Abstract
While the physiology of membrane-initiated estradiol signaling in the nervous system has remained elusive, a great deal of progress has been made toward understanding the activation of cell signaling. Membrane-initiated estradiol signaling activates G proteins and their downstream cascades, but the identity of membrane receptors and the proximal signaling mechanism(s) have been more difficult to elucidate. Mounting evidence suggests that classical intracellular estrogen receptor-α (ERα) and ERβ are trafficked to the membrane to mediate estradiol cell signaling. Moreover, an interaction of membrane ERα and ERβ with metabotropic glutamate receptors has been identified that explains the pleomorphic actions of membrane-initiated estradiol signaling. This review focuses on the mechanism of actions initiated by membrane estradiol receptors and discusses the role of scaffold proteins and signaling cascades involved in the regulation of nociception, sexual receptivity and the synthesis of neuroprogesterone, an important component in the central nervous system signaling.
Keywords: Estradiol receptor, mGluR, caveolin, β-arrestin, lordosis, nociception, neuroprogesterone
Introduction
It is well known that estrogens are involved in a wide range of physiological events from reproduction to development to cognition to neural and cardiovascular protection. As an extracellular signaling molecule, estrogen's actions are mediated through receptors. An estrogen receptor (ER) is a molecule that transduces estrogenic signals into cell-relevant events. The best characterized of the ERs belong to a nuclear receptor superfamily that includes the androgen receptor, Vitamin D receptor and thyroid hormone receptor.
Classically, ERs have been characterized as nuclear ligand-gated transcription factors of which there are two isoforms, ERα and ERβ. These isoforms have a high sequence homology and a conserved structure consisting of: an N-terminal A/B domain responsible for the transacting function 1 (AF-1); domain C, consisting of two zinc-fingers, responsible for DNA binding; domain D, the hinge region with the nuclear translocation signal; and domain E/F, the ligand binding region that has the transcription regulating activation function 2 (AF-2). The different domain regions of the receptor appear to be involved in specific actions, but their precise functions continue to remain incompletely elucidated [155; 188]. Upon binding 17β-estradiol (estradiol), the major circulating estrogen, intracellular ERs homo- or hetero-dimerize, associate with a specific part of the promoter region of DNA, the estrogen-response-element (ERE), and attract transcriptional machinery containing RNA polymerase and various co-factors to regulate gene expression [33]. Since ERα and ERβ differ in their AF-1 and AF-2 domains, it has been suggested that they can subserve different cellular events [59; 76]. The preferential ligand for the nuclear ER is estradiol and the two isoforms, ERα and ERβ, in spite of having only a moderate homology in the ligand binding domain, both bind to estradiol with a similar affinity [17; 83]. The chiral enantiomer, 17α-estradiol, binds with much lower affinity and has generally been considered to be biologically inactive. Recent evidence suggests, however, that 17α-estradiol may activate a novel ER, ER-X [201].
Interestingly, ligand activated nuclear ERs can also modulate the expression of genes through an ERE independent mechanism. Through the stabilization of protein interactions, estradiol stimulated ERs bind early immediate genes Fos/Jun to the activated protein-1 (AP-1) site [89]. Such interactions have been used to explain the agonist actions of selective estrogen receptor modulators (SERMs) such as tamoxifen. When ERs act through the ERE to upregulate transcription, tamoxifen, a nonsteroidal triphenylethylene derivative, is an antagonist. When tamoxifen has agonist actions, the ER is acting through the AP-1 site [89]. On the other hand, the so-called pure ER antagonist ICI 182,780 (Faslodex/Fulvestrant), a 7α-alkylsulphinyl analogue of estradiol, competitively inhibits estradiol binding to the ER [213]. The ICI 182,780 binding affinity is 89% of estradiol and, once bound to the receptor, it prevents dimerization and nucleo-cytoplasmic shuttling, inhibits AF-1 and AF-2 activity and increases proteasome degradation [37; 50]. Reports now suggest that ICI 182,780 may not be a “pure” antiestrogen as evidenced by its modulation of non-classical pathways [70; 215].
A second category of estradiol signaling is mediated by receptors associated with the cell membrane, the subject of the present review. Though initially not well-accepted, evidence has accumulated over the past 40 years that these actions are not dependent on translation or transcription, but may influence them. These estradiol actions are rapid (<5 minutes) and transient (∼1-4 hours) and can be mediated through membrane localized ERs [210]. Estradiol membrane-initialized actions stimulate a variety of signal transduction pathways that are involved in neuronal signaling, differentiation and survival. Recent experiments have focused on novel ERs being responsible for membrane estradiol signaling however most evidence supports that ERα and ERβ are involved in estradiol membrane-initialized actions. This review will concentrate on studies related to ERα and ERβ and discuss membrane estradiol signaling in the brain, and other tissues, where some of the questions about the mechanism of estradiol signaling are: what is/are the membrane estrogen receptor protein(s)? Is it a G protein-coupled receptor (GPCR)? How does it activate cell signaling pathways? What is/are the physiological significance of membrane-initiated estradiol action?
Localization of ER in the Brain
In the brain, the initial approach to studying ERs was binding studies that identified the areas involved in estradiol receptivity. For autoradiography experiments, animals were injected with 3H-estradiol or 125I-estradiol, which accumulated in cells within hypothalamic and limbic nuclei of the brain, consistent with their role in sexual reproduction and behavior [69; 127; 138; 172; 175]. Physiological studies demonstrated the essential importance of estradiol action in the brain for inducing lordosis, the stereotypic sexually receptive behavior in female rodents [104]. Similarly, estradiol priming is needed for progesterone induction of proceptive or solicitation behaviors [48; 109; 130]. Cells that regulate these behaviors are distributed in a sexual receptive—lordosis-regulating circuit that includes the posterodorsal medial amygdala, bed nucleus of the stria terminalis, medial preoptic area, arcuate nucleus and ventromedial nucleus of the hypothalamus [182; 207]. This circuit signals to downstream estradiol receptive areas, including the periaquaductal gray and the spinal nucleus of the vestibular complex that eventually innervate the medial motor neurons in the spinal cord that innervate axial muscles that affect the behavior [80].
The cloning of the ERα and then the discovery of another isoform, ERβ [63; 83], allowed for in situ hybridization studies that revealed the distribution of ERα and ERβ mRNA throughout the neuraxis. Although these studies largely confirmed previous results, new regions were demonstrated to have ER message that did not have a significant autoradiographic signal [176; 178; 179]. Moreover, ERα and ERβ have differential distribution in the brain between sexes and across species [24; 168; 177; 214; 224]. For example, in the hypothalamus, ERα and ERβ neurons are found in many of the same areas. On the other hand, in the supraoptic and paraventricular nuclei, a paucity of ERα is replaced by tremendous levels of ERβ [7; 193]. Another example is the distribution of ERβ mRNA in the hippocampus of humans, rats and mice, which is more readily detected than ERα mRNA. Alternatively, ERα mRNA has been found to be more abundant in the prefrontal cortex of non-human primates [66; 154; 176]. To add to the complexity, both ERα and ERβ are found in glial cells, pointing to a non-neuronal role for estradiol [12; 29; 146]. While the distribution of ERα and ERβ have been well-worked out, the fundamental significance of ERβ remains more difficult to elucidate.
Novel ERs
If ERα or ERβ mediated all estradiol action, then knocking out these receptors should eliminate all effects of estrogens. In many systems, this is what is observed. For example, in the control of reproduction, both behavior and regulation of ovulation are eliminated in ERα-/- knockout mice [108; 128; 158; 159; 219]. In dorsal root ganglion (DRG) neurons, estradiol attenuates the adenosine triphosphate (ATP)-induced intracellular ([Ca2+]i) flux, an action dependent on ERα [29]. Social discrimination is severely compromised in ERα-/- and ERβ-/- knockout mice [34], as is neuroprotection in cortex [43] and nigrostriatal dopamine system [87], intracellular signaling [1] and feeding [196].
However, removal of classic ER proteins does not eliminate all estradiol binding. 125I-estradiol binding is still observed in the hypothalamus and amygdala of double-knockout ERα-/-/ERβ-/- mice [173], suggesting the existence of other estrogen binding proteins that are not coded by ERα (ESR1) or ERβ (ESR2) genes. Moreover, estradiol actions on events such as synaptic transmission remain in the ERα-/-/ERβ-/- double-knockout mice [41; 54]. To explain these results, other estrogen binding proteins have been hypothesized [74; 97; 143; 152; 197; 200].
One such protein is ER-X, a novel membrane ER that has been observed in neocortex, uterus and lung plasma membrane microdomains associated with caveolin proteins [135; 199; 200]. Using antibodies against ERα (C1355, MC-20) and ERβ from Zymed, ER-X was immunoprecipitated, fractionated by SDS-PAGE and determined to have an apparent molecular weight of 62-63 kDa. Interestingly, ER-X preferentially binds 17α-estradiol and is not antagonized by ICI 182,780. Ligand stereospecificity and blockade with the ER antagonist ICI 182,780 are two important features of the classical receptors, ERα and ERβ. As with ERα, ER-X is developmentally regulated in the cortex. Expression peaks at post-natal days 7-10, and then drops off over the next month. In the normal adult, the expression of ER-X is almost undetectable, but re-emerges after ischemic injury or in animal models of Alzheimer's disease. The developmental profile and the response to both estradiol isoforms strongly suggest that ER-X is not the ER mediating functions that are affected by gonadectomy (e.g. reproduction).
Recently, it has been reported that estradiol stimulates a membrane-localized protein with features resembling a GPCR [49]. G protein-coupled receptor 30 (GPR30) was originally identified in a screen for neurotransmitter receptors in a Burkitt's lymphoma cell line and subsequently cloned [28]. GPR30 has significant sequence homology to the angiotensin II 1A, interleukin 8A and chemokine type 1 receptors, suggesting that the protein might be the receptor for a peptide or glycoprotein. However, the ligand for this orphan receptor appears to be estradiol [51]. GPR30 is an integral membrane protein with seven transmembrane domains expressed throughout the brain and periphery and in cancer cells [142]. At the cellular level, GPR30 was initially thought to be expressed on the plasma membrane, suggesting that it could serve as a membrane ER, but more recent studies have found it restricted to the Golgi apparatus and endoplasmic reticulum [61; 103; 132]. In cell lines, estradiol stimulation of GPR30 resulted in the rapid activation of signaling cascades that were similar to the response mediated by ERα through adenylyl cyclase pathways [156; 197]. In addition, estradiol stimulation of GPR30-transfected cells was blocked by ICI 182,780. Although others have shown that ICI 182,780 acts as an agonist [197]. To add to the confusion, others reported that endogenously expressing GPR30 cells did not respond to estradiol, while cells expressing endogenous ERα and ERβ responded [132; 136]. The results are consistent with GPR30 knock out mice in which estradiol was still fully capable of modulating the electrical properties of γ-aminobutyric (GABA)-ergic neurons in arcuate neurons [131; 143; 161]. Moreover, in vivo studies carried out with the selective GPR30 agonist G1 failed to demonstrate estrogenic properties [132]. Thus, current pharmacological and immunohistochemical data do not strongly support a role of GPR30 as a mediator of sexual reproduction.
Kelly and colleagues have suggested another membrane ER candidate. This protein has been characterized pharmacologically. It is activated by the diphenylacrylamide compound, STX, and estradiol [143; 144]. Interestingly, STX-induced activation of the phospholipase C/inositol triphosphate (PLC/IP3) signaling cascade remains in the ERα-/-/ERβ-/- mouse. Consistent with its role as an ER, the STX-binding protein is stereospecific for estradiol and is blocked with ICI 182,780. The STX-binding protein may regulate gonadotrophin-release hormone (GnRH) secretion through its attenuation of β-endorphin (β-END) and GABA synapses directly onto GnRH neurons leading to an increase in excitability [75; 212]. Since GnRH neurons do not express ERα, the STX-binding protein may mediate direct actions of estradiol on these neurons. Moreover, STX mimics the anorexic action of estradiol by attenuating the ovariectomy-induced increase in neuropeptide-Y (NPY) expression in the arcuate nucleus of female guinea pigs [144]. On the other hand, mice with ERα deleted from neurons do not have a physiological luteinizing hormone (LH) surge [219]. One explanation is that a cooperative role between ERα and the STX-binding protein is required for the LH surge. As with ER-X, the molecular characterization of this STX-binding protein remains to be elucidated.
Other potential estrogen binding proteins also await characterization. On western blots, ER immunoreactive bands with different molecular weights suggest that splice variants of ERα and/or ERβ receptors may be expressed. For example, a 46 kDa variant, ER46, was identified with the H222 C-terminal directed ERα antibody [97]. ER46 triggers nitric oxide synthase (NOS) activation in vascular endothelial cells. Others report 25 and 18 kDa ER-immunoreactive proteins that appear to be mitochondrial ATPase subunits expressed in cerebellum, olfactory bulb and hypothalamic membranes [148]. Interestingly, membrane ERs with apparent molecular weight of higher than 67 kDa has also been reported, but it is unclear whether these are artifacts or functional receptors [97]. In CHO-K1, COS-7, and Rat2 fibroblast cell lines, both estradiol and 17α-estradiol activate extracellular-signal regulated kinase (ERK) signaling, but probing these tissues using antibodies directed against ERα (MC-20, C1355, 6F11) and ERβ does not reveal proteins corresponding to native ERα, ERβ or ER-X [125]. These putative membrane ERs remain to be characterized, but in many assays ERα and ERβ appear to mediate membrane-initiated estradiol action.
Extranuclear ERα and ERβ Expression
In addition to nuclear and cytoplasmic immunoreactivity, ERα and ERβ have been associated with plasma membranes [3; 67; 72; 118; 119]. This along with numerous reports of rapid actions of estradiol strongly implies that ERs have actions apart from their long-established function of regulating transcription [74; 113]. This was dramatically demonstrated by Levin and colleagues using Chinese hamster ovary (CHO) cells [152]. CHO cells transfected with single cDNA transcripts for either ERα or ERβ yielded a single product for each transcript. Significantly, ERα and ERβ proteins were localized in the nucleus and the plasma membrane, providing strong evidence that the same receptor found intracellularly is also associated with the plasma membrane and may be responsible for the rapid actions of estradiol [152]. Moreover, estradiol binding affinity is similar for the nuclear and plasma localized receptors, ERα ∼0.2 nM and ERβ ∼1 nM. In breast cancer cells, 5% of endogenous ERα and ERβ are located in the cell membrane, which is similar to cells transfected with ERα and ERβ cDNAs [151].
On balance, overwhelming biochemical, molecular and pharmacological evidence reinforces the idea that the major membrane ERs are ERα and ERβ. The presence of membrane ERs explains the observation of estradiol rapidly modulating neuronal physiology in hippocampal, neostriatal and hypothalamic tissue [65]. A membrane impermeable estrogen, estradiol conjugated to bovine serum albumin (E-6-BSA) mimics the action of free estradiol [65]. Though ERα and ERβ are present in the membrane [152], it is still not well understood how ERs are trafficked to the membrane and promote rapid estradiol effects. ERα and ERβ appear to undergo post-transcriptional modification that allows for their insertion into the membrane [2; 22; 101].
Estradiol Regulation of ERα and ERβ
If membrane ERs are products of ERα and ERβ genes, then the regulation of their expression is an important question for understanding their physiology. Estradiol regulation of its cognate receptors is observed during the estrous cycle. For example, ER mRNA levels in the medial preoptic nucleus are highest during estrus and metestrus, attenuated at diestrus and low during proestrus [174]. Estradiol can also downregulate ERα and ERβ protein; extranuclear ER immunoreactivity parallels the loss of ER mRNA [92; 93; 96; 153; 169; 174; 180; 195; 208]. In an ovariectomized preparation, estradiol treatment of less than 20 minutes caused the disappearance of cytoplasmic ER immunostaining in the hypothalamus [18; 19; 106]. In cortical neurons, expression of green fluorescent protein tagged ER (ER-GFP) is downregulated by estradiol, and increased by ICI 182,780 [222]. The processes responsible for controlling the expression of ER mRNA and protein in the brain is unknown but it is likely that a posttranscriptional mechanism(s) is/are involved in their regulation.
Besides transcriptional regulation, estradiol regulation of ER degradation may account for change in expression. In cell lines, chronic or acute exposure to estradiol rapidly induced a 50-60% loss of intracellular ERα in the presence of protein synthesis and translation inhibitors [5; 209]. A viable explanation for these results is that proteolytic degradation of ERs is responsible for the downregulation. Estradiol transiently increases ubiquitination of intracellular ERs [126] and estradiol binding and estradiol-dependent sexually receptive behavior is increased in the presence of proteasome pathway inhibitors [5; 60]. This suggests that proteasome pathways are involved in maintaining ER levels in the brain and possibly involved in regulating the tissue response to estradiol. Whether membrane ERs are regulated by the proteasomal degradations is unknown. However, regulation of ER-GFP expression is dependent on ERK activation, suggesting membrane-initiated estradiol signaling has a role in ER expression [221].
Membrane Associated ERα and ERβ is Regulated by Estradiol
Membrane receptors, as a group, are regulated in a number of complex ways, only one of which is through transcriptional regulation. Other regulatory mechanisms include posttranslational modification, phosphorylation and trafficking of receptors into and out of the membrane. Removal of receptors from the cell membrane by internalization is a well-characterized mechanism of desensitization. For example, estradiol treatment induces the internalization of μ-opioid receptors (MOR) and the NPY-Y1 receptors through the release of β-END and NPY, respectively. The internalized receptors are transported to endosomes where the ligands are released from their receptors which are then sorted for either recycling or degradation. Such endosomal trafficking has been reported for many, if not all, membrane receptors [122; 163].
In the uterus, acute estradiol stimulation resulted in the internalization of a plasma membrane-localized ER, and in hypothalamic neurons, estradiol application rapidly increased the appearance of pits in the plasma membrane, an event associated with endocytosis. Taken together, these studies suggest that estradiol treatment induces ER internalization [56; 73; 129; 191]. The internalization of membrane receptors, such as during desentization, involves several cellular components associated with endocytosis including GTP-ases, adaptor proteins and ubiquitin. A well-characterized mechanism of desensitization involves the phosphorylation of activated GPCRs by G protein receptor kinases (GRKs), which can lead to binding of arrestins and adaptor/scaffolding proteins, and deter signaling by preventing any further G protein coupling [55]. For example, β-arrestin bound to β2-adrenoreceptors acts as an adaptor for binding with clathrin or caveolin proteins to help assemble the components needed for the endocytosis of β2-adrenoreceptors [90; 91; 149]. GRKs are activated by the Gβγ subunit which initiates the binding of β-arrestin to the activated GPCR in order to initiate internalization [46; 116]. It is unknown whether activation of ERs involves their phosphorylation but estradiol has been reported to modulate GRK expression and activation [9; 42; 47].
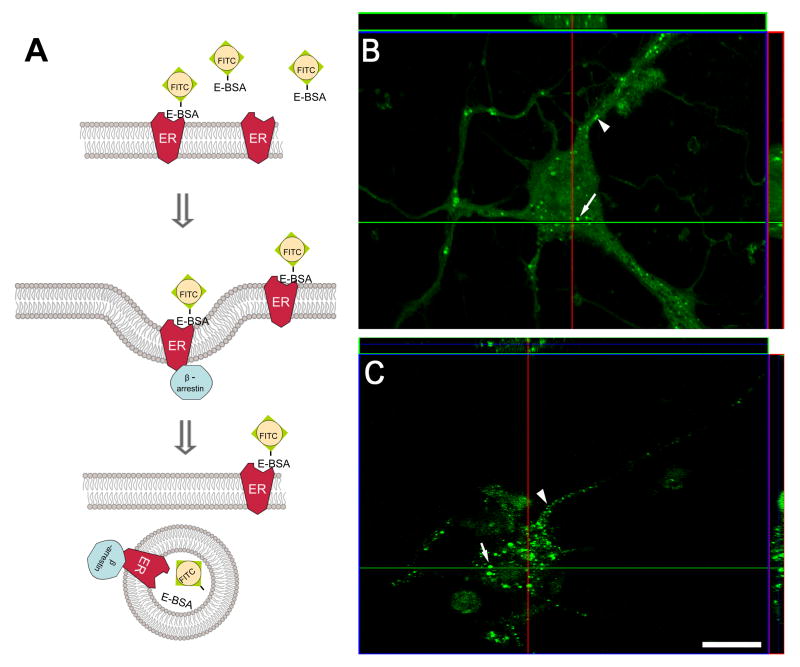
Estradiol can induce internalization of membrane ERs. Within 5-60 minutes after treatment with membrane impermeable estradiol constructs, the conjugated molecules have been visualized within cells [15; 42; 100; 120; 121]. One interpretation of the data is that ligand-bound membrane ER is internalized carrying the membrane impermeable estradiol (Fig. 1A). Upon agonist binding, the agonist-receptor complex is phosphorylated and β-arrestins are attached to the receptor and rapidly internalized into early endosomes. In this low pH intracellular compartment, the receptor is dissociated from its agonist and either returned to the plasma membrane or degraded (downregulation) [182]. The two events are distinguishable in terms of their time-course and effect on receptor number [10; 45; 98; 211]. Desensitization does not alter receptor number, whereas downregulation reduces receptor number. Desensitization is associated with the rapid internalization (translocation) of receptors following agonist binding while downregulation is a slower process. The internalization and recycling to the membrane occurs without loss of receptor number. Thus, internalization of GPCRs visualized by immunocytochemistry can be used as a marker of receptor activation [182; 183]. For ER, fluorescein-tagged E-6-BSA (E-6-BSA-FITC; Fig. 1B) and the membrane-constrained estradiol E-6-biotin (Fig. 1C) was observed to be internalized [42]. After 60 minutes, E-6-BSA-FITC and E-6-biotin were seen associated with plasma membranes and within the cytoplasm, suggesting these conjugated hormones are internalized in cortical neurons. These observations suggest that membrane ERs are internalized.
Figure 1. E-6-BSA-FITC and E-6-biotin are internalized in primary cortical neurons.
(A) Ligand bound receptors are internalized and transported to endosomes to be sorted for recycling or degradation by a β-arrestin mediated mechanism. (B) Cortical neuronal cultures were prepared on glass coverslips and treated with 1 μg/ml E-6-BSA-FITC for 60 min at 37 °C, fixed, and prepared for confocal microscopy. Analysis of reconstructed confocal z-stack slices (side panels) show that E-6-BSA-FITC binding was localized on plasma membranes (arrowheads) and within subcellular compartments (arrows) in several neuronal profiles. (C) Cortical neurons were prepared as described above but were treated with 50 nM E-6-biotin and permeablized after fixation. Biotin conjugated-estradiol was labeled with 1 mg/ml Alexa488-strepavidin to visualize internalization of the ligand. Reconstructed confocal z-stack slices (side panels) demonstrate that the fluorescein labeled E-6-biotin/strepavidin complex was internalized in a similar manner as E-6-BSA-FITC in several neuronal profiles. These findings suggest ligand bound ERs are internalized. Scale bar = 20 μm. [these data redrawn from 42]
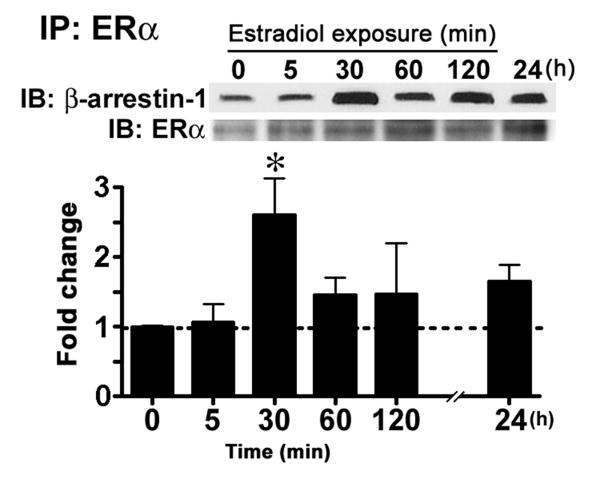
What is the mechanism by which the membrane ERα is internalized? As mentioned above, a general mechanism by which GPCRs are sequestered is modeled after the β2-adrenergic receptor and requires the binding of β-arrestin proteins to the receptor's cytoplasmic tail after agonist-induced activation and phosphorylation by GRKs [82; 223]. Co-immunoprecipitation after acute estradiol stimulation of primary cortical neuronal cultures showed an increased interaction between β-arrestin-1 and ERα, indicating that membrane ERα is internalized through a β-arrestin-mediated mechanism (Fig. 2) [42]. These results strongly support the idea that membrane ERα is regulated like other membrane receptors and that constant exposure to its natural ligand, estradiol regulates the number of receptors in the membrane, lending support to the idea that constant estradiol attenuates cellular response.
Figure 2. Estradiol treatment increased the interaction between ERα and β-arrestin-1 in cortical neuronal cultures.
Cortical neuronal cultures were treated with 10 nM estradiol for the times indicated and collected. An antibody raised against the C-terminal of ERα (MC-20) was used to immunoprecipitate (IP) receptors from cellular extracts. To determine the levels of co-immunoprecipitated β-arrestin-1 western immunoblot (IB) analysis (upper panel) was used. The bar graph shows that estradiol treatment increased the interaction between β-arrestin-1 and ERα over time (n = 4). Immunoblot analysis of ERα was used to verify loading. (Tukey's post hoc test, *p < 0.05) [these data redrawn from 42]
ERα and Receptor Trafficking
Insertion and internalization of ERs to and from the membrane has been more difficult to parse but these actions raises their own set of questions. Is there a stable population of membrane ERs and does exposure to estradiol cause internalization, or does estradiol cause the insertion of ERs into the membrane and then their internalization? While there is a dispute over whether ERα and ERβ can be trafficked to the membrane, a rapid translocation of ERα and ERβ has been observed within 5-60 min of estradiol exposure in HT22 cells and in cortical neurons [42; 171]. Since neither membrane targeting sequences nor stretches of hydrophobic residues have been identified within ERα and ERβ [190], the predominant hypothesis is that ERs are localized to the membrane via palmitoylation. ERα mutated to prevent palmitoylation, (e.g., Cys 477 to Ala), does not associate with calveolin-1 (CAV1) nor is it targeted to the membrane [2]. A conserved nine amino acid membrane targeting sequence has been identified in several steroid receptors including: the ligand binding domain of ERα and ERβ (that includes Cys477), as well as in the androgen receptor and progesterone receptors A and B [78; 137]. The association with CAV1 is important because it is a scaffolding protein that aids in membrane trafficking to lipid rafts [58; 100; 167]. Lipid rafts are membrane microdomains consisting of high concentrations of specific proteins and lipids. Among the most prominent of these proteins are caveolins. These microdomains function as regions in which membrane receptors and trimeric G proteins are clustered to concentrate membrane signaling.
One way to establish that ERα or ERβ are intrinsic membrane proteins that have a portion of the molecule exposed to the extracellular space is through surface biotinylation. With this process, surface proteins are labeled by chemically attaching a biotin molecule to exposed amine groups. The reagent, sulfo-biotin, is membrane impermeable and thus only proteins exposed on the extracellular surface are labeled. Once the proteins are biotinylated, the labeled proteins can be isolated using avidin conjugated beads and examined using western immunoblot analysis to determine the amount of cell surface protein. Recently, such an experiment was done with embryonic hypothalamic neurons and demonstrated a biotinylated ERα with a molecular weight of 50 kDa [61; 150]. Similar studies demonstrated a transmembrane ERα in astrocytes as well as the full length 66 kDa ERα [21]. Others have suggested that membrane ERs are attached to the inner leaflet of the cell membrane [189]. Stimulation of hypothalamic cultures with estradiol for 48 hours increased levels of a 50 kDa surface biotinylated ERα immunoreactive protein [61]. The presence of higher molecular weight biotinylated ER proteins in cortical tissue has also been observed [150].
Regardless of the timing, trafficking of ERs into and out of the cell membrane reveals a level of regulation not previously appreciated. These recent observations indicate that ERs in the membrane may not be a stable population, but rather inserted as needed and then sequestered following activation. The fact that estradiol causes ERs to be inserted into the membrane, but only transiently, suggests that continuing exposure to estradiol does not continually activate membrane ERs. Their activity, based on the levels in the membrane, may peak within minutes and then, as the ERs are removed, estradiol may no longer be able to signal through the membrane-initiated steroid signaling mechanism Thus, it is the nuclear localized ERs that primarily shape the long-term response to estradiol. On the other hand, signaling from the membrane to the nucleus demonstrates that membrane action will have long-term consequences, such as has been demonstrated for rapid estradiol activation of sexually receptive behavior [38; 81].
ERα and G proteins
In neurons and cell lines, estradiol has been shown to activate several second messenger signaling pathways coupled to G proteins. Activation of these pathways rapidly change synaptic and cellular responses, suggesting they are mediated by membrane ERs, but it is unclear whether these effects are mediated through a direct interaction with ERs and G proteins or through estradiol sensitive GPCRs. Estradiol rapidly modulates potassium and calcium membrane currents through activation of cyclic AMP (cAMP) and protein kinase A (PKA) pathways, suggesting that estradiol signaling is mediated through a Gαs coupled mechanism [11; 65; 123].
The membrane ER also appears to be coupled to a Gαq coupled mechanism. For example, estradiol modulates a Gαq coupled membrane ER that activates the PLC/protein kinase C (PKC) and PKA pathways [22; 23; 39; 40; 143]. Alternatively, a membrane ERα coupled to Gαi/o may also explain estradiol-induced activation of downstream G protein signaling cascades. In immortalized hypothalamic and COS-7 cell lines, a putative interaction between ERα and Gαi/o is reduced within 5 minutes of estradiol treatment [124; 220]. ICI 182,780 and pertussis toxin blocked the dissociation of ERα and Gαi/o. In cerebellar neurons, Gαi/o coupled ER is linked to ERK signaling and modulates striatal dopamine D2 receptor activation in estradiol primed ovariectomized rats [14; 198]. Activation of downstream G protein signaling cascades may also be induced by the Gβγ coupled ER mechanism [52; 151]. The mechanism by which ERs interact with G proteins is unknown. However, mutagenesis of ERα and use of G protein blocking peptides reveal that the ligand binding domain is necessary for the interaction [84]. These data suggest that estradiol-activation of these various signaling pathways involves ERs G proteins activation to initiate cell signaling and that the proximal events in this signaling may involve interaction with another membrane receptor that is a GPCR.
ER Signaling Through Metabotropic Glutamate Receptors
Classical ERα and ERβ are transcription factors that have extremely limited structural similarity with membrane GPCRs. Surface biotinylation studies show that ERα is inserted in the membrane and has an exposed extracellular portion [61; 150], but how this molecule initiates cell signaling is not clear. Boulware et al. [23] provided an alternative explanation to the “ER as a GPCR hypothesis” [113]. Upon estradiol binding to the ER, the ER promotes transactivation of the metabotropic glutamate receptors (mGluR), initiating mGluR signaling without the need for glutamate [23; 39; 85]. Estradiol binding to the ER activates mGluR, initiating downstream G protein signaling. A similar indirect activation of cell signaling has been proposed for ERs and tyrosine kinase receptors [115; 164; 187; 192; 204]. ER/tyrosine kinase receptors are activated following estradiol treatment [71]. Thus, the idea that membrane ERs may use other receptors to initiate cell signaling including tyrosine kinase receptors, insulin-like growth hormone receptor and mGluRs has emerged [23; 47; 145]. Such a receptor-receptor interaction of ERs and mGluRs is supported by co-immunoprecipitation experiments that indicate ERα can directly interact with mGluR1a [38]. In female hippocampal neurons, estradiol induces the phosphorylation of cAMP response-element binding (CREB) protein via stimulation of group I (Gαq-coupled) mGluRs [23]. In neurons from male hippocampus, estradiol did not increase CREB phosphorylation. Use of an mGluR agonist and an ER antagonist strongly suggest that a putative protein-protein interaction can alter the function of mGluR signaling. Activation of mGluR1a with S-3,5-dihydroxyphenylglycine (DHPG) induces CREB phosphorylation, but the response is attenuated following treatment with the ER antagonist ICI 182,780. ICI 182,780 does not appear be acting at the mGluR1a since male neurons that do not respond to estradiol respond to DHPG by increasing CREB phosphorylation levels.
The ER/mGluR interactions are dependent upon caveolin proteins [22] that are essential for the trafficking and clustering of signaling molecules. Along with palmitoylation, the interaction of caveolin proteins with ERα is critical for the insertion of the receptor to the membrane [151]. Interestingly, there is a brain region-specific ER-caveolin interaction. In hippocampal neurons, ERα interaction with either mGluR1 or mGluR2/3 was dependent upon caveolin-3 (CAV3) or CAV1 respectively. Conversely, ERβ interacts with mGluR2/3 via CAV3[22]. In striatal neurons, ERα via CAV1 activates mGluR5 [64]. Functional isolation of different ERs with mGluRs suggests a diverse array of potential estrogen-sensitive signaling pathways at the disposal of individual cells. The generation of specific ER/mGluR pairs via caveolin function may eventually be found to be responsible for many of the diverse observations of novel estrogen signaling in the central nervous system [113].
Physiology of Membrane ERs
Another of the continuing questions about rapid, membrane-initiated estradiol signaling relates to its physiological significance. A putative plasma membrane ER rapidly stimulated prolactin release from pituitary carcinoma cells (GH3/B6). Administration of E-6-BSA to GH3/B6 cells released prolactin after 1 minute [135], and the release of prolactin could also be modulated by antibodies directed towards ERα [216]. Here we describe three separate estrogen-sensitive processes that require a “novel” mechanism of estradiol action: regulation of sexual receptivity, neuroprogesterone synthesis and its influence on the hypothalamic-pituitary-gonadal (HPG) axis and signaling in DRG neurons associated with nociception. In these systems, we find a rapid component of estradiol signaling that is dependent upon ER/mGluR signaling.
Sexual receptivity
Arguably the best studied and most robust actions of estradiol in the brain have been on neural circuits controlling the HPG axis that regulates reproduction. In the female rat, estradiol acts on a limbic-hypothalamic circuit to allow the expression of lordosis, a stereotypic behavior indicative of (or reflecting) sexual receptivity [110; 186]. Although lordosis can be elicited by implanting estradiol directly into the hypothalamus [139; 166], attempts to induce lordosis behavior exclusively through membrane actions of estradiol have not been successful. The assumption is that gene transcription is needed to elicit lordosis behavior. In a normally cycling rat, estradiol rises slowly for several days before peaking on the afternoon of proestrus prior to the onset of sexual receptivity. Experimentally, this is mimicked in ovariectomized rats treated with estradiol, which induces lordosis behavior 30-48 hours later. Lordosis behavior, a measure of sexual receptivity, depends on the transcription of new proteins [62; 147], including enkephalin, β-END and oxytocin [13; 36; 111; 140; 141; 160; 217]. Both the demonstration of new protein synthesis and the time course of estradiol action pointed to an estradiol transcriptional regulation of sexual receptivity. A role of rapid, membrane-initiated signaling gradually emerged. Priming with E-6-BSA followed with a behaviorally-ineffective dose of estradiol was as efficacious as two injections of free estradiol at inducing lordosis behavior [81]. These results suggest that membrane-initiated estradiol signaling facilitates nuclear ER-stimulated transcriptional events, signifying cooperation between membrane- and nuclear-initiated actions of estradiol and indicating that rapid actions are involved in the estradiol induction of sexual receptivity.
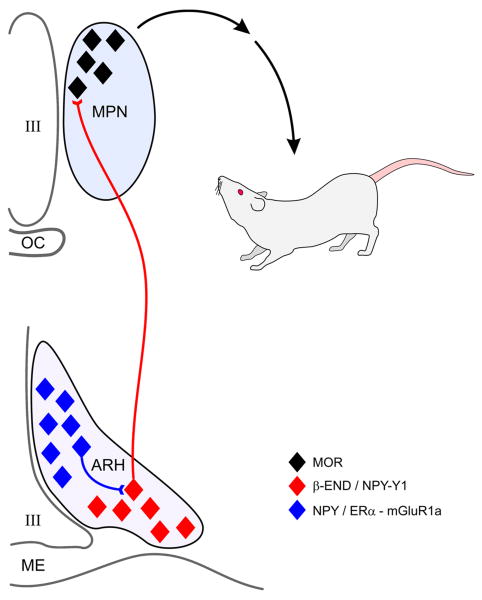
One of the best studied neuropeptides that regulates sexual receptivity is the endogenous opioid peptide, β-END (Fig. 3). β-END is synthesized in the arcuate nucleus and has an extensive projection throughout the forebrain, including the medial preoptic area [117]. Passive immunoneutralization experiments in the medial preoptic area with antibodies directed against β-END hinted that an endogenous opioid is rapidly activated by estradiol to regulate lordosis [203]. To examine the rapid estradiol component involved in the facilitation of lordosis, estradiol activation in the arcuate to medial preoptic nucleus projection was studied [117]. A hallmark of estradiol activation of this circuit is the rapid activation and internalization of MOR in the medial preoptic nucleus, an area associated with the regulation of lordosis behavior [45; 184]. Without MOR activation, lordosis behavior is significantly attenuated [181; 202]. E-6-biotin injected directly into the arcuate nucleus activated MOR facilitated lordosis behavior, providing further evidence that this was membrane-initiated signaling [39]
Figure 3. Regulation of sexual receptivity through the arcuate-medial preoptic nucleus projection.
Estradiol acts in the arcuate nucleus of the hypothalamus (ARH) to activate NPY expression cells. This membrane initiated estradiol signaling requires the interaction of ERα with mGluR1a to phosphorylate PKCθ. NPY released within the ARH activates NPY-Y1 receptors on β-END neurons that project to the medial preoptic nucleus (MPN) where released β-END activates MOR. This circuit enhances the lordosis behavior of the rat.
The potential that the estradiol activation of the arcuate to medial preoptic nucleus projection involves membrane-initiated signaling and an interaction with the mGluR1a was systematically examined and it was determined that: 1) indirect estradiol activation of MOR depends on ERα [114]; 2) ERα colocalizes with mGluR1a [39]; 3) ERα co-immunoprecipitates with mGluR1a in a membrane preparation from arcuate nucleus tissue [40]; 4) antagonism of mGluR1a attenuates the estradiol-induced MOR activation and lordosis [39]; 5) mGluR1a blockade of lordosis behavior is only effective at the time of estradiol treatment. These results suggest a model for ER/mGluR interaction that mediates behavior (Fig. 3) [113]. During low systemic estradiol levels, the arcuate-medial preoptic circuit is quiescent and the animal is not sexually receptive. In the medial preoptic nucleus, MORs are localized to the cell membrane, an indication these receptors are not activated. During proestrus, systemic estradiol reaches levels that induce behavior and increase the levels of ERα on the cell plasma membrane. In the arcuate nucleus, the new membrane-inserted ERα is stimulated, leading to MOR internalization and subsequent full lordosis behavior. Membrane ERα can be bypassed by directly stimulating mGluR1a under low estradiol conditions, resulting in MOR internalization and facilitation of lordosis. Conversely, when estradiol levels are high, antagonizing mGluR1a blocks estradiol-induced MOR internalization and attenuates sexual behavior. These data are consistent with the in vitro demonstration of ERα/mGluR1a signaling in hippocampal neurons and provided the first in vivo evidence that estradiol can signal through activation of mGluR1a. Further evidence of this rapid estradiol signaling is the demonstration that estradiol in vivo stimulates the phosphorylation of a novel, calcium independent PKCθ in the arcuate nucleus [40]. Pharmacological stimulation of PKC overcame ER antagonism with ICI 182,780 or mGluR1a antagonism with LY367485 and stimulated lordosis. This set of experiments demonstrates that lordosis behavior, a classical assay of estradiol action, has a rapid non-genomic component and underscores the importance of ER/mGluR interactions in the brain.
Neuroprogesterone synthesis
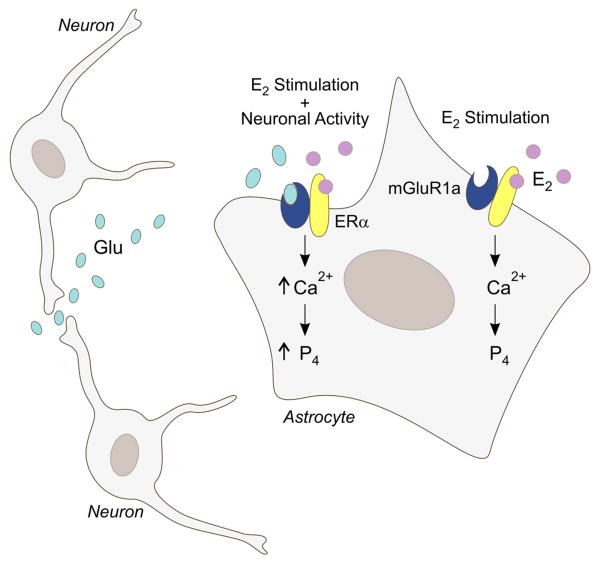
The brain, like the gonads and adrenal cortex, is a steroidogenic organ. All of the necessary steroidogenic enzymes needed to synthesize sex steroids from cholesterol have been isolated in various parts of the brain [170]. Steroids synthesized de novo in the nervous system are considered neurosteroids. The steroidogenic capacity in the cells of the central nervous system is widespread, but different cell types appear to preferentially produce specific steroids [226]. One of the most intriguing steroids synthesized in the brain is progesterone. Neuroprogesterone, progesterone produced by nervous tissue, is a product of astrocytes and its synthesis is widely distributed in the rat brain [108; 112; 185]. In addition to the myriad of progesterone-mediated actions, its metabolite, allopregnenalone, has profound effects on neuronal excitation through actions at the GABAA receptor [27; 99]. Since progesterone is involved in the estrogen positive feedback of the LH surge, an intriguing observation was that estradiol stimulates the synthesis of neuroprogesterone in the hypothalamus of adult female rats [108]. To reach an integrative understanding of the LH surge, the interaction between circulating estradiol and neuroprogesterone synthesis in astrocytes was demonstrated (Fig. 4). Estradiol rapidly increased free cytoplasmic calcium flux by releasing intracellular stores of calcium [29]. This calcium flux is dependent on activation of the PLC/IP3 pathway and was blocked by an inhibitor of the IP3 receptor. Similarly, estradiol increased the synthesis of neuroprogesterone that was dependent on robust [Ca2+]i [112]. To mimic the actions of estradiol on releasing IP3 receptor sensitive intracellular calcium stores, thapsigargin was used to induce the release of [Ca2+]i. The effect was a stimulation of neuroprogesterone synthesis that was as robust as estradiol. Moreover, the increase of progesterone synthesis was seen after one hour of treatment, the earliest time point examined [112]. Thus, in astrocytes, stimulation of neuroprogesterone synthesis is dependent on calcium flux.
Figure 4. Proposed mechanism through which estradiol signaling in astrocytes is integrated with local neuronal activity involved in the synthesis of neuroprogesterone.
Estradiol (E2), typically of ovarian origin, binds to membrane ERα and activates mGluR1a. This increases levels of free cytoplasmic calcium (Ca2+) through the inositol trisphosphate (IP3) receptor mediated release of intracellular stores of calcium. Elevated levels of intracellular Ca2+ are needed for neuroprogesterone (P4) synthesis in astrocytes. Studies in vitro demonstrate that E2 alone or an agonist mGluR1a alone increase intracellular calcium levels. However, when both an mGluR1a agonist and E2 are applied to astrocytes, the resulting Ca2+ flux is significantly greater, suggesting that P4 synthesis is also augmented. We propose that in vivo when E2-stimulated astrocytes are in the proximity of active nerve terminals, the released glutamate (Glu) activates astrocyte mGluR1a, resulting in significantly greater Ca2+ responses. This elevated Ca2+ response is hypothesized to produce a greater P4 synthesis in astrocytes [113].
How does estradiol signal though the PLC/IP3 pathway to stimulate [Ca2+]i flux and neuroprogesterone synthesis? As in neurons, astrocytes also express mGluR1a, and like neurons, co-immunoprecipitation demonstrates a potential interaction between ERα and mGluR1a in astrocytes, but not between ERβ and mGluR1a [85]. This observation is consistent with data that the ERα selective agonist, 4,4″,4”-(4-Propyl-[1H]-pyrazole-1,3,5-triyl)trisphenol (PPT), but not the ERβ selective, 2,3-bis(4-Hydroxyphenyl)-propionitrile (DPN), stimulates [Ca2+]i flux in astrocytes. As in the arcuate nucleus, the mGluR1a antagonist LY367385 blocked estradiol-induced [Ca2+]i flux, suggesting that in astrocytes the same ER/mGluR1a interaction exists between membrane ERα and mGluR1a [86]. Activation of the mGluR1a without estradiol induced a robust [Ca2+]i flux. When estradiol and DHPG were applied together, the [Ca2+]i flux was greatly amplified. Dual stimulation of astrocytic mGluR1a and ERα produced a significantly greater [Ca2+]i flux and in preliminary experiments a greater synthesis of neuroprogesterone, suggesting that for maximal neuroprogesterone signaling activation of both receptors is necessary. Micevych and Mermelstein [113] proposed that in vivo, estradiol acts most effectively on astrocytes that are near active glutaminergic terminals. Although this intriguing hypothesis requires testing, it suggests an integration of neuronal and astrocytic functions in terms of initiating the activation of GnRH neurons.
ATP signaling in DRG neurons
Another example of rapid, membrane-initiated estradiol signaling was observed in the cell bodies of primary afferent neurons. The cell bodies of primary spinal afferent neurons are located in the DRG at each spinal segment. Primary afferents transmit information about chemical or mechanical stimulation from the periphery to the spinal cord. There are several distinct size-categories of DRG neurons, and nociceptors are small to medium sized DRG neurons whose peripheral processes detect potentially damaging physical and chemical stimuli. ATP is a putative visceral pain signal that is released by mechanical distortion, tissue damage or inflammation to activate high threshold nociceptors [20; 26]. Visceral nociceptive C-fibers are activated by ATP and excitatory amino acids that are released by noxious stimuli from cells in target organs [25]. ATP activates purinergic P2X receptors on primary afferent fibers [44]. Opening of P2X channels results in membrane depolarization sufficient to trigger action potentials and calcium influx through voltage-gated calcium channels (VGCC) associated with nociception [79]. The predominant ATP receptor in small diameter nociceptive DRG neurons is P2X3 [32; 206]. P2X3-null mice have reduced pain-related behavior in response to noxious stimuli [35; 225].
ERs are distributed in regions of the central and peripheral nervous system that mediate nociception. For example, ERs are expressed in dorsal horn neurons of the spinal cord [8; 218] and DRG neurons [133; 134; 194]. Both ERα and ERβ are present in DRG neurons including the small to medium diameter putative nociceptors [133]. In vitro, 85% of the ATP-sensitive DRG neurons that appear to be visceral afferents [105], respond to estradiol [30], which correlates well with the idea that visceral afferents are estradiol sensitive. Indeed, visceral pain is affected by hormonal levels in cycling females [157; 165; 205], and the prevalence of functional disorders involving the viscera is sex differentiated [95; 162]. Thus, in addition to central actions of estradiol [6], estradiol can also act in the periphery to modulate nociception.
Estradiol modulates neuronal L-type VGCC [30; 94; 107] and has a significant role in modulating visceral sensitivity, indicating that estradiol alterations in sensory processing may underlie sex-based differences in functional pain symptoms [4]. However, reports of estradiol modulation of visceral and somatic nociceptive sensitivity are conflicting. For example, elevated estradiol levels have been reported to increase the threshold to cutaneous stimuli [102], but decrease the percentage of escape responses to ureteral calculosis [57]. On the other hand, nociceptive sensitivity appears to increase when estradiol levels are elevated [68] and in clinical studies, women report more severe pain levels, more frequent pain, and longer duration of pain than men [16; 53].
In a primary culture of DRG neurons, estradiol inhibited the ATP-mediated calcium influx in response to ATP stimulation. The estradiol action was stereospecific and inhibited by ER antagonists, tamoxifen and ICI 182,780 [30]. ATP initiates two calcium currents, one through P2X channels and a secondary response due to the opening of VGCCs in response to membrane depolarization [79]. The entire calcium transient is blocked with the purine receptor antagonist PPADS, but the calcium response is only partially inhibited by estradiol, suggesting that estradiol does not directly antagonize P2X receptors. Blockade of the L-type VGCC with nifedipine, however, significantly attenuated the ATP-induced calcium influx, and estradiol treatment did not result in additional inhibition, suggesting that estradiol mediates the opening of the L-type VGCC. This result is consistent with estradiol blockade of L-type calcium channels in PC-12 cells [77], neostriatal and hippocampal neurons [88; 107].
Although both ERα and ERβ are expressed in DRG neurons, only ERα is necessary for the estradiol attenuation of ATP-induced calcium influx [31]. In DRG neurons from ERα-/- mice, estradiol was not able to attenuate the ATP-induced calcium influx. While in wild type and ERβ-/- mice, estradiol attenuated the ATP-induced calcium influx [31]. As in other neurons, DRG neurons express mGluRs, but in these cells estradiol did not activate [Ca2+]i through these receptors. Instead it was reported that activated ERα rapidly attenuates calcium influx through L-type VGCCs. Such an interaction was hypothesized when ERα interacted with mGluR2/3 [22; 23]. The estradiol attenuation of ATP-induced calcium signaling was disrupted if the mGluR2/3 was blocked with the inhibitor, LY341495. Thus, rapid estradiol inhibition of calcium influx through L-type VGCCs in DRG neurons is dependent on mGluR2/3 (Li, P. et al., submitted for publication).
Summary
Although ERs have been extensively studied, the more recently embraced membrane-initiated estradiol action has created a great deal of confusion in the field. While estradiol action has repeatedly been demonstrated at the cell surface, the nature of membrane ERs remains elusive. Several candidate membrane ERs have been proposed to exist in the brain, including: ER-X, GPR30 and STX-activated protein. All of these, except GPR30, are located in the cell membrane. ER-X appears to have a large homology to ERα, but is not antagonized by ICI 182,780 and is not stereospecific. The STX-binding protein is antagonized by ICI 182,780, but has not been cloned. The best and most extensive support for membrane ER is for ERα and ERβ, the same molecules that act as ligand-gated transcription factors in the nucleus. These receptors are palmitoylated, and in association with caveolin proteins, trafficked to the cell membrane. In the cell membrane, ERα and ERβ appear to act like GPCRs to activate a wide range of cell signaling pathways. Membrane ERs bind estradiol as demonstrated by experiments using membrane-impermeable estradiol constructs. Estradiol treatment induces β-arrestin binding to ERα and subsequent internalization into endosomes. All of these results are consistent with the ER as a GPCR hypothesis, but what has been more difficult to demonstrate is the direct interaction of ERα and ERβ with G proteins. Indeed, it is clear that ERα and ERβ are not GPCRs. They initiate cell signaling by interacting with mGluRs. With or without glutamate, estradiol-activated ERs transactivate mGluRs stimulating them to activate G proteins. Co-immunoprecipitation studies demonstrate the potential interactions of ERα and ERβ with specific groups of mGluRs to signal through Gαq or Gαi/o pathways, explaining estradiol actions in different cells and in activated or quiescent cells. Pharmacological blockade of mGluRs abrogate membrane-initiated estradiol actions, including activation of cytoplasmic calcium flux, PKC and nuclear CREB, further suggesting that such interactions may be critical for ER signaling at the membrane.
In spite of this evidence, some membrane-initiated estradiol action remain in animals missing both ERα and ERβ, the so-called double knock outs (ERα-/-/ERβ-/-). Whether one of the known ER candidates or an as yet unknown ER is ultimately found to mediate this remaining estradiol action remains to be determined. It is likely, however, that whichever protein is added to the ER family, membrane-initiated estradiol signaling will involve interactions with mGluRs to modulate cell signaling in the nervous system. Many questions remain to be answered about membrane ERs, but research during the past decade has proven to be extremely valuable in beginning to define the parameters of membrane-initiated estradiol signaling.
Acknowledgments
Thanks to A.K. Christensen for help with the editing of this manuscript. This work was supported by DA013185, HD042635, and AG14751.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abraham IM, Todman MG, Korach KS, Herbison AE. Critical in vivo roles for classical estrogen receptors in rapid estrogen actions on intracellular signaling in mouse brain. Endocrinology. 2004;145:3055–3061. doi: 10.1210/en.2003-1676. [DOI] [PubMed] [Google Scholar]
- 2.Acconcia F, Ascenzi P, Bocedi A, Spisni E, Tomasi V, Trentalance A, Visca P, Marino M. Palmitoylation-dependent estrogen receptor alpha membrane localization: regulation by 17beta-estradiol. Mol Biol Cell. 2005;16:231–237. doi: 10.1091/mbc.E04-07-0547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Adams MM, Fink SE, Shah RA, Janssen WG, Hayashi S, Milner TA, McEwen BS, Morrison JH. Estrogen and aging affect the subcellular distribution of estrogen receptor-alpha in the hippocampus of female rats. J Neurosci. 2002;22:3608–3614. doi: 10.1523/JNEUROSCI.22-09-03608.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Al-Chaer ED, Traub RJ. Biological basis of visceral pain: recent developments. Pain. 2002;96:221–225. doi: 10.1016/S0304-3959(02)00046-5. [DOI] [PubMed] [Google Scholar]
- 5.Alarid ET, Bakopoulos N, Solodin N. Proteasome-mediated proteolysis of estrogen receptor: a novel component in autologous down-regulation. Mol Endocrinol. 1999;13:1522–1534. doi: 10.1210/mend.13.9.0337. [DOI] [PubMed] [Google Scholar]
- 6.Aloisi AM, Ceccarelli I, Herdegen T. Gonadectomy and persistent pain differently affect hippocampal c-Fos expression in male and female rats. Neurosci Lett. 2000;281:29–32. doi: 10.1016/s0304-3940(00)00819-3. [DOI] [PubMed] [Google Scholar]
- 7.Alves SE, Lopez V, McEwen BS, Weiland NG. Differential colocalization of estrogen receptor beta (ERbeta) with oxytocin and vasopressin in the paraventricular and supraoptic nuclei of the female rat brain: an immunocytochemical study. Proc Natl Acad Sci U S A. 1998;95:3281–3286. doi: 10.1073/pnas.95.6.3281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Amandusson A, Hallbeck M, Hallbeck AL, Hermanson O, Blomqvist A. Estrogen-induced alterations of spinal cord enkephalin gene expression. Pain. 1999;83:243–248. doi: 10.1016/s0304-3959(99)00109-8. [DOI] [PubMed] [Google Scholar]
- 9.Ansonoff MA, Etgen AM. Estrogen increases G protein coupled receptor kinase 2 in the cortex of female rats. Brain Res. 2001;898:186–189. doi: 10.1016/s0006-8993(01)02161-8. [DOI] [PubMed] [Google Scholar]
- 10.Arden JR, Segredo V, Wang Z, Lameh J, Sadee W. Phosphorylation and agonist-specific intracellular trafficking of an epitope-tagged mu-opioid receptor expressed in HEK 293 cells. J Neurochem. 1995;65:1636–1645. doi: 10.1046/j.1471-4159.1995.65041636.x. [DOI] [PubMed] [Google Scholar]
- 11.Aronica SM, Kraus WL, Katzenellenbogen BS. Estrogen action via the cAMP signaling pathway: stimulation of adenylate cyclase and cAMP-regulated gene transcription. Proc Natl Acad Sci U S A. 1994;91:8517–8521. doi: 10.1073/pnas.91.18.8517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Azcoitia I, Sierra A, Garcia-Segura LM. Localization of estrogen receptor beta-immunoreactivity in astrocytes of the adult rat brain. Glia. 1999;26:260–267. [PubMed] [Google Scholar]
- 13.Bale TL, Dorsa DM. Cloning, novel promoter sequence, and estrogen regulation of a rat oxytocin receptor gene. Endocrinology. 1997;138:1151–1158. doi: 10.1210/endo.138.3.4998. [DOI] [PubMed] [Google Scholar]
- 14.Belcher SM, Le HH, Spurling L, Wong JK. Rapid estrogenic regulation of extracellular signal- regulated kinase 1/2 signaling in cerebellar granule cells involves a G protein- and protein kinase A-dependent mechanism and intracellular activation of protein phosphatase 2A. Endocrinology. 2005;146:5397–5406. doi: 10.1210/en.2005-0564. [DOI] [PubMed] [Google Scholar]
- 15.Benten WP, Stephan C, Lieberherr M, Wunderlich F. Estradiol signaling via sequestrable surface receptors. Endocrinology. 2001;142:1669–1677. doi: 10.1210/endo.142.4.8094. [DOI] [PubMed] [Google Scholar]
- 16.Berkley KJ. Sex differences in pain. Behav Brain Sci. 1997;20:371–380. doi: 10.1017/s0140525x97221485. discussion 435-513. [DOI] [PubMed] [Google Scholar]
- 17.Bhat RA, Stauffer B, Unwalla RJ, Xu Z, Harris HA, Komm BS. Molecular determinants of ER alpha and ER beta involved in selectivity of 16 alpha-iodo-7 beta estradiol. J Steroid Biochem Mol Biol. 2004;88:17–26. doi: 10.1016/j.jsbmb.2003.10.009. [DOI] [PubMed] [Google Scholar]
- 18.Blaustein JD. Cytoplasmic estrogen receptors in rat brain: immunocytochemical evidence using three antibodies with distinct epitopes. Endocrinology. 1992;131:1336–1342. doi: 10.1210/endo.131.3.1380440. [DOI] [PubMed] [Google Scholar]
- 19.Blaustein JD, Turcotte JC. Estrogen receptor-immunostaining of neuronal cytoplasmic processes as well as cell nuclei in guinea pig brain. Brain Res. 1989;495:75–82. doi: 10.1016/0006-8993(89)91219-5. [DOI] [PubMed] [Google Scholar]
- 20.Bodin P, Burnstock G. Purinergic signalling: ATP release. Neurochem Res. 2001;26:959–969. doi: 10.1023/a:1012388618693. [DOI] [PubMed] [Google Scholar]
- 21.Bondar G, Kuo J, Hamid N, Micevych P. Estradiol-induced estrogen receptor-α trafficking. Neuroscience. doi: 10.1523/JNEUROSCI.2107-09.2009. Submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Boulware MI, Kordasiewicz H, Mermelstein PG. Caveolin proteins are essential for distinct effects of membrane estrogen receptors in neurons. J Neurosci. 2007;27:9941–9950. doi: 10.1523/JNEUROSCI.1647-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Boulware MI, Weick JP, Becklund BR, Kuo SP, Groth RD, Mermelstein PG. Estradiol activates group I and II metabotropic glutamate receptor signaling, leading to opposing influences on cAMP response element-binding protein. J Neurosci. 2005;25:5066–5078. doi: 10.1523/JNEUROSCI.1427-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brandenberger AW, Tee MK, Lee JY, Chao V, Jaffe RB. Tissue distribution of estrogen receptors alpha (ER-alpha) and beta (ER-beta) mRNA in the midgestational human fetus. J Clin Endocrinol Metab. 1997;82:3509–3512. doi: 10.1210/jcem.82.10.4400. [DOI] [PubMed] [Google Scholar]
- 25.Burnstock G. P2X receptors in sensory neurones. Br J Anaesth. 2000;84:476–488. doi: 10.1093/oxfordjournals.bja.a013473. [DOI] [PubMed] [Google Scholar]
- 26.Burnstock G. Purine-mediated signalling in pain and visceral perception. Trends Pharmacol Sci. 2001;22:182–188. doi: 10.1016/s0165-6147(00)01643-6. [DOI] [PubMed] [Google Scholar]
- 27.Callachan H, Cottrell GA, Hather NY, Lambert JJ, Nooney JM, Peters JA. Modulation of the GABAA receptor by progesterone metabolites. Proc R Soc Lond B Biol Sci. 1987;231:359–369. doi: 10.1098/rspb.1987.0049. [DOI] [PubMed] [Google Scholar]
- 28.Carmeci C, Thompson DA, Ring HZ, Francke U, Weigel RJ. Identification of a gene (GPR30) with homology to the G-protein-coupled receptor superfamily associated with estrogen receptor expression in breast cancer. Genomics. 1997;45:607–617. doi: 10.1006/geno.1997.4972. [DOI] [PubMed] [Google Scholar]
- 29.Chaban VV, Lakhter AJ, Micevych P. A membrane estrogen receptor mediates intracellular calcium release in astrocytes. Endocrinology. 2004;145:3788–3795. doi: 10.1210/en.2004-0149. [DOI] [PubMed] [Google Scholar]
- 30.Chaban VV, Mayer EA, Ennes HS, Micevych PE. Estradiol inhibits atp-induced intracellular calcium concentration increase in dorsal root ganglia neurons. Neuroscience. 2003;118:941–948. doi: 10.1016/s0306-4522(02)00915-6. [DOI] [PubMed] [Google Scholar]
- 31.Chaban VV, Micevych PE. Estrogen receptor-alpha mediates estradiol attenuation of ATP-induced Ca2+ signaling in mouse dorsal root ganglion neurons. J Neurosci Res. 2005;81:31–37. doi: 10.1002/jnr.20524. [DOI] [PubMed] [Google Scholar]
- 32.Chen CC, Akopian AN, Sivilotti L, Colquhoun D, Burnstock G, Wood JN. A P2X purinoceptor expressed by a subset of sensory neurons. Nature. 1995;377:428–431. doi: 10.1038/377428a0. [DOI] [PubMed] [Google Scholar]
- 33.Cheskis BJ, Greger JG, Nagpal S, Freedman LP. Signaling by estrogens. J Cell Physiol. 2007;213:610–617. doi: 10.1002/jcp.21253. [DOI] [PubMed] [Google Scholar]
- 34.Choleris E, Ogawa S, Kavaliers M, Gustafsson JA, Korach KS, Muglia LJ, Pfaff DW. Involvement of estrogen receptor alpha, beta and oxytocin in social discrimination: A detailed behavioral analysis with knockout female mice. Genes Brain Behav. 2006;5:528–539. doi: 10.1111/j.1601-183X.2006.00203.x. [DOI] [PubMed] [Google Scholar]
- 35.Cockayne DA, Hamilton SG, Zhu QM, Dunn PM, Zhong Y, Novakovic S, Malmberg AB, Cain G, Berson A, Kassotakis L, Hedley L, Lachnit WG, Burnstock G, McMahon SB, Ford AP. Urinary bladder hyporeflexia and reduced pain-related behaviour in P2X3-deficient mice. Nature. 2000;407:1011–1015. doi: 10.1038/35039519. [DOI] [PubMed] [Google Scholar]
- 36.Crowley RS, Insel TR, O'Keefe JA, Kim NB, Amico JA. Increased accumulation of oxytocin messenger ribonucleic acid in the hypothalamus of the female rat: induction by long term estradiol and progesterone administration and subsequent progesterone withdrawal. Endocrinology. 1995;136:224–231. doi: 10.1210/endo.136.1.7828535. [DOI] [PubMed] [Google Scholar]
- 37.Dauvois S, White R, Parker MG. The antiestrogen ICI 182780 disrupts estrogen receptor nucleocytoplasmic shuttling. J Cell Sci. 1993;106(Pt 4):1377–1388. doi: 10.1242/jcs.106.4.1377. [DOI] [PubMed] [Google Scholar]
- 38.Dewing P, Boulware MI, Sinchack K, Christensen A, Mermelstein PG, Micevych P. Membrane ERα interacts with mGluR1a to modulate female sexual receptivity. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dewing P, Boulware MI, Sinchak K, Christensen A, Mermelstein PG, Micevych P. Membrane estrogen receptor-alpha interactions with metabotropic glutamate receptor 1a modulate female sexual receptivity in rats. J Neurosci. 2007;27:9294–9300. doi: 10.1523/JNEUROSCI.0592-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Dewing P, Christensen A, Bondar G, Micevych P. PKC signaling in the hypothalamic arcuate nucleus regulates sexual receptivity in female rats. Endocrinology. 2008 doi: 10.1210/en.2008-0847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dominguez-Salazar E, Shetty S, Rissman EF. Rapid neural Fos responses to oestradiol in oestrogen receptor alphabeta double knockout mice. J Neuroendocrinol. 2006;18:195–202. doi: 10.1111/j.1365-2826.2005.01408.x. [DOI] [PubMed] [Google Scholar]
- 42.Dominguez R, Hu E, Zhou M, Baudry M. 17beta-estradiol-mediated neuroprotection and ERK activation require a pertussis toxin-sensitive mechanism involving GRK2 and beta-arrestin-1. J Neurosci. 2009;29:4228–4238. doi: 10.1523/JNEUROSCI.0550-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Dubal DB, Zhu H, Yu J, Rau SW, Shughrue PJ, Merchenthaler I, Kindy MS, Wise PM. Estrogen receptor alpha, not beta, is a critical link in estradiol-mediated protection against brain injury. Proc Natl Acad Sci U S A. 2001;98:1952–1957. doi: 10.1073/pnas.041483198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dunn PM, Zhong Y, Burnstock G. P2X receptors in peripheral neurons. Prog Neurobiol. 2001;65:107–134. doi: 10.1016/s0301-0082(01)00005-3. [DOI] [PubMed] [Google Scholar]
- 45.Eckersell CB, Popper P, Micevych PE. Estrogen-induced alteration of mu-opioid receptor immunoreactivity in the medial preoptic nucleus and medial amygdala. J Neurosci. 1998;18:3967–3976. doi: 10.1523/JNEUROSCI.18-10-03967.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Eichmann T, Lorenz K, Hoffmann M, Brockmann J, Krasel C, Lohse MJ, Quitterer U. The amino-terminal domain of G-protein-coupled receptor kinase 2 is a regulatory Gbeta gamma binding site. J Biol Chem. 2003;278:8052–8057. doi: 10.1074/jbc.M204795200. [DOI] [PubMed] [Google Scholar]
- 47.Etgen AM, Ansonoff MA, Quesada A. Mechanisms of ovarian steroid regulation of norepinephrine receptor-mediated signal transduction in the hypothalamus: implications for female reproductive physiology. Horm Behav. 2001;40:169–177. doi: 10.1006/hbeh.2001.1676. [DOI] [PubMed] [Google Scholar]
- 48.Etgen AM, Shamamian P. Regulation of estrogen-stimulated lordosis behavior and hypothalamic progestin receptor induction by antiestrogens in female rats. Horm Behav. 1986;20:166–180. doi: 10.1016/0018-506x(86)90015-2. [DOI] [PubMed] [Google Scholar]
- 49.Evinger AJ, 3rd, Levin ER. Requirements for estrogen receptor alpha membrane localization and function. Steroids. 2005;70:361–363. doi: 10.1016/j.steroids.2005.02.015. [DOI] [PubMed] [Google Scholar]
- 50.Fawell SE, White R, Hoare S, Sydenham M, Page M, Parker MG. Inhibition of estrogen receptor-DNA binding by the “pure” antiestrogen ICI 164,384 appears to be mediated by impaired receptor dimerization. Proc Natl Acad Sci U S A. 1990;87:6883–6887. doi: 10.1073/pnas.87.17.6883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Filardo EJ. Epidermal growth factor receptor (EGFR) transactivation by estrogen via the G-protein-coupled receptor, GPR30: a novel signaling pathway with potential significance for breast cancer. J Steroid Biochem Mol Biol. 2002;80:231–238. doi: 10.1016/s0960-0760(01)00190-x. [DOI] [PubMed] [Google Scholar]
- 52.Filardo EJ, Quinn JA, Bland KI, Frackelton AR., Jr Estrogen-induced activation of Erk-1 and Erk-2 requires the G protein-coupled receptor homolog, GPR30, and occurs via trans-activation of the epidermal growth factor receptor through release of HB-EGF. Mol Endocrinol. 2000;14:1649–1660. doi: 10.1210/mend.14.10.0532. [DOI] [PubMed] [Google Scholar]
- 53.Fillingim RB, Ness TJ. Sex-related hormonal influences on pain and analgesic responses. Neurosci Biobehav Rev. 2000;24:485–501. doi: 10.1016/s0149-7634(00)00017-8. [DOI] [PubMed] [Google Scholar]
- 54.Fugger HN, Kumar A, Lubahn DB, Korach KS, Foster TC. Examination of estradiol effects on the rapid estradiol mediated increase in hippocampal synaptic transmission in estrogen receptor alpha knockout mice. Neurosci Lett. 2001;309:207–209. doi: 10.1016/s0304-3940(01)02083-3. [DOI] [PubMed] [Google Scholar]
- 55.Gainetdinov RR, Premont RT, Bohn LM, Lefkowitz RJ, Caron MG. Desensitization of G protein-coupled receptors and neuronal functions. Annu Rev Neurosci. 2004;27:107–144. doi: 10.1146/annurev.neuro.27.070203.144206. [DOI] [PubMed] [Google Scholar]
- 56.Garcia-Segura LM, Hernandez P, Olmos G, Tranque PA, Naftolin F. Neuronal membrane remodelling during the oestrus cycle: a freeze-fracture study in the arcuate nucleus of the rat hypothalamus. J Neurocytol. 1988;17:377–383. doi: 10.1007/BF01187859. [DOI] [PubMed] [Google Scholar]
- 57.Giamberardino MA, Affaitati G, Valente R, Iezzi S, Vecchiet L. Changes in visceral pain reactivity as a function of estrous cycle in female rats with artificial ureteral calculosis. Brain Res. 1997;774:234–238. doi: 10.1016/s0006-8993(97)81711-8. [DOI] [PubMed] [Google Scholar]
- 58.Gilad LA, Bresler T, Gnainsky J, Smirnoff P, Schwartz B. Regulation of vitamin D receptor expression via estrogen-induced activation of the ERK 1/2 signaling pathway in colon and breast cancer cells. J Endocrinol. 2005;185:577–592. doi: 10.1677/joe.1.05770. [DOI] [PubMed] [Google Scholar]
- 59.Gonzales KL, Tetel MJ, Wagner CK. Estrogen receptor (ER) beta modulates ERalpha responses to estrogens in the developing rat ventromedial nucleus of the hypothalamus. Endocrinology. 2008;149:4615–4621. doi: 10.1210/en.2008-0511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gonzalez-Flores O, Guerra-Araiza C, Cerbon M, Camacho-Arroyo I, Etgen AM. The 26S proteasome participates in the sequential inhibition of estrous behavior induced by progesterone in rats. Endocrinology. 2004;145:2328–2336. doi: 10.1210/en.2003-1162. [DOI] [PubMed] [Google Scholar]
- 61.Gorosito SV, Lorenzo AG, Cambiasso MJ. Estrogen receptor alpha is expressed on the cell-surface of embryonic hypothalamic neurons. Neuroscience. 2008;154:1173–1177. doi: 10.1016/j.neuroscience.2008.05.001. [DOI] [PubMed] [Google Scholar]
- 62.Gorski RA, Yanase M. Estrogen facilitation of lordosis behavior in the female rat. Exp Brain Res. 1981 3:222–237. doi: 10.1007/978-3-642-45525-4_18. [DOI] [PubMed] [Google Scholar]
- 63.Green S, Walter P, Kumar V, Krust A, Bornert JM, Argos P, Chambon P. Human oestrogen receptor cDNA: sequence, expression and homology to v-erb-A. Nature. 1986;320:134–139. doi: 10.1038/320134a0. [DOI] [PubMed] [Google Scholar]
- 64.Grove-Strawser D, Mermelstein PG. Estrogen receptors activate different mGluRs across distinct brain regions. Abstract 195.13/10. Society for Neuroscience Annual Meeting; 2007; San Diego, CA. 2007. [Google Scholar]
- 65.Gu Q, Moss RL. 17 beta-Estradiol potentiates kainate-induced currents via activation of the cAMP cascade. J Neurosci. 1996;16:3620–3629. doi: 10.1523/JNEUROSCI.16-11-03620.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gundlah C, Kohama SG, Mirkes SJ, Garyfallou VT, Urbanski HF, Bethea CL. Distribution of estrogen receptor beta (ERbeta) mRNA in hypothalamus, midbrain and temporal lobe of spayed macaque: continued expression with hormone replacement. Brain Res Mol Brain Res. 2000;76:191–204. doi: 10.1016/s0006-8993(99)02475-0. [DOI] [PubMed] [Google Scholar]
- 67.Hart SA, Snyder MA, Smejkalova T, Woolley CS. Estrogen mobilizes a subset of estrogen receptor-alpha-immunoreactive vesicles in inhibitory presynaptic boutons in hippocampal CA1. J Neurosci. 2007;27:2102–2111. doi: 10.1523/JNEUROSCI.5436-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Holdcroft A. Hormones and the gut. Br J Anaesth. 2000;85:58–68. doi: 10.1093/bja/85.1.58. [DOI] [PubMed] [Google Scholar]
- 69.Hrabovszky E, Shughrue PJ, Merchenthaler I, Hajszan T, Carpenter CD, Liposits Z, Petersen SL. Detection of estrogen receptor-beta messenger ribonucleic acid and 125I-estrogen binding sites in luteinizing hormone-releasing hormone neurons of the rat brain. Endocrinology. 2000;141:3506–3509. doi: 10.1210/endo.141.9.7788. [DOI] [PubMed] [Google Scholar]
- 70.Jakacka M, Ito M, Weiss J, Chien PY, Gehm BD, Jameson JL. Estrogen receptor binding to DNA is not required for its activity through the nonclassical AP1 pathway. J Biol Chem. 2001;276:13615–13621. doi: 10.1074/jbc.M008384200. [DOI] [PubMed] [Google Scholar]
- 71.Kahlert S, Nuedling S, van Eickels M, Vetter H, Meyer R, Grohe C. Estrogen receptor alpha rapidly activates the IGF-1 receptor pathway. J Biol Chem. 2000;275:18447–18453. doi: 10.1074/jbc.M910345199. [DOI] [PubMed] [Google Scholar]
- 72.Kalita K, Szymczak S, Kaczmarek L. Non-nuclear estrogen receptor beta and alpha in the hippocampus of male and female rats. Hippocampus. 2005;15:404–412. doi: 10.1002/hipo.20066. [DOI] [PubMed] [Google Scholar]
- 73.Karthikeyan N, Thampan RV. Plasma membrane is the primary site of localization of the nonactivated estrogen receptor in the goat uterus: hormone binding causes receptor internalization. Arch Biochem Biophys. 1996;325:47–57. doi: 10.1006/abbi.1996.0006. [DOI] [PubMed] [Google Scholar]
- 74.Kelly MJ, Ronnekleiv OK. Membrane-initiated estrogen signaling in hypothalamic neurons. Mol Cell Endocrinol. 2008;290:14–23. doi: 10.1016/j.mce.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Kelly MJ, Ronnekleiv OK, Ibrahim N, Lagrange AH, Wagner EJ. Estrogen modulation of K(+) channel activity in hypothalamic neurons involved in the control of the reproductive axis. Steroids. 2002;67:447–456. doi: 10.1016/s0039-128x(01)00181-7. [DOI] [PubMed] [Google Scholar]
- 76.Kian Tee M, Rogatsky I, Tzagarakis-Foster C, Cvoro A, An J, Christy RJ, Yamamoto KR, Leitman DC. Estradiol and selective estrogen receptor modulators differentially regulate target genes with estrogen receptors alpha and beta. Mol Biol Cell. 2004;15:1262–1272. doi: 10.1091/mbc.E03-06-0360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Kim YJ, Hur EM, Park TJ, Kim KT. Nongenomic inhibition of catecholamine secretion by 17beta-estradiol in PC12 cells. J Neurochem. 2000;74:2490–2496. doi: 10.1046/j.1471-4159.2000.0742490.x. [DOI] [PubMed] [Google Scholar]
- 78.Klinge CM, Wickramasinghe NS, Ivanova MM, Dougherty SM. Resveratrol stimulates nitric oxide production by increasing estrogen receptor alpha-Src-caveolin-1 interaction and phosphorylation in human umbilical vein endothelial cells. FASEB J. 2008;22:2185–2197. doi: 10.1096/fj.07-103366. [DOI] [PubMed] [Google Scholar]
- 79.Koshimizu TA, Van Goor F, Tomic M, Wong AO, Tanoue A, Tsujimoto G, Stojilkovic SS. Characterization of calcium signaling by purinergic receptor-channels expressed in excitable cells. Mol Pharmacol. 2000;58:936–945. doi: 10.1124/mol.58.5.936. [DOI] [PubMed] [Google Scholar]
- 80.Kow LM, Pfaff DW. Mapping of neural and signal transduction pathways for lordosis in the search for estrogen actions on the central nervous system. Behav Brain Res. 1998;92:169–180. doi: 10.1016/s0166-4328(97)00189-7. [DOI] [PubMed] [Google Scholar]
- 81.Kow LM, Pfaff DW. The membrane actions of estrogens can potentiate their lordosis behavior-facilitating genomic actions. Proc Natl Acad Sci U S A. 2004;101:12354–12357. doi: 10.1073/pnas.0404889101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Krupnick JG, Benovic JL. The role of receptor kinases and arrestins in G protein-coupled receptor regulation. Annu Rev Pharmacol Toxicol. 1998;38:289–319. doi: 10.1146/annurev.pharmtox.38.1.289. [DOI] [PubMed] [Google Scholar]
- 83.Kuiper GG, Enmark E, Pelto-Huikko M, Nilsson S, Gustafsson JA. Cloning of a novel receptor expressed in rat prostate and ovary. Proc Natl Acad Sci U S A. 1996;93:5925–5930. doi: 10.1073/pnas.93.12.5925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Kumar P, Wu Q, Chambliss KL, Yuhanna IS, Mumby SM, Mineo C, Tall GG, Shaul PW. Direct Interactions with G alpha i and G betagamma mediate nongenomic signaling by estrogen receptor alpha. Mol Endocrinol. 2007;21:1370–1380. doi: 10.1210/me.2006-0360. [DOI] [PubMed] [Google Scholar]
- 85.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych P. Membrane Estrogen Receptor-Alpha Interacts with Metabotropic Glutamate Receptor 1a to Mobilize Intracellular Calcium in Hypothalamic Astrocytes. Endocrinology. 2008 doi: 10.1210/en.2008-0994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Kuo J, Hariri OR, Bondar G, Ogi J, Micevych PE. Membrane estradiol receptors interact with metabotropic glutamate receptors to mobilize intracellular calcium in hypothalamic astrocytes. Endocrinology. 2008 doi: 10.1210/en.2008-0994. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Kuppers E, Krust A, Chambon P, Beyer C. Functional alterations of the nigrostriatal dopamine system in estrogen receptor-alpha knockout (ERKO) mice. Psychoneuroendocrinology. 2008;33:832–838. doi: 10.1016/j.psyneuen.2008.03.007. [DOI] [PubMed] [Google Scholar]
- 88.Kurata K, Takebayashi M, Kagaya A, Morinobu S, Yamawaki S. Effect of beta-estradiol on voltage-gated Ca(2+) channels in rat hippocampal neurons: a comparison with dehydroepiandrosterone. Eur J Pharmacol. 2001;416:203–212. doi: 10.1016/s0014-2999(01)00880-9. [DOI] [PubMed] [Google Scholar]
- 89.Kushner PJ, Agard DA, Greene GL, Scanlan TS, Shiau AK, Uht RM, Webb P. Estrogen receptor pathways to AP-1. J Steroid Biochem Mol Biol. 2000;74:311–317. doi: 10.1016/s0960-0760(00)00108-4. [DOI] [PubMed] [Google Scholar]
- 90.Laporte SA, Oakley RH, Holt JA, Barak LS, Caron MG. The interaction of beta-arrestin with the AP-2 adaptor is required for the clustering of beta 2-adrenergic receptor into clathrin-coated pits. J Biol Chem. 2000;275:23120–23126. doi: 10.1074/jbc.M002581200. [DOI] [PubMed] [Google Scholar]
- 91.Laporte SA, Oakley RH, Zhang J, Holt JA, Ferguson SS, Caron MG, Barak LS. The beta2-adrenergic receptor/betaarrestin complex recruits the clathrin adaptor AP-2 during endocytosis. Proc Natl Acad Sci U S A. 1999;96:3712–3717. doi: 10.1073/pnas.96.7.3712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Lauber AH, Mobbs CV, Muramatsu M, Pfaff DW. Estrogen receptor messenger RNA expression in rat hypothalamus as a function of genetic sex and estrogen dose. Endocrinology. 1991;129:3180–3186. doi: 10.1210/endo-129-6-3180. [DOI] [PubMed] [Google Scholar]
- 93.Lauber AH, Romano GJ, Mobbs CV, Howells RD, Pfaff DW. Estradiol induction of proenkephalin messenger RNA in hypothalamus: dose-response and relation to reproductive behavior in the female rat. Brain Res Mol Brain Res. 1990;8:47–54. doi: 10.1016/0169-328x(90)90008-2. [DOI] [PubMed] [Google Scholar]
- 94.Lee DY, Chai YG, Lee EB, Kim KW, Nah SY, Oh TH, Rhim H. 17Beta-estradiol inhibits high-voltage-activated calcium channel currents in rat sensory neurons via a non-genomic mechanism. Life Sci. 2002;70:2047–2059. doi: 10.1016/s0024-3205(01)01534-x. [DOI] [PubMed] [Google Scholar]
- 95.Lee SJ, McEwen BS. Neurotrophic and neuroprotective actions of estrogens and their therapeutic implications. Annu Rev Pharmacol Toxicol. 2001;41:569–591. doi: 10.1146/annurev.pharmtox.41.1.569. [DOI] [PubMed] [Google Scholar]
- 96.Lee YJ, Gorski J. Estrogen receptor down-regulation is regulated noncooperatively by estrogen at the transcription level. Mol Cell Endocrinol. 1998;137:85–92. doi: 10.1016/s0303-7207(97)00235-9. [DOI] [PubMed] [Google Scholar]
- 97.Li L, Haynes MP, Bender JR. Plasma membrane localization and function of the estrogen receptor alpha variant (ER46) in human endothelial cells. Proc Natl Acad Sci U S A. 2003;100:4807–4812. doi: 10.1073/pnas.0831079100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Lohse MJ, Benovic JL, Caron MG, Lefkowitz RJ. Multiple pathways of rapid beta 2-adrenergic receptor desensitization. Delineation with specific inhibitors. J Biol Chem. 1990;265:3202–3211. [PubMed] [Google Scholar]
- 99.Majewska MD, Harrison NL, Schwartz RD, Barker JL, Paul SM. Steroid hormone metabolites are barbiturate-like modulators of the GABA receptor. Science. 1986;232:1004–1007. doi: 10.1126/science.2422758. [DOI] [PubMed] [Google Scholar]
- 100.Marin R, Ramirez CM, Gonzalez M, Gonzalez-Munoz E, Zorzano A, Camps M, Alonso R, Diaz M. Voltage-dependent anion channel (VDAC) participates in amyloid beta-induced toxicity and interacts with plasma membrane estrogen receptor alpha in septal and hippocampal neurons. Mol Membr Biol. 2007;24:148–160. doi: 10.1080/09687860601055559. [DOI] [PubMed] [Google Scholar]
- 101.Marino M, Ascenzi P. Steroid hormone rapid signaling: the pivotal role of S-palmitoylation. IUBMB Life. 2006;58:716–719. doi: 10.1080/15216540601019485. [DOI] [PubMed] [Google Scholar]
- 102.Martinez-Gomez M, Cruz Y, Salas M, Hudson R, Pacheco P. Assessing pain threshold in the rat: changes with estrus and time of day. Physiol Behav. 1994;55:651–657. doi: 10.1016/0031-9384(94)90040-x. [DOI] [PubMed] [Google Scholar]
- 103.Matsuda K, Sakamoto H, Mori H, Hosokawa K, Kawamura A, Itose M, Nishi M, Prossnitz ER, Kawata M. Expression and intracellular distribution of the G protein-coupled receptor 30 in rat hippocampal formation. Neurosci Lett. 2008;441:94–99. doi: 10.1016/j.neulet.2008.05.108. [DOI] [PubMed] [Google Scholar]
- 104.McEwen BS, Pfaff DW, Chaptal C, Luine VN. Brain cell nuclear retention of [3H]estradiol doses able to promote lordosis: temporal and regional aspects. Brain Res. 1975;86:155–161. doi: 10.1016/0006-8993(75)90649-6. [DOI] [PubMed] [Google Scholar]
- 105.McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- 106.Meredith JM, Auger CJ, Blaustein JD. Down-regulation of estrogen receptor immunoreactivity by 17 beta-estradiol in the guinea pig forebrain. J Neuroendocrinol. 1994;6:639–648. doi: 10.1111/j.1365-2826.1994.tb00630.x. [DOI] [PubMed] [Google Scholar]
- 107.Mermelstein PG, Becker JB, Surmeier DJ. Estradiol reduces calcium currents in rat neostriatal neurons via a membrane receptor. J Neurosci. 1996;16:595–604. doi: 10.1523/JNEUROSCI.16-02-00595.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Micevych P, Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK. The Luteinizing Hormone Surge Is Preceded by an Estrogen-Induced Increase of Hypothalamic Progesterone in Ovariectomized and Adrenalectomized Rats. Neuroendocrinology. 2003;78:29–35. doi: 10.1159/000071703. [DOI] [PubMed] [Google Scholar]
- 109.Micevych P, Soma KK, Sinchak K. Neuroprogesterone: key to estrogen positive feedback? Brain Res Rev. 2008;57:470–480. doi: 10.1016/j.brainresrev.2007.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Micevych P, Ulibarri C. Development of the limbic-hypothalamic cholecystokinin circuit: a model of sexual differentiation. Dev Neurosci. 1992;14:11–34. doi: 10.1159/000111643. [DOI] [PubMed] [Google Scholar]
- 111.Micevych PE, Abelson L, Fok H, Ulibarri C, Priest CA. Gonadal steroid control of preprocholecystokinin mRNA expression in the limbic-hypothalamic circuit: comparison of adult with neonatal steroid treatments. J Neurosci Res. 1994;38:386–398. doi: 10.1002/jnr.490380404. [DOI] [PubMed] [Google Scholar]
- 112.Micevych PE, Chaban V, Ogi J, Dewing P, Lu JK, Sinchak K. Estradiol stimulates progesterone synthesis in hypothalamic astrocyte cultures. Endocrinology. 2007;148:782–789. doi: 10.1210/en.2006-0774. [DOI] [PubMed] [Google Scholar]
- 113.Micevych PE, Mermelstein PG. Membrane estrogen receptors acting through metabotropic glutamate receptors: an emerging mechanism of estrogen action in brain. Mol Neurobiol. 2008;38:66–77. doi: 10.1007/s12035-008-8034-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Micevych PE, Rissman EF, Gustafsson JA, Sinchak K. Estrogen receptor-alpha is required for estrogen-induced mu-opioid receptor internalization. J Neurosci Res. 2003;71:802–810. doi: 10.1002/jnr.10526. [DOI] [PubMed] [Google Scholar]
- 115.Migliaccio A, Di Domenico M, Castoria G, de Falco A, Bontempo P, Nola E, Auricchio F. Tyrosine kinase/p21ras/MAP-kinase pathway activation by estradiol-receptor complex in MCF-7 cells. EMBO J. 1996;15:1292–1300. [PMC free article] [PubMed] [Google Scholar]
- 116.Miller WE, Maudsley S, Ahn S, Khan KD, Luttrell LM, Lefkowitz RJ. beta-arrestin1 interacts with the catalytic domain of the tyrosine kinase c-SRC. Role of beta-arrestin1-dependent targeting of c-SRC in receptor endocytosis. J Biol Chem. 2000;275:11312–11319. doi: 10.1074/jbc.275.15.11312. [DOI] [PubMed] [Google Scholar]
- 117.Mills RH, Sohn RK, Micevych PE. Estrogen-induced mu-opioid receptor internalization in the medial preoptic nucleus is mediated via neuropeptide Y-Y1 receptor activation in the arcuate nucleus of female rats. J Neurosci. 2004;24:947–955. doi: 10.1523/JNEUROSCI.1366-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Milner TA, Ayoola K, Drake CT, Herrick SP, Tabori NE, McEwen BS, Warrier S, Alves SE. Ultrastructural localization of estrogen receptor beta immunoreactivity in the rat hippocampal formation. J Comp Neurol. 2005;491:81–95. doi: 10.1002/cne.20724. [DOI] [PubMed] [Google Scholar]
- 119.Milner TA, McEwen BS, Hayashi S, Li CJ, Reagan LP, Alves SE. Ultrastructural evidence that hippocampal alpha estrogen receptors are located at extranuclear sites. J Comp Neurol. 2001;429:355–371. [PubMed] [Google Scholar]
- 120.Moats RK, 2nd, Ramirez VD. Electron microscopic visualization of membrane-mediated uptake and translocation of estrogen-BSA:colloidal gold by hep G2 cells. J Endocrinol. 2000;166:631–647. doi: 10.1677/joe.0.1660631. [DOI] [PubMed] [Google Scholar]
- 121.Moats RK, 2nd, Ramirez VD. Rapid uptake and binding of estradiol-17beta-6-(O-carboxymethyl)oxime:125I-labeled BSA by female rat liver. Biol Reprod. 1998;58:531–538. doi: 10.1095/biolreprod58.2.531. [DOI] [PubMed] [Google Scholar]
- 122.Mundell SJ, Matharu AL, Pula G, Roberts PJ, Kelly E. Agonist-induced internalization of the metabotropic glutamate receptor 1a is arrestin- and dynamin-dependent. J Neurochem. 2001;78:546–551. doi: 10.1046/j.1471-4159.2001.00421.x. [DOI] [PubMed] [Google Scholar]
- 123.Nabekura J, Oomura Y, Minami T, Mizuno Y, Fukuda A. Mechanism of the rapid effect of 17 beta-estradiol on medial amygdala neurons. Science. 1986;233:226–228. doi: 10.1126/science.3726531. [DOI] [PubMed] [Google Scholar]
- 124.Navarro CE, Abdul Saeed S, Murdock C, Martinez-Fuentes AJ, Arora KK, Krsmanovic LZ, Catt KJ. Regulation of cyclic adenosine 3′,5′-monophosphate signaling and pulsatile neurosecretion by Gi-coupled plasma membrane estrogen receptors in immortalized gonadotropin-releasing hormone neurons. Mol Endocrinol. 2003;17:1792–1804. doi: 10.1210/me.2003-0040. [DOI] [PubMed] [Google Scholar]
- 125.Nethrapalli IS, Tinnikov AA, Krishnan V, Lei CD, Toran-Allerand CD. Estrogen activates mitogen-activated protein kinase in native, nontransfected CHO-K1, COS-7, and RAT2 fibroblast cell lines. Endocrinology. 2005;146:56–63. doi: 10.1210/en.2004-1106. [DOI] [PubMed] [Google Scholar]
- 126.Nirmala PB, Thampan RV. Ubiquitination of the rat uterine estrogen receptor: dependence on estradiol. Biochem Biophys Res Commun. 1995;213:24–31. doi: 10.1006/bbrc.1995.2093. [DOI] [PubMed] [Google Scholar]
- 127.Nordeen EJ, Yahr P. A regional analysis of estrogen binding to hypothalamic cell nuclei in relation to masculinization and defeminization. J Neurosci. 1983;3:933–941. doi: 10.1523/JNEUROSCI.03-05-00933.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ogawa S, Gordan JD, Taylor J, Lubahn D, Korach K, Pfaff DW. Reproductive functions illustrating direct and indirect effects of genes on behavior. Horm Behav. 1996;30:487–494. doi: 10.1006/hbeh.1996.0052. [DOI] [PubMed] [Google Scholar]
- 129.Olmos G, Aguilera P, Tranque P, Naftolin F, Garcia-Segura LM. Estrogen-induced synaptic remodelling in adult rat brain is accompanied by the reorganization of neuronal membranes. Brain Res. 1987;425:57–64. doi: 10.1016/0006-8993(87)90483-5. [DOI] [PubMed] [Google Scholar]
- 130.Olster DH, Blaustein JD. Progesterone facilitation of lordosis in male and female Sprague-Dawley rats following priming with estradiol pulses. Horm Behav. 1988;22:294–304. doi: 10.1016/0018-506x(88)90002-5. [DOI] [PubMed] [Google Scholar]
- 131.Otto C, Fuchs I, Kauselmann G, Kern H, Zevnik B, Andreasen P, Schwarz G, Altmann H, Klewer M, Schoor M, Vonk R, Fritzemeier KH. GPR30 Does Not Mediate Estrogenic Responses in Reproductive Organs in Mice. Biol Reprod. 2008 doi: 10.1095/biolreprod.108.071175. [DOI] [PubMed] [Google Scholar]
- 132.Otto C, Rohde-Schulz B, Schwarz G, Fuchs I, Klewer M, Brittain D, Langer G, Bader B, Prelle K, Nubbemeyer R, Fritzemeier KH. G protein-coupled receptor 30 localizes to the endoplasmic reticulum and is not activated by estradiol. Endocrinology. 2008;149:4846–4856. doi: 10.1210/en.2008-0269. [DOI] [PubMed] [Google Scholar]
- 133.Papka RE, Storey-Workley M. Estrogen receptor-alpha and -beta coexist in a subpopulation of sensory neurons of female rat dorsal root ganglia. Neurosci Lett. 2002;319:71–74. doi: 10.1016/s0304-3940(01)02562-9. [DOI] [PubMed] [Google Scholar]
- 134.Papka RE, Storey-Workley M, Shughrue PJ, Merchenthaler I, Collins JJ, Usip S, Saunders PT, Shupnik M. Estrogen receptor-alpha and beta- immunoreactivity and mRNA in neurons of sensory and autonomic ganglia and spinal cord. Cell Tissue Res. 2001;304:193–214. doi: 10.1007/s004410100363. [DOI] [PubMed] [Google Scholar]
- 135.Pappas TC, Gametchu B, Watson CS. Membrane estrogen receptors identified by multiple antibody labeling and impeded-ligand binding. FASEB J. 1995;9:404–410. doi: 10.1096/fasebj.9.5.7896011. [DOI] [PubMed] [Google Scholar]
- 136.Pedram A, Razandi M, Levin ER. Nature of functional estrogen receptors at the plasma membrane. Mol Endocrinol. 2006;20:1996–2009. doi: 10.1210/me.2005-0525. [DOI] [PubMed] [Google Scholar]
- 137.Pedram A, Razandi M, Sainson RC, Kim JK, Hughes CC, Levin ER. A conserved mechanism for steroid receptor translocation to the plasma membrane. J Biol Chem. 2007;282:22278–22288. doi: 10.1074/jbc.M611877200. [DOI] [PubMed] [Google Scholar]
- 138.Pfaff DW. Uptake of 3H-estradiol by the female rat brain. An autoradiographic study. Endocrinology. 1968;82:1149–1155. doi: 10.1210/endo-82-6-1149. [DOI] [PubMed] [Google Scholar]
- 139.Pfaff DW, Sakuma Y. Facilitation of the lordosis reflex of female rats from the ventromedial nucleus of the hypothalamus. J Physiol. 1979;288:189–202. [PMC free article] [PubMed] [Google Scholar]
- 140.Priest CA, Eckersell CB, Micevych PE. Estrogen regulates preproenkephalin-A mRNA levels in the rat ventromedial nucleus: temporal and cellular aspects. Brain Res Mol Brain Res. 1995;28:251–262. doi: 10.1016/0169-328x(94)00213-x. [DOI] [PubMed] [Google Scholar]
- 141.Priest CA, Vink KL, Micevych PE. Temporal regulation by estrogen of beta-preprotachykinin mRNA expression in the rat ventromedial nucleus of the hypothalamus. Brain Res Mol Brain Res. 1995;28:61–71. doi: 10.1016/0169-328x(94)00184-g. [DOI] [PubMed] [Google Scholar]
- 142.Prossnitz ER, Oprea TI, Sklar LA, Arterburn JB. The ins and outs of GPR30: a transmembrane estrogen receptor. J Steroid Biochem Mol Biol. 2008;109:350–353. doi: 10.1016/j.jsbmb.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Qiu J, Bosch MA, Tobias SC, Grandy DK, Scanlan TS, Ronnekleiv OK, Kelly MJ. Rapid signaling of estrogen in hypothalamic neurons involves a novel G-protein-coupled estrogen receptor that activates protein kinase C. J Neurosci. 2003;23:9529–9540. doi: 10.1523/JNEUROSCI.23-29-09529.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Qiu J, Bosch MA, Tobias SC, Krust A, Graham SM, Murphy SJ, Korach KS, Chambon P, Scanlan TS, Ronnekleiv OK, Kelly MJ. A G-protein-coupled estrogen receptor is involved in hypothalamic control of energy homeostasis. J Neurosci. 2006;26:5649–5655. doi: 10.1523/JNEUROSCI.0327-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Quesada A, Micevych PE. Estrogen interacts with the IGF-1 system to protect nigrostriatal dopamine and maintain motoric behavior after 6-hydroxdopamine lesions. J Neurosci Res. 2004;75:107–116. doi: 10.1002/jnr.10833. [DOI] [PubMed] [Google Scholar]
- 146.Quesada A, Romeo HE, Micevych P. Distribution and localization patterns of estrogen receptor-beta and insulin-like growth factor-1 receptors in neurons and glial cells of the female rat substantia nigra: localization of ERbeta and IGF-1R in substantia nigra. J Comp Neurol. 2007;503:198–208. doi: 10.1002/cne.21358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Rainbow TC, Davis PG, McEwen BS. Anisomycin inhibits the activation of sexual behavior by estradiol and progesterone. Brain Res. 1980;194:548–555. doi: 10.1016/0006-8993(80)91240-8. [DOI] [PubMed] [Google Scholar]
- 148.Ramirez VD, Zheng J, Siddique KM. Membrane receptors for estrogen, progesterone, and testosterone in the rat brain: fantasy or reality. Cell Mol Neurobiol. 1996;16:175–198. doi: 10.1007/BF02088175. [DOI] [PubMed] [Google Scholar]
- 149.Rapacciuolo A, Suvarna S, Barki-Harrington L, Luttrell LM, Cong M, Lefkowitz RJ, Rockman HA. Protein kinase A and G protein-coupled receptor kinase phosphorylation mediates beta-1 adrenergic receptor endocytosis through different pathways. J Biol Chem. 2003;278:35403–35411. doi: 10.1074/jbc.M305675200. [DOI] [PubMed] [Google Scholar]
- 150.Raz L, Khan MM, Mahesh VB, Vadlamudi RK, Brann DW. Rapid estrogen signaling in the brain. Neurosignals. 2008;16:140–153. doi: 10.1159/000111559. [DOI] [PubMed] [Google Scholar]
- 151.Razandi M, Alton G, Pedram A, Ghonshani S, Webb P, Levin ER. Identification of a structural determinant necessary for the localization and function of estrogen receptor alpha at the plasma membrane. Mol Cell Biol. 2003;23:1633–1646. doi: 10.1128/MCB.23.5.1633-1646.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Razandi M, Pedram A, Greene GL, Levin ER. Cell membrane and nuclear estrogen receptors (ERs) originate from a single transcript: studies of ERalpha and ERbeta expressed in Chinese hamster ovary cells. Mol Endocrinol. 1999;13:307–319. doi: 10.1210/mend.13.2.0239. [DOI] [PubMed] [Google Scholar]
- 153.Ree AH, Landmark BF, Eskild W, Levy FO, Lahooti H, Jahnsen T, Aakvaag A, Hansson V. Autologous down-regulation of messenger ribonucleic acid and protein levels for estrogen receptors in MCF-7 cells: an inverse correlation to progesterone receptor levels. Endocrinology. 1989;124:2577–2583. doi: 10.1210/endo-124-5-2577. [DOI] [PubMed] [Google Scholar]
- 154.Register TC, Shively CA, Lewis CE. Expression of estrogen receptor alpha and beta transcripts in female monkey hippocampus and hypothalamus. Brain Res. 1998;788:320–322. doi: 10.1016/s0006-8993(98)00036-5. [DOI] [PubMed] [Google Scholar]
- 155.Reid G, Denger S, Kos M, Gannon F. Human estrogen receptor-alpha: regulation by synthesis, modification and degradation. Cell Mol Life Sci. 2002;59:821–831. doi: 10.1007/s00018-002-8470-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Revankar CM, Cimino DF, Sklar LA, Arterburn JB, Prossnitz ER. A transmembrane intracellular estrogen receptor mediates rapid cell signaling. Science. 2005;307:1625–1630. doi: 10.1126/science.1106943. [DOI] [PubMed] [Google Scholar]
- 157.Riley JL, 3rd, Robinson ME, Wise EA, Myers CD, Fillingim RB. Sex differences in the perception of noxious experimental stimuli: a meta-analysis. Pain. 1998;74:181–187. doi: 10.1016/s0304-3959(97)00199-1. [DOI] [PubMed] [Google Scholar]
- 158.Rissman EF, Early AH, Taylor JA, Korach KS, Lubahn DB. Estrogen receptors are essential for female sexual receptivity. Endocrinology. 1997;138:507–510. doi: 10.1210/endo.138.1.4985. [DOI] [PubMed] [Google Scholar]
- 159.Rissman EF, Wersinger SR, Taylor JA, Lubahn DB. Estrogen receptor function as revealed by knockout studies: neuroendocrine and behavioral aspects. Horm Behav. 1997;31:232–243. doi: 10.1006/hbeh.1997.1390. [DOI] [PubMed] [Google Scholar]
- 160.Romano GJ, Mobbs CV, Lauber A, Howells RD, Pfaff DW. Differential regulation of proenkephalin gene expression by estrogen in the ventromedial hypothalamus of male and female rats: implications for the molecular basis of a sexually differentiated behavior. Brain Res. 1990;536:63–68. doi: 10.1016/0006-8993(90)90009-z. [DOI] [PubMed] [Google Scholar]
- 161.Romano N, Lee K, Abraham IM, Jasoni CL, Herbison AE. Nonclassical estrogen modulation of presynaptic GABA terminals modulates calcium dynamics in gonadotropin-releasing hormone neurons. Endocrinology. 2008;149:5335–5344. doi: 10.1210/en.2008-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Sand P. Chronic Pain Syndromes of Gynecologic Origin. Journal of Reproductive Medicine. 2004;49:230–234. [PubMed] [Google Scholar]
- 163.Sandilands E, Frame MC. Endosomal trafficking of Src tyrosine kinase. Trends Cell Biol. 2008;18:322–329. doi: 10.1016/j.tcb.2008.05.004. [DOI] [PubMed] [Google Scholar]
- 164.Santen RJ, Song RX, Zhang Z, Kumar R, Jeng MH, Masamura A, Lawrence J, Jr, Berstein L, Yue W. Long-term estradiol deprivation in breast cancer cells up-regulates growth factor signaling and enhances estrogen sensitivity. Endocr Relat Cancer. 2005;12 1:S61–73. doi: 10.1677/erc.1.01018. [DOI] [PubMed] [Google Scholar]
- 165.Sapsed-Byrne S, Ma D, Ridout D, Holdcroft A. Estrous cycle phase variations in visceromotor and cardiovascular responses to colonic distension in the anesthetized rat. Brain Res. 1996;742:10–16. doi: 10.1016/s0006-8993(96)00989-4. [DOI] [PubMed] [Google Scholar]
- 166.Sawyer CH. Induction of estrus in the ovariectomized cat by local hypothalamic treatment with estrogen. Anat Rec. 1963;145:280. [Google Scholar]
- 167.Schlegel A, Wang C, Pestell RG, Lisanti MP. Ligand-independent activation of oestrogen receptor alpha by caveolin-1. Biochem J. 2001;359:203–210. doi: 10.1042/0264-6021:3590203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Schlenker EH, Hansen SN. Sex-specific densities of estrogen receptors alpha and beta in the subnuclei of the nucleus tractus solitarius, hypoglossal nucleus and dorsal vagal motor nucleus weanling rats. Brain Res. 2006;1123:89–100. doi: 10.1016/j.brainres.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 169.Schreihofer DA, Stoler MH, Shupnik MA. Differential expression and regulation of estrogen receptors (ERs) in rat pituitary and cell lines: estrogen decreases ERalpha protein and estrogen responsiveness. Endocrinology. 2000;141:2174–2184. doi: 10.1210/endo.141.6.7505. [DOI] [PubMed] [Google Scholar]
- 170.Schumacher M, Robert F. Progesterone: synthesis, metabolism, mechanisms of action, and effects in the nervous system. In: Pfaff DW, Arnold AP, Etgen AM, Fahrbach SE, Rubin RT, editors. Hormones, Brain and Behavior. Academic Press; Amsterdam: 2002. pp. 683–745. [Google Scholar]
- 171.Sheldahl LC, Shapiro RA, Bryant DN, Koerner IP, Dorsa DM. Estrogen induces rapid translocation of estrogen receptor beta, but not estrogen receptor alpha, to the neuronal plasma membrane. Neuroscience. 2008;153:751–761. doi: 10.1016/j.neuroscience.2008.02.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Sheridan PJ, Sar M, Stumpf WE. Autoradiographic localization of 3H-estradiol or its metabolites in the central nervous system of the developing rat. Endocrinology. 1974;94:1386–1390. doi: 10.1210/endo-94-5-1386. [DOI] [PubMed] [Google Scholar]
- 173.Shughrue PJ, Askew GR, Dellovade TL, Merchenthaler I. Estrogen-binding sites and their functional capacity in estrogen receptor double knockout mouse brain. Endocrinology. 2002;143:1643–1650. doi: 10.1210/endo.143.5.8772. [DOI] [PubMed] [Google Scholar]
- 174.Shughrue PJ, Bushnell CD, Dorsa DM. Estrogen receptor messenger ribonucleic acid in female rat brain during the estrous cycle: a comparison with ovariectomized females and intact males. Endocrinology. 1992;131:381–388. doi: 10.1210/endo.131.1.1612018. [DOI] [PubMed] [Google Scholar]
- 175.Shughrue PJ, Dellovade TL, Merchenthaler I. Estrogen modulates oxytocin gene expression in regions of the rat supraoptic and paraventricular nuclei that contain estrogen receptor-beta. Prog Brain Res. 2002;139:15–29. doi: 10.1016/s0079-6123(02)39004-6. [DOI] [PubMed] [Google Scholar]
- 176.Shughrue PJ, Lane MV, Merchenthaler I. Comparative distribution of estrogen receptor-alpha and -beta mRNA in the rat central nervous system. J Comp Neurol. 1997;388:507–525. doi: 10.1002/(sici)1096-9861(19971201)388:4<507::aid-cne1>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 177.Shughrue PJ, Lane MV, Scrimo PJ, Merchenthaler I. Comparative distribution of estrogen receptor-alpha (ER-alpha) and beta (ER-beta) mRNA in the rat pituitary, gonad, and reproductive tract. Steroids. 1998;63:498–504. doi: 10.1016/s0039-128x(98)00054-3. [DOI] [PubMed] [Google Scholar]
- 178.Shughrue PJ, Merchenthaler I. Evidence for novel estrogen binding sites in the rat hippocampus. Neuroscience. 2000;99:605–612. doi: 10.1016/s0306-4522(00)00242-6. [DOI] [PubMed] [Google Scholar]
- 179.Simerly RB, Chang C, Muramatsu M, Swanson LW. Distribution of androgen and estrogen receptor mRNA-containing cells in the rat brain: an in situ hybridization study. J Comp Neurol. 1990;294:76–95. doi: 10.1002/cne.902940107. [DOI] [PubMed] [Google Scholar]
- 180.Simerly RB, Young BJ. Regulation of estrogen receptor messenger ribonucleic acid in rat hypothalamus by sex steroid hormones. Mol Endocrinol. 1991;5:424–432. doi: 10.1210/mend-5-3-424. [DOI] [PubMed] [Google Scholar]
- 181.Sinchak K, Hendricks DG, Baroudi R, Micevych PE. Orphanin FQ/nociceptin in the ventromedial nucleus facilitates lordosis in female rats. Neuroreport. 1997;8:3857–3860. doi: 10.1097/00001756-199712220-00004. [DOI] [PubMed] [Google Scholar]
- 182.Sinchak K, Micevych P. Visualizing activation of opioid circuits by internalization of G protein-coupled receptors. Mol Neurobiol. 2003;27:197–222. doi: 10.1385/MN:27:2:197. [DOI] [PubMed] [Google Scholar]
- 183.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Sinchak K, Micevych PE. Progesterone blockade of estrogen activation of mu-opioid receptors regulates reproductive behavior. J Neurosci. 2001;21:5723–5729. doi: 10.1523/JNEUROSCI.21-15-05723.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sinchak K, Mills RH, Tao L, LaPolt P, Lu JK, Micevych P. Estrogen Induces de novo Progesterone Synthesis in Astrocytes. Dev Neurosci. 2003;25:343–348. doi: 10.1159/000073511. [DOI] [PubMed] [Google Scholar]
- 186.Sinchak K, Yang W, Lino S, Micevych P. Orphanin FQ infusion into the medial preoptic nucleus facilitates lordosis in estrogen primed female rats. New Orleans, LA: 2003. [Google Scholar]
- 187.Singh M, Setalo G, Jr, Guan X, Warren M, Toran-Allerand CD. Estrogen-induced activation of mitogen-activated protein kinase in cerebral cortical explants: convergence of estrogen and neurotrophin signaling pathways. J Neurosci. 1999;19:1179–1188. doi: 10.1523/JNEUROSCI.19-04-01179.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Skafar DF, Zhao C. The multifunctional estrogen receptor-alpha F domain. Endocrine. 2008;33:1–8. doi: 10.1007/s12020-008-9054-1. [DOI] [PubMed] [Google Scholar]
- 189.Song RX, Fan P, Yue W, Chen Y, Santen RJ. Role of receptor complexes in the extranuclear actions of estrogen receptor alpha in breast cancer. Endocr Relat Cancer. 2006;13 1:S3–13. doi: 10.1677/erc.1.01322. [DOI] [PubMed] [Google Scholar]
- 190.Song RX, Zhang Z, Santen RJ. Estrogen rapid action via protein complex formation involving ERalpha and Src. Trends Endocrinol Metab. 2005;16:347–353. doi: 10.1016/j.tem.2005.06.010. [DOI] [PubMed] [Google Scholar]
- 191.Sreeja S, Thampan RV. Estradiol-mediated internalisation of the non-activated estrogen receptor from the goat uterine plasma membrane: identification of the proteins involved. Mol Cell Biochem. 2004;259:131–140. doi: 10.1023/b:mcbi.0000021358.79359.c9. [DOI] [PubMed] [Google Scholar]
- 192.Stefanova I, Horejsi V, Ansotegui IJ, Knapp W, Stockinger H. GPI-anchored cell-surface molecules complexed to protein tyrosine kinases. Science. 1991;254:1016–1019. doi: 10.1126/science.1719635. [DOI] [PubMed] [Google Scholar]
- 193.Suzuki S, Handa RJ. Estrogen receptor-beta, but not estrogen receptor-alpha, is expressed in prolactin neurons of the female rat paraventricular and supraoptic nuclei: comparison with other neuropeptides. J Comp Neurol. 2005;484:28–42. doi: 10.1002/cne.20457. [DOI] [PubMed] [Google Scholar]
- 194.Taleghany N, Sarajari S, DonCarlos LL, Gollapudi L, Oblinger MM. Differential expression of estrogen receptor alpha and beta in rat dorsal root ganglion neurons. J Neurosci Res. 1999;57:603–615. [PubMed] [Google Scholar]
- 195.Thakur MK, Sharma PK. Transcription of estrogen receptor alpha and beta in mouse cerebral cortex: effect of age, sex, 17beta-estradiol and testosterone. Neurochem Int. 2007;50:314–321. doi: 10.1016/j.neuint.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 196.Thammacharoen S, Lutz TA, Geary N, Asarian L. Hindbrain administration of estradiol inhibits feeding and activates estrogen receptor-alpha-expressing cells in the nucleus tractus solitarius of ovariectomized rats. Endocrinology. 2008;149:1609–1617. doi: 10.1210/en.2007-0340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Thomas P, Pang Y, Filardo EJ, Dong J. Identity of an estrogen membrane receptor coupled to a G protein in human breast cancer cells. Endocrinology. 2005;146:624–632. doi: 10.1210/en.2004-1064. [DOI] [PubMed] [Google Scholar]
- 198.Thompson TL, Certain ME. Estrogen mediated inhibition of dopamine transport in the striatum: regulation by G alpha i/o. Eur J Pharmacol. 2005;511:121–126. doi: 10.1016/j.ejphar.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 199.Toran-Allerand CD. Estrogen and the brain: beyond ER-alpha, ER-beta, and 17beta-estradiol. Ann N Y Acad Sci. 2005;1052:136–144. doi: 10.1196/annals.1347.009. [DOI] [PubMed] [Google Scholar]
- 200.Toran-Allerand CD, Guan X, MacLusky NJ, Horvath TL, Diano S, Singh M, Connolly ES, Jr, Nethrapalli IS, Tinnikov AA. ER-X: a novel, plasma membrane-associated, putative estrogen receptor that is regulated during development and after ischemic brain injury. J Neurosci. 2002;22:8391–8401. doi: 10.1523/JNEUROSCI.22-19-08391.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Toran-Allerand CD, Tinnikov AA, Singh RJ, Nethrapalli IS. 17alpha-estradiol: a brain-active estrogen? Endocrinology. 2005;146:3843–3850. doi: 10.1210/en.2004-1616. [DOI] [PubMed] [Google Scholar]
- 202.Torii M, Kubo K. The effects of intraventricular injection of beta-endorphin on initial estrogen action to induce lordosis behavior. Physiol Behav. 1994;55:157–162. doi: 10.1016/0031-9384(94)90024-8. [DOI] [PubMed] [Google Scholar]
- 203.Torii M, Kubo K, Sasaki T. Differential effects of beta-endorphin and Met- and Leu-enkephalin on steroid hormone-induced lordosis in ovariectomized female rats. Pharmacol Biochem Behav. 1997;58:837–842. doi: 10.1016/s0091-3057(97)00018-x. [DOI] [PubMed] [Google Scholar]
- 204.Touhara K, Hawes BE, van Biesen T, Lefkowitz RJ. G protein beta gamma subunits stimulate phosphorylation of Shc adapter protein. Proc Natl Acad Sci U S A. 1995;92:9284–9287. doi: 10.1073/pnas.92.20.9284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Traub RJ, Murphy A. Colonic inflammation induces fos expression in the thoracolumbar spinal cord increasing activity in the spinoparabrachial pathway. Pain. 2002;95:93–102. doi: 10.1016/s0304-3959(01)00381-5. [DOI] [PubMed] [Google Scholar]
- 206.Ueno S, Tsuda M, Iwanaga T, Inoue K. Cell type-specific ATP-activated responses in rat dorsal root ganglion neurons. Br J Pharmacol. 1999;126:429–436. doi: 10.1038/sj.bjp.0702319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Ulibarri C, Micevych PE. Role of perinatal estrogens in sexual differentiation of the inhibition of lordosis by exogenous cholecystokinin. Physiol Behav. 1993;54:95–100. doi: 10.1016/0031-9384(93)90049-l. [DOI] [PubMed] [Google Scholar]
- 208.Un-no T, Hayami S, Nobata S, Sudoko H, Honma S, Fujita K, Ozono S. Neonatal exposure to estrogen in the Wistar rat decreases estrogen receptor-beta and induces epithelial proliferation of the prostate in the adult. Urol Int. 2007;79:345–351. doi: 10.1159/000109721. [DOI] [PubMed] [Google Scholar]
- 209.Valley CC, Solodin NM, Powers GL, Ellison SJ, Alarid ET. Temporal variation in estrogen receptor-alpha protein turnover in the presence of estrogen. J Mol Endocrinol. 2008;40:23–34. doi: 10.1677/JME-07-0067. [DOI] [PubMed] [Google Scholar]
- 210.Vasudevan N, Pfaff DW. Non-genomic actions of estrogens and their interaction with genomic actions in the brain. Front Neuroendocrinol. 2008;29:238–257. doi: 10.1016/j.yfrne.2007.08.003. [DOI] [PubMed] [Google Scholar]
- 211.von Zastrow M, Kobilka BK. Antagonist-dependent and -independent steps in the mechanism of adrenergic receptor internalization. J Biol Chem. 1994;269:18448–18452. [PubMed] [Google Scholar]
- 212.Wagner EJ, Ronnekleiv OK, Kelly MJ. The noradrenergic inhibition of an apamin-sensitive, small-conductance Ca2+-activated K+ channel in hypothalamic gamma-aminobutyric acid neurons: pharmacology, estrogen sensitivity, and relevance to the control of the reproductive axis. J Pharmacol Exp Ther. 2001;299:21–30. [PubMed] [Google Scholar]
- 213.Wakeling AE, Bowler J. Steroidal pure antioestrogens. J Endocrinol. 1987;112:R7–10. doi: 10.1677/joe.0.112r007. [DOI] [PubMed] [Google Scholar]
- 214.Wang J, Cheng CM, Zhou J, Smith A, Weickert CS, Perlman WR, Becker KG, Powell D, Bondy CA. Estradiol alters transcription factor gene expression in primate prefrontal cortex. J Neurosci Res. 2004;76:306–314. doi: 10.1002/jnr.20076. [DOI] [PubMed] [Google Scholar]
- 215.Wang MM, Traystman RJ, Hurn PD, Liu T. Non-classical regulation of estrogen receptor-alpha by ICI182,780. J Steroid Biochem Mol Biol. 2004;92:51–62. doi: 10.1016/j.jsbmb.2004.06.002. [DOI] [PubMed] [Google Scholar]
- 216.Watson CS, Norfleet AM, Pappas TC, Gametchu B. Rapid actions of estrogens in GH3/B6 pituitary tumor cells via a plasma membrane version of estrogen receptor-alpha. Steroids. 1999;64:5–13. doi: 10.1016/s0039-128x(98)00107-x. [DOI] [PubMed] [Google Scholar]
- 217.Wilcox JN, Roberts JL. Estrogen decreases rat hypothalamic proopiomelanocortin messenger ribonucleic acid levels. Endocrinology. 1985;117:2392–2396. doi: 10.1210/endo-117-6-2392. [DOI] [PubMed] [Google Scholar]
- 218.Williams SJ, Papka RE. Estrogen receptor-immunoreactive neurons are present in the female rat lumbosacral spinal cord. J Neurosci Res. 1996;46:492–501. doi: 10.1002/(SICI)1097-4547(19961115)46:4<492::AID-JNR11>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- 219.Wintermantel TM, Campbell RE, Porteous R, Bock D, Grone HJ, Todman MG, Korach KS, Greiner E, Perez CA, Schutz G, Herbison AE. Definition of estrogen receptor pathway critical for estrogen positive feedback to gonadotropin-releasing hormone neurons and fertility. Neuron. 2006;52:271–280. doi: 10.1016/j.neuron.2006.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Wyckoff MH, Chambliss KL, Mineo C, Yuhanna IS, Mendelsohn ME, Mumby SM, Shaul PW. Plasma membrane estrogen receptors are coupled to endothelial nitric-oxide synthase through Galpha(i) J Biol Chem. 2001;276:27071–27076. doi: 10.1074/jbc.M100312200. [DOI] [PubMed] [Google Scholar]
- 221.Xu Y, Traystman RJ, Hurn PD, Wang MM. Membrane restraint of estrogen receptor alpha enhances estrogen-dependent nuclear localization and genomic function. Mol Endocrinol. 2004;18:86–96. doi: 10.1210/me.2003-0262. [DOI] [PubMed] [Google Scholar]
- 222.Xu Y, Traystman RJ, Hurn PD, Wang MM. Neurite-localized estrogen receptor-alpha mediates rapid signaling by estrogen. J Neurosci Res. 2003;74:1–11. doi: 10.1002/jnr.10725. [DOI] [PubMed] [Google Scholar]
- 223.Zhang J, Ferguson SS, Barak LS, Aber MJ, Giros B, Lefkowitz RJ, Caron MG. Molecular mechanisms of G protein-coupled receptor signaling: role of G protein-coupled receptor kinases and arrestins in receptor desensitization and resensitization. Receptors Channels. 1997;5:193–199. [PubMed] [Google Scholar]
- 224.Zhang JQ, Cai WQ, Zhou DS, Su BY. Distribution and differences of estrogen receptor beta immunoreactivity in the brain of adult male and female rats. Brain Res. 2002;935:73–80. doi: 10.1016/s0006-8993(02)02460-5. [DOI] [PubMed] [Google Scholar]
- 225.Zhong Y, Dunn PM, Bardini M, Ford AP, Cockayne DA, Burnstock G. Changes in P2X receptor responses of sensory neurons from P2X3-deficient mice. Eur J Neurosci. 2001;14:1784–1792. doi: 10.1046/j.0953-816x.2001.01805.x. [DOI] [PubMed] [Google Scholar]
- 226.Zwain IH, Yen SS. Neurosteroidogenesis in astrocytes, oligodendrocytes, and neurons of cerebral cortex of rat brain. Endocrinology. 1999;140:3843–3852. doi: 10.1210/endo.140.8.6907. [DOI] [PubMed] [Google Scholar]