Abstract
We previously identified the aberrantly expressed cell cycle regulator cyclin B1 as a tumor antigen recognized by antibodies and T cells from patients with breast, lung, and head and neck cancers. Ordinarily expressed only transiently in the G2/M stage of the cell cycle in normal cells, cyclin B1 is constitutively expressed at high levels in the cytoplasm of these and many other tumor types, leading to its recognition by the cancer patient's immune system. We report here an unexpected observation that cyclin B1-specific antibody and memory CD4 and CD8 T cells are also found in many healthy individuals who have no history of cancer. Moreover, young as well as older healthy people have these responses suggesting that events other than cancer, which occur either early in life or throughout life, may lead to aberrant cyclin B1 expression and anti-cyclin B1 immunity. The role, if any, of immunity to this tumor-associated antigen is not known. We wanted to determine specifically whether immunity to cyclin B1 might be important in the immunosurveillance of cyclin B1+ tumors. We therefore tested in mice the effectiveness of vaccine-elicited anti-cyclin B1 immunity against a cyclin B1+ mouse tumor that was chosen based on our published observation that cyclin B1 overexpression is associated with the lack of p53 function. We found that cyclin B1 DNA prime-protein boost vaccine protected mice from a challenge with a tumor cell line that was established from a tumor arising in the p53−/− mouse that spontaneously overexpresses cyclin B1.
Keywords: cancer vaccines, human immunology, immunosurveillance, tumor immunology
Overexpression of cyclin B1 has been documented in many human cancers, including colorectal, lung, cervical, and head and neck carcinomas (1–5). Additionally, this overexpression has been shown to correlate with worse prognosis in lung, laryngeal, esophageal, and tongue cancers (1, 3, 5–8). Whereas cyclin B1 is expressed transiently in normal cells, in cancer cells, it is expressed constitutively in all stages of the cell cycle (9). Additionally, cancer-associated cyclin B1 overexpression is evident primarily in the cytoplasm where it can be subject to proteasomal processing and presentation in MHC class I. We reported isolation of cyclin B1 peptides from MHC class I molecules of tumor cells that were recognized by tumor-specific memory T cells present in patients with cyclin B1 overexpressing tumors (10). We later showed that patients with cancer and premalignant lesions that overexpress cyclin B1 have cyclin B1-specific antibodies of IgM, IgG and IgA isotypes (11).
Cyclin B1 overexpression in many tumors is secondary to the loss of p53 function either through p53 mutations or a deletion (9). Considering that alterations in p53 function happen in many tumors early in the process of transformation, cyclin B1 is a good candidate antigen for both immunotherapy and immunoprevention of a large number of human tumors. Additionally, because cyclin B1 is required for entry into mitosis, it is unlikely that a growing tumor could lose cyclin B1 expression and escape anti-cyclin B1 immunity.
While studying anti-cyclin B1 humoral responses in cancer patients, we discovered that many healthy individuals also had anti-cyclin B1 antibodies. To determine how frequently this may occur, and eventually to determine the potential significance of these immune responses, we collected blood samples from individuals in different age groups and with no known history of cancer. We found that in addition to antibodies, healthy individuals have both CD4+ and CD8+ antigen-experienced T cells specific for cyclin B1. This begged the question of whether these responses could be involved in immune surveillance of cyclin B1 overexpressing tumors. To directly test this, we used a transplantable cyclin B1 overexpressing tumor derived from a p53−/− mouse. We found that anti-cyclin B1 immunity elicited through a vaccine can inhibit tumor growth and significantly increase overall survival.
Results
Healthy Individuals Have Anti-Cyclin B1 Antibodies.
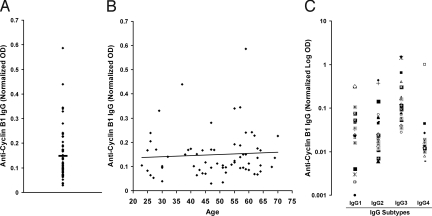
We studied individuals with no history of cancer to determine the prevalence of anti-cyclin B1 antibodies. Considering that there is a wide and continuous range of antibody titers in cancer patients, we wanted to find out whether this range would be recapitulated in the healthy population, or whether we would find instead individuals that either do or do not have anti-cyclin B1 antibody. Plasma was collected from 65 individuals ages 20–75 years and tested by ELISA for the presence of anti-cyclin B1 antibody. As shown in Fig. 1A, many healthy individuals had detectable levels of cyclin B1-specific IgG that ranged from very low to very high. Because even the lowest levels are positive values when calculated based on the antigen-negative background control values (as described in detail in the SI Text), we cannot determine where the cut-off point is between the true antibody positive and true antibody negative individuals and have chosen instead to divide the individuals as having antibody levels above or below the mean OD value of 0.149. Twenty-seven individuals, >40%, are found above the mean. Because cyclin B1 is overexpressed in cancer and the risk of neoplastic events is thought to increase with age, we sought to determine whether the prevalence or levels of anti-cyclin B1 IgG increased with age as well. We found no correlation between age and antibody levels in an adult population (Fig. 1B). To gain insight into the quality of cyclin B1-specific T cells that helped B cell isotype switching, we also looked for the predominant anti-cyclin B1 IgG subtypes. Although all subtypes were found, the anti-cyclin IgG was predominantly IgG3 (Fig. 1C), which has been suggested to be an indicator of Th1 T cell-mediated help (12, 13).
Fig. 1.
Healthy individuals have anti-cyclin B1 IgG. Plasma samples from healthy donors with no history of cancer (n = 65) were tested for anti-cyclin B1 IgG (dilution of 1:400) and IgG subtypes (1:20). (A) Anti-cyclin B1 IgG from all individuals was normalized to five standard controls. Bar, mean OD. (B) Levels of anti-cyclin B1 IgG are independent of age (Pearson correlation, P = 0.63). (C) Anti-cyclin B1 IgG subtypes were tested in 23 healthy individuals who had above the median levels of anti-cyclin B1 IgG. Each individual is represented by the same symbol in all four assays.
Healthy Individuals Have Cyclin B1-Specific T Cells.
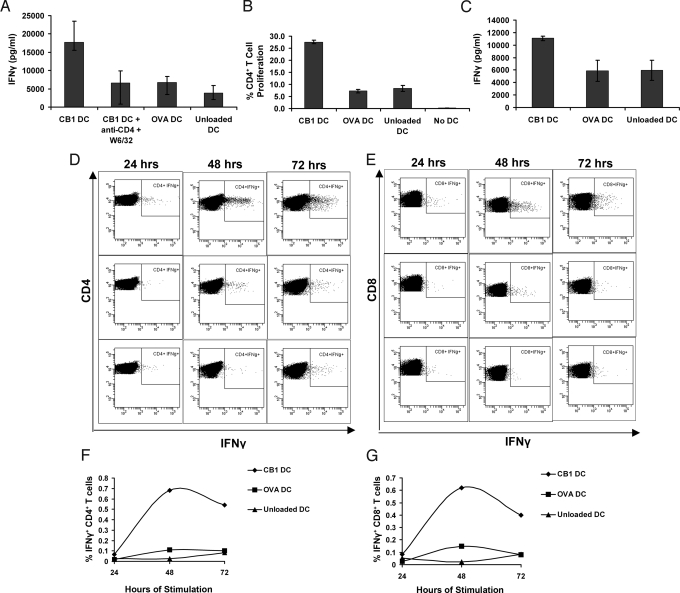
Because IgG is a T cell-dependent isotype, it can be expected, even though it is not always the case, that individuals positive for anti-cyclin B1 IgG would have cyclin B1 specific T cells. We had characterized cyclin B1 specific T-cell responses in cancer patients with cyclin B1 overexpressing tumors (10). Therefore, we sought to determine whether cyclin B1-specific T cells could be found in PBMC from healthy individuals. Plastic adherence was used to separate monocytes and grow dendritic cells (DCs), and lymphocytes were frozen until needed. DCs were loaded with cyclin B1, control antigen ovalbumin (OVA), or no antigen. DCs were then cultured in the presence of TNFα, IL-6, and PGE2 to promote their maturation before they were cocultured with autologous lymphocytes or with purified CD8+ T cells. Assessment of the culture supernatants by ELISA showed that lymphocytes from the healthy donor produced IFNγ in response to DCs presenting cyclin B1 but not OVA (Fig. 2A). This response depended on T-cell receptor signaling because blocking with antibodies against MHC class I and CD4 reduced IFNγ to control levels. PBL from the same individual were also labeled with CFSE before cocultuture with DC, and proliferation was assessed by flow cytometry on day seven. As shown in Fig. 2B, the CD4+ T cells proliferated significantly more in response to cyclin B1-loaded DCs than to DCs that were unloaded or loaded with OVA. We performed the same experiment with purified CD8+ T cells. After 10 days, the CD8+ T cells cocultured with cyclin B1-loaded DCs produced significantly more IFNγ than those with control DCs (Fig. 2C). Three additional healthy donors were tested with similar experimental protocols, and all three had cyclin B1-specific T-cell responses.
Fig. 2.
Healthy individuals have memory T cells specific for cyclin B1. (A) All T cells: Monocyte-depleted PBMC were cultured with autologous DCs that were loaded with ovalbumin (OVA), cyclin B1 (CB1), or unloaded. Supernatant from the seventh day of culture was tested by ELISA for IFNγ. W6/32: MHC class I blocking antibody. (B) CD4+ T cells: PBMC from the same donor as in A were labeled with CFSE and cultured with autologous DCs in the presence or absence of indicated antigen or without DC. Percentage of proliferating CD4+ T cells was assessed after seven days. (C) CD8+ T cells: CD8+ T cells were purified from PBMC (a second donor) and cultured with autologous DCs with and without indicated antigens. Supernatants were tested for IFNy after 10 days. Bars indicate standard deviation. (D–G) Brefeldin A was added for 11 h to one set of a triplicate culture at 6, 30, and 54 h after combination of DCs with PBL. After the incubation periods, CD4+ T cells (D and F) and CD8+ T cells (E and G) were assessed for intracellular IFNγ. (D and E) Flow cytometric measurement of IFNγ. (Top) PBL stimulated with cyclin B1-loaded DCs. (Center) PBL stimulated with OVA-loaded DCs. (Lower) PBL stimulated with unloaded DCs. (F and G) show a graphical representation of the percentage of IFNγ-positive cells for CD4+ (F) and CD8+ (G) T cells.
Cyclin B1-Specific T Cells in Healthy Individuals Are Antigen Experienced.
Because the T-cell responses we observed were after a week or longer of culture, which is theoretically sufficient to prime naïve cells, we designed additional experiments for detection of cyclin B1-specific T cells that had been primed in vivo. The PBL from the same healthy donor as in Fig. 2 A and B were cocultured with cyclin B1-loaded, OVA-loaded and unloaded DC; IFNγ production during the first, second, and third day of culture was assessed. Brefeldin A (BFA) was added to one set of the triplicate coculture for 11 h during the first day to trap newly synthesized proteins inside the cell by preventing their transport and secretion. Intracellular IFNγ accumulated during this 11 h incubation is indicated as the 24-h time period. (Fig. 2 D–G). The second and third set of the triplicates were then incubated with BFA for 11 h during the second and third day, indicated as the 48- and 72-h time periods, respectively. Already in the first 24 h, both CD4+ and CD8+ T cells produced more IFNγ in response to cyclin B1 than to controls. This effect was magnified over the 48- and 72-h periods.
Cyclin B1 Peptides Commonly Recognized by Human T Cells.
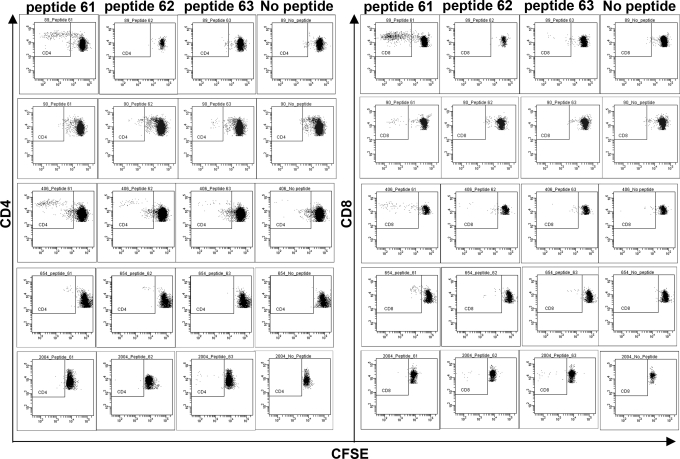
Cyclin B1 is a relatively large protein of 54 kDa and can be processed into numerous class I and class II restricted peptides that could serve as T-cell epitopes. We asked whether certain regions of the protein were more likely to be recognized by T cells, which might suggest a repertoire of preferred peptides to be used in vaccines to elicit or boost anti-tumor T-cell responses in patients. We used an overlapping cyclin B1 peptide library composed of peptides 12–15 aa in length, a size that has been shown to elicit both CD4+ and CD8+ T-cell responses (14, 15). We first tested pools of peptides (Fig. S1) and then individual peptides from pools that induced T-cell proliferation. Blood donors in these studies were healthy individuals enrolled in a lung cancer screening study who were confirmed to be free of lung cancer by CT scan. PBMC were isolated, labeled with CFSE, and cultured with peptide pools or individual peptides. Six days after stimulation, cells were tested for CFSE dilution. We identified three peptides, spanning amino acids 215–223, that stimulated T cells from multiple blood donors. Fig. 3 shows five individuals with CD4+ and CD8+ T cells that are specific for one or more of these three peptides.
Fig. 3.
Identification of commonly recognized cyclin B1 peptides. PBMC were labeled with CFSE and stimulated with 2 μg/mL recombinant cyclin B1 peptides. After six days of culture, PBMC were stained with cell surface markers and proliferation was assessed by flow cytometry. peptide 61: KFRLLQETMYMTVSI; peptide 62: LQETMYMTVSIIDRF; and peptide 63: MYMTVSIIDRFM.
Cyclin B1-Specific Immune Responses Can Prevent Tumor Growth.
Given that healthy individuals have both humoral and cellular immune responses that are specific for the self and tumor antigen cyclin B1, we explored the potential significance of having anti-cyclin B1 immunity before the onset of cancer. We used a transplantable mouse tumor model, a cyclin B1 overexpressing lymphoma cell line (LO2) that was derived from a p53−/− mouse and spontaneously overexpresses cyclin B1.
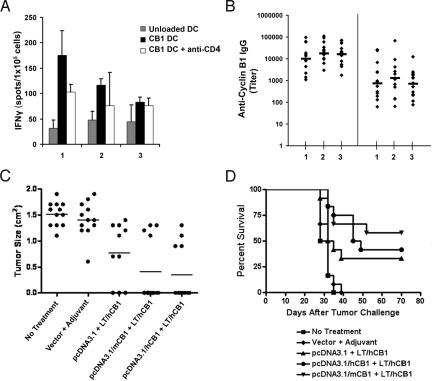
Because overexpressed cyclin B1 in tumors is neither mutated nor altered post-translationally, we expected that immune responses in healthy mice might be subject to self-tolerance and therefore difficult to elicit. For that reason we chose to test as immunogens both mouse (self) and human (85% homologous but could be considered nonself) cyclin B1 in 2 different forms, recombinant protein and cDNA. As adjuvant we chose transdermal delivery at the site of antigen injection of heat labile enterotoxin (LT) applied via an immunostimulatory (IS) patch (16, 17). The protein plus adjuvant vaccine elicited cyclin B1-specific CD4+ T cells and IgG, but there was no difference in tumor growth between vaccinated and unvaccinated groups challenged with LO2 (Fig. S2). In contrast, when we used the DNA prime-protein boost approach, we were able to induce effective anti-tumor immunity (Fig. 4). Fifteen mice per group were immunized with either pcDNA3.1 control vector (group 1) or gene expression vectors encoding either mouse cyclin B1 (group 2) or human cyclin B1 (group 3) cDNA. Three weeks and six weeks later, mice were boosted with recombinant human cyclin B1 protein followed by the application of the LT/IS patch, respectively. As controls we used untreated mice and mice primed with empty pcDNA3.1 vector and boosted with LT/IS patch only. Seventeen days after the last immunization, three mice per group were killed for assessment of in vitro T-cell responses and the remaining mice were challenged with LO2 tumor. Fig. 4A shows that the DNA prime-protein boost vaccination induces cyclin B1-specific T-cell responses that can only partially be blocked by anti-CD4 antibody (groups 2 and 3). These results implied successful priming of CD8+ T cells as well. The same results were obtained by boosting with mouse cyclin B1 protein (Fig. S3), and in those experiments we confirmed that cyclin B1 specific T-cell responses can also be blocked by anti-CD8 antibody. Cyclin B1 DNA prime-protein boost vaccination also successfully elicited both anti-human and anti-mouse cyclin B1 antibodies (Fig. 4B).
Fig. 4.
Cyclin B1 DNA prime-protein boost vaccination elicits cyclin B1-specific cellular and humoral responses and delays tumor growth. (A and B) Mice were primed with either pcDNA 3.1 empty vector (group 1), mouse cyclin B1 (mCB1, group 2), or human cyclin B1 (hCB1, group 3) cDNA. All three groups were boosted with human cyclin B1 recombinant protein and the LT/ IS patch two times in three-week intervals. (A) ELISPOT performed on mouse splenocytes. Error bars, SE. (B) ELISA for anti-human (Left) and anti-mouse (Right) cyclin B1 IgG. Bars, geometric mean. (C and D) Mice from groups 1, 2, and 3 with the addition of untreated and pcDNA3.1 empty vector + LT/IS patch controls were challenged with LO2 tumor cells. (C) Tumor growth on day 28 after tumor challenge. Bars, mean tumor size. (D) Survival after tumor challenge (Logrank test, P < 0.0001).
Importantly, the presence of anti-cyclin B1 immune responses before tumor challenge significantly delayed or completely prevented tumor growth. By day 28 after tumor challenge, groups that received the DNA prime-protein boost vaccine had significantly lower mean tumor volume (P < 0.0001) and significantly higher number of tumor-free mice (P = 0.0013) than no treatment and adjuvant only controls (Fig. 4C). By day 42, all mice in the control groups were killed due to excessive tumor burden whereas two mice in group 1, six mice in group 2, and five mice in group 3 remained tumor free. Fig. 4D shows that vaccination significantly enhanced survival. We saw no evidence of self-tolerance to mouse cyclin B1 because priming with mouse cDNA protected equally or slightly better than the human cyclin B1 DNA vaccine. Similar protection was observed when the mouse cyclin B1 DNA vaccine was boosted with mouse cyclin B1 protein (Fig. S3).
Discussion
We demonstrate here that healthy individuals with no history of cancer have an immune response against the self-protein cyclin B1 that was found to be abnormally expressed in tumor cells and thus characterized as a tumor associated antigen. Cyclin B1-specific IgG is present in many healthy individuals, independent of age. Furthermore, healthy individuals have cyclin B1-specific CD4+ and CD8+ memory T cells. There are many other self antigens that are expressed by normal adult tissues but abnormally expressed by tumors and thus considered tumor associated antigens. A partial list includes MUC1, expressed on normal ductal epithelial cells but overexpressed and hypoglycosylated on tumor cells (18); p53, expressed by normal cells undergoing genetic stress or apoptosis but constitutively overexpressed by tumor cells (19); melanocyte differentiation antigens overexpressed in melanoma cells (20); carcinoembryonic antigen (CEA), found in low levels in healthy colon (21) but overexpressed in tumor cells; the Bcl-2 family and other regulators of apoptosis (22); and cyclin B1, the subject of our studies.
Humoral and cellular immune responses elicited by overexpression, mislocalized expression, aberrant processing, or a combination of these, have been studied in cancer patients for evidence of their role in cancer immunosurveillance and disease outcome. We and others demonstrated that MUC1-specific antibodies are associated with a better prognosis in breast, pancreatic, lung, and ovarian cancer (23–26). Similarly, the presence of T cells in tumors has been associated with better prognosis in lung, ovarian, and colon cancer (27–29).
Although our study focused specifically on responses to tumor-associated antigens in healthy individuals, careful analysis of published papers dealing with these responses in cancer patients reveals that similar observations were made in the healthy controls in these studies but did not receive proper attention. T cells could be expanded in vitro for self antigens such as the melanoma-associated chondroitin sulfate proteoglycan (30), cytochrome p450 1B1 (31), survivin (32, 33), melanoma inhibitor of apoptosis protein (34), and tyrosinase related protein 1 (35). Additionally, detection of T cells specific for Melan-A/Mart-1 (CD8+, naïve), Her2/neu (CD8+, effector), CEA (CD4+, naïve/ignorant or suppressed), and wild-type p53 (CD8+, mostly CD45RA+) was possible even without in vitro expansion (36–39). These and similar studies focused primarily on the difference in responses to these molecules between healthy individuals and cancer patients or on showing that T cells were not centrally deleted and could potentially be expanded by vaccination. The question has not been asked how these responses are elicited in healthy people in the absence of cancer. We would like to propose that abnormal expression of self-molecules can occur during nonneoplastic events as well, such as infections and acute and chronic inflammation. We have published that several nonneoplastic events known to affect MUC1-expressing tissues, such as mumps virus infection of MUC1+ salivary glands or mastitis affecting MUC1+ breast ducts, correlate with the presence of anti-MUC1 antibody in healthy individuals (40). Compelling evidence for a nonneoplastic event eliciting immune responses to self antigens was obtained recently by studying mice recovering from viral infections (41). Mice infected with either vaccinia virus or lymphocytic choriomeningitis virus generated antibodies against the viral antigens against many host cell proteins involved in cell cycle progression and cell adhesion, some of which had been characterized as human tumor-associated antigens. Two recent reports show that infection of human fibroblasts with varicella zoster virus (VZV) (42) or human cytomegalovirus (HCMV) (43) induces overexpression of cyclin B1 in the cytoplasm of infected cells that strongly resembles its abnormal expression in cancer cells. In addition, this overexpressed cyclin B1 is packaged into VZV virions (44), potentially creating a natural viral vector for eliciting anti-cyclin B1 immunity. Anti-cyclin B1 immune memory developed in the context of this and potentially other viral infections could serve as effective immunosurveillance against tumors that overexpress cyclin B1.
The presence or induction of memory responses against self-antigens also known to be tumor associated antigens has been considered a case of broken self-tolerance. This has created an expectation that to elicit effective anti-tumor immunity, cancer vaccines must be able to break self-tolerance (45, 46). Although there is some evidence from clinical trials of cancer vaccines or other forms of immunotherapy that effective anti-tumor immunity sometimes correlates with autoimmunity (47, 48), most immune responses against tumor associated antigens do not recognize normal cells. We would like to propose that this is because the immune system maintains self-tolerance against the normal expression of these molecules and only responds to their abnormal expression brought about by infections or malignant transformation. Finding anti-cyclin B1 antibody and T cells in healthy humans suggests that this immune response if elicited or boosted should be safe and not expected to react against normal proliferating cells that have a normal pattern of cyclin B1 expression and regulation. Furthermore, our studies in mice show that anti-cyclin B1 immunity can be protective against tumors. Together, these data support further studies of cyclin B1 based vaccines as a useful strategy to boost preexisting anti-cyclin B1 immunity for cancer prevention in individuals who are at high risk for developing tumors that abnormally express cyclin B1.
Materials and Methods
The materials and methods for the synthesis of cyclin B1 recombinant proteins and cyclin B1 peptide library as well as the construction of mouse and human cyclin B1 pcDNA3.1 DNA vectors can be found in the SI Text.
Blood Donors.
Blood was collected from individuals ages 25–79 after receipt of informed consent. Blood collection at the University of Pittsburgh and the Rockefeller University was approved by the institutional review board of each university. Buffy coats were obtained from the Allegheny County Health Department Blood Bank. Blood used for peptide library stimulations was collected from individuals enrolled in a University of Pittsburgh biomarker study for lung cancer risk. Individuals for the biomarker study were ages 55–79, heavy smokers, and negative for lung cancer by computed tomography.
Measurement of Human Anti-Cyclin B1 Antibody Responses.
Anti-cyclin B1 antibody responses were measured using ELISA as detailed in the SI Text.
Generation and Loading of Dendritic Cells.
Dendritic cell generation is described in SI Text. DCs were loaded at 30 μg/mL for each protein. Both ovalbumin (OVA, Sigma) and cyclin B1 proteins were treated for LPS removal with Detoxi-Gel (Pierce, Thermo Fisher). After 4 h, a maturation mixture of 10 μg/mL TNFα (R&D), 10 μg/mL IL-6 (R&D), and 1 μg/mL PGE2 (Sigma) was added. DCs were allowed to mature for two days before use in assays.
T-Cell Stimulation with Dendritic Cells.
PBL remaining from monocyte isolation were either used directly in assays or subjected to CD8+ T-cell purification, following the manufacturer's protocol (Miltenyi). PBL or CD8+ T cells were plated with autologous, protein-loaded or unloaded DCs at a DC:T cell ratio of 1:10 in complete RPMI supplemented with 10% human serum (Gemini Bio-Products), 10 ng/mL IL-12 (R&D), and 5 ng/mL IL-6 (R&D). PBL used in proliferation assays were labeled with 5 μM CFSE, following the manufacturer's protocol (CellTrace Invitrogen) and as detailed for peptide library assays. Proliferation was assessed by flow cytometry on day seven. For assessment of secreted cytokines, blocking antibodies for MHC class I (W6/32) and CD4 (RPA-T4, 2.5 μg/mL; Biolegend) were added to the indicated T-cell cultures before the addition of DCs and refreshed every two days. IFNγ concentrations in the supernatant were assessed following the manufacturer's protocol (OptEIA; BD Biosciences). Cultures were in triplicates for supernatant and proliferation assays.
Intracellular Cytokine Staining.
Cyclin B1-loaded, OVA-loaded, and unloaded DCs were combined with autologous T cells in triplicate to perform the assay at three time points after the start of the coculture. Six hours after the coculture was begun, brefeldin A (BD Biosciences) was added to the first set of the triplicate for 11 h, after which cells were stained for intracellular IFNγ and cell surface markers, following the manufacturer's protocol (BD Cytofix/Cytoperm). This is indicated as the 24-h time period. Brefeldin A was then added to the remaining two sets of the triplicate at 30 and 54 h after culture (indicated as 48-h and 72-h time periods, respectively).
T-Cell Assays Employing the Cyclin B1 Peptide Library.
PBMCs were washed in PBS and labeled with 5 μM CFSE (CellTrace, Invitrogen) in warmed 0.1% FBS in PBS for 10 min at 37 °C. The CFSE was quenched with three volumes of ice-cold complete RPMI and washed with complete RPMI. PBMC were then resuspended in media containing anti-CD28 and anti-CD49d antibodies for additional costimualtion. Wells coated with anti-CD3 were used as positive controls. Peptides were added for a final concentration of 2 μg/mL. No peptide was added to negative control wells. After six days, cells were stained for cell surface markers and tested for proliferation by CFSE dilution using flow cytometry.
Animals and Cell Lines.
Six to eight-week-old C57BL/6 female mice were purchased from The Jackson Laboratory. All animals were housed in the University of Pittsburgh Animal Facility.
The LO2 cell line, a lymphoma derived from a p53 deficient mouse, was a kind gift from Dr. Soren Buus (University of Copenhagen, Denmark). It is further described in SI Text.
DNA Prime-Protein Boost Vaccination and Tumor Measurements.
C57BL/6 mice were shaved on the abdomen 24 h before treatments. Experimental groups were immunized with pcDNA3.1 vector alone or the vector carrying either mouse or human cyclin B1 cDNA. The cDNA (4 μg) was coated on 1–3-μm gold particles (Bio-Rad) and fired into the abdominal skin using a helium-powered gene gun. Three weeks later, mice were boosted with protein in the presence of heat-labile enterotoxin (LT) via an immunostimulatory (IS) patch (IOMAI Corporation). Briefly, 25 μg/100 μL per mouse of human or mouse cyclin B1 was injected s.c., with PBS injections used as controls. To prevent grooming during the immunization procedure, mice were anesthetized i.m. with 100 mg/kg ketamine (Phoenix Scientific) mixed with 11 mg/kg xylazine (Phoenix Scientific). The skin was abraded with sandpaper using gentle pressure 10 times in 1 direction. The area was then hydrated with saline-saturated gauze and blotted dry. The LT/IS patch was applied to the treated skin. Seventeen days after protein boost, 1 × 106 LO2 cells were injected s.c.
Measurement of Mouse Anti-Cyclin B1 T-Cell Responses.
Seventeen days after the last immunization, three mice in each group were killed for T-cell studies. IFNγ ELISPOT assays were performed as detailed in the SI Text.
Measurement of Mouse Anti-Cyclin B1 Antibody Responses.
Sera were drawn two weeks after the last immunization. Anti-cyclin B1 antibody titers were measured by ELISA as detailed in the SI Text.
Supplementary Material
Acknowledgments.
We thank Drs. Jill Siegfried, Joel Weissfeld, Madhav Dodhapkar, and Terri Guinipero for their collaboration, and Michelle Heid and Chen Xue Zhang for help with experiments. This work was supported by National Institutes of Health Grants 5P50 CA90440–07 (to O.J.F.), 5T32CA82084–08 (to L.A.V.), and the Dana Foundation.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/cgi/content/full/0903225106/DCSupplemental.
References
- 1.Hassan KA, et al. Clinical significance of cyclin B1 protein expression in squamous cell carcinoma of the tongue. Clin Cancer Res. 2001;7:2458–2462. [PubMed] [Google Scholar]
- 2.Wang A, Yoshimi N, Ino N, Tanaka T, Mori H. Overexpression of cyclin B1 in human colorectal cancers. J Cancer Res Clin Oncol. 1997;123:124–127. doi: 10.1007/BF01269891. [DOI] [PubMed] [Google Scholar]
- 3.Soria JC, et al. Overexpression of cyclin B1 in early-stage non-small cell lung cancer and its clinical implication. Cancer Res. 2000;60:4000–4004. [PubMed] [Google Scholar]
- 4.El-Ghobashy AA, et al. Overexpression of cyclins A and B as markers of neoplastic glandular lesions of the cervix. Gynecol Oncol. 2004;92:628–634. doi: 10.1016/j.ygyno.2003.11.010. [DOI] [PubMed] [Google Scholar]
- 5.Nozoe T, et al. Significance of cyclin B1 expression as an independent prognostic indicator of patients with squamous cell carcinoma of the esophagus. Clin Cancer Res. 2002;8:817–822. [PubMed] [Google Scholar]
- 6.Song Y, et al. Overexpression of cyclin B1 in human esophageal squamous cell carcinoma cells induces tumor cell invasive growth and metastasis. Carcinogenesis. 2008;29:307–315. doi: 10.1093/carcin/bgm269. [DOI] [PubMed] [Google Scholar]
- 7.Takeno S, et al. Prognostic value of cyclin B1 in patients with esophageal squamous cell carcinoma. Cancer. 2002;94:2874–2881. doi: 10.1002/cncr.10542. [DOI] [PubMed] [Google Scholar]
- 8.Dong Y, Sui L, Watanabe Y, Sugimoto K, Tokuda M. Clinical relevance of cyclin B1 overexpression in laryngeal squamous cell carcinoma. Cancer Lett. 2002;177:13–19. doi: 10.1016/s0304-3835(01)00770-4. [DOI] [PubMed] [Google Scholar]
- 9.Yu M, Zhan Q, Finn OJ. Immune recognition of cyclin B1 as a tumor antigen is a result of its overexpression in human tumors that is caused by non-functional p53. Mol Immunol. 2002;38:981–987. doi: 10.1016/s0161-5890(02)00026-3. [DOI] [PubMed] [Google Scholar]
- 10.Kao H, et al. Identification of cyclin B1 as a shared human epithelial tumor-associated antigen recognized by T cells. J Exp Med. 2001;194:1313–1323. doi: 10.1084/jem.194.9.1313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Suzuki H, Graziano DF, McKolanis J, Finn OJ. T cell-dependent antibody responses against aberrantly expressed cyclin B1 protein in patients with cancer and premalignant disease. Clin Cancer Res. 2005;11:1521–1526. doi: 10.1158/1078-0432.CCR-04-0538. [DOI] [PubMed] [Google Scholar]
- 12.Abbas AK, Murphy KM, Sher A. Functional diversity of helper T lymphocytes. Nature. 1996;383:787–793. doi: 10.1038/383787a0. [DOI] [PubMed] [Google Scholar]
- 13.Sahin U, Tureci O, Pfreundschuh M. Serological identification of human tumor antigens. Curr Opin Immunol. 1997;9:709–716. doi: 10.1016/s0952-7915(97)80053-2. [DOI] [PubMed] [Google Scholar]
- 14.Maecker HT, et al. Use of overlapping peptide mixtures as antigens for cytokine flow cytometry. J Immunol Methods. 2001;255:27–40. doi: 10.1016/s0022-1759(01)00416-1. [DOI] [PubMed] [Google Scholar]
- 15.Kern F, et al. Analysis of CD8 T cell reactivity to cytomegalovirus using protein-spanning pools of overlapping pentadecapeptides. Eur J Immunol. 2000;30:1676. doi: 10.1002/1521-4141(200006)30:6<1676::AID-IMMU1676>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 16.Glenn G. Vaccine adjuvants. Expert Rev Vaccines. 2003;2:163–164. doi: 10.1586/14760584.2.2.163. [DOI] [PubMed] [Google Scholar]
- 17.Glenn GM, et al. Transcutaneous immunization and immunostimulant strategies: Capitalizing on the immunocompetence of the skin. Expert Rev Vaccines. 2003;2:253–267. doi: 10.1586/14760584.2.2.253. [DOI] [PubMed] [Google Scholar]
- 18.Vlad AM, Kettel JC, Alajez NM, Carlos CA, Finn OJ. MUC1 immunobiology: From discovery to clinical applications. Adv Immunol. 2004;82:249–293. doi: 10.1016/S0065-2776(04)82006-6. [DOI] [PubMed] [Google Scholar]
- 19.Offringa R, Vierboom MP, van der Burg SH, Erdile L, Melief CJ. p53: A potential target antigen for immunotherapy of cancer. Ann N Y Acad Sci. 2000;910:223–233. doi: 10.1111/j.1749-6632.2000.tb06711.x. [DOI] [PubMed] [Google Scholar]
- 20.Boon T, Coulie PG, Van den Eynde BJ, van der Bruggen P. Human T cell responses against melanoma. Annu Rev Immunol. 2006;24:175–208. doi: 10.1146/annurev.immunol.24.021605.090733. [DOI] [PubMed] [Google Scholar]
- 21.Kuroki M, et al. Active production and membrane anchoring of carcinoembryonic antigen observed in normal colon mucosa. Cancer Lett. 1988;43:151–157. doi: 10.1016/0304-3835(88)90228-5. [DOI] [PubMed] [Google Scholar]
- 22.Andersen MH, Becker JC, Straten P. Regulators of apoptosis: Suitable targets for immune therapy of cancer. Nat Rev Drug Discov. 2005;4:399–409. doi: 10.1038/nrd1717. [DOI] [PubMed] [Google Scholar]
- 23.Hamanaka Y, et al. Circulating anti-MUC1 IgG antibodies as a favorable prognostic factor for pancreatic cancer. Int J Cancer. 2003;103:97–100. doi: 10.1002/ijc.10801. [DOI] [PubMed] [Google Scholar]
- 24.Richards ER, et al. Antibodies reactive with the protein core of MUC1 mucin are present in ovarian cancer patients and healthy women. Cancer Immunol Immunother. 1998;46:245–252. doi: 10.1007/s002620050484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hirasawa Y, et al. Natural autoantibody to MUC1 is a prognostic indicator for non-small cell lung cancer. Am J Respir Crit Care Med. 2000;161:589–594. doi: 10.1164/ajrccm.161.2.9905028. [DOI] [PubMed] [Google Scholar]
- 26.von Mensdorff-Pouilly S, et al. Survival in early breast cancer patients is favorably influenced by a natural humoral immune response to polymorphic epithelial mucin. J Clin Oncol. 2000;18:574–583. doi: 10.1200/JCO.2000.18.3.574. [DOI] [PubMed] [Google Scholar]
- 27.Johnson SK, et al. Immune cell infiltrates and prognosis in primary carcinoma of the lung. Lung Cancer. 2000;27:27–35. doi: 10.1016/s0169-5002(99)00095-1. [DOI] [PubMed] [Google Scholar]
- 28.Zhang L, et al. Intratumoral T cells, recurrence, and survival in epithelial ovarian cancer. N Engl J Med. 2003;348:203–213. doi: 10.1056/NEJMoa020177. [DOI] [PubMed] [Google Scholar]
- 29.Galon J, et al. Type, density, and location of immune cells within human colorectal tumors predict clinical outcome. Science. 2006;313:1960–1964. doi: 10.1126/science.1129139. [DOI] [PubMed] [Google Scholar]
- 30.Erfurt C, et al. Tumor-reactive CD4+ T cell responses to the melanoma-associated chondroitin sulphate proteoglycan in melanoma patients and healthy individuals in the absence of autoimmunity. J Immunol. 2007;178:7703–7709. doi: 10.4049/jimmunol.178.12.7703. [DOI] [PubMed] [Google Scholar]
- 31.Maecker B, et al. The shared tumor-associated antigen cytochrome P450 1B1 is recognized by specific cytotoxic T cells. Blood. 2003;102:3287–3294. doi: 10.1182/blood-2003-05-1374. [DOI] [PubMed] [Google Scholar]
- 32.Schmidt SM, et al. Survivin is a shared tumor-associated antigen expressed in a broad variety of malignancies and recognized by specific cytotoxic T cells. Blood. 2003;102:571–576. doi: 10.1182/blood-2002-08-2554. [DOI] [PubMed] [Google Scholar]
- 33.Schmitz M, et al. Generation of survivin-specific CD8+ T effector cells by dendritic cells pulsed with protein or selected peptides. Cancer Res. 2000;60:4845–4849. [PubMed] [Google Scholar]
- 34.Andersen MH, Reker S, Becker JC, thor Straten P. The melanoma inhibitor of apoptosis protein: A target for spontaneous cytotoxic T cell responses. J Invest Dermatol. 2004;122:392–399. doi: 10.1046/j.0022-202X.2004.22242.x. [DOI] [PubMed] [Google Scholar]
- 35.Touloukian CE, et al. Expression of a “self-”antigen by human tumor cells enhances tumor antigen-specific CD4(+) T-cell function. Cancer Res. 2002;62:5144–5147. [PMC free article] [PubMed] [Google Scholar]
- 36.Pickford WJ, Watson AJ, Barker RN. Different forms of helper tolerance to carcinoembryonic antigen: Ignorance and regulation. Clin Cancer Res. 2007;13:4528–4537. doi: 10.1158/1078-0432.CCR-07-0721. [DOI] [PubMed] [Google Scholar]
- 37.Cicinnati VR, et al. Increased frequencies of CD8+ T lymphocytes recognizing wild-type p53-derived epitopes in peripheral blood correlate with presence of epitope loss tumor variants in patients with hepatocellular carcinoma. Int J Cancer. 2006;119:2851–2860. doi: 10.1002/ijc.22251. [DOI] [PubMed] [Google Scholar]
- 38.Lee TV, et al. Identification of activated tumor antigen-reactive CD8+ cells in healthy individuals. Oncol Rep. 2000;7:455–466. doi: 10.3892/or.7.3.455. [DOI] [PubMed] [Google Scholar]
- 39.Pittet MJ, et al. High frequencies of naive Melan-A/MART-1-specific CD8(+) T cells in a large proportion of human histocompatibility leukocyte antigen (HLA)-A2 individuals. J Exp Med. 1999;190:705–715. doi: 10.1084/jem.190.5.705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cramer DW, et al. Conditions associated with antibodies against the tumor-associated antigen MUC1 and their relationship to risk for ovarian cancer. Cancer Epidemiol Biomarkers Prev. 2005;14:1125–1131. doi: 10.1158/1055-9965.EPI-05-0035. [DOI] [PubMed] [Google Scholar]
- 41.Ludewig B, et al. Molecular characterization of virus-induced autoantibody responses. J Exp Med. 2004;200:637–646. doi: 10.1084/jem.20040358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Leisenfelder SA, Moffat JF. Varicella-zoster virus infection of human foreskin fibroblast cells results in atypical cyclin expression and cyclin-dependent kinase activity. J Virol. 2006;80:5577–5587. doi: 10.1128/JVI.00163-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sanchez V, McElroy AK, Spector DH. Mechanisms governing maintenance of Cdk1/cyclin B1 kinase activity in cells infected with human cytomegalovirus. J Virol. 2003;77:13214–13224. doi: 10.1128/JVI.77.24.13214-13224.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Leisenfelder SA, Kinchington PR, Moffat JF. Cyclin-dependent kinase 1/cyclin B1 phosphorylates varicella-zoster virus IE62 and is incorporated into virions. J Virol. 2008;82:12116–12125. doi: 10.1128/JVI.00153-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Drake CG, Pardoll DM. Tumor immunology–towards a paradigm of reciprocal research. Semin Cancer Biol. 2002;12:73–80. doi: 10.1006/scbi.2001.0403. [DOI] [PubMed] [Google Scholar]
- 46.Overwijk WW. Breaking tolerance in cancer immunotherapy: Time to ACT. Curr Opin Immunol. 2005;17:187–194. doi: 10.1016/j.coi.2005.01.011. [DOI] [PubMed] [Google Scholar]
- 47.Phan GQ, Attia P, Steinberg SM, White DE, Rosenberg SA. Factors associated with response to high-dose interleukin-2 in patients with metastatic melanoma. J Clin Oncol. 2001;19:3477–3482. doi: 10.1200/JCO.2001.19.15.3477. [DOI] [PubMed] [Google Scholar]
- 48.Attia P, et al. Autoimmunity correlates with tumor regression in patients with metastatic melanoma treated with anti-cytotoxic T-lymphocyte antigen-4. J Clin Oncol. 2005;23:6043–6053. doi: 10.1200/JCO.2005.06.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Spisek R, et al. Frequent and specific immunity to the embryonal stem cell-associated antigen SOX2 in patients with monoclonal gammopathy. J Exp Med. 2007;204:831–840. doi: 10.1084/jem.20062387. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.