google Scholar]
- 16.Boutros M, Mlodzik M. Dishevelled: at the crossroads of divergent intracellular signaling pathways. Mech Dev. 1999;83:27–37. doi: 10.1016/s0925-4773(99)00046-5. [DOI] [PubMed] [Google Scholar]
- 17.Stubbs JL, Davidson L, Keller R, Kintner C. Radial intercalation of ciliated cells during Xenopus skin development. Development. 2006;133:2507–2515. doi: 10.1242/dev.02417. [DOI] [PubMed] [Google Scholar]
- 18.Twitty VC. Experimental Studies on the Ciliary Action of Amphibian Embryos. J Experimental Zoology. 1928;50:310–344. [Google Scholar]
- 19.Tung TC, Tung Y-FY. Experimental Studies on the determination of polarity of ciliary action in anuran embryos. Arch Biol (Liege) 1940;51:203–218. [Google Scholar]
- 20.Heisenberg CP, Tada M. Wnt signalling: a moving picture emerges from van gogh. Curr Biol. 2002;12:R126–128. doi: 10.1016/s0960-9822(02)00704-2. [DOI] [PubMed] [Google Scholar]
- 21.Wang Y, Guo N, Nathans J. The role of Frizzled3 and Frizzled6 in neural tube closure and in the planar polarity of inner-ear sensory hair cells. J Neurosci. 2006;26:2147–2156. doi: 10.1523/JNEUROSCI.4698-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strutt D, Strutt H. Differential activities of the core planar polarity proteins during Drosophila wing patterning. Dev Biol. 2007;302:181–194. doi: 10.1016/j.ydbio.2006.09.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Amonlirdviman K, Khare NA, Tree DR, Chen WS, Axelrod JD, Tomlin CJ. Mathematical modeling of planar cell polarity to understand domineering nonautonomy. Science. 2005;307:423–426. doi: 10.1126/science.1105471. [DOI] [PubMed] [Google Scholar]
- 24.Ross AJ, May-Simera H, Eichers ER, Kai M, Hill J, Jagger DJ, Leitch CC, Chapple JP, Munro PM, Fisher S, et al. Disruption of Bardet-Biedl syndrome ciliary proteins perturbs planar cell polarity in vertebrates. Nat Genet. 2005;37:1135–1140. doi: 10.1038/ng1644. [DOI] [PubMed] [Google Scholar]
- 25.Sive H, Grainger RM, Harland RM. The early development of Xenopus laevis: a laboratory manual. Cold Spring Harbor Press; Plainview, NY: 1998. [Google Scholar]
- 26.Goto T, Keller R. The planar cell polarity gene strabismus regulates convergence and extension and neural fold closure in Xenopus. Dev Biol. 2002;247:165–181. doi: 10.1006/dbio.2002.0673. [DOI] [PubMed] [Google Scholar]
- 27.Rasmussen JT, Deardorff MA, Tan C, Rao MS, Klein PS, Vetter ML. Regulation of eye development by frizzled signaling in Xenopus. Proc Natl Acad Sci U S A. 2001;98:3861–3866. doi: 10.1073/pnas.071586298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Davidson LA, Hoffstrom BG, Keller R, DeSimone DW. Mesendoderm extension and mantle closure in Xenopus laevis gastrulation: combined roles for integrin alpha(5)beta(1), fibronectin, and tissue geometry. Dev Biol. 2002;242:109–129. doi: 10.1006/dbio.2002.0537. [DOI] [PubMed] [Google Scholar]
- 29.Mitchell B, Jacobs R, Li J, Chien S, Kintner C. A positive feedback mechanism governs the polarity and motion of motile cilia. Nature. 2007;447:97–101. doi: 10.1038/nature05771. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.