Abstract
The Bill and Melinda Gates Foundation supports an ambitious portfolio of novel vaccines, drug regimens, and diagnostic tools for tuberculosis (TB). We elicited the expected efficacies and improvements of the novel interventions in discussions with the foundations managing their development. Using an age-structured mathematical model of TB, we explored the potential benefits of novel interventions under development and those not yet in the portfolio, focusing on the WHO Southeast Asia region. Neonatal vaccination with the portfolio vaccine decreases TB incidence by 39% to 52% by 2050. Drug regimens that shorten treatment duration and are efficacious against drug-resistant strains reduce incidence by 10–27%. New diagnostics reduce incidence by 13–42%. A triple combination of a portfolio vaccine, drug regimen, and diagnostics reduces incidence by 71%. A short mass vaccination catch-up campaign, not yet in the portfolio, to augment the triple combination, accelerates the decrease, preventing >30% more cases by 2050 than just the triple combination. New vaccines and drug regimens targeted at the vast reservoir of latently infected people, not in the portfolio, would reduce incidence by 37% and 82%, respectively. The combination of preventive latent therapy and a 2-month drug treatment regimen reduces incidence by 94%. Novel technologies in the pipeline would achieve substantial reductions in TB incidence, but not the Stop TB Partnership target for elimination. Elimination will require new delivery strategies, such as mass vaccination campaigns, and new products targeted at latently infected people.
Keywords: latent infection, novel interventions, transmission model, latent therapy
The Bill and Melinda Gates Foundation (BMGF) supports an ambitious portfolio of novel vaccines, treatment regimens, and diagnostic tools for tuberculosis (TB). Funded by BMFG and other sources, the Aeras Global TB Vaccine Foundation oversees vaccine development (1), the TB Alliance seeks novel drug regimens (2), and the Foundation for Innovative New Diagnostics (FIND) looks for new diagnostic tools (3). The current cornerstone of TB intervention is directly observed short-course therapy (DOTS), lasting generally 6 months and prone to dropout (4). DOTS is currently implemented in the 184 countries where 99% of all estimated TB cases occurred and 93% of the world population lived in 2006 (5). Cases are passively ascertained. Sputum smear light microscopy has been the mainstay of TB diagnosis for more than a century, but has important limitations (3). Delays between the patient visit to the clinic and diagnosis often lead to delays in treatment. Neonatal vaccination with bacillus Calmette–Guérin (BCG) vaccine is part of the expanded program of immunization in many countries, but its efficacy against pulmonary TB is poor (1). Despite these efforts, in large parts of Asia, Africa, Eastern Europe, and Latin America, incidence remains 2 orders of magnitude (5) above the Stop TB Partnership goal to eliminate TB as a public health problem, defined as <1 case per million population per year by 2050 (6).
Vaccine development aims to replace neonatal BCG with a vaccine more effective in preventing active TB disease (1). Improved diagnostics would shorten the duration of infectiousness and increase the probability of case detection before death from TB disease (3). New drug regimens would shorten treatment and improve efficacy against resistant strains (2). The question of interest is, if these new technologies being supported by a large financial investment become available as expected, what decrease in TB morbidity and mortality would they actually achieve? Although other groups have modeled potential effects of new technologies (7–10), none has specifically assessed and compared the benefits of the vaccines, drug regimens, and diagnostics under development. Here, we report on research using a mathematical model of TB transmission based on previous TB models (11–14) to investigate potential epidemiological benefits of the novel interventions (Fig. 1). We focus here on the WHO Southeast Asia region, which includes India but not China. In 2006, the region accounted for 35% of incident TB cases in the world and 32% of the TB-related deaths (5). The region has generally successful TB intervention programs, with high (100%) DOTS coverage and high (87%) DOTS new smear-positive treatment success (5). Thus, operationally, the Southeast Asia region is well-positioned to introduce new technologies. Motivated by the somewhat modest results of some of the portfolio interventions, we also explored the potential benefits of technologies and strategies not yet in the BMGF portfolio, but that perhaps should be, specifically vaccines and drugs for latently infected people and mass vaccination.
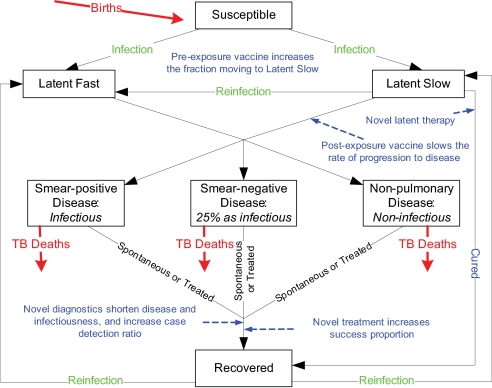
Fig. 1.
Streamlined flow chart of the TB natural history model. Novel interventions are indicated in purple text where they intervene in the natural history. None of the novel interventions directly protects against infection. Pre-exposure vaccination decreases the probability of developing disease once infected. After infection, vaccinated individuals have a higher probability of becoming long-term latently infected with a low lifetime risk of developing disease. Latent postexposure vaccination decreases the lifetime probability of developing disease. Latent treatment cures a person, but a person could be reinfected. Once a person develops disease, better diagnostics decreases the time that a person has disease and thus reduces the exposure of others to infection. Once diagnosed, better treatment regimens shorten treatment, achieving a higher success rate, also reducing exposure of others to infection. Adults and children, for whom the natural history of TB is qualitatively similar but quantitatively different, are distinguished in the model but not in this diagram. People are lost by death from all groups, but only TB deaths are shown.
Results
The fitted model generated a steady-state incidence rate of 1,755 new TB cases per million and 309 TB-related deaths per million per year in the Southeast Asia region. With no novel interventions, 101.7 million new TB cases and 17.9 million TB-related deaths are expected between 2015 and 2050. Table 1 presents the absolute number and percentage of cases and TB-related deaths prevented by the novel interventions and their combinations by 2050.
Table 1.
Cumulative number of TB cases and TB-related deaths prevented between 2015 and 2050 by using different novel interventions with various strategies in the Southeast Asia region
| Intervention strategy | Prevented active TB cases, millions (%) | Prevented TB-related deaths, millions (%) |
|---|---|---|
| Portfolio interventions | ||
| Vaccination | ||
| Neonatal vaccination with adolescent boost | ||
| Basic pre-exposure vaccine only | 18.2 (18) | 3.0 (17) |
| Basic pre-exposure vaccine with the additional effects | 23.8 (23) | 3.9 (22) |
| Active disease treatment regimens | ||
| 1. 4 months | 8.3 (8) | 2.0 (11) |
| 2. 2 months + 90% efficacy against drug-resistant strains | 19.3 (19) | 4.5 (25) |
| 3. 10 days + 90% efficacy against drug-resistant strains | 22.9 (23) | 5.3 (30) |
| Diagnostics | ||
| LED | 11.7 (12) | 4.2 (23) |
| NAAT | 24.4 (24) | 4.2 (23) |
| Dipstick | 39.4 (39) | 8.0 (45) |
| Combination | ||
| Neonatal vaccination with basic pre-exposure effects plus active- disease regimen 2 plus NAAT | 55.3 (54) | 10.4 (58) |
| Nonportfolio strategies and combinations | ||
| Mass vaccination (includes neonatal vaccination) | ||
| Basic pre-exposure vaccine only | 68.2 (67) | 11.5 (64) |
| Basic pre-exposure vaccine with the additional effects | 74.0 (73) | 12.4 (69) |
| Neonatal vaccination with 3-year mass catch-up, basic pre-exposure vaccine with additional effects | 67.9 (67) | 11.4 (64) |
| Mass vaccination with only a postexposure effect | 30.1 (30) | 5.0 (28) |
| Neonatal vaccination, basic pre-exposure with additional effects, plus mass vaccination with postexposure effect | 73.2 (72) | 12.3 (69) |
| Mass vaccination with basic pre-exposure vaccine, plus mass vaccination with postexposure effect | 80.2 (79) | 13.5 (75) |
| Mass vaccination with basic pre-exposure vaccine with additional effects, plus mass vaccination with postexposure effect | 85.9 (84) | 14.5 (81) |
| Neonatal vaccination with 3-year mass catch-up, basic pre-exposure with additional effects, plus mass vaccination with postexposure effect | 83.8 (82) | 14.1 (79) |
| Mass latent therapy | 64.8 (64) | 10.8 (60) |
| Active-disease regimen 2 plus mass latent therapy | 75.6 (74) | 13.1 (73) |
| Mass vaccination with basic pre-exposure effects plus 2-month active disease treatment regimen 2 plus NAAT | 77.8 (76) | 13.9 (78) |
| Neonatal vaccination with 3-year mass catch-up with basic pre-exposure effects plus active-disease regimen 2 plus NAAT | 73.5 (72) | 13.2 (74) |
Without novel intervention, cumulative incidence for 2015–2050 is 101.7 million active TB cases and 17.9 million TB-related deaths.
Novel Vaccination.
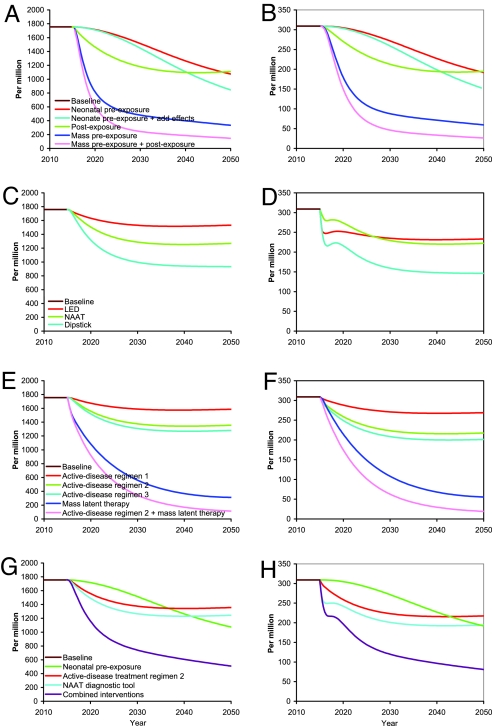
Neonatal vaccination with the basic pre-exposure vaccine at vaccine efficacy for progression (VEP) of 60% achieves a gradual 39% reduction in TB incidence at 2050 compared with 2015 (Fig. 2 A and B). The pre-exposure vaccine with additional effects achieves a 52% reduction. After ≈100 years, neonatal vaccination with the basic pre-exposure vaccine achieves a 55% reduction in incidence. In contrast, mass vaccination with the basic pre-exposure vaccine results in a much faster, nearly 80% decrease in incidence by 2050. Neonatal vaccination augmented by a 3-year mass catch-up program has a similar rapid drop in incidence, with the cumulative number of cases and deaths prevented by 2050 closer to that of sustained mass vaccination than to that of the neonatal strategy alone (Table 1).
Fig. 2.
Effect by year up to 2050 of interventions and strategies begun in 2015 on TB (all-types) incidence per million (A, C, E, and G) and TB (all types) related mortality per million (B, D, F, and H). (A and B) Vaccination. Shown are neonatal vaccination with basic pre-exposure vaccine, neonatal vaccination with pre-exposure vaccine with additional effects; postexposure vaccination of latently infected people; mass vaccination with basic pre-exposure vaccination; mass vaccination with pre-exposure vaccine with additional effects. (C and D) Novel TB diagnostic tools. (E and F) Five treatment scenarios: active-disease regimens 1, 2 and 3; mass latent therapy, and the active-disease regimen 2 combined with mass latent therapy. (G and H) Combination of active-disease treatment regimen 2, neonatal vaccination with basic pre-exposure vaccine, and NAAT diagnostic tool.
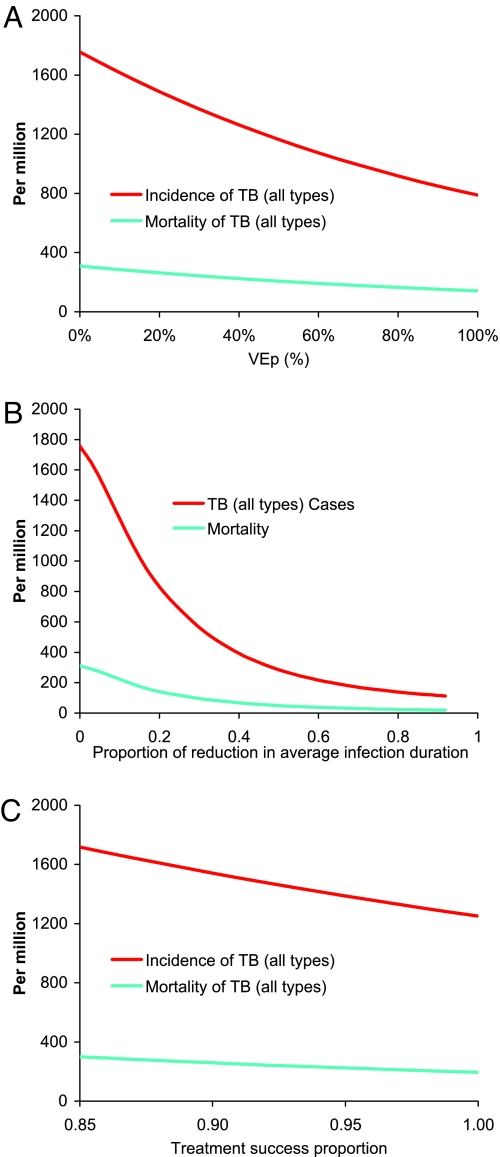
Vaccination of latently infected people with a nonportfolio postexposure vaccine achieves a rapid initial reduction, but incidence plateaus at only a 37% reduction, near the incidence achieved by neonatal vaccination alone at 2050 (Fig. 2 A and B). Combining vaccination of latently infected people using the postexposure vaccine and mass vaccination of uninfected people using the basic pre-exposure vaccine achieves a 92% reduction in TB incidence by 2050. In the sensitivity analysis of neonatal vaccination, TB incidence at 2050 decreases linearly, although not steeply, with increasing VEP (Fig. 3A). For example, if VEP were just 40% instead of the targeted 60%, incidence would be reduced 28% rather than 39%. Even if VEP were 100%, at which every vaccinated person who becomes infected is a slow progressor, incidence at 2050 would be reduced just 58%.
Fig. 3.
The sensitivity of TB incidence and TB-related mortality at 2050 to assumptions about interventions started in 2015. (A) Neonatal vaccination with basic pre-exposure vaccine varying VEP, the reduction in probability of becoming a fast progressor, from 0 to 100%. (B) Novel NAAT diagnostic, varying the proportion of reduction in average duration of infectiousness from 0 to 1.0 (C) Varying the treatment success proportion, the effect of novel active-disease treatment regimens from the current 0.84 to 1.00.
Novel Diagnostics.
The 3 diagnostic products achieve their maximum improvements in TB incidence and mortality fairly rapidly and plateau (Fig. 2 C and D). The light-emitting diode (LED) microscopy achieves a 13% reduction in incidence at 2050. The Dipstick for antigens or antibodies method reduces incidence by ≈42% at 2050 compared with 2015, with the nucleic acid amplification test (NAAT) in between with a 28% reduction. The reduction in incidence is caused by the indirect effect of shortening the duration of infectiousness. The mortality curves show a transient dip in mortality caused by a transient cohort effect in the diagnostic model that dissociates the duration of infectiousness from the case detection ratio. The time between disease onset and detection is shortened instantaneously by cutting the 2 middle infective stages. The people in these 2 compartments are moved into the recovered state, so transiently fewer people are available to die of TB. Within 5 years, the curves have returned to their expected behavior with the passage of the transient cohort from the dynamics. The increased case detection ratio under LED provides an enhanced decrease in mortality, so that NAAT and LED have a similar mortality at 2050, even though incidence at 2050 is considerably higher under LED compared with NAAT. In the sensitivity analysis (Fig. 3B), TB incidence and mortality at 2050 decrease quasi-linearly with proportional reduction in duration of infectiousness in the range considered for the proposed diagnostic tools, but they then plateau with decreasing duration of infectiousness. Although incidence at 2050 is not greatly affected if the average rollout time of NAAT varies from 0 to 20 years, the cumulative number of cases averted substantially decreases (Fig. S2D in SI Appendix).
Novel Treatment Regimens.
The 4-month, 2-month, and 10-day active disease treatment regimens 1, 2, and 3 produce 10%, 23%, and 27% reductions, respectively, in TB incidence by 2050 compared with 2015 (Fig. 2 E and F). Once cases are found, treatment in the Southeast Asia region is already on average 84% successful, leaving little room for improvement with drug regimens alone (Fig. 3C). In contrast, a drug that would enable mass latent preventive therapy would reduce incidence 82% by 2050. The combination of mass latent therapy and the 2-month active disease treatment regimen 2 reduces annual TB incidence in 2050 by nearly 94%.
Combinations.
The number of TB cases prevented by 2050 by the individual portfolio technologies neonatal vaccination with the basic pre-exposure vaccine, a 2-month treatment regimen effective against drug-resistant strains, and NAAT are similar at 18.2, 19.2, and 24.4 million, respectively (Table 1). The triple combination of the three prevents 55.3 million cases (Table 1) and lowers TB incidence by 71% (to 509.2 cases per million) (Fig. 2 G and H) by 2050, far more than any of them individually. Augmenting the triple combination with the nonportfolio mass vaccination catch-up campaign prevents 73.5 million cases (Table 1) and lowers incidence by 79% in 2050. As seen in Table 1, all of the nonportfolio technologies and strategies, alone or in combination with portfolio interventions, with the exception of mass latent therapy, do better than any of the portfolio interventions or their combination.
Sensitivity and Uncertainty Analysis.
Of the natural history parameters tested, the model is most sensitive to a change in the proportion of new latent infections who are fast progressors (Fig. S1 in SI Appendix). For example, a ±15% change in the proportion of new latent infections who are fast progressors results in a range of TB incidence of 1,032 (−41%) to 2,532 (+45%) (Fig. S1A in SI Appendix). The sensitivity is easy to understand, because decreasing the proportion of fast progressors is equivalent to vaccination with the basic pre-exposure vaccine. In the novel interventions (Fig. S2 A–C in SI Appendix), ±15% variation of the reduction of fast progressors, duration of protection, and treatment success proportion all produced <8% deviation in the results.
Other WHO Regions.
In the WHO Western Pacific, Eastern European, Eastern Mediterranean, and Latin American regions where HIV incidence is low, the novel interventions produced qualitatively similar results as in the Southeast Asia region (Table S3 in SI Appendix), with similar relative reduction in cases and deaths. An exception is that the novel treatments regimens prevented 1.5 times the percentage of cases and deaths in the Eastern European region and only approximately half the percentage of cases and deaths in the Western Pacific region as in the Southeast Asia region. The reason is that the current success proportion in the Eastern European region is just 72%, and in the Western Pacific region 92%, so that there is, respectively, more and less room for improvement over the current success proportion of 84% in the Southeast Asia region. The absolute number of cases and deaths prevented is smaller than in the Southeast Asia region because the baseline number of cumulative cases and deaths is smaller.
Discussion
Our results demonstrate that the novel vaccines, drug regimens, and diagnostics currently under development each offer substantial reductions in TB incidence and TB-related mortality, and more so as a triple combination, compared with current approaches. The achieved incidence would not be <1 case per million population, the goal of Stop TB Partnership, but it would be at more manageable rates. Augmenting neonatal vaccination by a short-term mass vaccination catch-up campaign, not in the current portfolio of delivery strategies, would accelerate the drop in incidence, preventing more deaths and cases. New technologies targeted at the vast reservoir of latently infected people would be of great benefit, particularly if combined with one or more of the novel technologies in the current pipeline. The 2 combinations, a 2-month treatment regimen combined with mass latent therapy and mass vaccination with pre-exposure vaccine combined with postexposure vaccine administered to latently infected people (15), provide similarly powerful reductions in TB mortality, preventing 73% and 75% of deaths, respectively.
Our approach differs from previous models in being based on the actual targeted or expected efficacies of the technologies under development. Remarkably, in none of our discussions about novel interventions did anyone suggest an approach, either currently under development or in the future, including vaccination, that would directly protect uninfected people against infection. To represent the input of the developers, vaccination in our model alters the postinfection natural history. Thus, our results cannot be compared with others (12, 16, 18) that assumed pre-exposure vaccine would protect against infection. In contrast to others (7, 16), novel diagnostics in our model can shorten the duration of infectiousness of both smear-positive and smear-negative cases, independently of the increase of the proportion of cases detected and cured.
In our model, latent therapy cures people in the latent state, but latent vaccination only reduces the lifetime risk of developing active disease. Under these assumptions, mass preventive therapy prevents twice as many TB cases and deaths by 2050 as postexposure vaccination of latently infected people (Table 1). Our results differ from other models (12, 16) that assume that both latent treatment and latent vaccination cure people. If a therapeutic vaccine that cured latently infected people were developed, it would have a similar effect as the preventive therapy modeled here.
Further exploration of the benefits of novel interventions in regions where HIV plays an important role in TB epidemiology is future research. The effect of HIV prevalence on the benefits of novel TB therapies that shorten treatment duration and improve cure rates was studied in detail by Sanchez et al. (9) using a model of Kenyan epidemiology specifically designed for that purpose. They found that at 3–20% HIV prevalence shortening treatment duration to 2 months achieved a 6–20% decrease in incidence and mortality in 25 years. The benefits vary substantially depending on the assumed HIV infection prevalence. To study the effects of novel vaccines and diagnostics on HIV-infected individuals will require further assumptions about their efficacy and models designed for that purpose. Preventive therapy of latent TB infection with currently available drugs in HIV-infected people may become routine. Trials are underway to assess the efficacy of preventive treatment for latent TB in HIV-infected people (18). The results of that and other similar trials could inform future modeling work. Even without taking HIV into account, in the sub-Saharan region with high HIV incidence, and to a lesser extent, in the sub-Saharan region with low HIV incidence, caused by the higher TB incidence and prevalence, in our model, mass vaccination with a pre-exposure vaccine prevents relatively fewer cases and deaths than in the other regions (Table S3 in SI Appendix).
We focused on the epidemiological outcomes of potential improvements in TB interventions. Further benefits would likely result from shorter treatment regimens, for both the patients being treated and the health care infrastructure. Shorter DOTS treatments, or eventually single contact treatments, could remove much of the stigma associated with TB treatment. People with active TB might seek health care earlier, enhancing the effects of improved diagnostics and treatments.
One limitation of the research is the quality of the data for establishing parameter values. Better and more complete data collection is crucial to improving modeling efforts. Regional transmission models average over local and country-level differences in epidemiology. All of the results presented here are in one sense optimistic, assuming nearly complete coverage of the target populations once novel technologies are introduced. Regardless of these limitations, the overall conclusions of the broad picture are not changed in the sensitivity analysis. Our goal was to gain general insight into the relative benefits of the novel vaccines, drug regimens, and diagnostics currently in the pipeline, and some not in the pipeline, not to predict the precise number of cases or deaths prevented.
Combinations of the new technologies currently under development could have an enormous benefit. However, there is a big difference between what is in the current portfolio and what could be achieved by going beyond it. To achieve further improvements, technologies and delivery strategies not currently under development should be considered. The findings could alter the practice of tuberculosis control, particularly if mass vaccination catch-up campaigns were added to the current practice of vaccinating neonates within the Expanded Program on Immunization. In this case, clinical trials for different target age groups need to be prepared. Vaccines and drug regimens targeting the vast reservoir of latently infected people would be potentially important investments. This study expands the universe of interventions to be seriously considered for support and development.
Methods
Natural History of Tuberculosis.
We developed an age-structured mathematical model similar to previous TB models (11–14) described by a series of differential equations (Fig. 1, SI Appendix, and SI Technical Appendix). Once infected, individuals enter 1 of 2 latent states. Infected people in the latent slow state have an ≈5% lifetime risk of developing active disease (slow progressors) (16, 19–26). Individuals in the latent fast state develop active TB disease with a mean of 7.3 months (fast progressors) (16, 19–26). TB cases arise as primary disease from the newly infected fast progressors, as endogenous reactivation of the slow progressors, or by exogenous reinfection of slow progressors so that they become fast progressors.
Active cases of TB are infectious pulmonary sputum smear-positive, infectious pulmonary sputum smear-negative (0.25 as infectious) (27), or noninfectious nonpulmonary, with age-dependent rates of developing each of the 3 types. Active TB cases can recover either naturally or by being treated once detected or die. People with untreated TB have a higher death rate than the general population, the death rate from smear-positive pulmonary disease being higher than from the other 2 forms. Successfully treated cases are noninfectious once they begin treatment. Recovered cases can be reinfected. The case detection rate (actually a ratio) is defined as the proportion of cases detected before they die or recover spontaneously. The Southeast Asia region has a low 1.2% HIV prevalence in adult incident TB cases (5). HIV prevalence levels in India in particular have been found to be half those estimated earlier (28). Thus, we did not include HIV in the model.
Novel Portfolio Interventions.
Discussions with FIND, Aeras, and the TB Alliance about the expected efficacies and improvements over current approaches provided the base case scenarios for our models of the novel portfolio interventions. We analyzed the sensitivity of the results to these values.
Vaccines.
The novel vaccination concept under development by Aeras is based on a prime-boost strategy, the prime likely being a recombinant BCG, the boost likely being a vector carrying TB antigens (1). The goal is to replace neonatal BCG vaccination with the new vaccine combination, providing an additional boost in adolescence to prolong duration of efficacy. The target efficacy of the portfolio vaccine combination is to decrease by 60% the proportion of infected people becoming fast progressors, VEP (29). The vaccine is expected to confer no direct protection against infection and be effective only if administered before a person becomes infected. We call this the pre-exposure vaccine. In the base case model, the basic pre-exposure vaccine has a VEP of 60%, lasting, as expected with adolescent boost, on average 33 years. In the natural history model, 5% of infected children (<15 years), and 15% of infected adults become fast progressors (23, 24, 30–37). At a VEP of 60%, vaccinated children have a 2% and vaccinated adults a 6% probability of becoming fast progressors rather than slow progressors if they become infected. In a sensitivity analysis, we varied the value of VEP from 0 to 100%.
We also modeled a pre-exposure vaccine with additional effects that reduce the lifetime risk of developing disease in slow progressors by half to ≈2.5% and the infectiousness of the smear-positive and smear-negative cases, called the vaccine efficacy for infectiousness, VEI (29), by half.
Diagnostics.
Practical considerations of introducing novel diagnostics, including the point of health care and sensitivity (3), were condensed to 2 key points relevant for the transmission model. Better diagnostics might (i) reduce the time during which a person is infectious for others by decreasing the time from onset of disease to diagnosis and treatment, and (ii) increase the case detection rate. FIND is developing 3 main diagnostic tools (3). The LED fluorescence microscopy is a simple technology that essentially changes the light bulb in current microscopes. NAAT technologies include loop-mediated isothermal amplification (also known as Eiken NAAT), and Cepheid, which incorporates an integrated platform for specimen processing, real-time PCR, and probing for rifampin resistance. These diagnostics would be administered at the level of the microscopy laboratory. Dipstick would be administered at the level of the health post and would provide results immediately. LED-fluorescence microscopy is expected to produce a 10% (8–12%) improvement in the case detection rate and, relative to an average duration between onset of illness and diagnosis of 24 months, achieve a 1-month reduction (4%) in average duration of smear-positive cases relative to Ziehl Neelsen microscopy. Because of the absence of definitive evidence, this tool is assumed to have no increased sensitivity to both smear-negative and nonpulmonary forms of TB. NAAT is expected to reduce a 24-month duration from onset of disease to detection by 3 months (12.5%) to 21 months for all 3 types of active TB cases. The Dipstick is expected to reduce a 24-month duration to detection by 4 months (16.5%) to 20 months for all 3 types of TB cases. A similar relative shortening is expected for other baseline durations between onset of illness and detection. In the Southeast Asia region, the relative shortenings for the novel diagnostics were computed based on 15.4 months of average duration from onset of disease to detection in smear-positive cases and 23.0 months in smear-negative cases (15). In a sensitivity analysis, we varied the proportionate reduction in average duration of infectiousness from 0 to 1.0.
In the models of novel diagnostics, to dissociate duration of infectiousness from the case detection ratio, untreated disease occurs in 4 stages. The 3 rates in the case detection rate apply only to the last stage, but all 4 stages are infectious (SI Technical Appendix). The duration of infectiousness is decreased by cutting the 2 middle stages, which are designed to correspond to the expected shortening of time from disease onset to detection of each novel diagnostic tool without changing the case detection rate.
Treatment Regimens.
Two goals of novel treatment regimens for active TB disease are to shorten treatment duration and improve efficacy against resistant strains. TB Alliance is developing 3 main approaches to active disease treatment regimens (2, 38, 39). The first is expected to shorten treatment duration from the current 6 months to 4, with little effect on drug-resistant strains. The second, a possible triple combination of therapies, would shorten duration to 2 months, with expected 90% efficacy against extensively and multidrug-resistant strains. A third regimen, requiring considerably more basic research, would shorten treatment to 10 days, similar to other antibiotic courses, and be 90% effective against extensively and multidrug-resistant strains. The first two would replace current regimens in DOTS. The third could be a single contact treatment for which DOTS would no longer be necessary. The corresponding models are called active disease treatment regimens 1, 2, and 3.
The shorter drug treatments and efficacy against extensively and multidrug-resistant strains are modeled by increasing the treatment success proportion, that is, those who are cured and completed treatment. Increased success proportion caused by shorter treatment is modeled by the relative reduction in the duration of the novel drug regimen compared with the current 6 months multiplied by the proportions of failure caused by the WHO categories default, transfer, death during treatment, or loss to follow-up (40) (Table S2 in SI Appendix). The increased success proportion against drug-resistant TB disease is modeled by reducing the proportions in the 2 WHO categories of treatment failure and death during treatment by the assumed efficacy against drug-resistant strains. After starting current treatment, people are quickly no longer infectious (41), so new drug regimens are not expected to shorten materially the infectious period compared with current therapy. In the Southeast Asia region, the average success proportion was 84% (40). The models of active treatment regimens 1, 2, and 3 achieve success proportions of 89%, 96%, and 99%, respectively. In a sensitivity analysis, we varied the success proportion up to 100%.
Nonportfolio Novel Interventions.
Based on initial results, we considered further novel interventions and strategies not yet in the portfolio but that perhaps should be. Once available, an effective pre-exposure vaccine would likely be administered widely, not just to neonates. We considered a mass vaccination strategy with the pre-exposure vaccine. We also considered neonatal vaccination augmented by a 3-year mass vaccination catch-up program. We then modeled vaccines and drug regimens targeted at the vast reservoir of latently infected slow progressors. The modeled postexposure vaccine reduces by half the lifetime risk of latently infected slow progressors developing disease. The modeled novel drug regimen cures latently infected slow progressors.
Sources of Data and Model Fitting.
Key indicators and variables for the natural history parameters of the model were obtained from a review of published research (42) and discussions with TB researchers (Table S1 in SI Appendix). In the few instances when no information was available, assumptions were made. Using the Downhill Simplex method (43), both the contact rate and the case detection rate were permitted to vary while fitting the model to the annual incidence rates of pulmonary smear-positive disease and TB-related mortality reported by the WHO for 2006 (5) (Table S2 in SI Appendix). The case detection rate was permitted to vary because of suspected underreporting of treatment among cases. The fitted incidence was assumed to capture the current BCG vaccination, treatment, and diagnostics. Novel intervention effects were included as improvements on the current status. We focus here on the Southeast Asia region. Results for other WHO regions are in Table S3 in SI Appendix.
To model the most optimistic scenario, interventions were assumed introduced in 2015 with immediate adoption everywhere and 100% coverage of the target population. For technical reasons, diagnostics are introduced with a rollout time of 1.5 years (see SI Technical Appendix). Results are presented as totals of all 3 forms of active TB cases and TB-related deaths, although the model generates the 3 separate disease forms.
Uncertainty and Sensitivity Analyses.
In sensitivity analyses, we varied the VEP and the shortening of the infectious period from 0 to 100% and the treatment success proportion from the current 84% to 100%. In further univariate sensitivity analyses, we investigated how TB incidence in 2050 varied with changes in the natural history parameters and novel intervention parameters by varying each parameter uniformly over the range of ±15% of the original parameter value.
Supplementary Material
Acknowledgments.
We thank Peter Small for support and helpful suggestions. This work was supported by the Bill and Melinda Gates Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0901720106/DCSupplemental.
References
- 1.Skeiky YA, Sadoff JC. Advances in tuberculosis vaccine strategies. Nat Rev Microbiol. 2006;4:469–476. doi: 10.1038/nrmicro1419. [DOI] [PubMed] [Google Scholar]
- 2.TB Alliance. Confronting TB: What It Takes, 2008 Annual Report. New York: Global Alliance for TB Drug Development; 2008. [Google Scholar]
- 3.Keeler E, et al. Reducing the global burden of tuberculosis: the contribution of improved diagnostics. Nature. 2006;444(Suppl 1):49–57. doi: 10.1038/nature05446. [DOI] [PubMed] [Google Scholar]
- 4.Raviglione MC, Uplekar MW. WHO's new stop TB strategy. Lancet. 2006;367:952–955. doi: 10.1016/S0140-6736(06)68392-X. [DOI] [PubMed] [Google Scholar]
- 5.WHO. WHO Report 2008. Geneva: WHO; 2008. Global tuberculosis control: Surveillance, planning, financing. [Google Scholar]
- 6.Dye C, Maher D, Weil D, Espinal M, Raviglione M. Targets for global tuberculosis control. Int J Tuberc Lung Dis. 2006;10:460–462. [PubMed] [Google Scholar]
- 7.Dowdy DW, Chaisson RE, Maartens G, Corbett EL, Dorman SE. Impact of enhanced tuberculosis diagnosis in South Africa: A mathematical model of expanded culture and drug susceptibility testing. Proc Natl Acad Sci USA. 2008;105:11293–11298. doi: 10.1073/pnas.0800965105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Salomon JA, et al. Prospects for advancing tuberculosis control efforts through novel therapies. PLoS Med. 2006;3:e273. doi: 10.1371/journal.pmed.0030273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sanchez MS, et al. Impact of HIV on novel therapies for tuberculosis control. AIDS. 2008;22:963–972. doi: 10.1097/QAD.0b013e3282f7cb4b. [DOI] [PubMed] [Google Scholar]
- 10.Young D, Dye C. The development and impact of tuberculosis vaccines. Cell. 2006;124:683–687. doi: 10.1016/j.cell.2006.02.013. [DOI] [PubMed] [Google Scholar]
- 11.Vynnycky E, Fine PE. The annual risk of infection with Mycobacterium tuberculosis in England and Wales since 1901. Int J Tuberc Lung Dis. 1997;1:389–396. [PubMed] [Google Scholar]
- 12.Dye C, Williams BG. Eliminating human tuberculosis in the twenty-first century. J R Soc Interface. 2008;5:653–662. doi: 10.1098/rsif.2007.1138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Blower SM, et al. The intrinsic transmission dynamics of tuberculosis epidemics. Nat Med. 1995;1:815–821. doi: 10.1038/nm0895-815. [DOI] [PubMed] [Google Scholar]
- 14.Blower SM, Small PM, Hopewell PC. Control strategies for tuberculosis epidemics: New models for old problems. Science. 1996;273:497–500. doi: 10.1126/science.273.5274.497. [DOI] [PubMed] [Google Scholar]
- 15.Okada M, et al. Novel prophylactic and therapeutic vaccine against tuberculosis. Vaccine. 2009;27:3267–3270. doi: 10.1016/j.vaccine.2009.01.064. [DOI] [PubMed] [Google Scholar]
- 16.Murray CJ, Salomon JA. Modeling the impact of global tuberculosis control strategies. Proc Natl Acad Sci USA. 1998;95:13881–13886. doi: 10.1073/pnas.95.23.13881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ziv E, Daley CL, Blower S. Potential public health impact of new tuberculosis vaccines. Emerg Infect Dis. 2004;10:1529–1535. doi: 10.3201/eid1009.030921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Moulton L, et al. Statistical design of THRio: A phased implementation clinic-randomized study of a tuberculosis preventive therapy intervention. Clin Trials J. 2007;4:190–199. doi: 10.1177/1740774507076937. [DOI] [PubMed] [Google Scholar]
- 19.Vynnycky E, Fine PE. Lifetime risks, incubation period, and serial interval of tuberculosis. Am J Epidemiol. 2000;152:247–263. doi: 10.1093/aje/152.3.247. [DOI] [PubMed] [Google Scholar]
- 20.Styblo K, Enarson DA. Epidemiology of Tuberculosis. The Hague: Royal Netherlands Tuberculosis Association; 1991. [Google Scholar]
- 21.Smith PG, Moss AR. Epidemiology of tuberculosis. In: Bloom BR, editor. Tuberculosis: Pathogenesis, Protection, and Control. Washington DC: Am Soc Microbiol; 1994. [Google Scholar]
- 22.Resch SC, Salomon JA, Murray M, Weinstein MC. Cost effectiveness of treating multidrug-resistant tuberculosis. PLoS Med. 2006;3:e241. doi: 10.1371/journal.pmed.0030241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sutherland I, Svandova E, Radhakrishna S. The development of clinical tuberculosis following infection with tubercle bacilli. 1. A theoretical model for the development of clinical tuberculosis following infection, linking from data on the risk of tuberculous infection and the incidence of clinical tuberculosis in the Netherlands. Tubercle. 1982;63:255–268. doi: 10.1016/s0041-3879(82)80013-5. [DOI] [PubMed] [Google Scholar]
- 24.Comstock GW. Epidemiology of tuberculosis. Am Rev Respir Dis. 1982;125:8–15. doi: 10.1164/arrd.1982.125.3P2.8. [DOI] [PubMed] [Google Scholar]
- 25.Murphy BM, Singer BH, Kirschner D. On treatment of tuberculosis in heterogeneous populations. J Theor Biol. 2003;223:391–404. doi: 10.1016/s0022-5193(03)00038-9. [DOI] [PubMed] [Google Scholar]
- 26.Murphy BM, Singer BH, Anderson S, Kirschner D. Comparing epidemic tuberculosis in demographically distinct heterogeneous populations. Math Biosci. 2002;180:161–185. doi: 10.1016/s0025-5564(02)00133-5. [DOI] [PubMed] [Google Scholar]
- 27.Small PM, et al. The epidemiology of tuberculosis in San Francisco. A population-based study using conventional and molecular methods. N Engl J Med. 1994;330:1703–1709. doi: 10.1056/NEJM199406163302402. [DOI] [PubMed] [Google Scholar]
- 28.UNAIDS Joint Programme on HIV/AIDS. AIDS Epidemic Update: December 2007. Geneva: UNAIDS; 2007. [Google Scholar]
- 29.Halloran ME, Struchiner CJ, Longini IM., Jr Study designs for evaluating different efficacy and effectiveness aspects of vaccines. Am J Epidemiol. 1997;146:789–803. doi: 10.1093/oxfordjournals.aje.a009196. [DOI] [PubMed] [Google Scholar]
- 30.Dye C, Garnett GP, Sleeman K, Williams BG. Prospects for worldwide tuberculosis control under the WHO DOTS strategy. Directly observed short-course therapy. Lancet. 1998;352:1886–1891. doi: 10.1016/s0140-6736(98)03199-7. [DOI] [PubMed] [Google Scholar]
- 31.Vynnycky E, Fine PE. The natural history of tuberculosis: The implications of age-dependent risks of disease and the role of reinfection. Epidemiol Infect. 1997;119:183–201. doi: 10.1017/s0950268897007917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Vynnycky E. London: University of London; 1996. An investigation of the transmission dynamics of M. tuberculosis. PhD dissertation. [Google Scholar]
- 33.Sutherland I. Recent studies in the epidemiology of tuberculosis, based on the risk of being infected with tubercle bacilli. Adv Tuberc Res. 1976;19:1–63. [PubMed] [Google Scholar]
- 34.Styblo K. Tuberculosis control and surveillance. In: Flenley DRJ, editor. Advances in Respiratory Medicine. Edinburgh: Churchill Livingstone; 1986. pp. 77–108. [Google Scholar]
- 35.Krishnamurthy VNS, et al. Incidence of tuberculosis among newly infected populations and in relation to the duration of infected status. Indian J Tuberc. 1976;33:1–3. [Google Scholar]
- 36.Krishnamurthy VCK. Risk of pulmonary tuberculosis associated with exogenous reinfection and endogenous reactivation in a south Indian rural population: A mathematical estimate. Indian J Tuberc. 1990;37:63–67. [Google Scholar]
- 37.Ferebee SH. Controlled chemoprophylaxis trials in tuberculosis. A general review. Bibl Tuberc. 1970;26:28–106. [PubMed] [Google Scholar]
- 38.Spigelman MK. New tuberculosis therapeutics: A growing pipeline. J Infect Dis. 2007;196(Suppl 1):S28–S34. doi: 10.1086/518663. [DOI] [PubMed] [Google Scholar]
- 39.Williams KJ, Duncan K. Current strategies for identifying and validating targets for new treatment-shortening drugs for TB. Curr Mol Med. 2007;7:297–307. doi: 10.2174/156652407780598575. [DOI] [PubMed] [Google Scholar]
- 40.WHO. Global Tuberculosis Control: Surveillance, Planning, Financing. Geneva: WHO; 2007. [Google Scholar]
- 41.Jindani A, Aber VR, Edwards EA, Mitchison DA. The early bactericidal activity of drugs in patients with pulmonary tuberculosis. Am Rev Respir Dis. 1980;121:939–949. doi: 10.1164/arrd.1980.121.6.939. [DOI] [PubMed] [Google Scholar]
- 42.Achterberg JT. Seattle: University of Washington; 2006. Mathematical models of tuberculosis: Applications and bases. MA thesis. [Google Scholar]
- 43.Press W. Numerical Recipes in C: The Art of Scientific Computing. New York: Cambridge Univ Press; 1997. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.