Abstract
The purpose of this study was to investigate the expression of platelet-derived growth factor receptor-alpha (PDGFR–α) in the myofibroblasts of corneas with stromal haze. Central corneal sections from rabbit eyes that had -9 diopter PRK were analyzed by immunocytochemistry (IHC) for the expression of PDGFR-α at 4 week after surgery. PDGFR-α was expressed immediately beneath the epithelial basement membrane in the anterior stroma. Double IHC studies revealed the expression of PDGFR-α in the anterior stroma co-localized with alpha-smooth muscle actin (SMA) marker for myofibroblasts. In vitro studies have suggested that PDGF is important in the development and viability of myofibroblasts after corneal injury. Expression of PDGFR-α in myofibroblasts supports these findings.
Keywords: Myofibroblasts, α-SMA (alpha – smooth muscle actin), PDGFR-α, wound healing, photorefractive keratectomy (PRK
INTRODUCTION
Transforming growth factor-beta (TGF β) and platelet-derived growth factor (PDGF) are thought to have important roles in the development and viability of myofibroblasts associated with corneal stromal opacity after injury (Masur, et al., 1996; Jester, et al., 1995, 1999, 2002; Wilson, et al., 1994; Shephard, et al., 2004; Funderburgh, et al., 2001). Studies by Jester and coworkers (2002) suggested that TGF β induces keratocyte proliferation and myofibroblast differentiation through activation of a PDGF autocrine loop. Our recent gene transfer studies in which PDGF effects were blocked in the stroma supported a role for PDGF in myofibroblast generation (Kaur, et al., in press). Surprisingly, however, there have been no studies confirming that corneal α-smooth muscle actin (α-SMA)-positive myofibroblast cells express PDGF receptors in situ. Therefore, this study was performed in a rabbit photorefractive keratectomy haze model to determine whether myofibroblasts express PDGF receptor α.
METHODS
The Animal Control Committee at the Cleveland Clinic Foundation approved the animal studies included in this work. All animals were treated in accordance with the tenets of the ARVO statement for the Use of Animals in Ophthalmic and Vision Research. A total of four 12- to 15 week-old female New Zealand white rabbits weighing 2.5– 3.0 kg each were included in this study. Anesthesia was obtained with intramuscular injection of ketamine hydrochloride (30mg/kg) and xylazine hydrochloride (5mg/kg). In addition, topical 1% proparacaine hydrochloride 1% (Alcon, Ft. Worth, TX, USA) was applied to each eye just prior to surgery. One eye of each rabbit, selected at random, had PRK for correction of 9 diopters of myopia with a 6.5 mm ablation diameter and 106 μm ablation depth using an Apex Summit Laser (Alcon, Fort Worth, TX, USA) to generate myofibroblasts and haze (Mohan, et al., 2003).
Rabbits were euthanized at 4 weeks after surgery by intravenous injection of 100 mg/kg pentobarbital while the animal was under general anesthesia. The corneo-scleral rims of ablated and unablated, contralateral control eyes were removed with 0.12 forceps and sharp Westcott scissors. The corneo-scleral rims were embedded in liquid OCT compound (Sakura Finetek, Torrance, CA, USA) in a 24 mm × 24mm × 5mm mould (Fisher, Pittsburgh, PA, USA), snap frozen on dry ice and stored at −80° C until sectioning was performed. Central corneal sections (7μm thick) were cut with a cryostat (HM 505M, Micron GmbH, Walldorf, Germany) and placed on 25 mm × 75 mm × 1mm microscope slides (Superfrost Plus, Fisher). Sections were maintained at −80° C until staining was performed.
Immunohistochemistry was performed as previously published (Zieske and Wasson, 1993; Netto, et al., 2006). Briefly, goat polyclonal anti-human PDGFR-α antibody (sc-12911, Santa Cruz Biotechnology, Inc CA, USA) was placed on sections (1:100 dilution in phosphate buffered saline [PBS]) and incubated at room temperature for 90 minutes. Sections were washed with PBS and then incubated in NL–577 (a red fluorophore) conjugated donkey anti-goat IgG (R&D Systems, Minneapolis, MN, USA) secondary antibody (diluted 1:100 in PBS) for 1 hour at room temperature. Negative controls were included using secondary antibody alone or control blocking peptide. In the latter experiments, 20X blocking peptide (sc-12911P, Santa Cruz Biotechnology, Inc., CA, USA) was incubated with the primary antibody (p-PDGFR-α (Tyr 754): sc-12911, Santa Cruz Biotechnology, Inc CA, USA) at 4° C for overnight prior to application to tissue sections.
Double immunofluorescent staining was then performed to study the co-expression of PDGFR-α and α–SMA in corneas after PRK. Sections were first stained for PDGFR–α using the goat polyclonal anti human PDGFR-α and NL–577 conjugated donkey anti-goat IgG, as described above. This was followed by staining for α-smooth muscle actin (α-SMA) expression. α-SMA was detected using a monoclonal mouse anti-human smooth muscle actin clone1A4 (Dako, M0851, Carpinteria, CA) at concentration of 1:50 for 90 minutes and Alexa Fluor 488 (Invitrogen, A11001, Carlsbad, California) goat anti-mouse IgG (H+L) conjugated secondary antibody (a green fluorophore), at a dilution of 1:100 in PBS for 60 minutes at room temperature. Coverslips were mounted with Vectashield containing DAPI (Vector Laboratories, Inc., Burlingame, CA, USA) to allow visualization of cell nuclei in the tissue sections. The sections were viewed and photographed with a Nikon Eclipse E 800 microscope equipped with digital SPOT camera (Micro Video Instruments; Avon, MA, USA).
RESULTS
Imunocytochemistry for PDGFR-α alone demonstrated high levels of expression of the receptor in cells immediately beneath the epithelial basement membrane, with much lower staining in keratocyte cells deeper in the stroma or in the epithelium (not shown). There was no detectible expression of PDGFR-α in the epithelium or stroma of unwounded corneas (not shown).
Double IHC for PDGFR-α and α-SMA (Figure) demonstrated that PDGFR-α expression in the stroma of wounded corneas was predominately in α-SMA-positive myofibroblast cells beneath the epithelial basement membrane. Very low and difficult to photograph levels of PDGFR-α staining were noted in the keratocytes of either wounded (Figure) or unwounded corneas. Staining was similar in all four wounded corneas with haze. No staining for PDGFR-α was noted in controls where excess antigen was included with the primary antibody or primary antibody was omitted (not shown).
Figure.
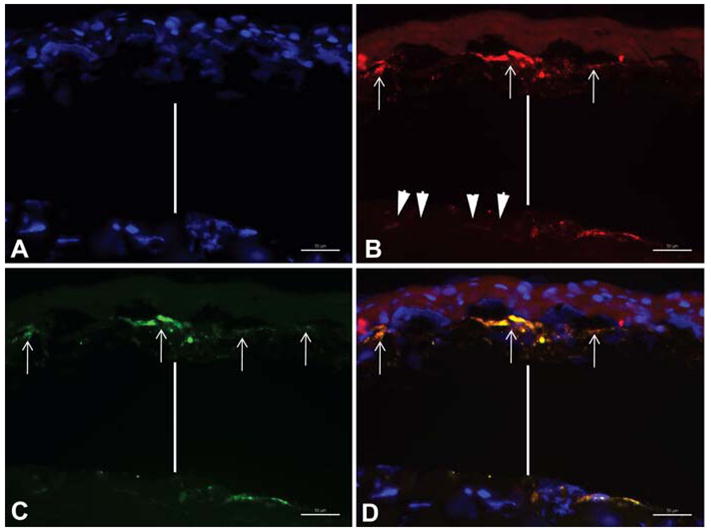
Double immunohistochemistry for PDGF receptor–α and α-smooth muscle actin in rabbit corneas that developed haze after -9D PRK. The vertical bar in each panel represents an artifactual separation beneath disordered extracellular matrix in the sub-epithelial stroma and the deeper stroma that nearly always occurs during sectioning of rabbit corneas with haze. A. Cell nuclei stained blue with DAPI. B. PDGFR–α positive cells beneath the epithelial basement membrane (arrows) stained for PDGFR-α (red). Note that keratocyte cells that are deeper in the stroma (arrowheads) appeared to have very low levels of PDGFR-α. C. SMA-positive cells beneath the epithelial basement membrane (arrows) stained for α-smooth muscle actin (green). D. Overlay of red, blue and green fluorescence shows co-localization of PDGFR–α and α-SMA in cells (arrows) in the sub-epithelial stroma. Mag. 400X.
DISCUSSION
PDGF is a cytokine that functions as a homodimer (AA or BB) or heterodimer (AB) of two related, but distinct, polypeptides (Hart, et al., 1995). PDGF mediates its effects through tyrosine kinase receptors that are also expressed as homodimers or heterodimers (Hart, et al., 1995). Two PDGF receptors, α and β, dimerize after ligand exposure. The α-receptor binds both the A and B chains, whereas the β receptor preferentially binds the B chain (Hart, et al., 1995).
The present study demonstrates that PDGFR-α is expressed predominately in myofiboroblasts in corneas with injuries that predispose to the development of haze. This suggests that PDGF plays an important role in myofibroblast development or function, likely via the TGF β-stimulated PDGF autocrine loop reported by Jester and coworkers (2002). PDGF that modulates myofibroblasts could also be derived from corneal epithelial cells (Kim, Mohan, and Wilson, 1999). The expression of PDGFR-β could not be evaluated in this study since available antibodies to PDGFR-β do not recognize rabbit antigen.
It is unknown when PDGF receptor expression is first noted in myofibroblast precursor cells. Thus, further studies will be needed to determine whether PDGF receptor expression is present in vimentin+, but a-SMA- and desmin-, myofibroblast precursor cells (Chaurasia, et al., in press). Our recent gene transfer studies (Kaur, et al., in press), in which PDGF effects were blocked in the stroma, supported a role for PDGF in myofibroblast generation, but yielded the intriguing result that, although a-smooth muscle actin (α-SMA)-positive myofibroblasts detected by IHC in the stroma were markedly decreased after PDGF blockade, the level of stromal opacity at the slit lamp was not significantly different from controls treated with empty plasmid. One hypothesis to account for this finding is that PDGF functions relatively late in myofibroblast development—after precursor cells have gained the capacity to produce extracellular matrix but before the expression of a-SMA. Further work is needed to characterize this time course and to explore the specific functions regulated by PDGF in myofibroblasts during corneal wound healing.
Acknowledgments
This study was supported by EY10056, EY015638, and Research to Prevent Blindness. Steven E. Wilson is a recipient of the RPB physician-scientist award.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Chaurasia SS, Kaur H, Medeiros FW, Smith SD, Wilson SE. Dynamics of the expression of intermediate filaments vimentin and desmin during myofibroblast differentiation after corneal injury. Exp Eye Res. doi: 10.1016/j.exer.2009.02.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Funderburgh JL, Funderburgh ML, Mann MM, Corpuz L, Roth MR. Proteoglycan expression during transforming growth factor beta induced keratocyte-myofibroblast transdifferentiation. J Biol Chem. 2001;276:44173–44178. doi: 10.1074/jbc.M107596200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hart KC, Galvin BD, Donoghue DJ. Structure and function of the platelet derived growth factor family and their receptors. Genet Eng. 1995;17:181–208. [PubMed] [Google Scholar]
- Jester JV, Huang J, Barry-Lane PA, Kao WW, Petroll WM, Cavanagh HD. Transforming growth factor (beta)- mediated corneal myofibroblast differentiation requires actin and fibronectin assembly. Invest Ophthalmol Vis Sci. 1999;40:1959–1967. [PubMed] [Google Scholar]
- Jester JV, Huang J, Petroll WM, Cavanagh HD. TGF beta induced myofibroblast differentiation of rabbit keratocytes requires synergistic TGF beta, PDGF and integrin signaling. Exp Eye Res. 2002;75:645–657. doi: 10.1006/exer.2002.2066. [DOI] [PubMed] [Google Scholar]
- Jester JV, Petroll WM, Barry PA, Cavanagh HD. Expression of alpha- smooth muscle (alpha SM) actin during corneal stromal wound healing. Invest Ophthalmol Vis Sci. 1995;36:809–819. [PubMed] [Google Scholar]
- Kaur H, Chaurasia’ SS, Medeiros FW, Agrawal A, Salomao MQ, Singh N, Ambati BK, Wilson SE. Corneal stroma PDGF blockade and myofibroblast development. Exp Eye Res. doi: 10.1016/j.exer.2008.12.006. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim WJ, Mohan RR, Wilson SE. Effect of PDGF, IL-1 alpha, and BMP2/4 on corneal fibroblast chemotaxis: expression of the platelet-derived growth factor system in the cornea. Invest Ophthalmol Vis Sci. 1999;40:1364–1372. [PubMed] [Google Scholar]
- Masur SK, Dewal HS, Dinh TT, Erenberg I, Petridou S. Myofibroblasts differentiate from fibroblasts when plated at low density. Proc Natl Acad Sci USA. 1996;93:4219–4223. doi: 10.1073/pnas.93.9.4219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohan RR, Hutcheon AE, Choi R, Hong J, Lee J, Mohan RR, Ambrosio R, Jr, Zeiske JD, Wilson SE. Apoptosis, necrosis, proliferation, and myofibroblast generation in the stroma following LASIK and PRK. Exp Eye Res. 2003;76:71–87. doi: 10.1016/s0014-4835(02)00251-8. [DOI] [PubMed] [Google Scholar]
- Netto MV, Mohan RR, Sinha S, Sharma A, Dupps W, Wilson SE. Stromal haze, myofibroblasts, and surface irregularity after PRK. Exp Eye Res. 2006;82:788–797. doi: 10.1016/j.exer.2005.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shephard P, Martin G, Smola-Hess S, Brunner G, Kreig T, Smola H. Myofibroblast differentiation is induced in keratinocyte-fibroblast co-cultures and is antagonistically regulated by endogenous transforming growth factor-beta and interleukin-1. Am J Pathol. 2004;164:2055–2066. doi: 10.1016/s0002-9440(10)63764-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson SE, Schultz GS, Chegini N, Weng J, He YG. Epidermal growth factor, transforming growth factor alpha, transforming growth factor beta, acidic fibroblast growth factor, basic fibroblast growth factor, and interleukin-1 proteins in the cornea. Exp Eye Res. 1994;59:63–71. doi: 10.1006/exer.1994.1081. [DOI] [PubMed] [Google Scholar]
- Zieske JD, Wasson M. Regional variation in distribution of EGF receptor in developing and adult corneal epithelium. J Cell Sci. 1993;106:145–152. doi: 10.1242/jcs.106.1.145. [DOI] [PubMed] [Google Scholar]


