Abstract
Recently, signaling changes in the FcεRI pathway involving inositol lipid phosphatases have been identified in the basophils of chronic idiopathic urticaria (CIU) subjects. Based on the profile of basophil FcεRI-mediated histamine degranulation, we have segregated CIU subjects into two groups, CIU Responder (CIU R) or CIU Nonresponder (CIU NR). In the present study, we compared expression of SHIP-1, SHIP-2, and Syk protein to histamine release (HR) from mast cells (MC) cultured from the peripheral blood of CIU R, CIU NR, and normal subjects. The MC of CIU R donors contained significantly increased Syk and decreased SHIP-2 as compared to CIU NR (Syk: p=0.038: SHIP-2: p=0.038) and normals (Syk: p=0.042: SHIP-2: p=0.027). Spontaneous HR from CIU donors was increased two-fold compared to normals (p=0.04). In summary, our results suggest a possible predilection for urticarial MC to spontaneously degranulate upon IgE sensitization contributing to the increased pruritis associated with CIU.
Keywords: FcεRI, CD34+ stem cell, signal transduction, urticaria, mast cell
INTRODUCTION
Urticaria is a common disorder affecting up to 25% of the United States population. Acute urticarial eruptions can be triggered by allergic reactions, viral infections, or physical stimuli. If lesions recur for over 6 weeks the condition is termed chronic urticaria (CU) and in the vast majority of cases no specific cause is determined, hence the term chronic idiopathic urticaria (CIU). Since urticaria resembles the lesions induced by injection of histamine or allergen into the skin, a role for MC activation in the generation of urticarial lesions has been proposed [1]. Previous studies have indicated that skin MC have heightened releasability to compound 48/80 in active CU that resolves in disease remission while subjects with both active and inactive CU demonstrate hyperreleasability to codeine sulfate [1; 2]. Although several groups have described the presence of circulating antibodies to IgE or the alpha subunit of the high affinity IgE receptor (FcεRI α) in up to 40% of CIU subjects [3; 4] their role in disease pathogenesis remains controversial[5].
Phosphoinositide lipid phosphatases are well-established as negative regulators of hematopoietic cell activation, survival and proliferation[6]. In particular, Src homology 2 (SH2)- containing inositol phosphatase (SHIP-1) is known to associate with the FcεRI β subunit and is activated upon stimulation of rodent MC with supraoptimal concentrations of antigen or IgE [7; 8]. MC of SHIP-1 knockout mice more readily degranulated after IgE receptor activation or even sensitization with a highly cytokinergic IgE alone [9]. Similarly, in hyper-releasable human basophils a five-fold reduction in SHIP-1 protein levels was associated with heightened response to Histamine Releasing Factor (HRF)[10]. SHIP-1 has also been shown to have a regulatory role in the kinetics of IgE-mediated signaling and mediator release in primary human basophils [11]. Moreover, a homologous protein, SHIP-2, has been found to limit MC degranulation as well as IL-4 and IL-13 gene expression upon FcεRI stimulation that is independent of SHIP-1 actions [12].
We have reported that changes in the amount of FcεRI signaling molecules contributes to the changes in releasability of urticarial basophils observed by other investigators and ourselves [13; 14; 15; 16; 17]. We have previously compared anti-IgE-induced histamine release from CIU basophils to their corresponding SHIP-1 and SHIP-2 levels. We have noted that CIU subjects’ basophils can be divided on the degree of histamine release after optimal anti-IgE stimulation (0.1 mg/ml) into anti-IgE responders (> 10% HR) or non-responders (<10% HR) [14]. In parallel, we have demonstrated that SHIP-2 is increased in the basophils of CIU anti-IgE non-responders (CIU NR) whereas SHIP-1 levels are reduced in the CIU anti-IgE responder (CIU R) donors Degranulation in human basophils appears to be very sensitive to the amount of cellular SHIP-1 as ≥49% knockdown of SHIP-1 protein in basophils cultured from CD34+ stem cells was sufficient to enhance HRF-mediated HR [18]. Furthermore, if the changes in expression of SHIP-1 or SHIP-2 proteins are functional they should be inversely proportional to the cellular phosphatidylinositol 3,4,5 trisphosphate concentration ([PI 3,4,5 P3]) after anti-IgE stimulation of the CIU basophils. In fact the heightened SHIP-2 expression observed in CIU NR basophils compared to normal basophils results in decreased anti-IgE-induced Akt phosphorylation, a surrogate measure of cellular [PI 3,4,5 P3] [14]. In addition, CIU R basophils were found to have constitutive phosphorylation of Akt consistent with their reduced expression of SHIP-1.
Spleen tyrosine kinase (Syk) is recruited to the FcεRI and B cell antigen receptor after antigen stimulation. Inhibition of Syk by R112 (an ATP-dependent Syk inhibitor) or elimination of Syk protein by chemical ablation has been shown to completely inhibit all three pathways of FcεRI mediator secretion in MC: degranulation, lipid mediator production, and cytokine production[18; 19]. In a population survey of human basophils from normal donors, HR to an optimal dose of anti-IgE and protein levels of Syk were positively correlated suggesting that Syk expression is a major determinant of histamine release in human basophils [20]. In contrast, Syk expression correlated poorly with anti-IgE HR in CIU basophils, and we identified Syk-deficient CIU basophils with normal HR [21]. These studies suggest that key elements of releasability may differ between basophils from normal and disease populations.
Although MC as well as their secreted mediators, have been implicated in the generation of urticarial lesions, direct analysis of the signaling changes in the FcεRI pathway that may contribute to the enhanced releasability of CIU MC have not been reported. In the current study, we cultured MC from the peripheral blood of CIU R, CIU NR and normal donors. We then quantified the expression of the signaling molecules SHIP-1, SHIP-2 and Syk in these MC as well as their anti-IgE-induced and spontaneous degranulation.
MATERIALS AND METHODS
Study Subjects
Healthy adult volunteers and subjects with physician-diagnosed CIU were recruited from the Johns Hopkins Asthma and Allergy Center. After informed consent via a protocol approved by the Western Institutional review board, subjects underwent venipuncture. Subjects were specifically excluded if they had received systemic steroids in the month before venipuncture. All CIU subjects reported active urticarial disease at the time of the blood sample collection and for a minimum of 90 days. Subjects with a diagnosis of physical urticaria or urticarial vasculitis were excluded from the study. A total of 24 subjects (6 CIU R, 8 CIU NR and 10 normal subjects) were enrolled in the study for the generation of CD34+ MCs. The average age and sex of the study subjects by subgroup were CIU R (age 40, 100% female); CIU NR (age 43, 75% female); normal donors (age 33, 50% female).
Basophil and CD34+ stem cell isolation
Peripheral blood was sedimented through a discontinuous double Percoll gradient (53.2% and 62%, respectively) as described [14]. To generate MC, CD34+ stem cells were purified from the upper Percoll fraction of mononuclear cells using a positive selection kit from Miltenyi. Basophils were isolated from the lower Percoll fraction as before and were stimulated for histamine release as noted below [14]. CIU basophils releasing > 10% histamine after 0.1 mg/ml anti-IgE were classified as CIU R; while basophils releasing <10% HR were designated as CIU NR.
MC culture
Purified CD 34 + progenitors were cultured for up to 8 weeks to generate MC using reagents provided by Karl Nocka, UCB Pharma (C-kit ligand, IL-6 and the supernatant from a B cell line) as described [22]. MC cultures from CIU subjects were classified according to the basophil functional status of each donor as either CIU R or CIU NR. MC were stained with Wright-Giemsa to confirm their morphology
MC flow cytometry
Cultured MC suspensions were labeled with monoclonal antibodies for direct and indirect dual-color immunofluorescence and flow cytometry. The antibodies used were directed to the following antigens: ckit [95C3 antibody (Beckman Coulter, Fullerton, CA) or YB5.B8 antibody (Becton Dickinson Biosciences, San Diego, CA)] and FcεRIα (22E7, an IgG1antibody unaffected by FcεRI occupancy, kindly provided by J. Kochan (Roche Pharmaceuticals, NJ). Cells were incubated with monoclonal antibodies specific to each surface marker for 30 min at 4°C. Cells were then washed and, if necessary, labeled with PE conjugated anti-mouse for an additional 30 min. Samples were analyzed on a Becton Dickinson FACS Caliber flow cytometer employing Cell Quest software. Data are expressed as net median fluorescence intensity (actual MFI minus MFI of irrelevant IgG control).
Basophil and MC histamine release
Percoll–enriched basophils were stimulated for histamine release in suspension by polyclonal goat anti-human IgE (0.01–1 μg/ml) or formyl-met-leu-phe (FMLP)(10−6 M) for 45 min at 37° C. Histamine was quantified in cell-free supernatants using an automated fluorometric assay. MC (≥ 5 weeks of culture) were sensitized with PS myeloma human IgE for 1.5 hrs, seeded into 96 well plates and stimulated with polyclonal goat antihuman anti-IgE (0.03–10 μg/ml), FMLP- (1 μM) in media containing 0.8 mM calcium for 45 min at 37° C. Spontaneous release was quantified from cells incubated in buffer alone. Histamine content of cultured CD34 + cells was evaluated weekly, as well as pre- and post-sensitization, by lysing duplicates of 20,000 cells in perchloric acid. Occasionally, > 50% loss of histamine content was observed upon sensitization (in MC cultured from all donor types) and the resulting data was excluded.
Western blotting for SHIP-1, SHIP-2 and Syk
Whole cell SDS lysates were prepared from MC (≥ 5 × 105), separated by SDS-PAGE gel electrophoresis and transferred to nitrocellulose for immunoblotting of the indicated proteins. The lysates were examined by sequentially probing the same membrane for the relative presence of the indicated proteins using the following antibodies: SHIP-1 (N1, rabbit), SHIP-2 (I20, goat), and Syk (4D10, mouse), obtained from Santa Cruz Biotechnology. The blots were stripped by immersion in 69 mM SDS, 62 mM Trizma-base, pH 6.7, 0.7% β-mercaptoethanol. All gels included 2 doses of a standard lysate of human T cells to allow for comparisons of band intensity across gels within a previously determined linear range of antibody staining, as described previously [10; 14]. The detection of specific proteins was performed with the appropriate secondary antibody coupled to horseradish peroxidase (HRP; GE Healthcare Biosciences, Piscatway, NJ) and chemiluminescence (Supersignal, Pierce) film radiography. Digital images of detected protein bands were quantified using Kodak digital software (Kodak, New Haven, Conn).
Statistical Design
All data are expressed as the mean ± the standard error of the mean (SEM). All error bars represent SEM. Statistical analysis was performed using the one-tailed unpaired two-sample Student t-test. A p value was considered significant if <0.05. Multivariate linear regression was used to investigate the relationship between spontaneous HR and Syk in the CIU R subgroup (Pearson’s Correlation Coefficient). All donors were evaluated for both degranulation as well as signaling protein expression over multiple weeks (5–8 weeks).
RESULTS
Cultured MC phenotype
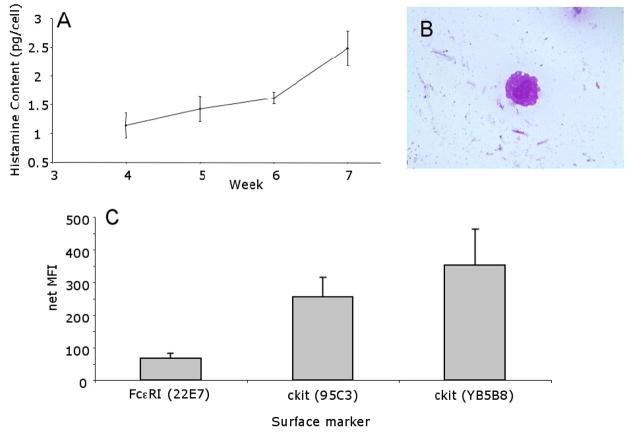
Cells cultured from peripheral blood CD34+ progenitor cells (from either CIU or normal donors) differentiated into MC, based on their histamine content (Figure 1A), granule staining characteristics, morphology (Figure 1B), and cell surface expression of both FcεRI and c-kit (Figure 1C). We found that MC after 5–6 weeks of culture expressed the histamine content, granule characteristics and surface phenotype typical of mature MC. No significant differences were observed between CIU R, CIU NR or normal MCs in these parameters.
Figure 1. Characterization of Cultured Human MC.
Panel A. Histamine Content. Aliquots of CD34+ derived MC were harvested weekly and examined for average total cell histamine content. (n=14). MC histamine content increased as expected, indicating that the MC were maturing and incorporating increasing histamine content on a per cell basis. Panel B. Morphology of Cultured MCs. MC (6 weeks) were stained with Wright-Giemsa (1000 X) (1 donor representative of 13). Panel C. Cell Surface Marker Expression on MCs after 6 weeks of Culture. Surface expression of FcεRIα (22e7, n = 12) and c-kit (95C3, n= 4; YB5B8, n = 7) was determined by flow cytometry. No differences were seen between CIU subgroups and normal MC.
Cultured MC expression of FcεRI signaling proteins
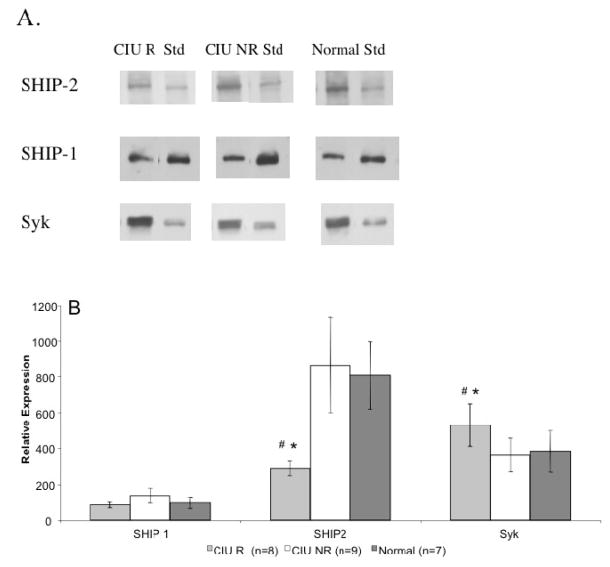
Previously, CIU donors were characterized as CIU R if their basophils released > 10% total histamine content to 0.1 mg/ml anti-IgE whereas CIU NR basophils released ≤10% histamine content [14]. Given the evidence for longitudinal stability of these basophil phenotypes in active disease [23], CIU cultured MC were classified relative to their basophil functional phenotype. Previous findings implicating SHIP-1, SHIP-2 and Syk in regulating basophil releasability prompted us to quantify the expression of these signaling molecules in our cultured MCs (Figure 2A). Cultured MC examined between weeks 5–8 revealed that the Syk levels were increased in CIU R subgroup (529 ± 119, n=8) as compared to CIU NR (363 ± 92, n=10, p=0.038) or normal subjects’ MCs (384 ± 115, n=8, p=0.042). In contrast SHIP-2 levels were significantly reduced in CIU R MCs (290 ± 40, n=8) as compared to CIU NR MCs (864 ± 264, n=10, p=0.038) and normal MCs (807 ± 189, n=8, p=0.027) (Figure 2B). No differences were seen in the levels of SHIP-1 expression between the MC subgroups.
Figure 2. Expression of SHIP-1, SHIP-2 and Syk Protein in Cultured MCs from CIU and Normal Donors.
Panel A. Representative Western Blot showing that MC of CIU R donors contained increased Syk and decreased SHIP-2 compared to CIU NR and normal donors in comparison to a standard lysate of human T cells. No difference was observed in the levels of SHIP-1. Panel B. Mean protein expression for weeks 5–8 of cultureshowing increased Syk in CIU R (mean ± SEM, n=8 determinations) compared to CIU NR ( n=10) and normals ( n=8). CIU R donors also contained decreased SHIP-2 ( n=8) compared to the MC of either CIU NR (n=10) or normals (n=8). # p < .05, CIU R to CIU NR; * p < .05, CIU R to normals.
Cultured MC degranulation
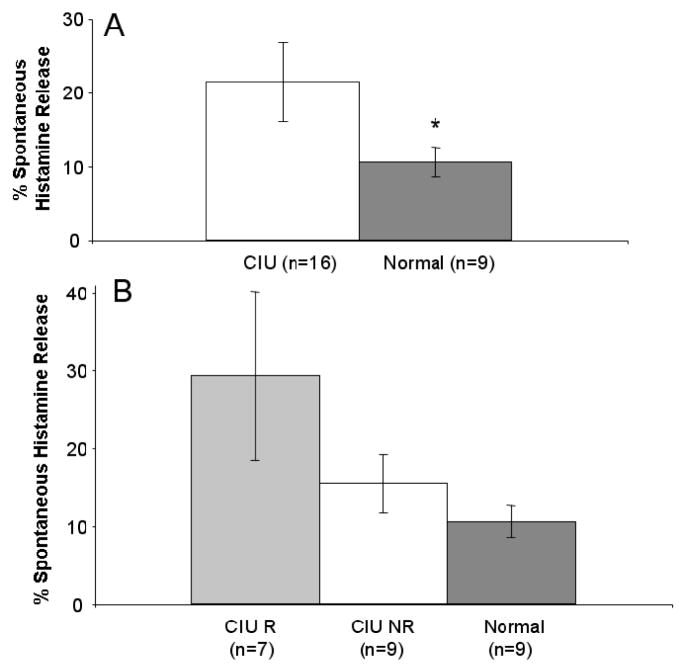
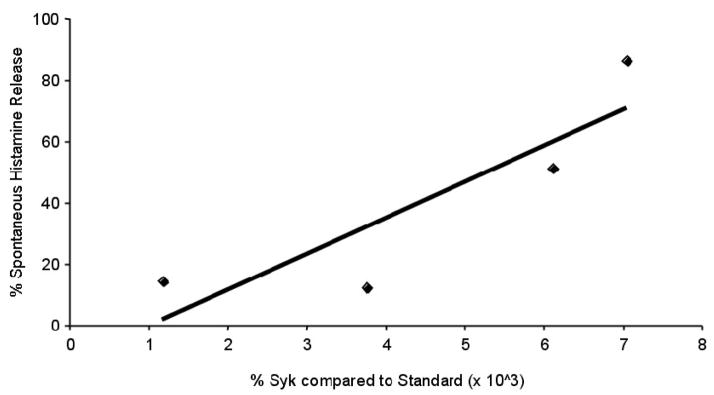
As a group, spontaneous HR in MC (weeks 5 to 8) cultured from CIU donors (21.4 ± 5.31%, n = 16) was significantly increased in comparison to MC (weeks 6 to 8) cultured from normal donors (10.5 ± 2.03%, n = 9, p=0.04) (Figure 3A). Among CIU subsets, CIU R MCs trended to a higher average spontaneous HR (29.2 ± 10.91, n=7) as compared to CIU NR (15.4 ± 3.77, n=9, p=0.14) and normal MCs (10.5 ± 2.03, n=9, p=0.07) (Figure 3B). Based on the observation that spontaneous HR and Syk content was increased in the CIU R group as compared to the CIU NR, we further examined the relationship between Syk and spontaneous HR. We found a trend towards spontaneous histamine release being dependant on Syk (R2= 0.7669, n = 4, p = 0.12, Pearson’s correlation coefficient, Figure 4). Further, the anti-IgE stimulated histamine response of the cultured MCs was examined at various concentrations of anti-IgE (0.3– 3 μg/ml) and ranged from 3 to 15% beyond spontaneous release; however we found no differences between the groups (data not shown).
Figure 3. Spontaneous Histamine Release in Cultured MC.
Panel A. Spontaneous HR in MC (weeks 5–8) cultured from CIU and normal donors. Spontaneous HR in MC cultured from CIU donors ( n = 16) is increased compared to MC cultured from normal donors ( n = 9). Panel B. Spontaneous HR in MC (weeks 5–8) cultured from CIU R (n = 7), CIU NR (n =9), and Normal donors (n = 9). Within the CIU donor group the CIU R trended towards the highest amount of spontaneous HR (n=7) compared to the CIU NR (n=9) and normal donors( n=9) * p < .05, CIU to normals.
Figure 4. Relationship between Spontaneous Histamine Release and Syk in Cultured MC from the CIU R subgroup.
Cells cultured from weeks 6–8 were analyzed for Syk and spontaneous histamine release. Multivariate analysis shows a linear relationship with an R=0.7669. (p = 0.12, Pearson’s correlation coefficient).
DISCUSSION
In this study, we examined the phenotype of cultured MC grown from the CD34+ cells of subjects with defined CIU disease in comparison with healthy controls. We classified CIU subjects MC relative to their blood basophil functional phenotype, CIU R or CIU NR, and found elevated Syk and reduced SHIP-2 levels in CIU R MC as compared to CIU NR and normal donor MC. Overall, spontaneous HR was increased two-fold overall in CIU subjects versus normal MCs after IgE sensitization. However, in contrast to their distinct basophil FcεRI-mediated degranulation phenotypes, the magnitude and dose-response to anti-IgE stimulation was nearly identical in both populations of cultured CIU MC.
We utilized the basophil phenotype as determined by ex vivo IgE receptor activation for classification of our CIU MCs several reasons. We have found stability of the basophil functional phenotype with repeated sampling in CIU subjects with persistent disease. Furthermore, we have noted a shift towards increased sensitivity to anti-IgE-mediated basophil HR (CIU R) or the recovery of normal HR (CIU NR subjects) with disease remission suggesting a mechanism operates to suppress basophil HR during active CIU [23]. We are currently investigating the nature of this suppressive mechanism. However, we note that basophil functional phenotypes are independent of the presence of autoantibodies to IgE or the FcεR1 [23].
In terms of clinical disease outcomes, CIU R subjects report significantly higher itch scores as compared to CIU NR donors [24]. A trend towards heightened spontaneous HR was observed in CIU R as compared to CIU NR cultured MCs consistent with an increased sensitivity to IgE sensitization rather than FcεRI aggregation. On a very limited basis we tested donors with a mouse anti-DNP IgE and saw a similar profile of spontaneous release. Of note, we attempted to culture MC from the CD34+ stem cells of cold-induced urticaria subjects (n=3) and in all three cases, the cells spontaneously degranulated prior to reaching maturity based on their morphology and the high histamine content of the culture supernatant.
The protein expression profile of a tissue-resident MC likely reflects the local microenvironment. While the protein expression in a prolonged cultured cell may not reflect the mature tissue cell, significant limitations still exist in being able to determine protein expression in skin MCs microdissected from biopsies or cultured from biopsies [25].
Our results with cultured CIU MC suggest they have undergone a change in the phosphatase-kinase balance that prevents activation of normal MC upon IgE sensitization. In addition, an IgE subtype may be produced in active CIU that enhances the releasability of the patient’s MC and/or basophils. Murine IgEs have been classified as highly (HC) or poorly cytokinergic (PC) based on their ability to stimulate cytokine production and promote survival of MC. Monomeric IgE stimulation of murine bone marrow-derived MC with SPE7, an HC IgE, resulted in up to 80% degranulation in SHIP-1 deficient BMMCs[9]. Two studies have highlighted the ability of IgE alone to promote secretion in human cord-derived or cultured human lung MC [26; 27].
Given that considerable controversy exists as to the role of basophils and MCs in CIU and that many of the current therapies (anti-histamines, LTRAs) are directed to the secretory products of the two cells, the fact that we identified molecular differences between the basophils and MCs in the CIU R subgroup vs. CIU NR and normal donors suggests both cell types may contribute in different ways to the CIU phenotype. Specifically, the CIU R basophils demonstrate constitutive phosphorylation of Akt, an important signal leading to secretion of cytokines, including IL-3 recently shown to be an autocrine priming factor for basophils [28]. However it is the elevated Syk expression in the MCs from CIU R donors that appears to correlate with their spontaneous histamine release. Both cell types may be responsible for the increased pruritis reported by CIU R donors [24]. In addition, the current study has illuminated a defect in spontaneous HR (in cultured mast cells) rather than IgE receptor triggered HR as previously reported in basophils [14].
When exposed to a common culture environment, the expression of signaling molecules implicated in the regulation of FcεRI-induced degranulation are differentially modulated in CIU R MC versus CIU NR and normal MC, implying a genetic disposition in CD34+ progenitor cells to respond to a cytokine milieu. The growth factors required for differentiation of CD34+ progenitors into MC may also modulate expression of the signaling molecules studied. Intracellular signal transduction typically occurs by post-translational modification of signaling proteins or lipids as well as changes in subcellular location. More recent data suggest that cellular stimulation can affect expression of kinases and phosphatases with accompanying changes in cell function [29]. The expression of all three signaling molecules examined in this study has been shown to be specifically regulated by cytokines and endotoxin. For example, Syk protein expression is upregulated while SHIP-1 protein expression is downregulated upon culturing basophils with IL-3 [30] [Langdon and MacDonald, unpublished observations]. Recently, LPS treatment of murine bone marrow derived macrophages or MC was shown to increase TGF-β which acts in an autocrine fashion to upregulate SHIP-1 but not SHIP-2 protein levels[31]. LPS treatment of human monocytes upregulates SHIP-2 while both SHIP-1 and SHIP-2 protein levels are increased in LPS treated B cells [32; 33].
As reviewed recently, the elucidation of changes in the expression of key regulatory elements in the FcεRI pathway of CIU subjects provides an opportunity for treatment with newer, more specific immunomodulators developed originally for the treatment of other immune-mediated diseases [34]. Specifically, the stratification of CIU patients into subgroups such as CIU R and CIU NR in conjunction with the profiling of even a limited number of signaling proteins in basophils and cultured MCs may prove beneficial. The increased expression of Syk kinase coupled with increased spontaneous HR upon IgE sensitization in CIU R mast cells suggests treatment with a Syk inhibitor such as R112, proven effective in the treatment of allergic rhinitis, may be useful in CIU [35; 36]. Furthermore, a second Syk inhibitor, R406, blocks anti-IgE mediated secretion in cultured human MCs prepared from cord blood CD34+ cells, is bioavailable and blocks anti-IgE induced upregulation of the cell-surface activation marker CD63 on human basophils [37].
In summary, the heightened spontaneous HR observed in MC from CIU donors may contribute to the increased pruritis associated with CIU as well as expose a possible common predilection for urticarial MC to spontaneously degranulate upon IgE sensitization in the subset of CIU R donors. Notably, CIU patients with active disease despite standard antihistamine treatment who received omalizumab, a humanized monoclonal anti-IgE, showed significantly decreased urticaria severity scores and increased symptom-free days in support of a pathogenic role for IgE and the FcεRI in activating skin MC in CIU [38].
Acknowledgments
Funded by AAAAI MOA Interest Section grant and K08 grant to SS and NIH K22 grant to BMV. We thank Karl Nocka and Sudhir Rao for reagents and helpful advice on culturing MCs. We thank Ms. Nancy Van Keuren for administrative assistance.
ABBREVIATIONS
- CIU
chronic idiopathic urticaria
- CIU NR
CIU anti-IgE non-responder
- CIU R
CIU anti-IgE responder
- HR
histamine release
- HRF
histamine releasing factor
- MC
mast cell
- [PI 3,4,5 P3]
phosphatidylinositol 3,4,5 trisphosphate concentration
- Syk
spleen tyrosine kinase
- SHIP
Src homology 2 (SH2)-containing inositol phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacques P, Lavoie A, Bedard PM, Brunet C, Hebert J. Chronic idiopathic urticaria: profiles of skin mast cell histamine release during active disease and remission. J Allergy Clin Immunol. 1992;89:1139–43. doi: 10.1016/0091-6749(92)90297-f. [DOI] [PubMed] [Google Scholar]
- 2.Cohen RW, Rosenstreich DL. Discrimination between urticaria-prone and other allergic patients by intradermal skin testing with codeine. J Allergy Clin Immunol. 1986;77:802–7. doi: 10.1016/0091-6749(86)90377-5. [DOI] [PubMed] [Google Scholar]
- 3.Gruber BL, Baeza ML, Marchese MJ, Agnello V, Kaplan AP. Prevalence and functional role of anti-IgE autoantibodies in urticarial syndromes. J Invest Dermatol. 1988;90:213–7. doi: 10.1111/1523-1747.ep12462239. [DOI] [PubMed] [Google Scholar]
- 4.Hide M, Francis DM, Grattan CE, Hakimi J, Kochan JP, Greaves MW. Autoantibodies against the high-affinity IgE receptor as a cause of histamine release in chronic urticaria. N Engl J Med. 1993;328:1599–604. doi: 10.1056/NEJM199306033282204. [DOI] [PubMed] [Google Scholar]
- 5.Brodell LA, Beck LA, Saini SS. Pathophysiology of chronic urticaria. Ann Allergy Asthma Immunol. 2008;100:291–7. doi: 10.1016/S1081-1206(10)60588-1. [DOI] [PubMed] [Google Scholar]
- 6.Harris SJ, Parry RV, Westwick J, Ward SG. Phosphoinositide lipid phosphatases: natural regulators of phosphoinositide 3-kinase signaling in T lymphocytes. J Biol Chem. 2008;283:2465–9. doi: 10.1074/jbc.R700044200. [DOI] [PubMed] [Google Scholar]
- 7.Kimura T, Sakamoto H, Appella E, Siraganian RP. The negative signaling molecule SH2 domain-containing inositol-polyphosphate 5-phosphatase (SHIP) binds to the tyrosine-phosphorylated beta subunit of the high affinity IgE receptor. J Biol Chem. 1997;272:13991–6. doi: 10.1074/jbc.272.21.13991. [DOI] [PubMed] [Google Scholar]
- 8.Gimborn K, Lessmann E, Kuppig S, Krystal G, Huber M. SHIP down-regulates FcepsilonR1-induced degranulation at supraoptimal IgE or antigen levels. J Immunol. 2005;174:507–16. doi: 10.4049/jimmunol.174.1.507. [DOI] [PubMed] [Google Scholar]
- 9.Huber M, Helgason CD, Damen JE, Liu L, Humphries RK, Krystal G. The src homology 2-containing inositol phosphatase (SHIP) is the gatekeeper of mast cell degranulation. Proc Natl Acad Sci U S A. 1998;95:11330–5. doi: 10.1073/pnas.95.19.11330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Vonakis BM, Gibbons S, Jr, Sora R, Langdon JM, MacDonald SM. Src homology 2 domain-containing inositol 5′ phosphatase is negatively associated with histamine release to human recombinant histamine- releasing factor in human basophils. J Allergy Clin Immunol. 2001;108:822–31. doi: 10.1067/mai.2001.119159. [DOI] [PubMed] [Google Scholar]
- 11.Gibbs BF, Rathling A, Zillikens D, Huber M, Haas H. Initial Fc epsilon RI-mediated signal strength plays a key role in regulating basophil signaling and deactivation. J Allergy Clin Immunol. 2006;118:1060–7. doi: 10.1016/j.jaci.2006.07.022. [DOI] [PubMed] [Google Scholar]
- 12.Leung WH, Bolland S. The inositol 5′-phosphatase SHIP-2 negatively regulates IgE-induced mast cell degranulation and cytokine production. J Immunol. 2007;179:95–102. doi: 10.4049/jimmunol.179.1.95. [DOI] [PubMed] [Google Scholar]
- 13.Sabroe RA, Francis DM, Barr RM, Black AK, Greaves MW. Anti-Fc(episilon)RI auto antibodies and basophil histamine releasability in chronic idiopathic urticaria. J Allergy Clin Immunol. 1998;102:651–8. doi: 10.1016/s0091-6749(98)70283-0. [DOI] [PubMed] [Google Scholar]
- 14.Vonakis BM, Vasagar K, Gibbons J, Gober SCL, Sterba P, Chang H, Saini SS. Basophil FceRI histamine release parallels expression of Src-homology2-containing inositol phosphatases in chronic idiopathic urticaria. J Allergy Clin Immunol. 2007;119:441–8. doi: 10.1016/j.jaci.2006.09.035. [DOI] [PubMed] [Google Scholar]
- 15.Luquin E, Kaplan AP, Ferrer M. Increased responsiveness of basophils of patients with chronic urticaria to sera but hypo-responsiveness to other stimuli. Clin Exp Allergy. 2005;35:456–60. doi: 10.1111/j.1365-2222.2005.02212.x. [DOI] [PubMed] [Google Scholar]
- 16.Greaves MW, Plummer VM, McLaughlan P, Stanworth DR. Serum and cell bound IgE in chronic urticaria. Clin Allergy. 1974;4:265–71. doi: 10.1111/j.1365-2222.1974.tb01384.x. [DOI] [PubMed] [Google Scholar]
- 17.Kern F, Lichtenstein LM. Defective histamine release in chronic urticaria. J Clin Invest. 1976;57:1369–77. doi: 10.1172/JCI108405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rossi AB, Herlaar E, Braselmann S, Huynh S, Taylor V, Frances R, Issakani SD, Argade A, Singh R, Payan DG, Masuda ES. Identification of the Syk kinase inhibitor R112 by a human mast cell screen. J Allergy Clin Immunol. 2006;118:749–55. doi: 10.1016/j.jaci.2006.05.023. [DOI] [PubMed] [Google Scholar]
- 19.Zhang J, Berenstein EH, Evans RL, Siraganian RP. Transfection of Syk protein tyrosine kinase reconstitutes high affinity IgE receptor-mediated degranulation in a Syk-negative variant of rat basophilic leukemia RBL-2H3 cells. J Exp Med. 1996;184:71–9. doi: 10.1084/jem.184.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Macglashan DW., Jr Relationship between spleen tyrosine kinase and phosphatidylinositol 5′ phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–33. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 21.Vonakis BM, Saini SS. Syk-deficient basophils from donors with chronic idiopathic urticaria exhibit a spectrum of releasability. J Allergy Clin Immunol. 2008;121:262–4. doi: 10.1016/j.jaci.2007.10.010. [DOI] [PubMed] [Google Scholar]
- 22.Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J Immunol. 2006;177:2755–9. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- 23.Eckman J, Hamilton RG, Gober LM, Sterba PM, Saini SS. Basophil Phenotypes in Chronic Idiopathic Urticaria in Relation to Disease Activity and Autoantibodies. J Invest Dermatol. 2008;128:1956–63. doi: 10.1038/jid.2008.55. [DOI] [PubMed] [Google Scholar]
- 24.Baker R, Vasagar K, Ohameje N, Gober L, Chen SC, Sterba PM, Saini SS. Basophil histamine release activity and disease severity in chronic idiopahtic urticaria. Ann Allergy Asthma Immunol. 2008;100:244–249. doi: 10.1016/S1081-1206(10)60449-8. [DOI] [PubMed] [Google Scholar]
- 25.Kambe N, Kambe M, Kochan JP, Schwartz LB. Human skin-derived mast cells can proliferate while retaining their characteristic functional and protease phenotypes. Blood. 2001;97:2045–52. doi: 10.1182/blood.v97.7.2045. [DOI] [PubMed] [Google Scholar]
- 26.Cruse G, Kaur D, Yang W, Duffy SM, Brightling CE, Bradding P. Activation of human lung mast cells by monomeric immunoglobulin E. Eur Respir J. 2005;25:858–63. doi: 10.1183/09031936.05.00091704. [DOI] [PubMed] [Google Scholar]
- 27.Matsuda K, Piliponsky AM, Iikura M, Nakae S, Wang EW, Dutta SM, Kawakami T, Tsai M, Galli SJ. Monomeric IgE enhances human mast cell chemokine production: IL-4 augments and dexamethasone suppresses the response. J Allergy Clin Immunol. 2005;116:1357–63. doi: 10.1016/j.jaci.2005.08.042. [DOI] [PubMed] [Google Scholar]
- 28.Schroeder JT, Chichester KL, Bieneman AP. Human basophils secrete IL-3: evidence of autocrine priming for phenotypic and functional responses in allergic disease. J Immunol. 2009;182:2432–8. doi: 10.4049/jimmunol.0801782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Rauh MJ, Krystal G. Of mice and men: elucidating the role of SH2-containing inositol 5- phosphatase (SHIP) in human disease. Clin Invest Med. 2002;25:68–70. [PubMed] [Google Scholar]
- 30.Kepley CL, Youssef L, Andrews RP, Wilson BS, Oliver JM. Multiple defects in Fc epsilon RI signaling in Syk-deficient nonreleaser basophils and IL-3-induced recovery of Syk expression and secretion. J Immunol. 2000;165:5913–20. doi: 10.4049/jimmunol.165.10.5913. [DOI] [PubMed] [Google Scholar]
- 31.Sly LM, Rauh MJ, Kalesnikoff J, Buchse T, Krystal G. SHIP, SHIP2, and PTEN activities are regulated in vivo by modulation of their protein levels: SHIP is up-regulated in macrophages and mast cells by lipopolysaccharide. Exp Hematol. 2003;31:1170–81. doi: 10.1016/j.exphem.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 32.Pengal RA, Ganesan LP, Fang H, Marsh CB, Anderson CL, Tridandapani S. SHIP-2 inositol phosphatase is inducibly expressed in human monocytes and serves to regulate Fcgamma receptor-mediated signaling. J Biol Chem. 2003;278:22657–63. doi: 10.1074/jbc.M302907200. [DOI] [PubMed] [Google Scholar]
- 33.Brauweiler A, Tamir I, Marschner S, Helgason CD, Cambier JC. Partially distinct molecular mechanisms mediate inhibitory FcgammaRIIB signaling in resting and activated B cells. J Immunol. 2001;167:204–11. doi: 10.4049/jimmunol.167.1.204. [DOI] [PubMed] [Google Scholar]
- 34.Vonakis BM, Saini SS. New concepts in chronic urticaria. Curr Opin Immunol. 2008;20:709–16. doi: 10.1016/j.coi.2008.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Meltzer EO, Berkowitz RB, Grossbard EB. An intranasal Syk-kinase inhibitor R112 improves the symptoms of seasonal allergic rhinitis in a park environment. J Allergy Clin Immunol. 2005;115:791–6. doi: 10.1016/j.jaci.2005.01.040. [DOI] [PubMed] [Google Scholar]
- 36.Guyer BJ, Shimamoto SR, Bradhurst AL, Grossbard EB, Dreskin SC, Nelson HS. Mast cell inhibitor R112 is well tolerated and affects prostaglandin D2 but not other mediators, symptoms, or nasal volumes in a nasal challenge model of allergic rhinitis. Allergy Asthma Proc. 2006;27:208–13. doi: 10.2500/aap.2006.27.2861. [DOI] [PubMed] [Google Scholar]
- 37.Braselmann S, Taylor V, Zhao H, Wang S, Sylvain C, Baluom M, Qu K, Herlaar E, Lau A, Young C, Wong BR, Lovell S, Sun T, Park G, Argade A, Jurcevic S, Pine P, Singh R, Grossbard EB, Payan DG, Masuda ES. R406, an orally available spleen tyrosine kinase inhibitor blocks fc receptor signaling and reduces immune complex-mediated inflammation. J Pharmacol Exp Ther. 2006;319:998–1008. doi: 10.1124/jpet.106.109058. [DOI] [PubMed] [Google Scholar]
- 38.Gober L, Sterba P, Eckman J, Saini S. Effect of Anti-IgE (Omalizumab) in Chronic Idiopathic Urticaria Patients. J Allergy Clin Immunol. 2008;121:S147. [Google Scholar]