Abstract
Protective immunity against avian influenza (AI) virus was elicited in chickens by single-dose vaccination with a replication competent adenovirus (RCA) -free human adenovirus (Ad) vector encoding an H7 AI hemagglutinin (AdChNY94.H7). Chickens vaccinated in ovo with an Ad vector encoding an AI H5 (AdTW68.H5) previously described, which were subsequently vaccinated intramuscularly with AdChNY94.H7 post-hatch, responded with robust antibody titers against both the H5 and H7 AI proteins. Antibody responses to Ad vector in ovo vaccination follow a dose-response kinetic. The use of a synthetic AI H5 gene codon optimized to match the chicken cell tRNA pool was more potent than the cognate H5 gene. The use of Ad-vectored vaccines to increase resistance of chicken populations against multiple AI strains could reduce the risk of an avian-originating influenza pandemic in humans.
1. Introduction
Highly pathogenic (HP) avian influenza (AI) viruses belonging to the H5 or H7 types continue to threaten the world poultry industry and are zoonotic agents with pandemic potential for humans [19]. In December 2005 the World Organization for Animal Health and the Food and Agriculture Organization of the United Nations recommended that vaccination of poultry be considered for the control of AI viruses [21]. We reported previously that protective immunity against AI can be elicited in chickens in a single-dose regimen by in ovo vaccination with a replication competent adenovirus (RCA)-free human adenovirus serotype 5 (Ad5) vector encoding an avian H5 hemagglutinin (HA). Vaccinated chickens were protected against both H5N1 and H5N2 HPAI virus challenges [18]. RCA-free Ad5 vectored AI vaccines can be produced in large-scale in the well-characterized PER.C6 cell line in serum-free suspension bioreactors [6] in conjunction with chromatography-mediated purification [4]. Safe and cost effective vaccine delivery to large chicken populations is feasible via routinely used automated in ovo injectors [11, 20] in response to an emerging AI pandemic. This Ad5-vectored AI vaccine is in compliance with a DIVA (differentiation between infected and vaccinated animals) strategy because the vector only encodes the viral HA. Thus, the routinely used serologic tests detecting antibodies against conserved AI viral proteins allows easy discrimination between chickens exposed to field AI viruses and chickens subjected to vaccination only.
Multiple experimental recombinant vaccines have been developed in recent years, some of which, have been reported to efficiently protect chickens against HPAI challenge [1, 5, 9, 16]. However, live recombinant vectors have the risk of generating revertants and allow spread of genetically modified organisms in both target and non-target species in the environment. Another aspect to be considered is the possible presence of immunity against a particular vector in the chicken population because maternally derived antibodies reduce [8] and active immunization precludes [15] the development of an efficacious immune response against the antigen expressed by the transgene in this specie. Thus, the use of vectors such as Newcastle disease virus [1] that are endemic in the poultry industry, require prove that they will elicit the desired immune response in chickens immune against Newcastle disease (vaccines against Newcastle disease are widely and repeatedly used in the poultry industry worldwide). In contrast to live virus vaccines that may generate undesirable new reassortants with concurrently circulating wild influenza viruses [2], it is not possible for the DNA genome of Ad5 to undergo reassortment with the segmented RNA genome of an influenza virus. The Ad5 vector system overcomes these issues, and the RCA-free Ad5 vector will not propagate even in human cells in the absence of expression of the Ad E1 gene.
In the present study, we developed an RCA-free human Ad5 vector encoding a North American lineage avian H7 HA (AdChNY94.H7) and demonstrate that immunized chickens are protected against H7 HPAI challenge. We also show that chickens vaccinated in ovo with AdTW68.H5 and subsequently vaccinated with AdChNY94.H7 after hatch develop antibodies against both the H5 and H7 HA proteins. Antibody responses to Ad vector vaccination follow a dose-response kinetic. Moreover, we demonstrate that the use of a synthetic AI H5 gene with codons optimized to match the chicken tRNA pool is more immunogenic than its counterpart without codon optimization.
2. Materials and Methods
2.1. Construction of the AdChNY94.H7 vector
The Ad5 recombinant vaccine with the H7 transgene was developed as previously described [18]. In brief, a fragment containing the full-length H7 gene of AI strain A/Chicken/New York/13142-5/94 was inserted into the HindIII-BamHI site of the shuttle plasmid pAdApt (Crucell Holland BV; Leiden, The Netherlands) to generate the plasmid pAdApt-NY94.H7. An RCA-free E1/E3-defective Ad5 vector encoding this H7 HA gene was subsequently constructed in human PER.C6 cells (Crucell) by cotransfection of pAdApt-NY94.H7 with the Ad5 backbone plasmid pJM17, followed by multiple cycles of plaque purification. The AdChNY94.H7 vector was validated by DNA sequencing. Titer [infectious units/ml (ifu)] was determined by the Adeno-X rapid titer kit (BD Clontech; Mountain View, CA, U.S.A.).
2.2. Experimental design
The generated RCA-free Ad5 vector encoding the avian H7 HA (AdChNY94.H7) described above and the RCA-free vector encoding the H5 HA (AdTW68.H5) of AI strain A/Turkey/Wisconsin/68 (H5N9) previously described [18] were administered either to specific-pathogen-free (SPF) chickens or to 18-day SPF embryonated eggs to evaluate antibody responses and protection against challenge as described below. Hemagglutination inhibition (HI) antibody titers in serum samples were determined as described [17] against 4 hemagglutinating units of the low pathogenic A/Turkey/Oregon/71 (H7N3) or the A/Turkey/Wisconsin/68 (H5N9) strains. Control groups included 10 chickens vaccinated with an Ad5 vector (AdCMV-tetC) encoding an irrelevant antigen (tetanus toxin C-fragment) [12] and 10 chickens which were not exposed to Ad5 vectors. Birds were reared and handled according to Institutional Animal Care and Use Committee’s guidelines at Auburn University as well as at USDA Southeast Poultry Research Laboratory.
2.3. Trial 1
AdChNY94.H7 was delivered in ovo to 8 SPF embryos at a dose of 1.5 × 1011 ifu in a volume of 300 µl on day 18 of embryonation. Individual serum samples were obtained from all chickens on days 28 and 45 after hatch and tested for H7 HI antibodies against the A/Turkey/Oregon/71 (H7N3) AI strain.
2.4. Trial 2
We subsequently expanded the experiment to evaluate the intramuscular (IM) route for vaccine delivery and demonstrate protection against lethal challenge with a HPAI virus strain. For this purpose, 11 chickens were vaccinated IM at day 28 of age with 1.1 × 1011 ifu contained in 300 µl. Serum samples were obtained from all birds individually on days 20 and 35 post vaccination and assessed for HI antibody titers against the A/Turkey/Oregon/71 strain. Challenge was performed in a biosafety level 3+ facility by choanal inoculation of 105.5 embryo infective doses (EID50) of the H7N3 HPAI virus strain A/Chicken/Chile/4957/02 (H7N3) [14]. The HA of this challenge strain has 92.3 % deduced amino acid sequence similarity with the HA of the A/Chicken/New York/13142-5/94 strain expressed from the Ad5 vector (GenBank accessions AY303632 and AF072384). A total of 11 chickens, as well as 11 unvaccinated controls, were challenged on day 70 of age. Challenged birds were observed daily for mortality throughout an experimental period of 14 days. Oropharyngeal swabs from individual birds were obtained for quantitation of AI genomes by real time RT-PCR at days 2 and 5 after challenge, suspended in 1 ml of brain heart infusion medium (Difco; Kansas City, MO), and stored at −70°C. RNA was extracted by using the MagMax RNA extraction kit (Ambion, Austin, TX, U.S.A.) using a Kingfisher 24 extraction robot. Real-time RT-PCR was performed with primers specific for type A influenza virus matrix RNA as described [13]. Copy number of viral RNA was interpolated from the cycle thresholds by using standard curves generated from known amounts of control A/Chicken/Chile/4957/02 RNA (101.0 to 106.0 EID50/ml).
2.5. Trial 3
To determine dose response and persistence of specific antibodies in in ovo vaccinated chickens we performed two experiments. In the 1st experiment we used 105, 107 and 109 ifu of AdTW68.H5 per 18-day-old embryonating chicken egg with 14 eggs per dilution. Serum samples were obtained from all chickens at 10 day intervals from day 10 through 40 of age and tested for H5 HI antibodies against the A/Turkey/Wisconsin/68 (H5N9) strain. The percentage of chickens with detectable antibodies at each day was also examined. In a 2nd experiment the A/Turkey/Wisconsin/68 HA gene sequence (http://www.ncbi.nlm.nih.gov/entrez/viewer.fcgi?db=nucleotide&val=1840077) was optimized to the chicken codons [7, 10] and synthesized at GENEART (http://www.geneart.com/gene_technologies/faq.php). The synthetic HA gene was inserted into the multiple cloning sites of the expression plasmid in the correct orientation under transcriptional control of the CMV promoter. An Adderived Ad vector encoding the codon-optimized HA gene (AdTW68.H5ck) was constructed in human PER.C6 cells as described [18]. Three groups of 20 chickens were inoculated each with 4×105, 4×106, or 4×107 ifu of AdTW68.H5ck (200 µl per egg) at day 18 of embryonation. Serum samples were obtained at day 26 of age and tested for HI antibodies against the A/Turkey/Wisconsin/68 (H5N9) strain.
2.6. Trial 4
The codon optimized AdTW68.H5ch was delivered in ovo to 27 SPF embryos at a dose of 4 × 107 ifu in a volume of 300 µl (highest dose used in trial 3) on day 18 of embryonation. Serum samples were obtained at day 27 after hatch for HI determination of individual H5 antibody titers from all chickens. The same chickens vaccinated in ovo with AdTW68.H5ch were subsequently vaccinated IM on day 27 with 5.7 × 1010 ifu/300µl of the new AdChNY94.H7 construct (described above). Serum samples from all chickens were obtained at day 52 of age for H5 and H7 antibody testing against the low pathogenic A/Turkey/Oregon/71 (H7N3) or the A/Turkey/Wisconsin/68 (H5N9) strains. Eleven chickens were maintained until day 68 of age and tested only for H7 antibodies. Differences between antibody titers at different days after vaccination were analyzed by Mann Whitney test.
3. Results
3.1. Trial 1
H7 antibody levels in chickens vaccinated in ovo with AdChNY94.H7 achieved a median of 3 log2 on day 25 and 5 log2 on day 45 after hatch. A significant increase of the mean HI antibody titers was detected between day 25 and 45 (P=0.041). The standard error of the mean was 0.7319 and 0.9590 for days 25 and 45 respectively. Two vaccinated birds maintained an antibody negative status throughout the experimental period.
3.2. Trial 2
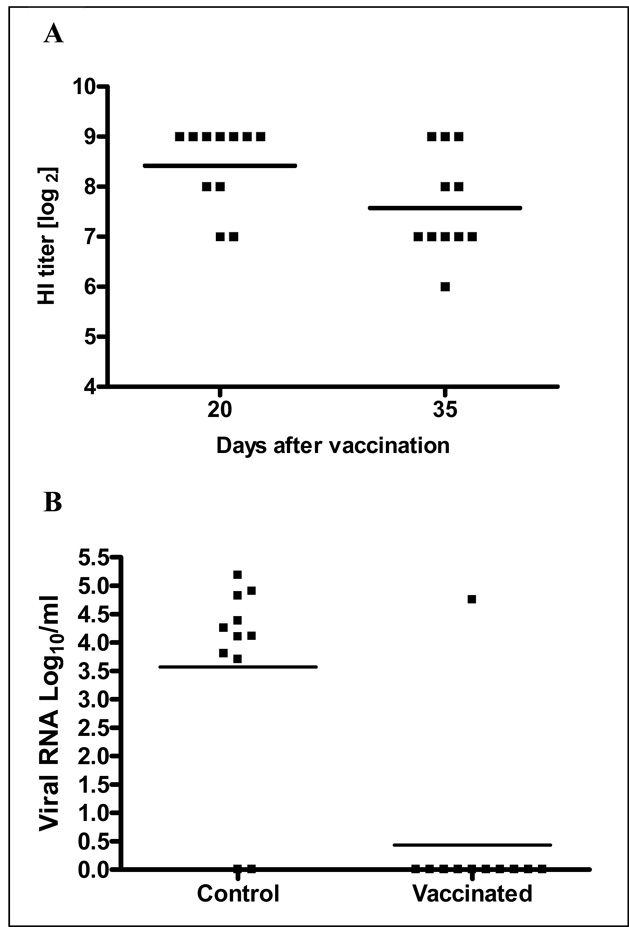
H7 antibody titers detected in chickens vaccinated IM with AdChNY94.H7 (Fig. 1A) were higher than the values obtained after in ovo delivery of a lower dose of the same vaccine in the previous trial (trial 1). Antibody titers in chickens immunized IM showed mean HI antibody titers of 8.5 log2 on day 25 and 7.6 log2 on day 35 after vaccination with no significant differences (P=0.05) between these days. Viral genomes of AI strain A/Chicken/Chile/4957/02 in challenged chickens were quantitatively determined by real-time RT-PCR in oropharyngeal swabs collected 2 and 5 days post-challenge. As seen in Fig. 1B viral RNA was detectable in 1 immunized bird at 2 days after challenge but was detected in all but two non-immunized control chickens. The survival rate of vaccinated chickens was 100% (11/11) while only 13.2% (2/11) of unvaccinated birds survived after challenge with HPAI strain A/Chicken/Chile/4957/02 (H7N3).
Figure 1.
Protection of chickens by immunization with Ad-vectored H7 AI vaccine against HPAI A/Chicken/Chile/4957/02 (H7N3) virus challenge. (A) Serum H7 antibodies induced by intramuscular immunization with an RCA-free Ad5 vector encoding H7 HA (AdChNY94.H7). Vaccination was performed on day 28 of age with 300µl of virus suspension containing with 1.1×1011 ifu. The Ad5 vector was purified by ultracentrifugation over cesium chloride gradient and resuspended in Ad buffer. HI titers were determined on days 20 and 35 after vaccination. HI titers are expressed as log2 [HI titer] in individual birds; bars represent the log2 [GMT] for each group. No HI titers were detected in any control chickens (n=11) (not shown). (B) On day 70 of age, chickens were challenged through the choanal slit with 105.5 EID50 of the HPAI A/Ch/Chile/4957/02 (H7N3) virus. A/Ch/Chile/4957/02 viral genomes in individual chickens expressed as log10 copies per ml determined by real-time RT-PCR in oropharyngeal samples collected 2 days post-challenge. A significant difference (P = 0.005) in viral load was detected between vaccinated and control birds 2 days after challenge using a paired t-test (Prism 4.03).
3.3. Trial 3
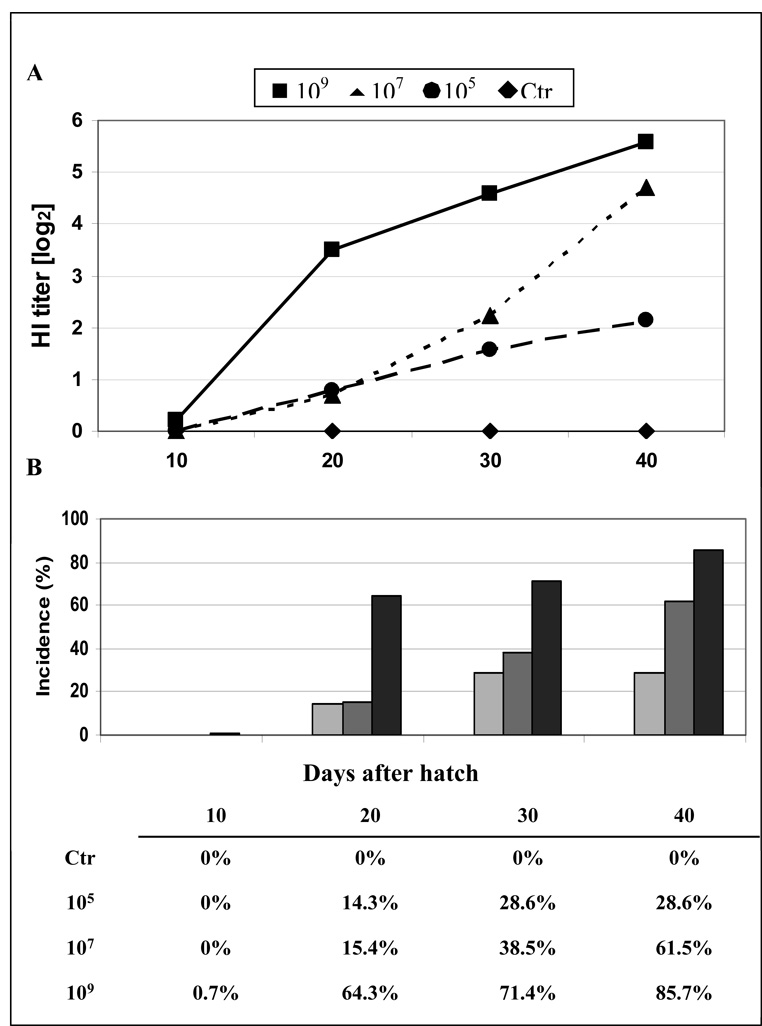
Fig. 2A shows the H5 antibody titers detected in chickens on days 10, 20, 30, and 40 of age which were vaccinated in ovo with increasing doses of AdTW68.H5. As seen in this figure, vaccine dosage varying between 105 and 109 ifu per egg induced detectable HI antibodies in the hatched chickens. However, significantly higher HI titers (P<0.05) were achieved by chickens vaccinated with the higher dose of AdTW68.H5. On day 40 no significant difference in antibody titers (P>0.05) was detected between chickens receiving 107 or 109 ifu per egg. As seen in Fig. 2B, the percentage of chickens with detectable HI antibody levels increased with dosage. While 105 ifu per egg induced detectable antibody in 29% of vaccinated chickens on day 40 after hatch, on the same day 86% of the chickens vaccinated with 109 ifu showed HI antibodies against A/Tk/WI/68 (H5N9). Codon optimization of the Ad vectored vaccine resulted in higher titers as compared with the codon non-optimized homologous counterpart. As seen in Fig. 3, in ovo administration of 107 ifu of AdTW68.H5ch induced mean HI titers of 4.3 log2 at day 26 after hatch. A higher dose (109 ifu per egg) of the non codon optimized AdTW68.H5 induced approximately the same HI titer on day 30 of age in the previous trial (see above). Similarly as with the non codon-optimized vaccine, a dose effect was observed with the highest dose (107 ifu) of AdTW68.H5ch induced significantly higher HI titers (P<0.001) in the chickens as compared with the lowest dose (105 ifu) of the vaccine.
Figure 2.
Serum H5 antibodies induced by in ovo immunization with increasing doses of RCA-free Ad5 vector encoding H5 HA (AdTW68.H5). A) Vaccination was performed on day 18 of embryonation with 300µl of virus suspension containing 105, 107, and 109 ifu of the AdTW68.H5 vector per egg. HI antibody titers were measured in chickens on days 10, 20, 30, and 40 of age. All control birds produced no measurable AI antibody titers. B) Percentage of chickens with detectable HI antibody levels resulting from in ovo immunization at 18 days of embryonation with increasing doses [105, 107, and 109 ifu per egg; n=14 per dilution] of RCA-free Ad5 vector encoding H5 HA (AdTW68.H5). HI antibody titers were measured in chickens on days 10, 20, 30, and 40 of age. All naÏve control birds and birds immunized by the control vector AdCMV-tetC produced no measurable AI antibody titers (not shown).
Figure 3.
The Ad vector encoding the codon-optimized A/Turkey/Wisconsin/68 HA gene (AdTW68.H5ck) was used for in ovo delivery. Chicken groups (n=20) were inoculated each with 4×105, 4×106, or 4×107 ifu of AdTW68.H5ck (200 µl per egg) at day 18 of embryonation. Serum H5 antibodies against the A/Turkey/Wisconsin/68 (H5N9) strain obtained at day 26 of age are shown. All control birds produced no measurable AI antibody titers (not shown).
3.4. Trial 4
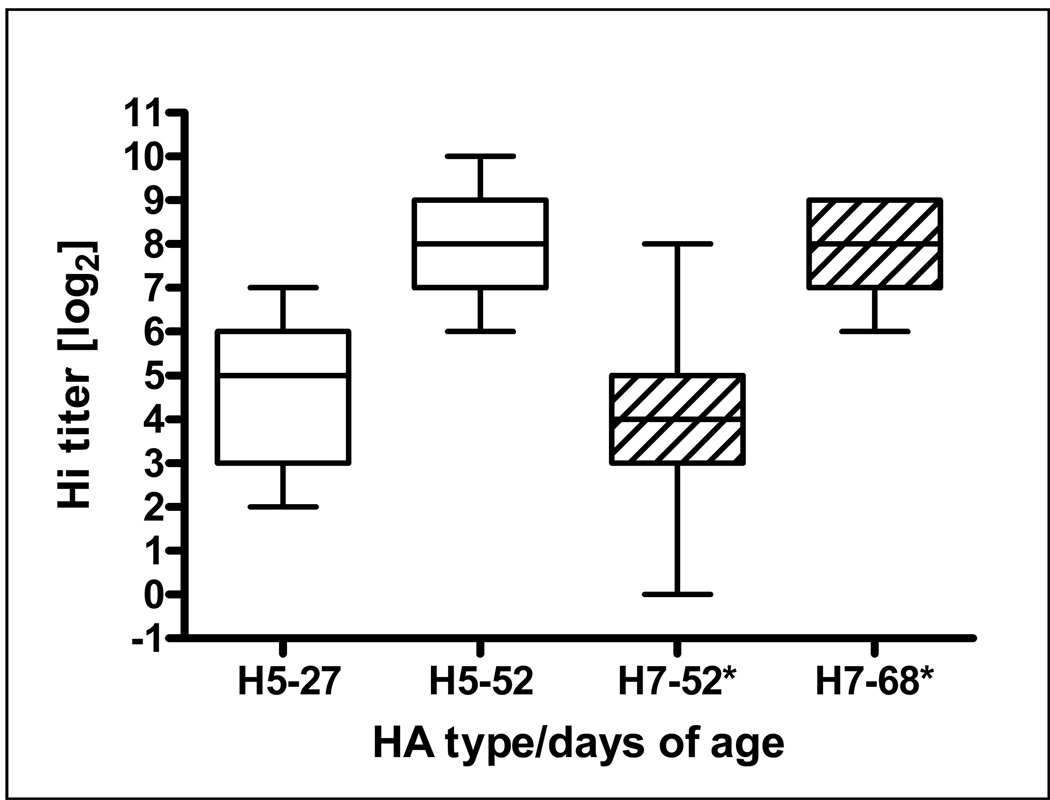
As shown in Fig. 4, in ovo immunization with AdTW68.H5ch elicited H5 antibody titers, as measured by HI against AI strain A/Turkey/Wisconsin/68 (H5N9), varying between 2 and 7 log2 with a mean of 4.6 log2 on day 27 of age. These birds continued to increase their H5 antibody levels achieving values varying between 6 and 10 log2 with a mean of 7.9 log2 (P<0.0001) on day 52 of age. On day 52 of age the same chickens had also developed H7 antibody levels, as measured against A/Turkey/Oregon/68 (H7N3), varying between 0.0 and 8 log2 with a mean of 4.0 log2 in response to the intramuscular vaccination with AdChNY94.H7 on day 27 of age. Chickens continue to increase their H7 antibody titers (P<0.0001) with time achieving a mean of 8 log2 on day 68 of age (41 days after vaccination).
Figure 4.
Serum HI antibodies (box plots: 25 – 75 percentile) against both the H5 and H7 AI proteins induced by in ovo vaccination on day 18 of embryonation with AdTW68.H5 and subsequent intramuscular vaccination with AdChNY94.H7 on day 27 after hatch. A significant increase (P<0.0001) (Mann Whitney test) of H5-specific antibody was detected between days 27 and 52 after hatch. Similarly, AdChNY94.H7 intramuscular vaccination also determined a significant increase of H7 antibody (P<0.0001) between days 52 and 68 of age. All control birds produced no measurable AI antibody titers (not shown)
4. Discussion
In ovo vaccination of chickens on day 18 of embryonation has been routinely practiced for the last two decades in commercial chickens in the U.S. [11, 20]. Thus, mass delivery of new vaccines by this route can be easily adopted by the industry. Previously, we developed an RCA-free Ad-vectored vaccine encoding the H5 HA protein of AI. We demonstrated that in ovo vaccinated chickens responded with high antibody levels against the H5 HA and that vaccinated chickens were protected against HPAI challenge [18]. A natural follow up of these studies was to develop an RCA-free recombinant Ad vector encoding the H7 gene of AI to confirm the protective capabilities of this novel type vaccine using a different and relevant HA subtype.
The results shown herein demonstrate that the newly developed AdChNY94.H7 vaccine efficiently induced a specific antibody response in chickens both vaccinated in ovo and by the intramuscular route. We also demonstrate that IM vaccinated chickens were protected against challenge with an HPAI H7N3 strain that caused severe mortality in the Chilean poultry industry in 2002 [14]. The fact that a few control chickens survived the challenge with the HPAI strain has been observed by one of us in numerous other challenge experiments with HPAI and cannot be explained in this study.
These results confirm that the RCA-free adenovirus vector efficiently expresses different HA genes in chickens vaccinated via different routes. Furthermore, the encoded proteins elicit a protective immune response in chickens.
In previous work we showed that chickens vaccinated with AdTW68.H5 with HI antibody titers of 5 log2 were protected (100%) against challenge with the HPAI A/Chicken/Queretaro/19/95 (H5N2) strain. However AI viral genomes were detected until day 7 after challenge in those birds [18]. In the current study chickens vaccinated with AdChNY94.H7 were also 100% protected against HPAI challenge but in addition reduction of viral shedding (as determined by quantitation of AI viral genomes in oropharyngeal swabs) was higher. The difference may be explained by the fact that chickens of the current work had mean HI antibody titers of ∼8.0 log2 at the time of challenge while chickens of the previous study had titers of ∼5.0 log2. Higher antibody titers are likely the result of IM vaccination in these birds.
We intentionally used the intramuscular route in the challenge trial because we were also interested in determining whether chickens immunized in ovo with an Ad-vectored vaccine can be vaccinated post-hatch with another Ad-vectored vaccine containing the same Ad backbone. This knowledge is relevant as it has been shown for other recombinant vaccines that pre-existing immunity to the vector in the host reduces or precludes the establishment of protective immune responses against exogenous antigens expressed by the same vector upon vaccination [8, 15]. Thus, in ovo vaccination with AdTW68.H5ch, which elicits an immune response against both the Ad vector and the H5 HA, could impair an efficient response against the H7 HA after subsequent vaccination with the same Ad vector encoding a different HA (AdChNY94.H7) after hatch. The present results showed that dually vaccinated chickens (in ovo with AdTW68.H5ch followed by intramuscular injection of AdChNY94.H7 on day 27 after hatch) responded with robust HI titers against both the H5 and H7 encoded proteins. Notably, the anti-H7 antibody titers achieved by chickens vaccinated with AdChNY94.H7 only (trial 2) were in the same range as those obtained by chickens vaccinated dually with AdTW68.H5ch and AdChNY94.H7 (trial 3). A possible explanation for this result may be that the immune response against the non-replicating adenovirus is rather limited in contrast to strong immune responses resulting from replicating viral vectors such as fowl pox virus [15].
From an applied perspective, this result is relevant as it demonstrates the feasibility of revaccinating chicken populations against a different AI hemagglutinin with the same Ad-vector technology. For example, chicken populations subjected to a regional in ovo vaccination program with the Ad-vector technology against H5-type AI strains could be emergency revaccinated intramuscularly against H7 using the same technology in case of an unexpected outbreak of an H7 strain in that region.
The results on antibody response following vaccination with different doses of the vaccine construct were expected. Higher doses induce not only higher antibody responses but also increased percentage of antibody positive chickens within the vaccinated group. Higher doses likely result in greater numbers of host cells being transduced by the RCA-free adenovirus vector and increased transgene expression. It is however interesting that higher antibody levels (over 4 log2) are detected around day 26 of age, which is relatively late in the life of a broiler chicken. Previous experiences and current results have shown that over 90% of chickens with robust antibody titers are protected against challenge but we have also observed vaccinated chickens with marginal antibody levels that were protected against HPAI challenge. Thus, it seems that more in depth research on cellular immunity after vaccination with this vaccine is required to improve our understanding of the induced protection. Also, it seems necessary to further evaluate protection against challenge at time points closer to hatch.
Higher H5 antibody titers were detected in chickens vaccinated with the codon optimized synthetic HA gene (AdTW68.H5ck) [7, 10] as compared with the antibody titers detected in chickens vaccinated with the non codon-optimized homologous counterpart of a previous trial (Fig. 2A). This result is in agreement recent with results by Jiang et al [3] which also show that optimization to the chicken codons improves the specific antibody responses of vaccinated chickens. The use of synthetic genes instead of natural genes provides a great advantage for vaccine production as it makes it feasible to produce vaccines against virus strains causing outbreaks of disease in distant regions of the world without the necessity of importing dangerous strains or their genetic material to the vaccine production sites.
The results presented herein further corroborate that immunization with an RCA-free human Ad5 vector encoding AI hemagglutinins elicits protective immunity against HPAI viruses in chickens. Unlike replicating recombinant vectors that are associated with the risk of generating revertants and allow spread of genetically modified organisms in both target and non-target species in the environment, the RCA-free Ad5 vectors will not propagate in the field. Ad-vectored in ovo AI vaccines can be produced rapidly and mass-administered into chicken populations within the context of a high standard safety profile in response to an emerging AI pandemic. The use of RCA-free Ad5-vectored AI vaccine technology in the world’s poultry industry will provide an immune barrier against multiple strains of avian influenza. Such a barrier will not only protect this important protein source for human consumption, but also would significantly reduce the risk of an avian-originating influenza pandemic in humans.
Acknowledgments
We thank Cassandra Breedlove, Z. Huang, Suzanne DeBlois, and Aniko Zsak for technical assistance. This work was supported by USDA grant USDA CSREES CAP Award #2005-35605-15388 and by NIH Award #1 R43 AI068285-01.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ge J, Deng G, Wen Z, Tian G, Wang Y, Shi J, Wang X, Li Y, Hu S, Jiang Y, Yang C, Yu K, Bu Z, Chen H. Newcastle disease virus-based live attenuated vaccine completely protects chickens and mice from lethal challenge of homologous and heterologous H5N1 avian influenza viruses. J Virol. 2007;81:150–158. doi: 10.1128/JVI.01514-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hilleman MR. Realities and enigmas of human viral influenza: pathogenesis, epidemiology and control. Vaccine. 2002;20:3068–3087. doi: 10.1016/s0264-410x(02)00254-2. [DOI] [PubMed] [Google Scholar]
- 3.Jiang Y, Yu K, Zhang H, Zhang P, Li C, Tian G, Li Y, Wang X, Ge J, Bu Z, Chen H. Enhanced protective efficacy of H5 subtype avian influenza DNA vaccine with codon optimized HA gene in a pCAGGS plasmid vector. Antiviral Res. 2007;75:234–241. doi: 10.1016/j.antiviral.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 4.Konz JO, Livingood RC, Bett AJ, Goerke AR, Laska ME, Sagar SL. Serotype specificity of adenovirus purification using anion-exchange chromatography. Hum Gene Ther. 2005;16:1346–1353. doi: 10.1089/hum.2005.16.1346. [DOI] [PubMed] [Google Scholar]
- 5.Lee CW, Senne DA, Suarez DL. Generation of reassortant influenza vaccines by reverse genetics that allows utilization of a DIVA (Differentiating Infected from Vaccinated Animals) strategy for the control of avian influenza. Vaccine. 2004;22:3175–3181. doi: 10.1016/j.vaccine.2004.01.055. [DOI] [PubMed] [Google Scholar]
- 6.Lewis JA, Brown EL, Duncan PA. Approaches to the release of a master cell bank of PER.C6 cells; a novel cell substrate for the manufacture of human vaccines. Dev Biol (Basel) 2006;123:165–176. [PubMed] [Google Scholar]
- 7.Mossadegh N, Gissmann L, Muller M, Zentgraf H, Alonso A, Tomakidi P. Codon optimization of the human papillomavirus 11 (HPV 11) L1 gene leads to increased gene expression and formation of virus-like particles in mammalian epithelial cells. Virology. 2004;326:57–66. doi: 10.1016/j.virol.2004.04.050. [DOI] [PubMed] [Google Scholar]
- 8.Paoletti E. Applications of pox virus vectors to vaccination: an update. PNAS USA. 1996;93:11349–11353. doi: 10.1073/pnas.93.21.11349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Park MS, Steel J, Garcia-Sastre A, Swayne D, Palese P. Engineered viral vaccine constructs with dual specificity: Avian influenza and Newcastle disease. PNAS USA. 2006;103:8203–8208. doi: 10.1073/pnas.0602566103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ramakrishna L, Anand KK, Mohankumar KM, Ranga U. Codon optimization of the tat antigen of human immunodeficiency virus type 1 generates strong immune responses in mice following genetic immunization. J Virol. 2004;78:9174–9189. doi: 10.1128/JVI.78.17.9174-9189.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Sharma JM, Burmester BR. Resistance to Marek's disease at hatching in chickens vaccinated as embryos with the turkey herpesvirus. Avian Dis. 1982;26:134–149. [PubMed] [Google Scholar]
- 12.Shi Z, Zeng M, Yang G, Siegel F, Cain LJ, Van Kampen KR, Elmets CA, Tang DC. Protection against tetanus by needle-free inoculation of adenovirus-vectored nasal and epicutaneous vaccines. J Virol. 2001;75:11474–11482. doi: 10.1128/JVI.75.23.11474-11482.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Spackman E, Senne DA, Myers TJ, Bulaga LL, Garber LP, Perdue ML, Lohman K, Daum LT, Suarez DL. Development of a real-time reverse transcriptase PCR assay for type A influenza virus and the avian H5 and H7 hemagglutinin subtypes. J Clin Microbiol. 2002;40:3256–3260. doi: 10.1128/JCM.40.9.3256-3260.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Suarez DL, Senne DA, Banks J, Brown IH, Essen SC, Lee C-W, Manwell RJ, Mathieu-Benson C, Moreno V, Pedersen JC, Panigraphy B, Rojas H, Spackman E, Alexander DJ. Recombination resulting in virulence shift in avian influenza outbreak, Chile. Emerg Infect Dis. 2004;10:693–699. doi: 10.3201/eid1004.030396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Swayne DE, Beck JR, Kinney N. Failure of a recombinant fowl poxvirus vaccine containing an avian influenza hemagglutinin gene to provide consistent protection against influenza in chickens preimmunized with a fowl pox vaccine. Avian Dis. 2000;44:132–137. [PubMed] [Google Scholar]
- 16.Swayne DE, Garcia M, Beck JR, Kinney N, Suarez DL. Protection against diverse highly pathogenic H5 avian influenza viruses in chickens immunized with a recombinant fowlpox vaccine containing an H5 avian influenza hemagglutinin gene insert. Vaccine. 2000;18:1088–1095. doi: 10.1016/s0264-410x(99)00369-2. [DOI] [PubMed] [Google Scholar]
- 17.Swayne DE, Senne DA, Beard CW. Influenza. In: Swayne DE, Glisson JR, Jackwood MW, Pearson JE, Reed WM, editors. Isolation and Identification of Avian Pathogens. 4th ed. American Assoc Avian Pathol, Kennett Square; 1998. pp. 150–155. [Google Scholar]
- 18.Toro H, Tang DC, Suarez DL, Sylte MJ, Pfeiffer J, Van Kampen KR. Protective avian influenza in ovo vaccination with non-replicating human adenovirus vector. Vaccine. 2007;25:2886–2891. doi: 10.1016/j.vaccine.2006.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ungchusak K, Auewarakul P, Dowell SF, Kitphati R, Auwanit W, Puthavathana P, Uiprasertkul M, Boonnak K, Pittayawonganon C, Cox NJ, Zaki SR, Thawatsupha P, Chittaganpitch M, Khontong R, Simmerman JM, Chunsutthiwat S. Probable person-to-person transmission of avian influenza A (H5N1) N Engl J Med. 2005;352:333–340. doi: 10.1056/NEJMoa044021. [DOI] [PubMed] [Google Scholar]
- 20.Wakenell PS, Bryan T, Schaeffer J, Avakian A, Williams C, Whitfill C. Effect of in ovo vaccine delivery route on herpesvirus of turkeys/SB-1 efficacy and viremia. Avian Dis. 2002;46:274–280. doi: 10.1637/0005-2086(2002)046[0274:EOIOVD]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 21.World Organization for Animal Health (OIE), a., Food and Agriculture Organization of the United Nations (FAO) Recommendations of the OIE/FAO International Scientific Conference on Avian Influenza; 7 – 8 April 2005; Paris. In, www.oie.int/eng/avian_influenza/OIE_FAO_Recom_05.pdf ed. [Google Scholar]