Abstract
The Hippo signaling pathway is a highly conserved potent regulator of cell growth, division, and apoptosis. YAP, the nuclear effector of the Hippo pathway, is a highly conserved component of this pathway in mammalian systems. In humans, amplification of the chromosome region containing the YAP gene (11q22) has been reported in several tumor types. This study was performed to determine if YAP expression was present in four common types of malignant tumors which have the highest lifetime risk of causing cancer death among men and women in the United States. YAP expression intensity and distribution were evaluated in normal tissues and compared to the most frequently occurring malignant tumors in these tissues (colonic adenocarcinoma, lung adenocarcinoma, ovarian serous cystadenocarcinoma, and ductal carcinoma of the breast). For each tissue, the nuclear and cytoplasmic YAP expression intensity was scored as negative, low, or high. We found focal expression of YAP in the progenitor and reparative cellular compartments of normal tissue. In contrast, there was strong and diffuse nuclear and cytoplasmic YAP expression in colonic adenocarcinoma, lung adenocarcinoma, and ovarian serous cystadenocarcinoma. We concluded that the potent Hippo growth regulatory pathway shows markedly different expression patterns in normal tissues of the colon, lung, and ovary compared to the three common malignant tumor types we examined in these tissues. Our findings suggest that activation of the Hippo signaling pathway may occur through YAP as part of cell proliferation in normal tissue homeostasis and also might be a frequently activated oncogenic pathway in three common malignant tumor types.
Keywords: Hippo, immunohistochemistry, tumorigenesis, Yes-associated protein
Introduction
Cancer presently ranks as the second highest cause of death in the United States (1). Currently among men and women living in the United States, cancers of the lung, breast, colon, and ovary are among the most common types of malignancies resulting in cancer death (2). The process of carcinogenesis is complex, and it is believed that multiple mechanisms contribute to the development of malignant tumors with disruption of the balance between cell proliferation and apoptosis being among the major mechanisms (3). Molecular pathways which have a role in the maintenance of tissue homeostasis are critical to the proper control of cell proliferation and apoptosis.
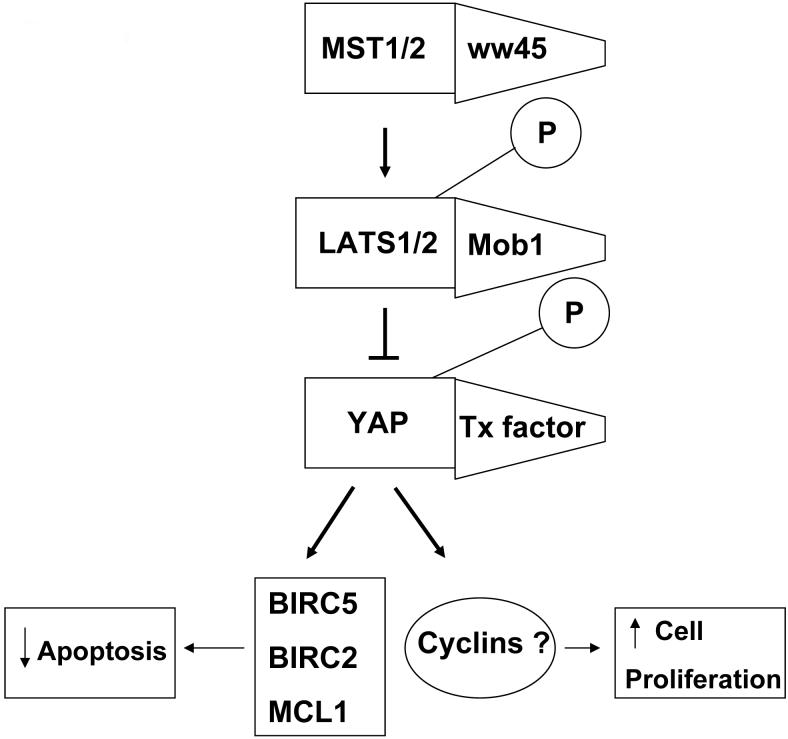
The Hippo pathway, a vital growth regulator of cell proliferation and apoptosis was initially identified by mosaic screens in Drosophila melanogaster (4,5). Information that is known about the Hippo pathway in Drosophila is likely directly applicable to mammalian systems as it has been shown that mammalian homologs are capable of rescuing Drosophila mutants defective in Hippo signaling (6-8). The components of this pathway which are highly conserved in mammals include the following: YAP (Yes-associated protein), Lats1/2, Mob, Mst1/2, Sav, Merlin, Expanded 1/2, and Fat 4 (Figure 1) (5). Hippo (Hpo) and Warts (Wts), two significant core pathway components, belong to the Sterile 20 (Ste20) and Nuclear Dbf2-related (NDR) serine/threonine protein kinase families, respectively (7,10-14). Salvador (Sav), a third major component of the Hippo pathway, is a scaffold protein with two WW domains and is homologous to the human ww45 protein (15,16). YAP, one of the highly conserved components in mammals, is considered to be a nuclear effector of the Hippo pathway (5,17). It has been noted that loss of critical components of the Hippo pathway can lead to uncontrolled cell growth, which implicates this as a tumor suppressor pathway (15).
Figure 1.
Studies from several groups, including our own, have suggested that the main genes involved in the Hippo pathway (Hpo, Sav, and Wts) must function in coordination to properly regulate cell proliferation and apoptosis. Given the growing attention to this novel growth regulatory and tumor suppressor pathway, we wanted to investigate the involvement of YAP, the nuclear effector of the Hippo pathway, for expression in human tumors which have a high lifetime risk of causing cancer death among men and women in the United States. The tumors we selected for the study were adenocarcinoma of the lung, ductal carcinoma of the breast (DCB), ovarian serous cystadenocarcinoma (OSC), and colonic adenocarcinoma. Adenocarcinoma of the lung was included in our study because it has currently replaced squamous cell carcinoma as the most frequently occurring form of lung cancer in both genders with women being more likely than men to have adenocarcinoma as opposed to other types (18). DCB was studied because it represents the most common histological type and accounts for up to 80% of all breast cancers (19). OSC and colonic adenocarcinoma were also included in the study as serous carcinoma is the most common type of ovarian cancer (20) and colonic adenocarcinoma is the most frequently occurring malignant neoplasm of the colon (21).
Materials and Methods
Tissue Samples
Tissue microarray (TMA) sections were constructed from paraffin-embedded tissue blocks of different types of malignant tumors and their companion normal tissues. Tissues were obtained from the pathology department at The Johns Hopkins Hospital in Baltimore, Maryland. The diagnoses were verified by evaluation of the histopathological and immunohistochemical results. All non tumor and tumor slides were reviewed by two reference pathologists.
Tissue Microarray Design
Core needle biopsies of pre-existing paraffin-embedded tissues were obtained and then re-embedded in an arrayed master block using techniques originally developed by Kononen, then later modified by Hedvat. Sample circular spots, 0.4mm in diameter, were obtained using the instrument's arraying device. Four core needle samples were obtained from each tumor and companion normal tissue specimen.
Immunohistochemical Analysis
Tissue Histology and Immunohistochemistry
Paraffin-embedded sections on polylysine coated slides were used for staining. Sections were cut at 4 μm. Immunohistochemistry for YAP was performed as follows: slides were deparaffinized in xylene and rehydrated in a graded alcohol series. Antigen retrieval was achieved by microwaving in 10mM sodium citrate buffer at pH 6 for 10 minutes. Peroxidase was blocked with 3% hydrogen peroxide in methanol, and nonspecific protein binding was blocked with 5% goat serum. The primary antibody for YAP, which is commercially available (Cell Signaling Tech. Inc. Danvers, MA) was diluted with phosphate buffered saline (PBS) containing 5% goat serum (1:100) and applied overnight in a humidity chamber at 4°C. Following washing with PBS, biotinylated secondary antibody was diluted in blocking reagent (1:100) and applied for 30 minutes at room temperature. Streptavidin was applied for 30 minutes at room temperature. Visualization was performed using DAB chromogen for 5-10 minutes. Control sections were incubated with isotype antibody diluents instead of primary antibody. Sections were counterstained with hematoxylin, dehydrated, and mounted.
YAP expression was both nuclear and cytoplasmic in normal tissues and in the four different types of cancer included in our study: colonic adenocarcinoma, lung adenocarcinoma, OSC, and DCB. The extent and pattern of staining varied among both normal tissues and tumors. The percentage of cells with strong staining in the cytoplasm was detected in all optical fields and a final score was made. Similarly, the percentage of cells with YAP nuclear reactivity was detected in all optical fields and a final score was made. The results for each tissue studied were classified as either nuclear or cytoplasmic based on the scoring.
Table 1 defines the scoring system used in grading the level of nuclear and cytoplasmic YAP expression. The definition of a YAP positive tissue is one in which the expression intensity has been scored as low or high.
Table 1.
|
Score |
Grading |
|---|---|
| Complete absence of reactivity | Negative |
| Weak cytoplasmic reactivity (regardless of extent) | Low |
| Strong cytoplasmic reactivity in less than 50% of cells | Low |
| Strong cytoplasmic reactivity in more than 50% of cells | High |
| Nuclear expression in sporadic cells (<10% of cells) | Low |
| Nuclear expression in >10% of cells | High |
Statistical Methods
In preparation for statistical analysis of staining intensity distribution, the data for each tissue studied were placed into three categories (negative, low, or high) based on the scoring results for level of nuclear and cytoplasmic YAP expression. The data from these three categories were compared and assessed for statistical significance. For example in colon tissue, the number of normal and colonic adenocarcinoma specimens with negative YAP expression, low YAP expression, and high YAP expression were compared and assessed for any statistically significant differences within the data from all three categories. The purpose of this analysis was to determine whether or not the staining intensity distribution of the four neoplastic tissues included in the study would tend to favor low more than negative YAP expression and favor high more than low YAP expression as compared to normal tissues. Statistical significance was determined using The Fisher's Exact Test to compute the p-values for YAP positivity data (negative vs. low + high) and YAP expression data across the distribution of intensities (negative vs. low vs. high) for normal tissues compared with the four types of neoplastic tissues examined. All p-values were calculated using a JavaScript calculator.
Results
Evaluation of YAP Expression in the Colon
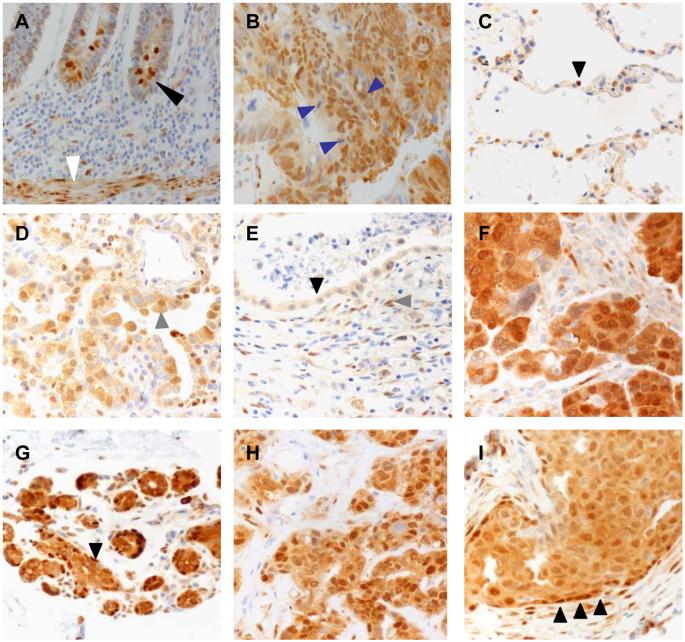
In the assessment of nuclear and cytoplasmic YAP expression intensity, the biopsies from 16 patients with normal colon tissue and 28 patients with neoplastic colonic adenocarcinoma were analyzed. YAP expression was assessed using immunohistochemistry methods and scored as detailed in the methods and Table 1. There was YAP expression in the nucleus and cytoplasm of the cells in the basal crypt zones (figure 2A black arrow head), which are areas that correlate with the position of the tissue progenitor cells for the crypt epithelium. The presence of YAP staining in the tissue progenitor cell regions supports what has been previously reported in animal studies (22). We also saw strong YAP staining in the muscularis mucosa smooth muscle cells, which are thought to be terminally differentiated (figure 2A white arrow head). Other studies have shown an association between Ki-67, a proliferation marker, and YAP (9,16,22). Ki-67 stains of control and YAP expressing tissues demonstrated that enhanced proliferation of the tissue was a result of YAP activation (22). In contrast to normal tissues, colonic adenocarcinoma tissues expressed strong nuclear and cytoplasmic YAP (figure 2B). Some of the mitotic malignant cells showed cytoplasmic expression of YAP (figure 2B blue arrow heads).
Figure 2.
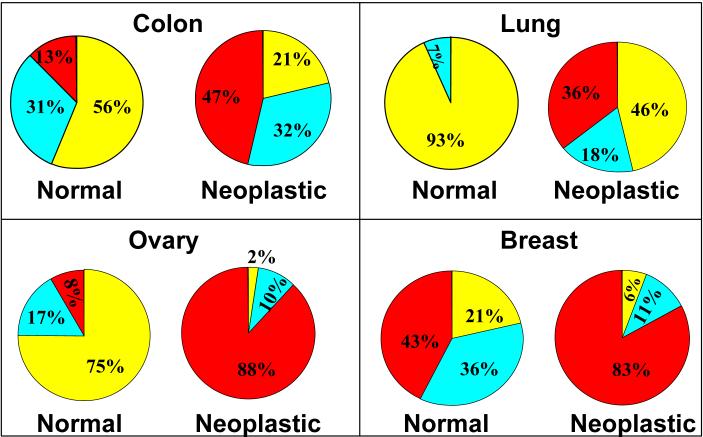
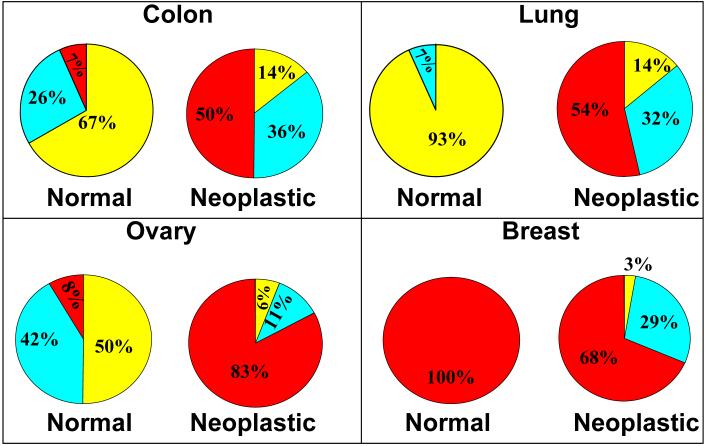
Any detectable (low or high) nuclear YAP staining was present in 7 of 16 (13%+31% = 44%) patients' normal colon tissue epithelial cells compared to 22 of 28 (47%+32% = 79%) patients' colonic adenocarcinoma tissues (figure 3 “colon”). The difference in any nuclear YAP expression between normal colon and colonic adenocarcinoma tissue was statistically significant (p<0.01). Cytoplasmic YAP expression intensity was determined from normal colon core biopsies of 15 patients and colonic adenocarcinoma tissue biopsies of 28 patients. In the 15 patients with normal colon biopsies, 5 of 15 (26% + 7% = 33%) were positive for cytoplasmic YAP compared to 24 of 28 patients (50% + 36% = 86%) with adenocarcinoma of the colon, which was a statistically significant (p<0.01) difference (figure 4 “colon”).
Figure 3.
Figure 4.
Evaluation of YAP Expression in the Lung
Core biopsies of 43 patients with normal lung tissue and neoplastic lung adenocarcinoma tissue were assessed for nuclear and cytoplasmic YAP expression intensity. We found that the non-neoplastic lung tissue showed YAP staining limited to type II pneumocytes, which compose the regenerative compartment of the alveolus (figure 2C black arrow head). There was diffuse, relatively strong staining of malignant lung epithelial cells (figure 2D gray arrow head). Only 1 of 15 (7%) patients' normal lung tissues expressed nuclear YAP while 15 of 28 (36% + 18% = 54%) patients' lung adenocarcinoma tissues showed any expression of nuclear YAP (figure 3 “lung”). The difference in any nuclear YAP expression between normal lung and lung adenocarcinoma tissue was statistically significant (p<0.01). In the group of patients with normal lung tissue, 1 of 15 (7%) expressed cytoplasmic YAP, whereas 24 of 28 (54% + 32% = 86%) patients with lung adenocarcinoma tissue expressed cytoplasmic YAP. This difference reached statistical significance (p<0.01) (figure 4 “lung”).
Evaluation of YAP Expression in the Ovary
Ovarian tissue biopsies from 54 patients were evaluated for nuclear and cytoplasmic YAP expression. In the normal ovary, we found scattered YAP expressing cells in the ovarian stroma (figure 2E gray arrow head) with light cytoplasmic staining of surface epithelial cells (figure 2E black arrow head). The OSC tissues showed generally strong cytoplasmic and nuclear staining (figure 2F). In those with normal ovarian tissue, 3 of 12 (8% + 17% = 25%) patients expressed nuclear YAP while 41 of 42 (88% + 10% = 98%) patients with OSC were positive for nuclear YAP (figure 3 “ovary”). This difference in any detectable nuclear YAP expression between normal ovary and OSC tissue was statistically significant (p<0.01). In the patients with normal ovarian tissue, 6 of 12 (8% + 42% = 50%) patients expressed cytoplasmic YAP in the surface epithelium while 41 of 42 (88% + 10% = 98%) patients with OSC had positive cytoplasmic YAP expression (figure 4 “ovary”). This difference in any detectable cytoplasmic YAP expression between normal ovary and OSC was statistically significant (p<0.01).
Evaluation of YAP Expression in Breast
Nuclear and cytoplasmic YAP expression was assessed in 49 patients with normal breast tissue biopsies and in neoplastic tissues of DCB. Normal breast tissue showed expression of YAP primarily in the cytoplasm of the ductal epithelium and in the cytoplasm and nuclei of myoepithelial cells (figure 2G black arrow head). DCB tissue showed both cytoplasmic and nuclear staining of malignant cells (figure 2H) and staining highlighted myoepithelial cells in cases of ductal carcinoma in situ (figure 2I black arrow heads). In the group with normal breast tissue, 11 of 14 (43%+36% = 79%) patients expressed nuclear YAP in the ductal epithelium compared to 33 of 35 (85%+11% = 94%) patients with DCB tissue (figure 3 “breast”). There was no statistically significant difference in any detectable nuclear YAP expression between the normal breast and DCB tissue (p=0.13). When we compare the difference in nuclear YAP expression across the distribution of intensities between the normal breast and DCB tissues, however, there was a statistically significant difference (p=0.01).
In those patients with normal breast tissue, 14 of 14 (100%) patients expressed cytoplasmic YAP in the ductal epithelium while 34 of 35 (68%+29%=97%) patients with DCB tissue had any detectable cytoplasmic YAP expression (figure 4 “breast”). The cytoplasmic YAP expression difference between the normal breast and DCB tissues was not statistically significant (p=0.29) even when we compared the cytoplasmic YAP expression across the distribution of intensities for normal and DCB tissues.
Discussion
Balancing cell proliferation and apoptosis is essential to proper tissue growth, development, and function. A disruption in this equilibrium can result in excessive tissue loss with subsequent loss of function as in the case of excessive apoptosis (23), or it can result in tumorigenesis if there is inadequate apoptosis coupled to uncontrolled cell proliferation. The Hippo pathway, which has been uncovered with the aid of Drosophila genetic screens, is a potent regulator of tissue homeostasis by controlling cell growth, division, and apoptosis. It is also considered to be a potent developmental pathway as mice deficient in YAP have an embryonic lethal phenotype (24). There is tremendous conservation of this pathway in mammals, such that mammalian homologs can rescue mutant phenotypes in Drosophila. Given that this pathway controls the fundamental process of cell division and death, it is a prime target for dysregulation in cancer.
We explored the expression of YAP, a highly conserved Hippo pathway component within mammalian systems, in four common tumor types with the highest lifetime risk of cancer death among men and women in the United States. In this study, we found YAP expression in the progenitor and reparative compartments of the human colon and lung. There was also significantly enhanced YAP expression and a change in the cellular distribution of YAP in 3 of 4 tissue types examined. Together, these findings suggest enhanced and altered YAP expression might be a common event in the carcinogenesis process of the following three types of tumors: colonic adenocarcinoma, lung adenocarcinoma, and OSC. Additional histological tumor types were not evaluated in this study, so it is unknown whether expression patterns of the YAP gene would be the same as in the histological tumor types we studied.
It is not clear if the expression of YAP directly induces tumorgenesis or contributes to a permissive cellular environment for the development of tumors. The Hippo pathway has been closely linked to cell proliferation. Several groups have correlated proliferation markers, such as Ki-67 to YAP or to other pathway members such as ww45 (9, 16, 22). In our study, hepatomegaly developed upon over expression of YAP in the murine liver (17). Inactivation of YAP results in regression of the liver to normal size, but continued YAP expression results in foci of malignant transformation to hepatocellular carcinoma (17). It is likely that additional genetic events contribute to the development of the malignant foci. There is evidence that implicates YAP in the development of hepatocellular carcinoma (26). The YAP gene is present on chromosome 11q22, a region which has been noted in previous studies to be amplified in several types of cancers (25,26).
It has been suggested that the development of malignancy requires six processes to occur: self-sufficiency in growth signals, insensitivity to antigrowth signals, ability to evade apoptosis, unlimited replicative potential of the tumor cells, ability to sustain angiogenesis, and an ability to invade tissues and metastasize (3). Genomic instability as a result of inactivation of tumor suppressors can enable the six processes of malignancy to occur in premalignant cells (3). With YAP being the nuclear effector of the Hippo tumor suppressor pathway, it is unlikely that YAP is responsible for each of these processes, but YAP may be involved in providing an environment favorable for inhibition of apoptosis and promoting cellular proliferation by increasing the genomic instability of premalignant cells and enabling the six critical processes in the development of malignancy to occur. The potent effects of YAP upon cell growth, division and apoptosis support the notion that YAP normally functions to maintain tissue homeostatis, however, once dysregulated, it can contribute to a malignant cellular phenotype.
The four malignant tissue types we examined demonstrated the presence of both cytoplasmic and nuclear staining suggesting that the changes in the cellular localization of YAP was a significant finding that might hint at the mechanism thought by which YAP might induce malignant transformation. From detailed studies in the fly, nuclear translocation appears to be a critical step in order to activate transcription (17). Our results showing expression of YAP in the nucleus of normal tissue likely represents the normal physiologic function of YAP. We found YAP expression in the nucleus of a few cells in the progenitor regions of the colon, which is in agreement with recent reports of intestinal progenitor cell expansion in YAP transgenic mice (22) (figure 2A). In normal breast tissue, YAP expression was apparent in the myoepithelial cells which are involved in ductal elongation and morphogenesis in mammary gland organization (figure 2G and I) (27). We also saw YAP expression in the type II pneumocytes of the non-neoplastic lung (figure 2C black arrow head). Type II pneumocytes are active in repair and tissue maintenance. The expression of YAP in the cells of normal tissues suggests this pathway may also be active in normal physiologic tissue homeostasis and repair.
We have also identified the expression of cytoplasmic YAP to best correlate with the neoplastic phenotypes we examined as compared to their respective normal tissues. In the colon, lung, and ovary, cytoplasmic YAP expression is significantly different in colonic adenocarcinoma, lung adenocarcinoma, and OSC compared to what is seen in normal tissues. We rarely see cytoplasmic expression of YAP without nuclear expression. This perhaps provides insight into the possible mechanisms that are involved in the alteration and enhancement of YAP activity. Malignant cells might produce excess YAP during genomic amplification that might overwhelm the normal physiologic regulatory systems and result in abnormal cytoplasmic accumulation. This accumulation of YAP within the cytoplasm maintains a constant pool of the protein for nuclear translocation. Another possibility is the stability of the YAP protein might be altered in the neoplastic tissues resulting in ineffective protein turnover and excessive YAP activity.
In conclusion, the outcome of the study suggests that YAP may be a component of an oncogenic pathway that is present in three common histological tumor types. Given the widespread expression of the nuclear effector YAP, it is likely that this pathway is commonly altered in the process of carcinogenesis. Therefore, this pathway could be a potential therapeutic target for the three common malignant tumor types (colonic adenocarcinoma, lung adenocarcinoma, and OSC) which demonstrated higher YAP expression compared to their respective normal tissues. These three histological tumor types included in our study are presently the most frequently occurring malignant tumors of the colon, lung, and ovary. Colonic adenocarcinoma, lung adenocarcinoma, and OSC are common lethal types of malignancies among men and women living in the United States. Further studies would need to be conducted on additional tumor types in order to determine if YAP expression is higher in other colon, lung, and ovarian tumor types not examined in our study.
Table 2.
| Tissue Type | Negative | Low | High |
|---|---|---|---|
| Normal Colon | 9/16 | 5/16 | 2/16 |
| Neoplastic Colon | 6/28 | 9/28 | 13/28 |
| Normal Lung | 14/15 | 1/15 | 0/15 |
| Neoplastic Lung | 13/28 | 5/28 | 10/28 |
| Normal Ovary | 9/12 | 2/12 | 1/12 |
| Neoplastic Ovary | 1/42 | 4/42 | 37/42 |
| Normal Breast | 3/14 | 5/14 | 6/14 |
| Neoplastic Breast | 2/35 | 4/35 | 29/35 |
Table 3.
| Tissue Type | Negative | Low | High |
|---|---|---|---|
| Normal Colon | 10/15 | 4/15 | 1/15 |
| Neoplastic Colon | 4/28 | 10/28 | 14/28 |
| Normal Lung | 14/15 | 1/15 | 0/15 |
| Neoplastic Lung | 4/28 | 9/28 | 15/28 |
| Normal Ovary | 6/12 | 5/12 | 1/12 |
| Neoplastic Ovary | 1/42 | 2/42 | 39/42 |
| Normal Breast | 0/14 | 0/14 | 14/14 |
| Neoplastic Breast | 1/35 | 10/35 | 24/35 |
Acknowledgments
MFG was sponsored by a channel scholarship from El-Minia University Department of Pathology, Egypt. RAA support DK06187. APK grant from Maryland Cigarette Restitution Fund GI Cancer SPORE CA62924.
We would like to thank the following individuals for their contributions in the preparation and provision of the tissue microarrays (TMA): Pedram Argani (breast TMA), Ie Ming Shih (ovarian TMA), Edward Gabrielson (lung TMA), and Robb Wilentz (colon TMA).
Footnotes
Disclosure/Conflict of Interest No conflict of interest to declare.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errorsmaybe discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jemal A, Siegel R, Ward E, Murray T, Xu J, Smigal C, Thun MJ. Cancer Statistics, 2007. CA Cancer J Clin. 2007;57:43–66. doi: 10.3322/canjclin.57.1.43. [DOI] [PubMed] [Google Scholar]
- 2.Jemal A, Ward E, Hao Y, Thun M. Trends in the Leading Causes of Death in the United States, 1970-2002. JAMA. 2005;294:1255–59. doi: 10.1001/jama.294.10.1255. [DOI] [PubMed] [Google Scholar]
- 3.Hanahan D, Weinberg RA. The Hallmarks of Cancer. Cell. 2000;100(1):57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 4.Edgar BA. From cell structure to transcription: Hippo forges a new path. Cell. 2006;124:267–73. doi: 10.1016/j.cell.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 5.Pan D. Hippo signaling in organ size control. Geve Dev. 2007;21(8):886–97. doi: 10.1101/gad.1536007. [DOI] [PubMed] [Google Scholar]
- 6.Justice RW, Zilian O, Woods DF, Noll M, Bryant PJ. The Drosophila tumor suppressor gene, warts, encodes a homolog of human myotonic dystrophy kinase and is required for the control of cell shape and proliferation. Genes Dev. 1995;9(5):534–46. doi: 10.1101/gad.9.5.534. [DOI] [PubMed] [Google Scholar]
- 7.Wu S, Huang J, Dong J, Pan D. Hippo encodes a Ste-20 family protein kinase that restricts cell proliferation and promotes apoptosis in conjunction with salvador and warts. Cell. 2003;114:445–56. doi: 10.1016/s0092-8674(03)00549-x. [DOI] [PubMed] [Google Scholar]
- 8.Lai ZC, Wei X, Shimizu T, Ramos E, Rohrbaugh M, Nikolaidis N, Ho L, Li Y. Control of cell proliferation and apoptosis by mob as tumor suppressor, mats. Cell. 2005;120:675–85. doi: 10.1016/j.cell.2004.12.036. [DOI] [PubMed] [Google Scholar]
- 9.Zhao B, Wei X, Li W, Udan RS, Yang Q, Kim J, Xie J, Ikenoue T, Yu J, Li L, Zheng P, Ye K, Chinnaiyan A, Halder G, Lai Z, Guan K. Inactivation of YAP oncoprotein by the Hippo pathway is involved in cell contact inhibition and tissue growth control. Genes & Dev. 2007;21:2747–61. doi: 10.1101/gad.1602907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Harvey KF, Pfleger CM, Hariharan IK. The Drosophila Mst ortholog, hippo, restricts growth and cell proliferation and promotes apoptosis. Cell. 2003;114:457–67. doi: 10.1016/s0092-8674(03)00557-9. [DOI] [PubMed] [Google Scholar]
- 11.Jia J, Zhang W, Wang B, Trinko R, Jiang J. The Drosophila Ste20 family kinase dMST functions as a tumor suppressor by restricting cell proliferation and promoting apoptosis. Genes Dev. 2003;17(20):2514–19. doi: 10.1101/gad.1134003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pantalacci S, Tapon N, Leopold P. The Salvador partner Hippo promotes apoptosis and cell-cycle exit in Drosophila. Nat Cell Biol. 2003;5:921–27. doi: 10.1038/ncb1051. [DOI] [PubMed] [Google Scholar]
- 13.Udan RS, Kango-Singh M, Nolo R, Tao C, Halder G. Hippo promotes proliferation arrest and apoptosis in the Salvador/Warts pathway. Nat Cell Biol. 2003;5:914–20. doi: 10.1038/ncb1050. [DOI] [PubMed] [Google Scholar]
- 14.Xu T, Wang W, Zhang S, Stewart RA, Yu W. Identifying tumor suppressors in genetic mosaics: the Drosophila lats gene encodes a putative protein kinase. Development. 1995;121(4):1053–63. doi: 10.1242/dev.121.4.1053. [DOI] [PubMed] [Google Scholar]
- 15.Olson Mf. Modeling human cancer: report on the eighth Beatson International Cancer Conference. Cancer Research. 2005;65:11247–50. doi: 10.1158/0008-5472.CAN-05-2713. [DOI] [PubMed] [Google Scholar]
- 16.Lee JH, Kim TS, Yang TH, Koo BK, Oh SP, Lee KP, Oh HJ, Lee SH, Kong YY, Kim JM, Lim DS. A crucial role of ww45 in developing epithelial tissues in the mouse. EMBO J. 2008;27(8):1231–42. doi: 10.1038/emboj.2008.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong J, Feldmann G, Huang J, Wu S, Zhang N, Comerford S, Gayyed M, Anders R, Maitra A, Pan D. Elucidation of a Universal Size Control Mechanism in Drosophila and Mammals. Cell. 2007;130:1120–33. doi: 10.1016/j.cell.2007.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Patel JD, Bach PB, Kris MG. Lung cancer in US women. JAMA. 2004;291(14):1763–68. doi: 10.1001/jama.291.14.1763. [DOI] [PubMed] [Google Scholar]
- 19.Lu D, Masood S, Khalbuss WE, Bul M. A Subset of Breast Invasive Ductal Carcinoma with Distinctive Cytomorphology, Aggressive Clinical Behavior, and Unique Immunologic Profiles. Cancer Cytopath. 2002;96(5):294–300. doi: 10.1002/cncr.10745. [DOI] [PubMed] [Google Scholar]
- 20.Santin AD, Zhan F, Bellone S, Palmieri M, Cane S, Gokden M, Roman JJ, O'Brien TJ, Tian E, Cannon MJ, Shaughnessy J, Jr, Pecorelli S. Discrimination between uterine serous papillary carcinoma and ovarian serous papillary tumours by gene expression profiling. Brit J of Cancer. 2004;90:1814–24. doi: 10.1038/sj.bjc.6601791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brahmania M, Kanthan CS, Kanthan R. Collision tumor of the colon-colonic adenocarcinoma and ovarian granulose cell tumor. World J of Surg Oncol. 2007;5:118. doi: 10.1186/1477-7819-5-118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Camargo FD, Gokhale S, Johnnidis J, Fu D, Bell G, Jaenisch R, Brummelkamp T, et al. YAP1 Increases Organ Size and Expands Undifferentiated Progenitor Cells. Curr Biol. 2007;17:2054–60. doi: 10.1016/j.cub.2007.10.039. [DOI] [PubMed] [Google Scholar]
- 23.Brunner T, Mueller C. Apoptosis in disease: about shortage and excess. Essays Biochem. 2003;39:119–30. doi: 10.1042/bse0390119. [DOI] [PubMed] [Google Scholar]
- 24.Morin-Kensicki EM, Boone BN, Howell M, Stonebraker JR, Teed J, Alb JG, Magnuson TR, O'Neal W, Milgram SL. Defects in yolk sac vasculogenesis, chorioallantoic fusion, and embryonic axis elongation in mice with targeted disruption of Yap65. Mol Cell Biol. 2006;26(1):77–87. doi: 10.1128/MCB.26.1.77-87.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Overholtzer M, Zhang J, Smolen GA, Muir B, Li W, Sgroi DC, Deng C, Brugge JS, Haber DA. Transforming properties of YAP, a candidate oncogene on the chromosome 11q22 amplicon. Proc Natl Acad Sci USA. 2006;103(33):12405–10. doi: 10.1073/pnas.0605579103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Zender L, Spector M, Xue W, Flemming P, Cordon-Cardo C, Silke J, Fan S, Luk J, Wigler M, Hannon G. Identification and validation of oncogenes in liver cancer using an integrative oncogenomic approach. Cell. 2006;125:1253–67. doi: 10.1016/j.cell.2006.05.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gudjonsson T, Adriance M, Sternlicht M, Petersen O, Bissell M. Myoepithelial cells: their origin and function in breast morphogenesis and neoplasia. J of Mam Gland Biol and Neopl. 2005;10(3):261–72. doi: 10.1007/s10911-005-9586-4. [DOI] [PMC free article] [PubMed] [Google Scholar]