Abstract
Current approaches to the development of regenerative therapies have been influenced by our understanding of embryonic development, stem cell biology, and tissue engineering technology. The ultimate goal of regenerative therapy is to develop fully functioning bioengineered organs which work in cooperation with surrounding tissues to replace organs that were lost or damaged as a result of disease, injury, or aging. Here, we report a successful fully functioning tooth replacement in an adult mouse achieved through the transplantation of bioengineered tooth germ into the alveolar bone in the lost tooth region. We propose this technology as a model for future organ replacement therapies. The bioengineered tooth, which was erupted and occluded, had the correct tooth structure, hardness of mineralized tissues for mastication, and response to noxious stimulations such as mechanical stress and pain in cooperation with other oral and maxillofacial tissues. This study represents a substantial advance and emphasizes the potential for bioengineered organ replacement in future regenerative therapies.
Keywords: regenerative therapy, transplantation
The current approaches being used to develop future regenerative therapies are influenced by our understanding of embryonic development, stem cell biology, and tissue engineering technology (1–4). One of the more attractive concepts under consideration in regenerative therapy is stem cell transplantation of enriched or purified tissue-derived stem cells (5), or in vitro manipulated embryonic stem (ES) and induced pluripotent stem (iPS) cells (6, 7). This therapy has the potential to restore the partial loss of organ function by replacing hematopoietic stem cells in hematopoietic malignancies (8), neural stem cells in Parkinson's disease (9), mesenchymal stem cells in myocardial infarction (10), and hepatic stem cells in cases of hepatic insufficiency (11).
The ultimate goal of regenerative therapy is to develop fully functioning bioengineered organs that can replace lost or damaged organs following disease, injury, or aging (4, 12–14). The feasibility of this concept has essentially been demonstrated by successful organ transplantations for various injuries and diseases (15). It is expected that bioengineering technology will be developed for the reconstruction of fully functional organs in vitro through the precise arrangement of several different cell species. However, these technologies have not yet achieved 3-dimensional reconstructions of fully functioning organs. To achieve the functional replacement of lost or damaged tissues and organs, the development of 3-dimensional bioengineered tissues comprising a single cell type is now being attempted using biodegradative materials (3), appropriate cell aggregation (16), or uniform cell sheets (17). These are now clinically applied for corneal dysfunction (18), myocardial infarction (19), and hepatic insufficiency (20) using oral mucosal epithelial cells, myocardial cells, and liver cells, respectively, with favorable clinical results.
A concept has also now been proposed to develop a bioengineered organ by reproducing the developmental processes during organogenesis (13, 21, 22). Almost all organs arise from their respective germs through reciprocal interactions between the epithelium and mesenchyme in the developing embryo (23–25). Therefore, it is predicted that a functional bioengineered organ could be produced by reconstituting organ germs between epithelial and mesenchymal cells in vitro, although the existence of organ-inductive stem cells in the adult body has not been fully elucidated yet with the exception of hair follicles (26) and the mammary gland (27). Tooth replacement regenerative therapy, which is also induced by typical reciprocal epithelial, and mesenchymal interactions (25, 28), is thought to be a feasible model system to evaluate the future clinical application of bioengineered organ replacement (13, 21). The strategy to develop a bioengineered third tooth after the loss of deciduous and permanent teeth is to properly reproduce the processes which occur during embryonic development through the reconstitution of a bioengineered tooth germ in vitro (21). We have recently developed a method for creating 3-dimensional bioengineered organ germ, which can be used as an ectodermal organ such as the tooth or whisker follicle (29). Our analyses have provided an effective method for reconstituting this organ germ and raised the possibility of tooth replacement with integrated blood vessels and nerve fibers in an adult oral environment (29). However, it remains to be determined whether a bioengineered tooth can achieve full functionality, including sufficient masticatory performance, biomechanical cooperation with tissues in the oral and maxillofacial regions, and proper responsiveness via sensory receptors to noxious stimulations in the maxillofacial region. There are currently no published reports describing successful replacement with a fully functional bioengineered organ.
In our current study, we describe a fully functioning tooth replacement achieved by transplantation of a bioengineered tooth germ into the alveolar bone of a lost tooth region in an adult mouse. We propose this as a model for future organ replacement therapy. The bioengineered tooth, which was erupted and reached occlusion in the oral environment, had the correct tooth structure, hardness of mineralized tissues for mastication, and responsiveness to experimental orthodontic treatment and noxious stimulation in cooperation with tissues in the oral and maxillofacial regions. Our results thus demonstrate the potential of bioengineered organ replacement for use in future regenerative therapies.
Results
Eruption and Occlusion of a Bioengineered Tooth.
We first investigated whether a bioengineered molar tooth germ, which was reconstituted from embryonic day 14.5 (ED14.5) molar tooth germ-derived epithelial and mesenchymal cells by our previously developed organ germ method, could erupt and reach occlusion with an opposing tooth in the mouse adult oral environment (Fig. 1A). After 5–7 days in an organ culture, a single bioengineered molar tooth germ, which had developed at the early bell stage of a natural tooth germ and was with a mean length of 534.4 ± 45.6 μm (Fig. 1B), was then transplanted with the correct orientation into a properly-sized bony hole in the upper first molar region of the alveolar bone in an 8-week-old adult murine lost tooth transplantation model. In this model, the upper first molar had been extracted, and the resulting wounds had been allowed to heal for 3 weeks (Fig. 1A and Fig. S1A). The cusp tip of the bioengineered tooth was exposed into the oral cavity at 36.7 ± 5.5 days after transplantation at a frequency of 34/60 (56.6%) (Fig. 1C Center and Fig. S1 B–D Center). In current transplantation model, the non-erupted explants also occurred at low frequency and were due to the microsurgery for the transplantation, such as transplantation with the reverse direction or the falling off the explants. The vertical dimension of the tooth crown continually increased and the bioengineered tooth finally reached the plane of occlusion with the opposing lower first molar at 49.2 ± 5.5 days after transplantation (Fig. 1C Right, and Fig. S1 B–D Right and E). During the course of eruption and occlusion, the alveolar bone at the bony hole gradually healed in the areas around the bioengineered tooth and the regenerated tooth had sufficient periodontal space between itself and the alveolar bone (Fig. 1D and Fig. S1D). The bioengineered tooth also formed a correct structure comprising enamel, ameloblast, dentin, odontoblast, dental pulp, alveolar bone, and blood vessels (Fig. 1D). It is known that mice have a considerable amount of cellular cementum that increases in thickness both on the sides of the roots and in the interradicular area and forms around the apex of the molar roots (30). The fully occluded bioengineered tooth was also observed to have a large amount of cellular cementum that was equivalent to a normal murine molar tooth (Fig. 1D and Fig. S1A). The root of the bioengineered tooth was also observed to be surrounded by sufficient periodontal ligaments (PDL) (Fig. 1D). Observations of the bioengineered tooth morphology revealed that the crown had plural cusp structure. The lengths and crown widths of the erupted bioengineered teeth were 1,474.4 ± 115.1 and 690.7 ± 177.7 μm, respectively. However, the bioengineered tooth was smaller than the other normal teeth, since at present we cannot regulate the crown width, cusp position, and tooth patterning including anterior/posterior and buccal/lingual structures using in vitro cell manipulation techniques.
Fig. 1.
Eruption and occlusion of a bioengineered tooth. (A) Schematic representation of the transplantation technology used for the generation of reconstituted tooth germ. (B) Phase contrast image of bioengineered tooth germ on day 5 of an organ culture. (Scale bar, 200 μm.) (C) Oral photographs of a bioengineered tooth during eruption and occlusion processes, including before eruption (Left), immediately after eruption (Center), and full occlusion (Right). (Scale bar, 200 μm.) (D) Histological analysis of the bioengineered tooth during the eruption and occlusion processes, including before eruption (Left), immediately after eruption (Center), and full occlusion (Right). (Scale bar, 100 μm.) (E) Oral photograph of a bioengineered tooth reconstituted using a combination of epithelial cells from normal mice and mesenchymal cells from GFP-transgenic mice (GFP bioengineered tooth). A merged image is shown. (Scale bar, 200 μm.) (F) A sectional image of a GFP bioengineered tooth. Fluorescent and DIC images are merged. (Scale bar, 100 μm.) (G) Oral photographs showing occlusion of normal (Upper) and bioengineered (Lower) teeth. (Scale bar, 200 μm.) (H) MicroCT images of the occlusion of normal (Left) and bioengineered (Right) teeth. External (Left) and cross section (Right) images are shown. The bioengineered tooth is indicated by the arrowhead.
We also transplanted green fluorescence protein (GFP)-labeled bioengineered tooth germ, which was reconstituted by normal epithelial cells and the mesenchymal cells from GFP-transgenic mice into non-transgenic mice as described above (29). A GFP-labeled bioengineered tooth was produced and could be observed in the bony hole in the alveolar bone of adult mice (Fig. 1E and Fig. S1F). GFP-positive mesenchymal cells were also detectable both in the odontoblasts and in the dental pulp and PDL, which differentiate from the dental papilla and dental follicle cells, respectively (Fig. 1F). Green fluorescence was also observed in the dentinal tubules of the GFP-positive odontoblasts in the regenerated tooth (Fig. 1F Lower).
We next investigated the gene expression profiles of colony-stimulating factor 1 (Csf1) and parathyroid hormone receptor (Pthr1), which are thought to regulate osteoclastogenesis during tooth eruption (31). Those genes were detectable in the eruption pathway and at the boundary surface between the dental follicle of the bioengineered tooth and osseous tissues, as is seen in normal teeth (Fig. S2). These observations suggest that the eruption of the bioengineered tooth germ faithfully reproduced the molecular mechanisms involved in the normal tooth eruption process.
We next analyzed the occlusion established between the bioengineered tooth and the opposing lower teeth. We often observed that the bioengineered tooth moved physiologically before achieving the occlusion during the transplantation experiments. The regenerated tooth achieved normal occlusion in harmony with other teeth in the recipient animal and had opposing cuspal contacts that maintained the proper occlusal vertical dimensions between the opposing arches (Fig. 1 G and H, and Fig. S1 B–E). Following the achievement of occlusion at 49.2 ± 5.5 days after transplantation, there was no excessive increase in the tooth length or perforation of the maxillary sinus by the erupted bioengineered tooth at up to 120 days after transplantation. These results indicated that the bioengineered tooth moved in response to mechanical stress and achieved functional occlusion with the opposing natural tooth.
Masticatory Potential of the Bioengineered Tooth.
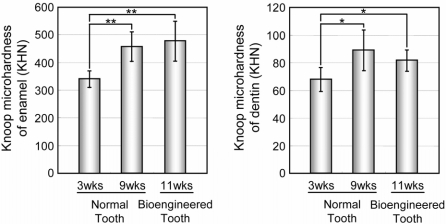
The masticatory potential of a bioengineered tooth is essential for achieving proper tooth function (32). We thus performed a Knoop hardness test, which is a test for mechanical hardness and is used in particular for very brittle materials or thin sheets. This was an important parameter for evaluating masticatory functions in our bioengineered tooth, including both the dentin and the enamel components. The Knoop hardness of both the enamel and dentin of normal teeth in 3-week-old and 9-week-old mice significantly increases in according to the postnatal period (Fig. 2). These values for enamel and dentin in the normal teeth of 9-week-old adult mice were measured at 447.7 ± 88.9 and 88.4 ± 10.2 Knoop hardness number (KHN), respectively (Fig. 2). The same measurements in the bioengineered tooth were 461.1 ± 83.2 and 81.4 ± 7.53 KHN, respectively (Fig. 2). These findings indicated that the hardness of the bioengineered tooth is in the normal range.
Fig. 2.
Assessment of the hardness of the bioengineered tooth. Knoop microhardness values of the enamel (Left) and dentin (Right) of the bioengineered tooth at 11-weeks post transplantation were compared with those of normal teeth from 3- and 9-week-old mice. Error bars show the standard deviation (n = 3). P < 0.001 (*) and <0.0001 (**) was regarded as statistically significant (t test).
Bioengineered Tooth Response to Mechanical Stress.
It has been postulated that regeneration of a fully functional tooth could be achieved by fulfilling critical functions in an adult oral environment such as the cooperation of the bioengineered tooth with the oral and maxillofacial regions through the PDL (31, 33). Histochemical analysis of the PDL of our bioengineered tooth (Fig. 1D) showed a positive connection between this tooth and the alveolar bone, and suggesting that this tooth may be responsive to mechanical stress. It has been demonstrated previously that alveolar bone remodeling is induced via the response of the PDL to mechanical stress such as the treatment of orthodontic movements (31, 33). These same studies have further demonstrated that the localization of osteoclasts for bone resorption and osteoblasts for bone formation can be observed in the area of compression and on the tension side, respectively (31, 33). Thus, we analyzed the movement of our bioengineered tooth and also the osteoclast and osteoblast localization for remodeling in the alveolar bone by inducing orthodontic movements experimentally.
When the bioengineered tooth was moved buccally for 17 days with a mechanical force in an experimental tooth movement model, it performed as well as a normal tooth (Fig. 3A and Fig. S3). Histochemical analysis additionally revealed morphological changes in the PDL in both the sides containing lingual tension and buccal compression following 6 days of treatment (Fig. 3A and Fig. S3). Osteoblast-like cells, which have a cuboidal shape and rounded nuclei, and osteoclast-like cells, which are multinucleated giant cells, were observed on the surface of the alveolar bone within the tension and compression sides, respectively (Fig. 3A and Fig. S3). During experimental tooth movement, tartrate-resistant acid phosphatase (TRAP)-positive staining was observed in the multinucleated cells, indicating that osteoclast-like giant cells were dominant on the compression side (Fig. 3B). In contrast, the localization of osteocalcin (Ocn) mRNA-positive cells was observed in the cells on the tension side, indicating that osteoblast-like cells were dominant (Fig. 3B). A fluorescent double-labeling experiment using calcein and tetracycline further showed that incorporation of these reagents into the alveolar bone on the tension side, but not the compression side, was clearly observable in the double-labeled line within 10 days of the orthodontic treatment (Fig. 3C). These findings suggest that the PDL of the bioengineered tooth successfully mediates bone remodeling via the proper localization of osteoclasts and osteoblasts in response to mechanical stress.
Fig. 3.
Experimental tooth movement. (A) Horizontal sections of the root of a normal tooth (Upper) and a bioengineered tooth (Lower) were analyzed by hematoxylin-eosin staining (HE) at days 0 (Left), 6 (Center), and 17 (Right) of experimental orthodontic treatment. (Scale bar, 100 μm.) (B) Sections of a normal and bioengineered tooth were analyzed by TRAP staining and in situ hybridization of Ocn at day 6 of the orthodontic treatment. TRAP-positive cells (arrow) and Ocn mRNA-positive cells (arrowhead) are indicated. (Scale bar, 100 μm.) (C) The root of the bioengineered tooth was analyzed for bone formation. The image in the box in Left is shown at higher magnification in Right. Tetracycline (arrowhead) and calcein (arrow) labeling was detectable on the tension side. (Scale bar, 50 μm.)
Perceptive Potential of Neurons Entering the Tissue of the Bioengineered Tooth.
The perception of noxious stimulations such as mechanical stress and pain, are important for the protection and proper functions of teeth (34). Neurons in the trigeminal ganglion, which innervate the pulp and PDL, can detect these stress events and transduce the corresponding perceptions to the central nervous system (34). We have previously reported that nerve fibers are detectable in the pulp of a developing bioengineered tooth in the oral cavity (29). In our current experiments, we evaluated the responsiveness of nerve fibers in the pulp and PDL of the bioengineered tooth to induced noxious stimulations.
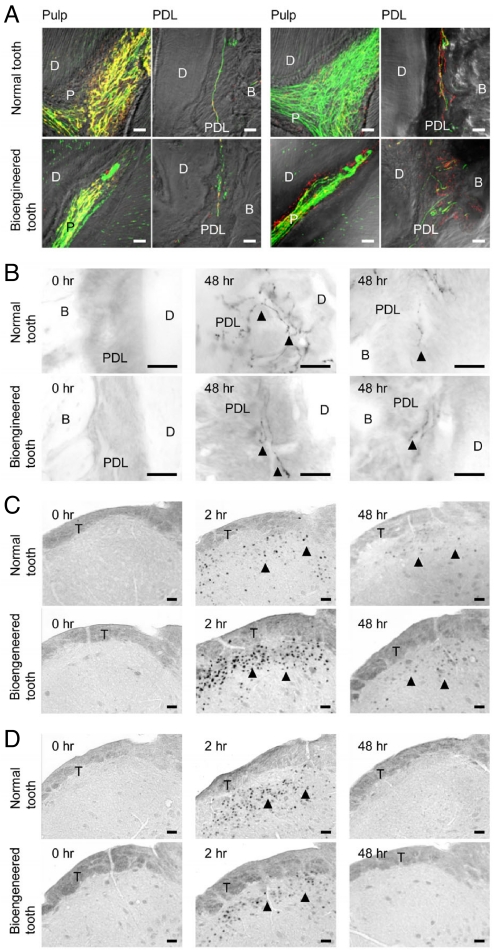
Anti-neurofilament (NF)-immunoreactive nerve fibers were detected in the pulp, dentinal tubules, and PDL of the bioengineered tooth as in a normal tooth (Fig. 4A and Fig. S4). Neuropeptide Y (NPY), which is synthesized in sympathetic nerves (34), was also detected in the pulp and PDL neurons (Fig. 4A and Fig. S4 C and D). Calcitonin gene-related peptide (CGRP), which is synthesized in sensory nerves and is involved in sensing tooth pain (34) was also observed in both pulp and PDL neurons (Fig. 4A and Fig. S4 E and F). NPY and CGRP were detected in both the anti-NF positive and negative-immunoreactive neurons (Fig. 4A and Fig. S4 C–F).
Fig. 4.
Pain response to mechanical stress. (A) Nerve fibers in the pulp and PDL in the normal (Upper) and bioengineered (Lower) tooth were analyzed immunohistochemically using specific antibodies for the combination (left 2 columns) of NF (green) and NPY (red) and the combination (right 2 columns) of NF and CGRP (red). (Scale bar, 25 μm.) (B) Analysis of galanin immunoreactivity in the PDL of a normal (Upper) and bioengineered (Lower) tooth for the assessment of orthodontic force. No galanin expression was evident in the untreated tooth (Left). Galanin expression (arrowhead) was detected in the PDL of a normal and bioengineered tooth after 48 h of orthodontic treatment (Right). (Scale bar, 25 μm.) (C) Analysis of c-Fos-immunoreactivity in the medullary dorsal horn of mice with a normal tooth (Upper) or a bioengineered tooth (Lower) after 0 h (Left), 2 h (Center), and 48 h (Right) of orthodontic treatment. c-Fos expression (arrowhead) was also detected. (Scale bar, 50 μm.) (D) Analysis of c-Fos immunoreactivity in the medullary dorsal horn of mice with a normal tooth (Upper) or a bioengineered tooth (Lower) after 0 h (Left), 2 h (Center), and 48 h (Right) of stimulation by pulp exposure. c-Fos expression (arrowhead) was evident in the medullary dorsal horn after 2 and 48 h of pulp exposure. (Scale bar, 50 μm.) D, dentin; P, pulp; B, bone; PDL, periodontal ligament; T, spinal trigeminal tract.
We next evaluated the perceptive potential of these neurons in the bioengineered tooth against noxious stimulations such as orthodontic treatment and pulp stimulation. The expression of galanin, which is a neuropeptide involved in pain transmission (35), increased in response to persistent painful stimulation of the nerve terminals within the PDL of the bioengineered tooth to the same extent as in a normal tooth (Fig. 4B). Thus PDL nerve fibers in the bioengineered tooth appear to respond to nociceptive stimulation caused by our experimental tooth movements. Previous studies have reported that neurons expressing the proto-oncogene c-Fos protein are detectable in the superficial layers of the medullary dorsal horn following noxious stimulations such as electrical, mechanical and chemical stimulation of intraoral receptive fields involving the tooth pulp, PDL, and peripheral nerves innervating the intraoral structures (34, 35). We found in our current analyses that the c-Fos-immunoreactive neurons present in both the normal tooth and the bioengineered tooth drastically increased at 2 h after experimental tooth movement, and then gradually decreased within 48 h (Fig. 4C). Following pulp stimulation, positive neurons in both normal and bioengineered teeth also increased at 2 h after stimulation, but could not be detected at 48 h (Fig. 4D). These data indicate that the nerve fibers innervating both the pulp and PDL of the bioengineered tooth have perceptive potential for nociceptive stimulations and can transduce these events to the central nervous system (the medullary dorsal horn).
Discussion
We successfully demonstrate herein that our bioengineered tooth germ develops into a fully functioning tooth with sufficient hardness for mastication and a functional responsiveness to mechanical stress in the maxillofacial region. We also show that the neural fibers that have re-entered the pulp and PDL tissues of the bioengineered tooth have proper perceptive potential in response to noxious stimulations such as orthodontic treatment and pulp stimulation. These findings indicate that bioengineered tooth generation techniques can contribute to the rebuilding of a fully functional tooth.
Critical issues in tooth regenerative therapy are whether the bioengineered tooth can reconstitute functions such as mastication (32) and responsive potential to mechanical stress (31, 33) and noxious stimulations (34), including cooperation of the regenerated tooth with both the oral and maxillofacial regions. Eruption and occlusion are essential first steps toward dental organ replacement therapy and successful incorporation into the oral and maxillofacial region (21, 36). Our laboratory has demonstrated previously that a bioengineered tooth germ can develop into a tooth with the correct structure in an adult mouse (29). It has also been reported previously that normal tooth germ isolated from murine embryos and a bioengineered tooth constructed from cultured tooth bud cells can develop and erupt in a toothless oral soft tissue region (diastema) of adult mice and in the tooth extraction sockets of an adult rat (37–42). In our current study, we provide evidence that a bioengineered tooth with the same hardness as an adult natural tooth can erupt with normal gene expression, including Csf1 and Pthr1, which are thought to regulate osteoclastogenesis, and achieve functional occlusion with the opposing natural teeth. Previous reports have suggested that the eruption of tooth germ is generally induced at the site of tooth development and by the gubernacular cord, which is derived from the epithelium of the dental lamina (43). Hence, our findings provide significant insights into tooth eruption mechanisms and strongly suggest that masticatory potential can be successfully restored by the transplantation of bioengineered tooth germ.
To establish cooperation between the bioengineered tooth and the maxillofacial region, 1 critical issue to address is whether a functional PDL is achieved and thereby the restoration of interactions between the bioengineered tooth and the alveolar bone (31, 33). The PDL has essential roles in tooth support, homeostasis, and repair, and is involved in the regulation of periodontal cellular activities such as cell proliferation, apoptosis, the secretion of extracellular matrices, the resorption and repair of the root cementum, and remodeling of the alveolar bone proper (31, 33). Although implant therapy has been established and is effective for replacement of a missing tooth, this therapy involves osseointegration into the alveolar bone that does not reconstitute the PDL (44). The regeneration of PDL has been studied previously using cell sheets (17) and stem cells (22), but has not yet been fully successful. It is thought that orthodontic tooth movement, a process involving pathogenic and physiologic responses to extreme forces applied to a tooth through bone remodeling controlled by osteogenesis and osteoclastgenesis (31), is a good assay model for the evaluation of PDL functions. In our present study, the PDL associated with the bioengineered tooth performed in complete cooperation with the oral and maxillofacial regions and bone remodeling successfully occurred following the application of orthodontic mechanical force. These findings indicate that it is possible to restore and re-establish cooperation between the bioengineered tooth and maxillofacial regions and thus regenerate critical dental functions.
The peripheral nervous system plays important roles in the regulation of organ functions and the perception of external stimuli such as pain and mechanical stress (45). During development of the peripheral nervous system, growing axons navigate and establish connections to their developing target organs (46). The recovery of the nervous system, which is associated with the reentry of nerve fibers, is critical for organ replacement (47). Although the functions of several internal organs, including the liver, kidney, and pancreas, are also mediated by specific humoral factors such as hormones and cytokines via blood circulation (45), perceptions of external stimuli are also essential to the functions of several organs, such as the eye, limbs, and teeth (45). The tooth is well recognized as a peripheral target organ for sensory trigeminal nerves, which are required for the function and protection of the teeth (46). It is known also that the perception of mechanical forces during mastication is limited in implant patients (48). Thus, the restoration of nerve functions is also critical for tooth regenerative therapy and future organ replacement therapy (13, 45). In our current study, we demonstrate that several species of nerve fibers, including NF, NPY, CGRP, and galanin-immunoreactive neurons, successfully re-entered both the pulp and/or PDL region of the bioengineered tooth. These nerves could thereby transduce the signals from noxious stimulations such as mechanical stress by orthodontic treatment and the exposure of pulp. Previous studies have also revealed that trigeminal nerve fibers navigate and establish their axonal projections into the pulp and PDL during early tooth development in a spatiotemporally controlled manner through expression of regulatory factors such as nerve growth factor, glial cell line-derived neurotrophic factor, and semaphorin 3a (46). Our present results suggest the possibility that the transplantation of regenerated tooth germ can induce trigeminal axon innervation and establishment in an adult jaw through the replication of trigeminal axon pathfinding and nerve fiber patterning during early tooth development (46).
In conclusion, this study provides evidence of a successful replacement of an entire and fully functioning organ in an adult body through the transplantation of bioengineered organ germ, reconstituted by single cell manipulation in vitro. Our study therefore makes a substantial contribution to the development of bioengineering technology for future organ replacement therapy. Further studies on the identification of available adult tissue stem cells for the reconstitution of a bioengineered tooth germ and the regulation of stem cell differentiation into odontogenic cell lineage will help to achieve the realization of tooth regenerative therapy for missing teeth.
Methods
Transplantation.
The upper first molars of 5-week-old C57BL/6 (SLC) mice were extracted under deep anesthesia. Mice were maintained for 3 weeks to allow for natural repair of the tooth cavity and oral epithelium. Before transplantation, we confirmed using microCT analysis that the remaining tooth root components and/or the tooth that had developed from them could not be observed in the bony holes (SI Methods). Following repair, an incision of approximately 1.5 mm in length was made through the oral mucosa at the extraction site with fine scissors to access the alveolar bone. A fine pin vice (Tamiya) was used to create a bony hole of about 0.5–1.0 mm in diameter in the exposed alveolar bone surface. Just before transplantation, we removed the collagen gel from the bioengineered tooth germ in the in vitro organ culture and marked the top of the dental epithelium with vital staining dye, such as methylene blue, to ensure the correct direction of the explants. The explants were then transplanted into the bony hole according to the dye. The incised oral mucosa was next sutured with 8–0 nylon (8–0 black nylon 4 mm 1/2R, Bear Medic Corp.) and the surgical site was cleaned. The mice containing the transplants were fed a powdered diet (Oriental Yeast) and skim milk until the regenerated tooth had erupted.
Supplementary Material
Acknowledgments.
We thank Dr. Masaru Okabe (Osaka University) for kindly providing the C57BL/6-TgN (act-EGFP) OsbC14-Y01-FM131 mice. We are also grateful to Dr. Toru Deguchi and Dr. Masahiro Seiryu (Tohoku University) for their analysis of the tooth perceptive potential; Dr. Nobuo Takeshita and Dr. Yuichi Sakai (Tohoku University) for analysis of experimental tooth movement; Dr. Masahiro Saito for critical reading of this manuscript and valuable discussions; and Mr K. Koga (Carl Zeiss) for providing technical support for our microscopic observations. This work was partially supported by Health and Labour Sciences Research Grants from the Ministry of Health, Labour, and Welfare (No. 21040101) to S.K. and T.T., a Grant-in Aid for Scientific Research in Priority Areas (No. 50339131) to T.T., a Grant-in-Aid for Scientific Research (A) and by an “Academic Frontier” Project for Private Universities to T.T. (2003–2007) from Ministry of Education, Culture, Sports and Technology, Japan.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0902944106/DCSupplemental.
References
- 1.Brockes JP, Kumar A. Appendage regeneration in adult vertebrates and implications for regenerative medicine. Science. 2005;310:1919–1923. doi: 10.1126/science.1115200. [DOI] [PubMed] [Google Scholar]
- 2.Watt FM, Hogan BL. Out of Eden: Stem cells and their niches. Science. 2000;287:1427–1430. doi: 10.1126/science.287.5457.1427. [DOI] [PubMed] [Google Scholar]
- 3.Langer RS, Vacanti JP. Tissue engineering: The challenges ahead. Sci Am. 1999;280:86–89. doi: 10.1038/scientificamerican0499-86. [DOI] [PubMed] [Google Scholar]
- 4.Atala A. Tissue engineering, stem cells and cloning: Current concepts and changing trends. Expert Opin Biol Ther. 2005;5:879–892. doi: 10.1517/14712598.5.7.879. [DOI] [PubMed] [Google Scholar]
- 5.Korbling M, Estrov Z. Adult stem cells for tissue repair–a new therapeutic concept? N Engl J Med. 2003;349:570–582. doi: 10.1056/NEJMra022361. [DOI] [PubMed] [Google Scholar]
- 6.Chien KR, Moretti A, Laugwitz KL. Development. ES cells to the rescue. Science. 2004;306:239–240. doi: 10.1126/science.1104769. [DOI] [PubMed] [Google Scholar]
- 7.Nishikawa S, Goldstein RA, Nierras CR. The promise of human induced pluripotent stem cells for research and therapy. Nat Rev Mol Cell Biol. 2008;9:725–729. doi: 10.1038/nrm2466. [DOI] [PubMed] [Google Scholar]
- 8.Copelan EA. Hematopoietic stem-cell transplantation. N Engl J Med. 2006;354:1813–1826. doi: 10.1056/NEJMra052638. [DOI] [PubMed] [Google Scholar]
- 9.Lindvall O, Kokaia Z. Stem cells for the treatment of neurological disorders. Nature. 2006;441:1094–1096. doi: 10.1038/nature04960. [DOI] [PubMed] [Google Scholar]
- 10.Segers VF, Lee RT. Stem-cell therapy for cardiac disease. Nature. 2008;451:937–942. doi: 10.1038/nature06800. [DOI] [PubMed] [Google Scholar]
- 11.Wang X, et al. The origin and liver repopulating capacity of murine oval cells. Proc Natl Acad Sci USA. 2003;100:11881–11888. doi: 10.1073/pnas.1734199100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Griffith LG, Naughton G. Tissue engineering–current challenges and expanding opportunities. Science. 2002;295:1009–1014. doi: 10.1126/science.1069210. [DOI] [PubMed] [Google Scholar]
- 13.Ikeda E, Tsuji T. Growing bioengineered teeth from single cells: Potential for dental regenerative medicine. Expert Opin Biol Ther. 2008;8:735–744. doi: 10.1517/14712598.8.6.735. [DOI] [PubMed] [Google Scholar]
- 14.Purnell B. New release: The complete guide to organ repair. Introduction. Science. 2008;322:1489. doi: 10.1126/science.322.5907.1489. [DOI] [PubMed] [Google Scholar]
- 15.Lechler RI, Sykes M, Thomson AW, Turka LA. Organ transplantation–how much of the promise has been realized? Nat Med. 2005;11:605–613. doi: 10.1038/nm1251. [DOI] [PubMed] [Google Scholar]
- 16.Layer PG, Robitzki A, Rothermel A, Willbold E. Of layers and spheres: The reaggregate approach in tissue engineering. Trends Neurosci. 2002;25:131–134. doi: 10.1016/s0166-2236(00)02036-1. [DOI] [PubMed] [Google Scholar]
- 17.Yang J, et al. Reconstruction of functional tissues with cell sheet engineering. Biomaterials. 2007;28:5033–5043. doi: 10.1016/j.biomaterials.2007.07.052. [DOI] [PubMed] [Google Scholar]
- 18.Nishida K, et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N Engl J Med. 2004;351:1187–1196. doi: 10.1056/NEJMoa040455. [DOI] [PubMed] [Google Scholar]
- 19.Miyahara Y, et al. Monolayered mesenchymal stem cells repair scarred myocardium after myocardial infarction. Nat Med. 2006;12:459–465. doi: 10.1038/nm1391. [DOI] [PubMed] [Google Scholar]
- 20.Ohashi K, et al. Engineering functional two- and three-dimensional liver systems in vivo using hepatic tissue sheets. Nat Med. 2007;13:880–885. doi: 10.1038/nm1576. [DOI] [PubMed] [Google Scholar]
- 21.Sharpe PT, Young CS. Test-tube teeth. Sci Am. 2005;293:34–41. doi: 10.1038/scientificamerican0805-34. [DOI] [PubMed] [Google Scholar]
- 22.Duailibi SE, Duailibi MT, Vacanti JP, Yelick PC. Prospects for tooth regeneration. Periodontol 2000. 2006;41:177–187. doi: 10.1111/j.1600-0757.2006.00165.x. [DOI] [PubMed] [Google Scholar]
- 23.Kuure S, Vuolteenaho R, Vainio S. Kidney morphogenesis: Cellular and molecular regulation. Mech Dev. 2000;92:31–45. doi: 10.1016/s0925-4773(99)00323-8. [DOI] [PubMed] [Google Scholar]
- 24.Hogan BL, Yingling JM. Epithelial/mesenchymal interactions and branching morphogenesis of the lung. Curr Opin Genet Dev. 1998;8:481–486. doi: 10.1016/s0959-437x(98)80121-4. [DOI] [PubMed] [Google Scholar]
- 25.Pispa J, Thesleff I. Mechanisms of ectodermal organogenesis. Dev Biol. 2003;262:195–205. doi: 10.1016/s0012-1606(03)00325-7. [DOI] [PubMed] [Google Scholar]
- 26.Claudinot S, Nicolas M, Oshima H, Rochat A, Barrandon Y. Long-term renewal of hair follicles from clonogenic multipotent stem cells. Proc Natl Acad Sci USA. 2005;102:14677–14682. doi: 10.1073/pnas.0507250102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shackleton M, et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 28.Tucker A, Sharpe P. The cutting-edge of mammalian development; how the embryo makes teeth. Nat Rev Genet. 2004;5:499–508. doi: 10.1038/nrg1380. [DOI] [PubMed] [Google Scholar]
- 29.Nakao K, et al. The development of a bioengineered organ germ method. Nat Methods. 2007;4:227–230. doi: 10.1038/nmeth1012. [DOI] [PubMed] [Google Scholar]
- 30.Klingsberg J, Butcher EO. Comparative histology of age changes in oral tissues of rat, hamster, and monkey. J Dent Res. 1960;39:158–169. doi: 10.1177/00220345600390011101. [DOI] [PubMed] [Google Scholar]
- 31.Wise GE, King GJ. Mechanisms of tooth eruption and orthodontic tooth movement. J Dent Res. 2008;87:414–434. doi: 10.1177/154405910808700509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Manly RS, Braley LC. Masticatory performance and efficiency. J Dent Res. 1950;29:448–462. doi: 10.1177/00220345500290040701. [DOI] [PubMed] [Google Scholar]
- 33.Shimono M, et al. Regulatory mechanisms of periodontal regeneration. Microsc Res Tech. 2003;60:491–502. doi: 10.1002/jemt.10290. [DOI] [PubMed] [Google Scholar]
- 34.Byers MR, Narhi MV. Dental injury models: Experimental tools for understanding neuroinflammatory interactions and polymodal nociceptor functions. Crit Rev Oral Biol Med. 1999;10:4–39. doi: 10.1177/10454411990100010101. [DOI] [PubMed] [Google Scholar]
- 35.Deguchi T, Takeshita N, Balam TA, Fujiyoshi Y, Takano-Yamamoto T. Galanin-immunoreactive nerve fibers in the periodontal ligament during experimental tooth movement. J Dent Res. 2003;82:677–681. doi: 10.1177/154405910308200904. [DOI] [PubMed] [Google Scholar]
- 36.Yen AH, Sharpe PT. Regeneration of teeth using stem cell-based tissue engineering. Expert Opin Biol Ther. 2006;6:9–16. doi: 10.1517/14712598.6.1.9. [DOI] [PubMed] [Google Scholar]
- 37.Ohazama A, Modino SA, Miletich I, Sharpe PT. Stem-cell-based tissue engineering of murine teeth. J Dent Res. 2004;83:518–522. doi: 10.1177/154405910408300702. [DOI] [PubMed] [Google Scholar]
- 38.Song Y, Yan M, Muneoka K, Chen Y. Mouse embryonic diastema region is an ideal site for the development of ectopically transplanted tooth germ. Dev Dyn. 2008;237:411–416. doi: 10.1002/dvdy.21427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Duailibi SE, et al. Bioengineered dental tissues grown in the rat jaw. J Dent Res. 2008;87:745–750. doi: 10.1177/154405910808700811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang W, et al. Tissue engineered hybrid tooth-bone constructs. Methods. 2009;47:122–128. doi: 10.1016/j.ymeth.2008.09.004. [DOI] [PubMed] [Google Scholar]
- 41.Abukawa H, et al. Reconstructing mandibular defects using autologous tissue-engineered hybrid tooth and bone constructs. J Oral Maxillofac Surg. 2009;67:335–347. doi: 10.1016/j.joms.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 42.Duailibi MT, et al. Bioengineered teeth from cultured rat tooth bud cells. J Dent Res. 2004;83:523–528. doi: 10.1177/154405910408300703. [DOI] [PubMed] [Google Scholar]
- 43.Carollo DA, Hoffman RL, Brodie AG. Histology and function of the dental gubernacular cord. Angle Orthod. 1971;41:300–307. doi: 10.1043/0003-3219(1971)041<0300:HAFOTD>2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 44.Voruganti K. Clinical periodontology and implant dentistry, 5th edition. Br Dent J. 2008;205:216. [Google Scholar]
- 45.Guyton AC. Textbook of Medical Physiology. Am J Med Sci. 1967;253:126. [Google Scholar]
- 46.Luukko K, Kvinnsland IH, Kettunen P. Tissue interactions in the regulation of axon pathfinding during tooth morphogenesis. Dev Dyn. 2005;234:482–488. doi: 10.1002/dvdy.20586. [DOI] [PubMed] [Google Scholar]
- 47.Bengel FM, et al. Effect of sympathetic reinnervation on cardiac performance after heart transplantation. N Engl J Med. 2001;345:731–738. doi: 10.1056/NEJMoa010519. [DOI] [PubMed] [Google Scholar]
- 48.Hammerle CH, et al. Threshold of tactile sensitivity perceived with dental endosseous implants and natural teeth. Clin Oral Implants Res. 1995;6:83–90. doi: 10.1034/j.1600-0501.1995.060203.x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.