Abstract
We recently found that genistein, a plant-derived natural compound, is a novel cAMP signaling agonist in pancreatic β-cells. In the present study, we further show that exposure of clonal insulin secreting (INS-1E) cells to genistein for 48 h enhanced glucose-stimulated insulin secretion (GSIS), whereas insulin content was not altered, suggesting that genistein-enhanced GSIS is not due to a modulation of insulin synthesis. This genistein’s effect is protein tyrosine kinase- and KATP channel-independent. In addition, genistein had no effect on glucose transporter-2 expression or cellular ATP production, but similarly augmented pyruvate-stimulated insulin secretion in INS-1E cells, indicating that the improvement of insulin secretory function by long-term genistein exposure is not related to an alternation in glucose uptake or the glycolytic pathway. The enhanced insulin secretion by genistein was associated with elevated intracellular Ca2+ concentration and dependent on protein kinase A and new protein synthesis as this effect was completely blocked by N-[2-(p-Bromocinnamylamino)ethyl]-5-isoquinolinesulfonamide or cycloheximide. Similarly, 48 h of genistein exposure also enhanced GSIS in freshly isolated mouse and human pancreatic islets, suggesting a non-species-specific and biologically relevant effect. These findings provide evidence that genistein may be a novel bioactive compound that has an anti-diabetic effect by improving insulin secretion from pancreatic β-cells.
Keywords: β-cells, cAMP, genistein, glucose-stimulated insulin secretion, protein kinase A
1. Introduction
Recently, phytochemical isoflavones have drawn wide attention for their potentially beneficial effects on some human degenerative diseases. Genistein, the primary isoflavone in legumes, has a well-known weak estrogenic effect by binding to estrogen receptors (Kim et al., 1998) and is widely used as a protein tyrosine kinase (PTK) inhibitor at pharmacological doses (Akiyama et al., 1987). Study show that dietary intake of genistein can relief several symptoms in postmenopausal women (Anderson et al., 1999; Crisafulli et al., 2004), and has beneficial effects on cardiovascular disease, cancer, hyperlipidemia, osteoporosis, and various forms of chronic renal disease (Atteritano et al., 2007; Hertrampf et al., 2007; Lamartiniere, 2000; Squadrito et al., 2002; Squadrito et al., 2000; Velasquez and Bhathena, 2001; Yang et al., 2006).
Studies on whether genistein on itself has an effect on diabetes are very limited. Recent studies performed in animals and humans have shown that ingestion of isoflavones containing soy protein moderates hyperglycemia (Jayagopal et al., 2002; Lavigne et al., 2000). However, it is not clear whether isoflavones primarily contributes to this beneficial effect. Emerging studies reported that administration of isoflavones lowered plasma glucose in diabetic animals (Ali et al., 2005; Mezei et al., 2003) and postmenopausal women (Cheng et al., 2004) independent of its effect on food intake or weight gain, suggesting that genistein may be a novel plant-derived anti-diabetic agent, although the mechanism whereby genistein exerts such an beneficial effect on diabetes is unknown. Most published trials using isoflavones or genistein have focused largely on elucidating the effect of isoflavones on lipid profiles, and therefore data from recent studies suggest an anti-diabetic effect of genistein presumably by a hypolipidemic effect (Mezei et al., 2003), thereby increasing insulin sensitivity. However, the results are not consistent. Studies demonstrated that isoflavone administration lowered plasma glucose, but lipid profile or insulin sensitivity was unaffected in obese and diabetic animals (Ali et al., 2005) and humans (Cheng et al., 2004). However, other studies found that soy protein extract with high content of isoflavones improves lipid profile and insulin sensitivity (Dyrskog et al., 2005; Jeppesen et al., 2006; Nordentoft et al., 2008). Neither antioxidant activity could explain genistein’s effect, though oxidative stress and reactive oxygen species play a potential role in the initiation of diabetes (Evans et al., 2002; Haskins et al., 2003; Lankin et al., 2005; Robertson, 2004), since genistein only exhibit an antioxidant effect at high concentrations ranging from 25–100 µM (Ruiz-Larrea et al., 1997; Wei et al., 1993). The achievable levels of total plasma genistein in both humans (King and Bursill, 1998; Xu et al., 1995) and rodents (Naaz et al., 2003; Santell et al., 1997) through dietary supplementation are usually no more than 5 µM.
Although studies are limited and the results are inconsistent, the available data suggest that genistein may have a direct effect on pancreatic β-cells. Several earlier studies demonstrated that genistein stimulates insulin secretion from a clonal β-cell line (Ohno et al., 1993) and cultured islets (Jonas et al., 1995; Sorenson et al., 1994) while other studies have found an inhibitory effect on insulin secretion (Jones and Persaud, 1994; Persaud et al., 1999). Considering higher concentrations (>30 µM) used in most of these studies it is unclear whether genistein at physiological doses (<5 µM) can act directly on pancreatic β-cells to modulate cellular function. We recently discovered that genistein at physiologically achievable concentrations (0.01–5.0 µM) acutely enhance insulin secretion both in clonal β-cells and mouse islets (Liu et al., 2006). But it is unknown if relatively long-term exposure to genistein can improve insulin secretory function of β-cells.
In the present study, we found that exposure to genistein for 48 h enhanced glucose-stimulated insulin secretion (GSIS) both in clonal β-cells and pancreatic islets without affecting insulin content. The enhanced insulin secretory function of β-cells by genistein is not mediated through a change in glucose metabolism or KATP channel activity, but dependent on the cAMP/ protein kinase A (PKA) pathway and new protein synthesis.
2. Materials and methods
2.1. Reagents
RPMI-1640 media were purchased from Sigma-Aldrich (St. Louis, MO), CMRL-1066 media were from Mediatech Inc. (Herndon, VA), heat-inactivated fetal bovine serum (FBS) were obtained from HyClone (Logan, UT), and medium supplements from Invitrogen (Carlsbad, CA); insulin ELISA kit was obtained from Mercodia Inc. (Winston-Salem, NC); protein assay kit was purchased from Bio-Rad (Hercules, CA); ATP assay kit was purchased from Promega (Madison, WI); glucose transporter-2 (Glut-2) antibody was from Santa Cruz Biotechnology (Santa Cruz, CA); Indo 1 penta-acetoxymethylester (Indo 1-AM) was from Invitrogen (Carlsbad, CA); all other reagents and chemicals were from Sigma-Aldrich (St. Louis, MO). Stock solution of genistein, at 20 mM dissolved in dimethyl sulfoxide (DMSO), was stored at –20 ºC before use.
2.2. Cell and islet culture
INS-1E cells (a generous gift from Dr. Pierre Maechler at University of Geneva, Switzerland) were cultured in RPMI-1640 medium containing 11.1 mM glucose and supplemented with 10% FBS, 1 mM sodium pyruvate, 10 mM HEPES, 2 mM L-glutamine, 50 µM β-mercaptoethanol, 100 units/ml penicillin, and 100 µg/ml streptomycin (Merglen et al., 2004). The medium was changed every other day until the cells became confluent. Mouse islets were isolated from male C57BL/6J mice by collagenase digestion as described (Yang et al., 2004) and maintained in RPMI-1640 at 37 °C and 5% CO2 for 12 hrs before treatment. The protocol of this study was reviewed and approved by the Institutional Animal Care and Use Committee At Virginia Polytechnic Institute and State University. Human islets were isolated from cadaver organ donors in the Islet Cell Resource Centers at Southern California Resources Center & Southern California Islet Consortium at National Medical Center (Duarte, CA), Washington University (St. Louis, MO), University of Minnesota (St. Paul, MN), University of Miami (Miami, FL), University of Illinois (Chicago, IL), University of Wisconsin, University of Pennsylvania, University of Alabama (Birmingham, AL), and Joslin Diabetes Center (Boston, MA). The islet purity was 80–90% and viability was 80–97%. Before the experiment, the islets were maintained in CMRL-1066 medium containing 10% FBS.
2.3. Insulin secretion and content
Confluent INS-1E cells or islets were cultured in RPMI-1640 containing 5.5 mM glucose and 2% FBS at 37 °C and 5% CO2 in the presence of various concentrations of genistein or vehicle for 48 h. In some experiments, cells were co-incubated with genistein and PKA or translation inhibitor. Cells and islets were then washed in Krebs-Ringer bicarbonate buffer (KRBB: 129 mM NaCl, 4.8 mM KCl, 1.2 mM MgSO4, 1.2 mM KH2PO4, 2.5 mM CaCl2, 5 mM NaHCO3, 0.1% BSA, and 10 mM HEPES, pH 7.4), followed by incubation in KRBB containing either glucose, sodium pyruvate or potassium chloride for 30 min. Insulin secreted in supernatants was measured by an ELISA kit. For insulin content measurement, treated cells and islets were lysed in lysis buffer (50 mM HEPES, 0.1% (v/v) Triton X-100, 1 mM PMSF, 10 mM E-64, 10 mM pepstatin A, 10 mM TLCK, 100 mM leupeptin, pH 8.0). Proteins were extracted and harvested by sonication and centrifugation. Insulin and protein contents were measured by assay kits. Our data showed that exposure of the cells to genistein for 48 h had no effect on insulin or protein content. All insulin data in the present study were therefore expressed as optical density recorded by a plate reader.
2.4. Intracellular Ca2+ measurements
The concentration of cytosolic free Ca2+ was measured using the intracellular fluorescent Ca2+ probe indo-1 AM as previously described (Chen et al., 2003; Florea and Blatter, 2008). Briefly, Confluent INS-1E cells were treated with genistein (5 µM) or vehicle for 48 h. The cells were then washed and incubated with indo-1-AM (10 µM) in KRBB supplemented with glucose (20 mM) and Ca2+ (2mM) for 5 min at 37°C. After washing in KRBB, Indo-1 AM was excited at a wavelength of 365 nm and fluorescence emission from the Ca2+-bound indo-1 AM was captured at 405 nm. The Ca2+ concentration was expressed as the relative fluorescence units.
2.5. ATP content
INS-1E cells were treated with genistein or vehicle as described above for 48 h. Cells were washed in KRBB, and then either lysed for measuring basal ATP content, or stimulated with 20 mM glucose for 30 min at 37 °C before protein extraction. Cellular ATP levels were measured using an ATP luminescence assay kit according to the manufacturer’s instruction.
2.6. Western blot analysis
After experimental treatments, INS-1E cells were harvested in lysis buffer and performed immunoblot analysis as previous described (Liu et al., 2005). Briefly, the tissues were sonicated and then centrifuged at 10,000 × g for 5 min. Protein levels of the extracts were measured using a Bio-Rad assay kit. Equal amounts of protein from cell extracts were subjected to immunoblot. Membranes were probed with antibody against Glut-2. The immunoreactive proteins were detected by chemiluminescence. Nitrocellulose membranes were stripped and re-probed with β-actin. The protein bands were digitally imaged for densitometric quantitation with a software program (Gene tools, Synoptics Ltd. UK). Glut-2 protein level was normalized to β-actin expression from the same sample.
2.7. Statistics
Data were analyzed with one-way ANOVA, using the Proc Mixed procedure of SAS program, and are expressed as mean ± standard error (S.E.M.). Treatment differences were subjected to Tukey’s multiple comparison test. Differences were considered significant at P< 0.05.
3. Results
3.1. Long-term exposure to genistein increases GSIS in INS-1E cells
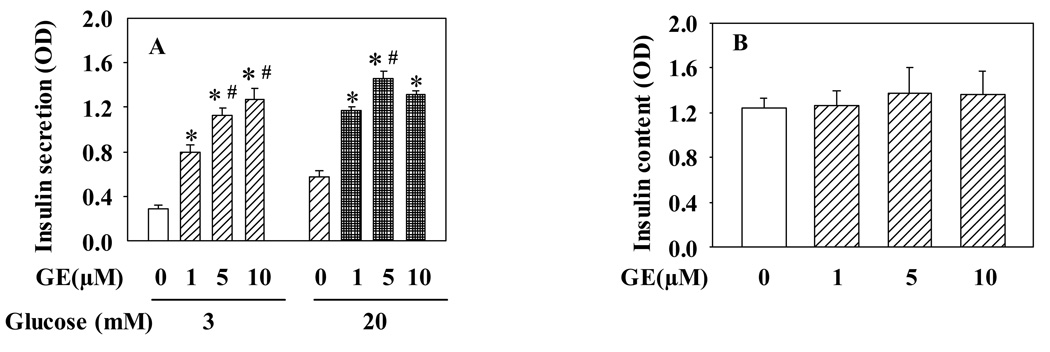
To investigate whether relative long-term exposure of pancreatic β-cells to genistein can modulate insulin secretory function, we first incubated INS-1E cells with various concentrations of genistein for 48 h, followed by GSIS assay without genistein. As shown in Fig. 1A, pretreatment of the cells with genistein significantly augmented both 3 and 20 mM glucose-induced insulin secretion, with 1 µM concentration already inducing a significant effect, although a maximal increase was observed at 5 µM genistein. To assess whether the effect of genistein on insulin secretion is due to its effect on insulin synthesis, we measured the total insulin content in control and genistein-treated cells. Data from these studies show that there was no significant difference in insulin content between the control and genistein-treated INS-1E cells (Fig. 1B).
Figure 1.
Long-term exposure to genistein potentiates GSIS in INS-1E cells. INS-1E cells were incubated in RPMI1640 medium containing various concentrations of genistein (GE) or vehicle for 48 h. Cells were then washed and further cultured in KRBB containing either 3 or 20 mM glucose for 30 min at 37°C. Insulin secreted into KRBB (A) and inside the cells (B) was measured by an ELISA kit. Values are means ± S.E.M. derived from four to seven separate experiments. *, P< 0.05 vs. vehicle alone-treated cells; #, P< 0.05 vs. 1µM genistein-treated cells.
3.2. Long-term exposure to genistein improves insulin secretory function of pancreatic islets
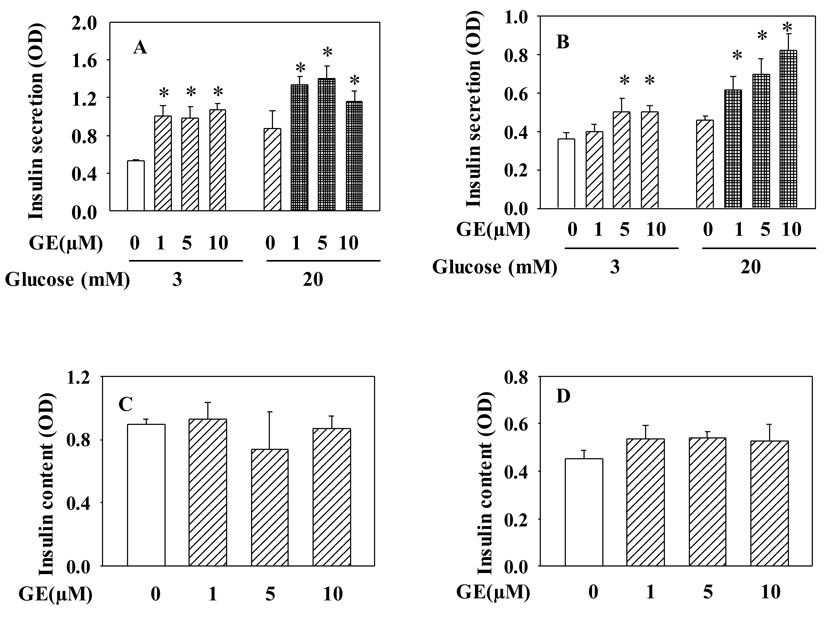
We also tested whether genistein has a similar effect on insulin secretion in pancreatic islets. As shown in Fig. 2, long-term exposure to genistein also dose dependently enhanced GSIS in both mouse (Fig. 2A) and human islets (Fig. 2B), suggesting physiological relevance of our in vitro findings. Consistent with the result seen in INS-1E cells, genistein had no effect on insulin content in islets (Fig. 2C, 2D).
Figure 2.
Long-term exposure to genistein potentiates GSIS in pancreatic islets. Mouse (A, C) and human (B, D) islets were incubated with genistein (GE) or vehicle for 48 h. GSIS and insulin assay protocols are the same as described in Fig. 1. Values are means ± S.E.M. from four to seven experiments. *, P< 0.05 vs. vehicle alone-treated cells.
3.3. The effect of genistein on GSIS is independent of PTK
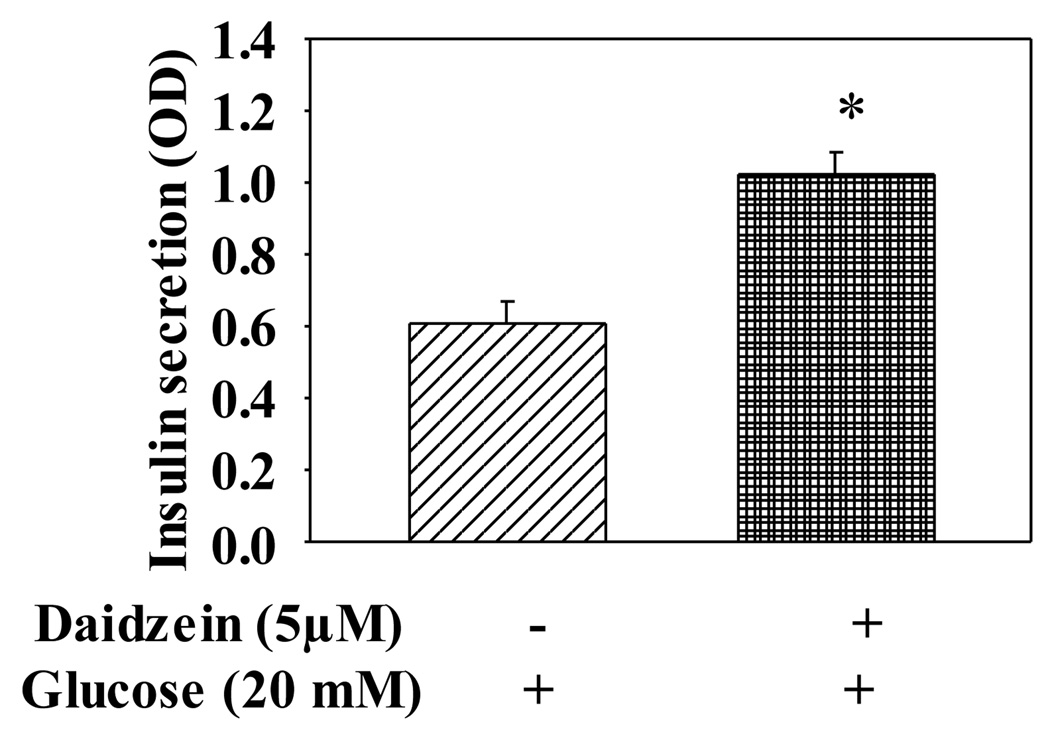
Since genistein is often used as a PTK inhibitor in studies of PTK-mediated cellular events, and PTK may be involved in regulation of insulin secretion (Persaud et al., 1999), we therefore evaluated whether genistein enhances GSIS by inhibition of PTK in INS-1E cells. We compared the effect of genistein with that of daidzein, an analogue of genistein that is inactive for PTK inhibition, on GSIS. As expected, exposure of cells to daidzein at 5 µM for 48 h also augmented GSIS (Fig. 3). Indeed, our recent study demonstrated that genistein, at the highest concentration used in the present study (10 µM), did not inhibit PTK activity (Liu et al., 2006).
Figure 3.
The effect of daidzein on GSIS in INS-1E cells. INS-1E cells were cultured with daidzein (5µM) or vehicle for 48 h. Cells were then incubated in KRBB containing 20 mM glucose for 30 min at 37°C. Insulin secreted into KRBB was determined by an EILSA kit. Values are means ± S.E.M. derived from seven experiments. *, P< 0.05 vs. vehicle alone-treated cells.
3.4. Genistein increases cytosolic free Ca2+ concentration in INS-1E cells
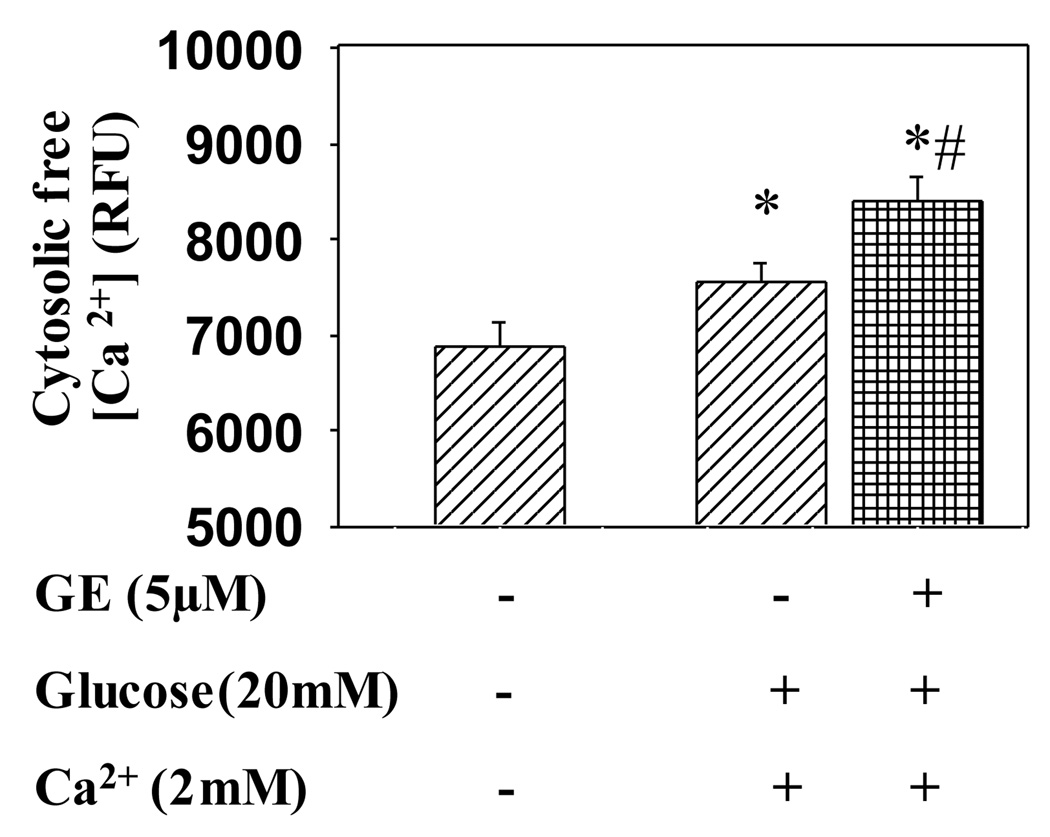
Since Ca2+ influx is the crucial step in insulin secretion, we tested whether genistein treatment increases intracellular Ca2+ level in β-cells. We found that genistein treatment significantly potentiated extracellular Ca2+ and glucose induced increase in intracellular Ca2+ concentration in INS-1E cells (Fig. 4).
Figure 4.
Genistein increases intracellular free Ca2+ concentration. INS-1E cells treated with or without genistein (5 µM) for 48 h were incubated in KRBB containing indo-1 AM (10 µM), glucose (20 mM) and Ca2+ (2 mM) at 37 °C for 5 min. The cells were then excited at a wavelength of 365 nm and fluorescence emission from the Ca2+-bound indo-1 AM was captured at 405 nm. The intracellular Ca2+ concentration was expressed as the relative fluorescence units (RFU). Values are means ± S.E.M. derived from six independent experiments. *, P< 0.05 vs. vehicle alone-treated cells; #, P<0.05 vs. glucose+ calcium+vehicle-treated cells.
3.5. The effect of genistein on insulin secretion in INS-1E cells is not due to a change in glucose metabolism or KATP channel sensitivity
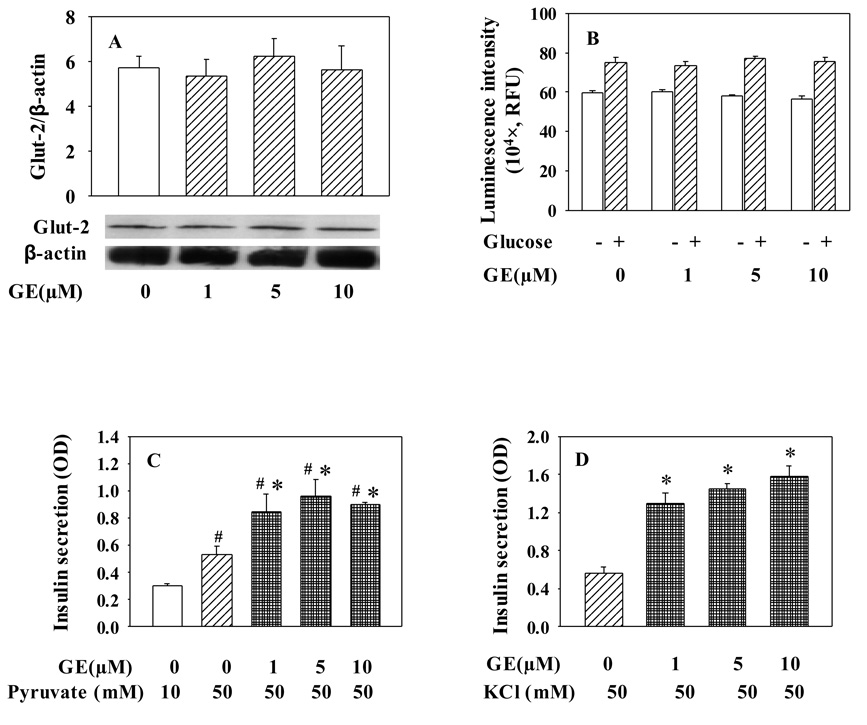
To determine whether genistein enhances GSIS through regulation of glucose metabolism, thereby modulation of ATP generation, we first measured β-cell protein expression of Glut-2, which is essential for β-cell glucose uptake and subsequent GSIS. However, we did not observe any significant change in Glut-2 protein expression after genistein treatment (Fig. 5A). We then examined the effects of genistein on intracellular ATP levels under the conditions for insulin secretion assay. Data in Fig. 5B indicated no significant effects of long-term genistein exposure on basal or high glucose-stimulated ATP production. Further, we determined whether long-term exposure to genistein could augment sodium pyruvate-stimulated insulin secretion, which circumvents the key step enzyme of glycolysis. As shown in Fig. 5C, genistein also enhanced sodium pyruvate-stimulated insulin secretion. These data suggest that the long-term effect of genistein on insulin secretion is not due to the regulation of components in the glucose metabolism pathways. Moreover, we found that long-term genistein exposure elevated KCl-stimulated insulin secretion at a comparable degree to those induced by high glucose and pyruvate (Fig. 5D), suggesting that long-term effect of genistein on GSIS from INS-1E cells is not mediated through modulating KATP channel activity.
Figure 5.
The effect of genistein on insulin secretion in INS-1E cells is not due to a change in glucose metabolism or KATP channel sensitivity. INS-1E cells were cultured in the presence of genistein (GE) at indicated concentrations for 48 h. Glut-2 protein was detected by Western blot using Gult-2 antibody, and normalized to β-actin (A). Basal and glucose (20 mM)-induced ATP production was determined as described in “Materials and Methods” (B). For insulin secretion assays, cells were washed and incubated with sodium pyruvate (C) or potassium chloride (D) in KRBB for 30 min at 37°C. Insulin secreted in KRBB was determined by an EILSA kit. Values are means ± S.E.M. from four to seven separate experiments. *, P< 0.05 vs. vehicle alone-treated cells; #, P< 0.05 vs.10 mM pyruvate-stimulated cells.
3.6. Genistein-enhanced insulin secretion is mediated by the cAMP/PKA signaling pathway requiring protein synthesis
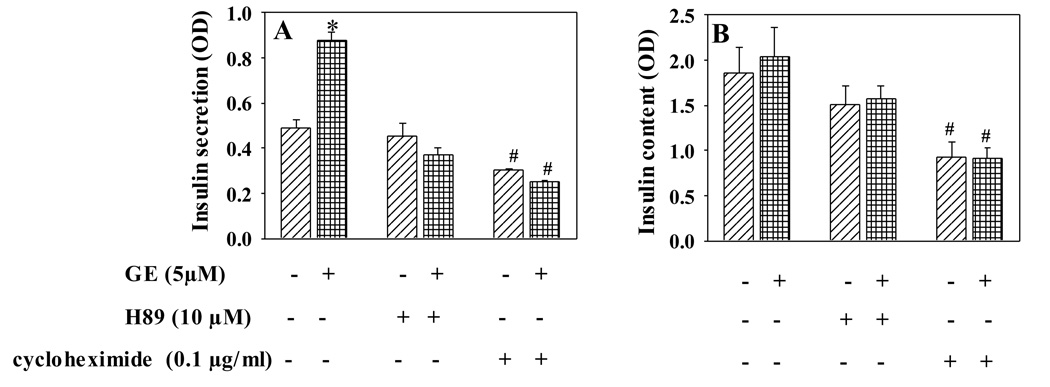
Next, we determined whether the cAMP/PKA signaling pathway mediates genistein’s effect. INS-1E cells were incubated with genistein or vehicle in the presence or absence of PKA inhibitor N-[2-(p-Bromocinnamylamino) ethyl]-5-isoquinolinesulfonamide (H89, 10 µM) for 48 h. H89 completely blocked the genistein-induced GSIS (Fig. 6A), whereas insulin content was not significantly altered by H89 (Fig. 6B). This result suggests an important role of the cAMP/PKA pathway in mediating enhanced insulin secretion from β-cells following long-term exposure to genistein. We further found that cycloheximide (0.1 µg/ml), an inhibitor of protein translation, which was confirmed to inhibit insulin synthesis (Fig. 6B), also abolished the effect of genistein on GSIS (Fig. 6A), suggesting that new protein synthesis is required for this genistein action in β-cells.
Figure 6.
Genistein improves insulin secretory function but not insulin content through a mechanism involving PKA and new protein synthesis. INS-1E cell were incubated with genistein (5 µM) in the presence or absence of PKA inhibitor H89 (10 µM), or translational inhibitor cycloheximide (0.1 µg/ml), for 48 h. Cells were then incubated in KRBB containing 20 mM glucose for 30 min at 37°C. Insulin secreted into KRBB (A) or insulin content (B) was determined by ELISA. Values are means ± S.E.M. derived from four independent experiments. *,#, P< 0.05 vs. vehicle alone-treated cells.
4. Discussion
Insulin is an important hormone required for normal metabolism. In healthy subjects, insulin is released in exquisitely exact amounts to meet the metabolic demand. Specifically, β-cells sense changes in plasma glucose concentration and response by releasing corresponding amount of insulin (Rutter and Parton, 2008). Decrease in both sensing and secreting capacity of β-cells results in abnormal glucose homeostasis. While no pharmacological agent can restore the exact kinetics of insulin secretion in response to glucose (Doyle and Egan, 2003), insulinotropic agents are still very important for effective glycemic control in diabetic patients. In the present study, we found that long-term exposure of β-cells to genistein, at physiologically relevant concentrations through dietary consumption (King and Bursill, 1998), improves insulin secretory function of pancreatic β-cells. Genistein is a widely used dietary supplement in the US and has a relative long metabolic half-life in plasma (King and Bursill, 1998). This finding therefore may provide a basic mechanism underlying the physiological effects of genistein on diabetes.
In this study, we first found that exposure of INS-1E cells to genistein for 48 h enhanced GSIS. A similar increase in GSIS was seen in both mouse and human pancreatic islets, showing that this effect of genistein is not species-specific and thus may be biologically relevant, given that the concentrations used in this study overlap with those of physiologically achievable following dietary consumption of genistein products. Unlike free fatty acids, which acutely increase both basal and GSIS (Gravena et al., 2002), but detrimentally reduce insulin synthesis after incubation with the cells for 48 h (Bollheimer et al., 1998), genistein had no effect on insulin content, suggesting that its effect on insulin secretion is not due to a modulation of insulin synthesis or an adverse effect on the cells, such as apoptosis, as seen in β-cells exposed to free fatty acids (Bollheimer et al., 1998; Gravena et al., 2002). Indeed, genistein had no effect on mitochondria metabolism as determined by ATP assay, further supporting that genistein-enhanced GSIS is not due to an abnormal effect on cellular function.
While genistein is a pharmacological inhibitor of PTK (Akiyama et al., 1987), we recently found that genistein at the concentrations used in the present study had no effect on basal or agonist-induced PTK activity in β-cells (Liu et al., 2006), which is consistent with previous reports that genistein only inhibits PTK at a much higher concentration (Peterson and Barnes, 1996), suggesting that genistein-improved insulin secretory function of β-cells is not related to PTK inhibition. Indeed, daidzein, an analogue of genistein that does not inhibit PTK (Liu et al., 2006; Vissac-Sabatier et al., 2003), also increased insulin secretion, an effect that is only slightly less potent than that of genistein, further supporting a PTK-independent effect of genistein.
It is well characterized that glucose induces insulin secretion through glycolysis and mitochondrial oxidation in the cells, which increase intracellular ATP/ADP ratio, sequentially leading to closure of KATP channels, depolarization of voltage-gated L-type Ca2+ channels on the plasma membrane, Ca2+ influx, and activation of exocytosis of insulin-containing granules (Iezzi et al., 2004; Newsholme et al., 2005; Rutter, 2001). Glut-2, the major glucose transporter expressed on the surface of pancreatic β-cells (Tiedge and Lenzen, 1991), primarily transports glucose into β-cells (Johnson et al., 1990; Permutt et al., 1989; Thorens et al., 1988). Following the entry into the cells, glucose is phosphorylated by the rate-limiting enzyme glucokinase and further hydrolyzed to generate pyruvate, which is oxidized through the tricarboxylic acid cycle by mitochondria in β-cells to produce ATP. Therefore, Glut-2 and glucokinase are two critical proteins that control the rate of glucose metabolism and thus the rate of insulin secretion from β-cells (Chen et al., 1990; Matschinsky, 1990; Meglasson et al., 1986; Terauchi et al., 1995). However, we provide the following evidence suggesting that the effect of genistein on insulin secretion is not mediated through regulating these proteins or other components in glucose metabolic pathway. First, we didn’t find that genistein-elevated insulin secretion was paralleled by increased Glut-2 protein level in β-cells. Second, genistein similarly enhanced insulin secretion stimulated by pyruvate, which bypassed glycolysis. Third, genistein exposure did not alter basal or glucose induced ATP generation in β-cells.
As aforementioned, KATP channels, which are present in the plasma membrane of pancreatic β-cells, play an integral role in mediating GSIS via regulation of cell membrane potential (Iezzi et al., 2004; Newsholme et al., 2005; Rutter, 2001). While genistein had no effect on intracellular ATP level, which determines the activity of KATP channels, thereby subsequent GSIS, it is still possible that genistein may directly inhibit KATP channel activity by binding to the sulfonylurea receptor 1 (Cyrino et al., 2003), thus resulting in closure of KATP channels, membrane depolarization, and insulin secretion. We considered this possibility and therefore examined insulin release elicited in the presence of KCl (50 mM), which directly causes membrane depolarization without altering KATP channel activity (Huang et al., 1998). We observed that pre-treatment of β-cells with genistein for 48 h also potentiated potassium-stimulated insulin secretion, which closely resembled its effect on glucose- and pyruvate-evoked insulin secretion. This result suggests that genistein enhances insulin release through a KATP channel-independent mechanism.
Recent studies showed that activation of several protein kinases, including calmodulin-dependent protein kinase (CaMK), PKA, and protein kinase C (PKC), can facilitate insulin exocytosis through various mechanisms (Blanpied and Augustine, 1999; Nesher et al., 2002; Zawalich et al., 1997). We recently discovered for the first time that genistein at physiologically achievable concentrations rapidly activates cAMP/PKA signaling by stimulating plasma membrane-associated adenylyl cyclase activity both in INS-1E cells and mouse islets (Liu et al., 2006). While it is unclear how genistein activates adenylyl cyclase which are differentially regulated by Ca2+, G-proteins and protein kinases (Portela-Gomes and Abdel-Halim, 2002; Thams et al., 1982; Tian and Laychock, 2001), genistein may directly act on the cell surface to facilitate cAMP production involving a non-genomic mechanism, which is an ongoing project in this laboratory. In the present study, we found that pharmacological inhibition of PKA activity completely abolished improved insulin secretion following long-term exposure to genistein, suggesting that the improved insulin secretory function of β-cells is through modulation of PKA.
It is well established that cAMP signaling plays an important role in incretin-stimulated insulin secretion in β-cells. Activation of PKA in response to elevated intracellular cAMP has an acute effect on insulin secretion through immediate interaction with L-type Ca2+ channel (Dyachok and Gylfe, 2004), increasing total number of insulin-containing secretory vesicles (Renstrom et al., 1997; Rorsman et al., 2000), and sensitization of secretory machineries to Ca2+ (Wan et al., 2004). Interestingly, our data show that intracellular Ca2+ concentration was elevated by genistein treatment in β-cells, suggesting that the improvement of insulin secretory function by genistein may be at least partially attributable to its modulation of Ca2+ signaling in β-cells. However, it is still unclear whether genistein regulates Ca2+ influx pathway or release of Ca2+ from intracellular stores, and whether cAMP and PKA are involved in this process. While it is unclear how PKA is involved in the effect of long-term genistein exposure on insulin secretion, it is unlikely that the observed genistein action in the present study is mediated through a rapid activation of PKA, because genistein was removed from cell cultures during insulin secretion assay. In fact, our result showed that this genistein effect on insulin secretion is dependent on new protein synthesis, suggesting that long-term genistein exposure improves β-cell function via a genomic mechanism. PKA activates transcriptional factor cAMP response element binding protein, which might stimulate the expression of Glut-2 and glucokinase that plays a role in glucose sensing and thereby insulin secretion. However, while we didn’t measure glucokinase in this study, we have not seen a significant effect of genistein on Glut-2 protein expression. Recent study demonstrated that hormone-sensitive lipase (HSL), the enzyme for acylglycerol hydrolysis that is expressed in β-cells (Fex and Mulder, 2008), plays an important role in insulin secretion (Fex and Mulder, 2008; Larsson et al., 2008). HSL can be directly activated by PKA (Kraemer and Shen, 2002). However, whether long-term exposure of β-cells to genistein improves insulin secretory function through increasing HSL activity or expression remains to be determined. Another candidate protein that may be involved in mediating genistein-enhanced insulin secretion is synaptosomal-associated protein of 25 kDa (SNAP-25). Insulin is released from β-cells through regulated exocytosis, which requires transport and docking of insulin secretory granules to the plasma membrane and subsequent fusion. Studies showed that SNAP-25, a membrane bound protein, is involved in this process of insulin exocytosis from β-cells (Blanpied and Augustine, 1999; Gonelle-Gispert et al., 2000), and its expression is up-regulated by PKA in oocytes and steroidogenic cells (Grosse et al., 2000). It is therefore tempting to speculate that genistein may enhance GSIS in β-cells through cAMP/PKA-mediated upregulation of SNAP-25 expression, an aspect that needs further investigation.
In conclusion, in the present study we found that 48 h of exposure to genistein improves insulin secretory function of β-cells, an effect that is not mediated through modification in glucose metabolism or KATP channel activity, but is cAMP/PKA mediated involving elevation of intracellular Ca2+ concentration and requires new protein synthesis.
Acknowledgments
This work was supported by grants from the American Diabetes Association (to D. Liu), and Virginia Commonwealth Health Research Board (to D. Liu), and fund from The Taishan Scholar Program (QDU-EYE) at Qingdao University, China. We thank Kathy Reynolds and Wei Zhen for their excellent technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement
The authors have no conflicts of interest.
Reference
- Akiyama T, Ishida J, Nakagawa S, Ogawara H, Watanabe S, Itoh N, Shibuya M, Fukami Y. Genistein, a specific inhibitor of tyrosine-specific protein kinases. J. Biol. Chem. 1987;262:5592–5595. [PubMed] [Google Scholar]
- Ali AA, Velasquez MT, Hansen CT, Mohamed AI, Bhathena SJ. Modulation of carbohydrate metabolism and peptide hormones by soybean isoflavones and probiotics in obesity and diabetes. J. Nutr. Biochem. 2005;16:693–699. doi: 10.1016/j.jnutbio.2005.03.011. [DOI] [PubMed] [Google Scholar]
- Anderson JJ, Anthony MS, Cline JM, Washburn SA, Garner SC. Health potential of soy isoflavones for menopausal women. Public Health Nutr. 1999;2:489–504. doi: 10.1017/s1368980099000671. [DOI] [PubMed] [Google Scholar]
- Atteritano M, Marini H, Minutoli L, Polito F, Bitto A, Altavilla D, Mazzaferro S, Anna RD, Cannata ML, Gaudio A, Frisina A, Frisina N, Corrado F, Cancellieri F, Lubrano C, Bonaiuto M, Adamo EB, Squadrito F. Effects of the phytoestrogen genistein on some predictors of cardiovascular risk in osteopenic, postmenopausal women: a 2-years randomized, double-blind, placebo-controlled study. J. Clin. Endocrinol. Metab. 2007;92:3068–3075. doi: 10.1210/jc.2006-2295. [DOI] [PubMed] [Google Scholar]
- Blanpied TA, Augustine GJ. Protein kinase A takes center stage in ATP-dependent insulin secretion. Proc. Natl. Acad. Sci. U. S. A. 1999;96:329–331. doi: 10.1073/pnas.96.2.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bollheimer LC, Skelly RH, Chester MW, McGarry JD, Rhodes CJ. Chronic exposure to free fatty acid reduces pancreatic beta cell insulin content by increasing basal insulin secretion that is not compensated for by a corresponding increase in proinsulin biosynthesis translation. J. Clin. Invest. 1998;101:1094–1101. doi: 10.1172/JCI420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Alam T, Johnson JH, Hughes S, Newgard CB, Unger RH. Regulation of beta-cell glucose transporter gene expression. Proc. Natl. Acad. Sci. U. S. A. 1990;87:4088–4092. doi: 10.1073/pnas.87.11.4088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen L, Koh DS, Hille B. Dynamics of calcium clearance in mouse pancreatic beta-cells. Diabetes. 2003;52:1723–1731. doi: 10.2337/diabetes.52.7.1723. [DOI] [PubMed] [Google Scholar]
- Cheng SY, Shaw NS, Tsai KS, Chen CY. The hypoglycemic effects of soy isoflavones on postmenopausal women. J. Womens Health (Larchmt) 2004;13:1080–1086. doi: 10.1089/jwh.2004.13.1080. [DOI] [PubMed] [Google Scholar]
- Crisafulli A, Marini H, Bitto A, Altavilla D, Squadrito G, Romeo A, Adamo EB, Marini R, D’Anna R, Corrado F, Bartolone S, Frisina N, Squadrito F. Effects of genistein on hot flushes in early postmenopausal women: a randomized, double-blind EPT- and placebo-controlled study. Menopause. 2004;11:400–404. doi: 10.1097/01.gme.0000109314.11228.e5. [DOI] [PubMed] [Google Scholar]
- Cyrino FZ, Bottino DA, Coelho FC, Ravel D, Bouskela E. Effects of sulfonylureas on K(ATP) channel-dependent vasodilation. J. Diabetes Complications. 2003;17:6–10. doi: 10.1016/s1056-8727(02)00273-8. [DOI] [PubMed] [Google Scholar]
- Doyle ME, Egan JM. Pharmacological agents that directly modulate insulin secretion. Pharmacol. Rev. 2003;55:105–131. doi: 10.1124/pr.55.1.7. [DOI] [PubMed] [Google Scholar]
- Dyachok O, Gylfe E. Ca(2+)-induced Ca(2+) release via inositol 1,4,5-trisphosphate receptors is amplified by protein kinase A and triggers exocytosis in pancreatic beta-cells. J. Biol. Chem. 2004;279:45455–45461. doi: 10.1074/jbc.M407673200. [DOI] [PubMed] [Google Scholar]
- Dyrskog SE, Jeppesen PB, Colombo M, Abudula R, Hermansen K. Preventive effects of a soy-based diet supplemented with stevioside on the development of the metabolic syndrome and type 2 diabetes in Zucker diabetic fatty rats. Metabolism. 2005;54:1181–1188. doi: 10.1016/j.metabol.2005.03.026. [DOI] [PubMed] [Google Scholar]
- Evans JL, Goldfine ID, Maddux BA, Grodsky GM. Oxidative stress and stress-activated signaling pathways: a unifying hypothesis of type 2 diabetes. Endocr Rev. 2002;23:599–622. doi: 10.1210/er.2001-0039. [DOI] [PubMed] [Google Scholar]
- Fex M, Mulder H. Lipases in the pancreatic beta-cell: implications for insulin secretion. Biochem. Soc. Trans. 2008;36:885–890. doi: 10.1042/BST0360885. [DOI] [PubMed] [Google Scholar]
- Florea SM, Blatter LA. The effect of oxidative stress on Ca2+ release and capacitative Ca2+ entry in vascular endothelial cells. Cell Calcium. 2008;43:405–415. doi: 10.1016/j.ceca.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Gonelle-Gispert C, Molinete M, Halban PA, Sadoul K. Membrane localization and biological activity of SNAP-25 cysteine mutants in insulin-secreting cells. J. Cell Sci. 2000;113:3197–3205. doi: 10.1242/jcs.113.18.3197. [DOI] [PubMed] [Google Scholar]
- Gravena C, Mathias PC, Ashcroft SJ. Acute effects of fatty acids on insulin secretion from rat and human islets of Langerhans. J. Endocrinol. 2002;173:73–80. doi: 10.1677/joe.0.1730073. [DOI] [PubMed] [Google Scholar]
- Grosse J, Bulling A, Brucker C, Berg U, Amsterdam A, Mayerhofer A, Gratzl M. Synaptosome-associated protein of 25 kilodaltons in oocytes and steroid-producing cells of rat and human ovary: molecular analysis and regulation by gonadotropins. Biol. Reprod. 2000;63:643–650. doi: 10.1095/biolreprod63.2.643. [DOI] [PubMed] [Google Scholar]
- Haskins K, Bradley B, Powers K, Fadok V, Flores S, Ling X, Pugazhenthi S, Reusch J, Kench J. Oxidative stress in type 1 diabetes. Ann. N. Y. Acad. Sci. 2003;1005:43–54. doi: 10.1196/annals.1288.006. [DOI] [PubMed] [Google Scholar]
- Hertrampf T, Gruca MJ, Seibel J, Laudenbach U, Fritzemeier KH, Diel P. The bone-protective effect of the phytoestrogen genistein is mediated via ERalpha-dependent mechanisms and strongly enhanced by physical activity. Bone. 2007;40:1529–1535. doi: 10.1016/j.bone.2007.02.006. [DOI] [PubMed] [Google Scholar]
- Huang X, Wheeler MB, Kang YH, Sheu L, Lukacs GL, Trimble WS, Gaisano HY. Truncated SNAP-25 (1–197), like botulinum neurotoxin A, can inhibit insulin secretion from HIT-T15 insulinoma cells. Mol. Endocrinol. 1998;12:1060–1070. doi: 10.1210/mend.12.7.0130. [DOI] [PubMed] [Google Scholar]
- Iezzi M, Kouri G, Fukuda M, Wollheim CB. Synaptotagmin V and IX isoforms control Ca2+ -dependent insulin exocytosis. J. Cell Sci. 2004;117:3119–3127. doi: 10.1242/jcs.01179. [DOI] [PubMed] [Google Scholar]
- Jayagopal V, Albertazzi P, Kilpatrick ES, Howarth EM, Jennings PE, Hepburn DA, Atkin SL. Beneficial effects of soy phytoestrogen intake in postmenopausal women with type 2 diabetes. Diabetes Care. 2002;25:1709–1714. doi: 10.2337/diacare.25.10.1709. [DOI] [PubMed] [Google Scholar]
- Jeppesen PB, Dyrskog SE, Agger A, Gregersen S, Colombo M, Xiao J, Hermansen K. Can stevioside in combination with a soy-based dietary supplement be a new useful treatment of type 2 diabetes? An in vivo study in the diabetic goto-kakizaki rat. Rev. Diabet. Stud. 2006;3:189–199. doi: 10.1900/RDS.2006.3.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson JH, Newgard CB, Milburn JL, Lodish HF, Thorens B. The high Km glucose transporter of islets of Langerhans is functionally similar to the low affinity transporter of liver and has an identical primary sequence. J. Biol. Chem. 1990;265:6548–6551. [PubMed] [Google Scholar]
- Jonas JC, Plant TD, Gilon P, Detimary P, Nenquin M, Henquin JC. Multiple effects and stimulation of insulin secretion by the tyrosine kinase inhibitor genistein in normal mouse islets. Br. J. Pharmacol. 1995;114:872–880. doi: 10.1111/j.1476-5381.1995.tb13285.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones PM, Persaud SJ. Tyrosine kinase inhibitors inhibit glucose-stimulated insulin secretion. Biochem. Soc. Trans. 1994;22:209S. doi: 10.1042/bst022209s. [DOI] [PubMed] [Google Scholar]
- Kim H, Peterson TG, Barnes S. Mechanisms of action of the soy isoflavone genistein: emerging role for its effects via transforming growth factor beta signaling pathways. Am. J. Clin. Nutr. 1998;68:1418S–1425S. doi: 10.1093/ajcn/68.6.1418S. [DOI] [PubMed] [Google Scholar]
- King RA, Bursill DB. Plasma and urinary kinetics of the isoflavones daidzein and genistein after a single soy meal in humans. Am. J. Clin. Nutr. 1998;67:867–872. doi: 10.1093/ajcn/67.5.867. [DOI] [PubMed] [Google Scholar]
- Kraemer FB, Shen WJ. Hormone-sensitive lipase: control of intracellular tri-(di-)acylglycerol and cholesteryl ester hydrolysis. J. Lipid Res. 2002;43:1585–1594. doi: 10.1194/jlr.r200009-jlr200. [DOI] [PubMed] [Google Scholar]
- Lamartiniere CA. Protection against breast cancer with genistein: a component of soy. Am. J. Clin. Nutr. 2000;71:1705S–1707S. doi: 10.1093/ajcn/71.6.1705S. [DOI] [PubMed] [Google Scholar]
- Lankin VZ, Lisina MO, Arzamastseva NE, Konovalova GG, Nedosugova LV, Kaminnyi AI, Tikhaze AK, Ageev FT, Kukharchuk VV, Belenkov YN. Oxidative Stress in Atherosclerosis and Diabetes. Bull. Exp. Biol. Med. 2005;140:41–43. doi: 10.1007/s10517-005-0406-z. [DOI] [PubMed] [Google Scholar]
- Larsson S, Wierup N, Sundler F, Eliasson L, Holm C. Lack of cholesterol mobilization in islets of hormone-sensitive lipase deficient mice impairs insulin secretion. Biochem. Biophys. Res. Commun. 2008;376:558–562. doi: 10.1016/j.bbrc.2008.09.045. [DOI] [PubMed] [Google Scholar]
- Lavigne C, Marette A, Jacques H. Cod and soy proteins compared with casein improve glucose tolerance and insulin sensitivity in rats. Am. J. Physiol. Endocrinol. Metab. 2000;278:E491–E500. doi: 10.1152/ajpendo.2000.278.3.E491. [DOI] [PubMed] [Google Scholar]
- Liu D, Jiang H, Grange RW. Genistein activates the 3',5'-cyclic adenosine monophosphate signaling pathway in vascular endothelial cells and protects endothelial barrier function. Endocrinology. 2005;146:1312–1320. doi: 10.1210/en.2004-1221. [DOI] [PubMed] [Google Scholar]
- Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55:1043–1050. doi: 10.2337/diabetes.55.04.06.db05-1089. [DOI] [PubMed] [Google Scholar]
- Matschinsky FM. Glucokinase as glucose sensor and metabolic signal generator in pancreatic beta-cells and hepatocytes. Diabetes. 1990;39:647–652. doi: 10.2337/diab.39.6.647. [DOI] [PubMed] [Google Scholar]
- Meglasson MD, Manning CD, Najafi H, Matschinsky FM. Glucose transport by radiation-induced insulinoma and clonal pancreatic beta-cells. Diabetes. 1986;35:1340–1344. doi: 10.2337/diab.35.12.1340. [DOI] [PubMed] [Google Scholar]
- Merglen A, Theander S, Rubi B, Chaffard G, Wollheim CB, Maechler P. Glucose sensitivity and metabolism-secretion coupling studied during two-year continuous culture in INS-1E insulinoma cells. Endocrinology. 2004;145:667–678. doi: 10.1210/en.2003-1099. [DOI] [PubMed] [Google Scholar]
- Mezei O, Banz WJ, Steger RW, Peluso MR, Winters TA, Shay N. Soy isoflavones exert antidiabetic and hypolipidemic effects through the PPAR pathways in obese Zucker rats and murine RAW 264.7 cells. J. Nutr. 2003;133:1238–1243. doi: 10.1093/jn/133.5.1238. [DOI] [PubMed] [Google Scholar]
- Naaz A, Yellayi S, Zakroczymski MA, Bunick D, Doerge DR, Lubahn DB, Helferich WG, Cooke PS. The soy isoflavone genistein decreases adipose deposition in mice. Endocrinology. 2003;144:3315–3320. doi: 10.1210/en.2003-0076. [DOI] [PubMed] [Google Scholar]
- Nesher R, Anteby E, Yedovizky M, Warwar N, Kaiser N, Cerasi E. Beta-cell protein kinases and the dynamics of the insulin response to glucose. Diabetes 51 Suppl. 2002;1:S68–S73. doi: 10.2337/diabetes.51.2007.s68. [DOI] [PubMed] [Google Scholar]
- Newsholme P, Brennan L, Rubi B, Maechler P. New insights into amino acid metabolism, beta-cell function and diabetes. Clin. Sci. (Lond) 2005;108:185–194. doi: 10.1042/CS20040290. [DOI] [PubMed] [Google Scholar]
- Nordentoft I, Jeppesen PB, Hong J, Abudula R, Hermansen K. Increased insulin sensitivity and changes in the expression profile of key insulin regulatory genes and beta cell transcription factors in diabetic KKAy-mice after feeding with a soy bean protein rich diet high in isoflavone content. J. Agric. Food Chem. 2008;56:4377–4385. doi: 10.1021/jf800504r. [DOI] [PubMed] [Google Scholar]
- Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S, Kawazu S. Genistein augments cyclic adenosine 3’5’-monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocr. Res. 1993;19:273–285. doi: 10.1080/07435809309026682. [DOI] [PubMed] [Google Scholar]
- Permutt MA, Koranyi L, Keller K, Lacy PE, Scharp DW, Mueckler M. Cloning and functional expression of a human pancreatic islet glucose-transporter cDNA. Proc. Natl. Acad. Sci. U. S. A. 1989;86:8688–8692. doi: 10.1073/pnas.86.22.8688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Persaud SJ, Harris TE, Burns CJ, Jones PM. Tyrosine kinases play a permissive role in glucose-induced insulin secretion from adult rat islets. J. Mol. Endocrinol. 1999;22:19–28. doi: 10.1677/jme.0.0220019. [DOI] [PubMed] [Google Scholar]
- Peterson G, Barnes S. Genistein inhibits both estrogen and growth factor-stimulated proliferation of human breast cancer cells. Cell Growth Differ. 1996;7:1345–1351. [PubMed] [Google Scholar]
- Portela-Gomes GM, Abdel-Halim SM. Overexpression of Gs proteins and adenylyl cyclase in normal and diabetic islets. Pancreas. 2002;25:176–181. doi: 10.1097/00006676-200208000-00011. [DOI] [PubMed] [Google Scholar]
- Renstrom E, Eliasson L, Rorsman P. Protein kinase A-dependent and -independent stimulation of exocytosis by cAMP in mouse pancreatic B-cells. J. Physiol. 1997;502:105–118. doi: 10.1111/j.1469-7793.1997.105bl.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robertson RP. Chronic oxidative stress as a central mechanism for glucose toxicity in pancreatic islet beta cells in diabetes. J. Biol. Chem. 2004;279:42351–42354. doi: 10.1074/jbc.R400019200. [DOI] [PubMed] [Google Scholar]
- Rorsman P, Eliasson L, Renstrom E, Gromada J, Barg S, Gopel S. The Cell Physiology of Biphasic Insulin Secretion. News Physiol. Sci. 2000;15:72–77. doi: 10.1152/physiologyonline.2000.15.2.72. [DOI] [PubMed] [Google Scholar]
- Ruiz-Larrea MB, Mohan AR, Paganga G, Miller NJ, Bolwell GP, Rice-Evans CA. Antioxidant activity of phytoestrogenic isoflavones. Free Radic. Res. 1997;26:63–70. doi: 10.3109/10715769709097785. [DOI] [PubMed] [Google Scholar]
- Rutter GA. Nutrient-secretion coupling in the pancreatic islet beta-cell: recent advances. Mol. Aspects Med. 2001;22:247–284. doi: 10.1016/s0098-2997(01)00013-9. [DOI] [PubMed] [Google Scholar]
- Rutter GA, Parton LE. The beta-cell in type 2 diabetes and in obesity. Front Horm. Res. 2008;36:118–134. doi: 10.1159/000115360. [DOI] [PubMed] [Google Scholar]
- Santell RC, Chang YC, Nair MG, Helferich WG. Dietary genistein exerts estrogenic effects upon the uterus, mammary gland and the hypothalamic/pituitary axis in rats. J. Nutr. 1997;127:263–269. doi: 10.1093/jn/127.2.263. [DOI] [PubMed] [Google Scholar]
- Sorenson RL, Brelje TC, Roth C. Effect of tyrosine kinase inhibitors on islets of Langerhans: evidence for tyrosine kinases in the regulation of insulin secretion. Endocrinology. 1994;134:1975–1978. doi: 10.1210/endo.134.4.8137766. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Morabito N, Crisafulli A, D’Anna R, Corrado F, Ruggeri P, Campo GM, Calapai G, Caputi AP, Squadrito G. The effect of the phytoestrogen genistein on plasma nitric oxide concentrations, endothelin-1 levels and endothelium dependent vasodilation in postmenopausal women. Atherosclerosis. 2002;163:339–347. doi: 10.1016/s0021-9150(02)00013-8. [DOI] [PubMed] [Google Scholar]
- Squadrito F, Altavilla D, Squadrito G, Saitta A, Cucinotta D, Minutoli L, Deodato B, Ferlito M, Campo GM, Bova A, Caputi AP. Genistein supplementation and estrogen replacement therapy improve endothelial dysfunction induced by ovariectomy in rats. Cardiovasc. Res. 2000;45:454–462. doi: 10.1016/s0008-6363(99)00359-4. [DOI] [PubMed] [Google Scholar]
- Terauchi Y, Sakura H, Yasuda K, Iwamoto K, Takahashi N, Ito K, Kasai H, Suzuki H, Ueda O, Kamada N, et al. Pancreatic beta-cell-specific targeted disruption of glucokinase gene. Diabetes mellitus due to defective insulin secretion to glucose. J. Biol. Chem. 1995;270:30253–30256. doi: 10.1074/jbc.270.51.30253. [DOI] [PubMed] [Google Scholar]
- Thams P, Capito K, Hedeskov CJ. Differential effects of Ca2+-calmodulin on adenylate cyclase activity cyclase activity in mouse and rat pancreatic islets. Biochem. J. 1982;206:97–102. doi: 10.1042/bj2060097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thorens B, Sarkar HK, Kaback HR, Lodish HF. Cloning and functional expression in bacteria of a novel glucose transporter present in liver, intestine, kidney, and beta-pancreatic islet cells. Cell. 1988;55:281–290. doi: 10.1016/0092-8674(88)90051-7. [DOI] [PubMed] [Google Scholar]
- Tian Y, Laychock SG. Protein kinase C and calcium regulation of adenylyl cyclase in isolated rat pancreatic islets. Diabetes. 2001;50:2505–2513. doi: 10.2337/diabetes.50.11.2505. [DOI] [PubMed] [Google Scholar]
- Tiedge M, Lenzen S. Regulation of glucokinase and GLUT-2 glucose-transporter gene expression in pancreatic B-cells. Biochem. J. 1991;279:899–901. doi: 10.1042/bj2790899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Velasquez MT, Bhathena SJ. Dietary phytoestrogens: a possible role in renal disease protection. Am. J. Kidney Dis. 2001;37:1056–1068. doi: 10.1016/s0272-6386(05)80025-3. [DOI] [PubMed] [Google Scholar]
- Vissac-Sabatier C, Bignon YJ, Bernard-Gallon DJ. Effects of the phytoestrogens genistein and daidzein on BRCA2 tumor suppressor gene expression in breast cell lines. Nutr. Cancer. 2003;45:247–255. doi: 10.1207/S15327914NC4502_15. [DOI] [PubMed] [Google Scholar]
- Wan QF, Dong Y, Yang H, Lou X, Ding J, Xu T. Protein kinase activation increases insulin secretion by sensitizing the secretory machinery to Ca2+ J. Gen. Physiol. 2004;124:653–662. doi: 10.1085/jgp.200409082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wei H, Wei L, Frenkel K, Bowen R, Barnes S. Inhibition of tumor promoter-induced hydrogen peroxide formation in vitro and in vivo by genistein. Nutr. Cancer. 1995;20:1–12. doi: 10.1080/01635589309514265. [DOI] [PubMed] [Google Scholar]
- Xu X, Harris KS, Wang HJ, Murphy PA, Hendrich S. Bioavailability of soybean isoflavones depends upon gut microflora in women. J. Nutr. 1995;125:2307–2315. doi: 10.1093/jn/125.9.2307. [DOI] [PubMed] [Google Scholar]
- Yang JY, Lee SJ, Park HW, Cha YS. Effect of genistein with carnitine administration on lipid parameters and obesity in C57Bl/6J mice fed a high-fat diet. J. Med. Food. 2006;9:459–467. doi: 10.1089/jmf.2006.9.459. [DOI] [PubMed] [Google Scholar]
- Yang Z, Chen M, Ellett JD, Fialkow LB, Carter JD, Nadler JL. The novel anti-inflammatory agent lisofylline prevents autoimmune diabetic recurrence after islet transplantation. Transplantation. 2004;77:55–60. doi: 10.1097/01.TP.0000104844.48064.81. [DOI] [PubMed] [Google Scholar]
- Zawalich WS, Bonnet-Eymard M, Zawalich KC. Signal transduction in pancreatic beta-cells: regulation of insulin secretion by information flow in the phospholipase C/protein kinase C pathway. Front. Biosci. 1997;2:d160–d172. doi: 10.2741/a180. [DOI] [PubMed] [Google Scholar]