The 5-year survival rate for children diagnosed with medulloblastoma, the most common form of pediatric central nervous system malignancy, was recently reported as 85% for average-risk patients and 70% for high-risk patients (Gottardo and Gajjar, 2006). The encouraging survival rates can be traced to extensive modifications in the medical treatment, such as risk-adapted strategies and adoption of 3-dimensional conformal radiation methods (Gottardo and Gajjar, 2006, Merchant et al., 2006, Packer et al., 1999). Due to the growing number of pediatric brain tumor survivors, an area of research determined to better understand the long-term outcomes and treatment related late-effects experienced by this population has been put forth. The concept of survival now includes a need to monitor and respond to those patients considered at risk for continued morbidities associated with cancer and its treatment (Oeffinger and Robison, 2007).
This article will first describe the nature of impairment experienced by a large number of survivors of pediatric posterior fossa tumors. Predominant risk factors that have been identified within the literature, including patient age at treatment, time from treatment, surgery, radiation and chemotherapy, will be explored and the related impact on the survivor's school life will be discussed. In the final sections of the paper, risk-based care will be examined. A multi-disciplinary team approach is emphasized.
The nature of impairment
While the survival rate continues to improve and in many cases can be considered favorable (Gottardo and Gajjar, 2006), a large majority of survivors experience significant impairments following treatment (Ness et al., 2008). Declines in overall intellectual ability and problems in academic achievement following treatment plague this group of children and challenge their families (Mabbott et al., 2005, Mulhern et al., 2005). In addition, a large number eventually require some form of special education service (Dennis et al., 1996, Mitby et al., 2003). Although considered broad spectrum abilities, monitoring intellect and academic achievement remains important. Deficits in these areas can produce limitations in education attainment, employment goals, and independent living as the patient ages into adulthood (Maddrey et al., 2005, Moore, 2005, Mostow et al., 1991). To further our understanding of the etiology of these changes additional studies have examined underlying specific cognitive abilities. Thought to be contributing to the decline in intellect and academic achievement is a slowed rate of information processing speed, impaired memory capacity and a decline in the ability to sustain attention (Maddrey et al., 2005, Mulhern and Palmer, 2003, Nagel et al., 2006, Palmer et al., 2007, Reeves et al., 2007, Schatz et al., 2000).
Not all patients experience the same type of challenges. The nature and severity of deficits vary among patients with both patient and treatment factors attributing to eventual outcomes. These factors help to identify who is at higher risk and must be considered when examining late-effects following treatment for pediatric posterior fossa tumors.
Patient Age and Time from Treatment
Among the healthy, general intellect, as measured by an overall quotient score (IQ), is expected to remain stable across time. However, a decline in patient IQ as time from treatment increases is one of the most frequently documented outcomes among those with posterior fossa tumors (Mulhern et al., 1992, Mulhern et al., 1998, Ris et al., 2001). Cross sectional studies demonstrate older age at the time of treatment acts as a protective variable against the severity of decline (Mulhern et al., 1992). More recent longitudinal studies demonstrate that the pattern of decline over time differs depending on age of the patient at the time of treatment (Palmer et al., 2003, Ris et al., 2001). The intellectual ability of 50 patients was prospectively monitored over a 7 year period resulting in the analysis of 188 psychological evaluations (Palmer et al., 2003). For patients who were older at the time of treatment (M = 11.05 years) the model predicts baseline intellect to remain unchanged until approximately 2 years post-diagnosis when a slight decline begins. At approximately 4 years post-diagnosis this rate of decline significantly increases. The younger patient however (M = 5.86 years) experiences an immediate decline that does not plateau until several years post treatment. It has also been shown that the decline in intellect is due to a failure to learn and acquire new information at an expected rate rather than the loss of previously acquired information (Palmer et al., 2001).
Intelligence within the healthy population is strongly supported by normal age related development of working memory and processing speed (Fry and Hale, 2000). This association was also demonstrated among cancer survivors where it was reported that those with weaker working memory ability and slower processing speed exhibited lower intellect (Schatz et al., 2000). Rather than seeing the expected age-related development of verbal memory ability, those with posterior fossa tumors exhibit declines over time (Copeland et al., 1999, Mulhern et al., 2001) with difficulties in both retrieval and recognition of verbal information (Nagel et al., 2006).
Age-related challenges are also reflected within the academic domains of reading, spelling and math among those treated for posterior fossa tumors. Older age at diagnosis was associated with better reading and higher ratings of school performance by parents of survivors (Mabbott et al., 2005), while younger age at diagnosis was predictive of poorer reading ability among survivors (Mabbott et al., 2005, Mulhern et al., 2005).
Surgery
Extent of tumor removal has been considered when determining a patient's risk classification and assigning risk-based treatment options for patients with posterior fossa tumors (Gajjar et al., 2006). Since residual tumor leads to the determination of high-risk disease and a need for higher radiation treatment doses, complete surgical resection of the tumor is desired whenever possible. The impact of the surgery can include damage to the proximal structures such as the cerebellum and has been related to eventual cognitive outcome.
The influence of surgical resection on cognitive outcome was studied by following 24 patients with posterior fossa tumors treated with surgery alone (Steinlin et al., 2003). While it was found that post-operatively patients exhibited normal verbal, performance and general intelligence, over half showed significant performance deficits for attention, memory, processing speed and visual-constructive ability. Damage to the vermis area of the cerebellum was related to more severe performance deficits. The authors concluded the results highlight the importance of considering the role of the cerebellum in cognitive development and how surgery may interrupt that development.
A recent review outlined evidence within the literature to suggest a relationship between abnormalities of the cerebellum and cognitive processes among children and adolescents (Steinlin, 2007). In addition to studies of patients with pediatric posterior fossa tumors, studies involving children with a variety of conditions including Dandy Walker syndrome, Fragile X syndrome, Down's syndrome, Williams syndrome, dyslexia, attention deficit disorder, brain trauma, and leukemia were also included. However, an accompanying review highlights the need to consider discrepancies in methodology, such as motor components of measures and co-morbid conditions such as increased intracranial pressure, when interpreting results supporting the role of the cerebellum in cognition (Frank et al., 2007). A call was made for more controlled lesions studies that eliminate confounding variables in order to determine what specific functions are supported by the cerebellum, how, and to what extent (Frank et al., 2007).
A thorough study of cognitive function among a group of child and adolescent survivors of cerebellar astrocytoma was recently completed (Vaquero et al., 2008). Among the outcomes measured were planning ability, conceptual capacity, semantic verbal fluency, memory, selective attention, response inhibition, and visual-spatial organization. The astrocytoma group, treated with surgery alone, showed deficits in short term memory, semantic verbal fluency, and selective attention. The level of impairment was greater for those patients with tumor, and therefore surgery, involving the vermis area of the cerebellum. The authors conclude that surgery clearly generated an impact on executive function and that perhaps these areas of function are further compromised by additional treatments such as radiation and chemotherapy.
Radiation
Of the modalities used in the treatment of posterior fossa tumors, the impact of receiving radiation has been most studied. An early review of studies examining the neuropsychological status of patients showed receiving irradiation had an adverse effect on intellectual development (Mulhern et al., 1992). Those who received radiation therapy had IQ levels 12-14 points lower than those who had not received irradiation. Six years later the impact of two levels of radiation dose exposure (36 Gy versus 23.4 Gy) was studied in combination with the age of the patient: younger versus older, split at median age at diagnosis = 8.85 years (Mulhern et al., 1998). Four groups of long term survivors were formed for purpose of analyses: Young/36 Gy, Young/23.4 Gy, Older/36 Gy, Older 23.4 Gy. Tests of global intellect, non-verbal intellect, reading, math and attention were completed by all patients. Demonstrating that neuropsychological outcome was both age and dose dependent, group outcomes for all measures were ordered from lowest to highest as follows: Young/36 Gy < Young/23.4 Gy < Older/36 Gy < Older 23.4 Gy.
An interaction between age of the patient and radiation dose exposure was also shown to be significant in a large longitudinal study of 111 patients prospectively followed over a 7 year period (Mulhern et al., 2005). Three areas of academic achievement were studied including reading decoding, spelling and math. Those who were treated as high risk, therefore receiving greater doses of radiation, and those who were younger, experienced greater declines over time in intellect and academic abilities (Mulhern et al., 2005).
The relationships between two levels of cranial radiation (25 Gy and 35 Gy) and several cognitive outcomes among a group of adolescents surviving standard risk medulloblastoma were examined (Kieffer-Renaux et al., 2000). Those who received standard dose radiation treatment (35 GY) showed significantly lower verbal IQ than those who had received reduced dose (25 Gy). Similar relationships were found for verbal fluency, and comprehension of written words. While both groups showed decreased processing speed and impaired verbal memory, those who had received the higher standard dose irradiation had greater impairments.
Cognitive outcome has also been found to differ significantly across groups when comparing patients that received varying types of treatment for posterior fossa tumors. Those who had received surgery and cranial radiation were compared to those who had received surgery alone, and to those who had non-CNS tumors (Mabbott et al., 2008). No significant differences between groups for working memory and sustained attention were found, with each group performing within normal range. However, there was a significant difference between groups for information processing speed. Those who had received radiation as part of their treatment showed significantly slower information processing speed than those who received surgery alone or were treated for non-CNS tumors.
Chemotherapy
For those diagnosed with posterior fossa tumors such as medulloblastoma or PNET, treatment with surgery, radiation and adjuvant chemotherapy is a familiar approach producing favorable outcomes (Gajjar et al., 2006, Gottardo and Gajjar, 2006, Packer et al., 1999, Thomas et al., 2000). This is not always the approach however for patients diagnosed with posterior fossa tumors when under three years of age, or for those within European protocols. Studies exist among this group of patients where treatment includes surgery and chemotherapy, thereby delaying the need for radiation therapy. Unfortunately, evaluation of cognitive outcome within these studies is uncommon. One exception is an examination of 43 patients who were <3 years of age when diagnosed with classic or desmoplastic medulloblastoma and treated with surgery followed by three cycles of chemotherapy (Rutkowski et al., 2005). Fourteen of these patients completed neuropsychological evaluation an average of 4.8 years after diagnosis. The general intelligence and visual-motor integration of this group of patients was significantly lower than healthy controls of the same age. Patients receiving chemotherapy were compared to an earlier cohort of patients that had also received radiation following surgery. The group receiving chemotherapy showed higher general intelligence.
A recent study reported follow-up data for 108 patients involved in a European trial who received either cranial spinal irradiation alone or chemotherapy prior to cranial spinal irradiation as treatment for standard-risk medulloblastoma. Patients were diagnosed between 3 and 15 years of age and were contacted for follow-up an average of 7.2 years following diagnosis (Bull et al., 2007). Depending on the age of the participant, they were asked to complete, or have their parent complete, a variety of questionnaires regarding quality of survival including health status, behavior and quality of life. Those who had received chemotherapy in addition to radiation treatment reported significantly poorer health status, greater physical restrictions and increased need for therapeutic services. While providing a thought provoking look at the additive impact of chemotherapy, caution in the interpretation of these results has been noted (Packer, 2008). Patients were not always randomized to the treatment arms. In specific cases physicians and parents were able to choose which arm of the study their patient was assigned. When looking at the results among only those who were randomized (n=50), no significant differences in quality of life measures were evident. In addition, no direct assessment of cognitive function was completed. However, those who had received chemotherapy plus radiation reported an increased need for educational support when compared to those who received radiation therapy alone.
Impact on School Life
Synthesis of the research identifies a particular risk and challenge for adolescent and young adult survivors. Depending on the survivor's cancer history and treatment, the cognitive risks and the need for support will differ. Examining utilization of special education services among a large cohort of pediatric cancer survivors (n=11,425), those who had been treated for central nervous system malignancies (n=1637) exhibited the greatest rates of need (Mitby et al., 2003). Analyzed by age at diagnosis, over 70% of those diagnosed between 0 and 5 years of age reported requiring special education services, over 55% for those diagnosed between 6 and 10 years of age, over 32% for those diagnosed between 11 and 15 years of age, and over 23% for those diagnosed between 16 and 20 years of age. The length of required services also increased for those who were diagnosed at earlier ages and who had received higher levels of radiation dosage (Mitby et al., 2003).
For the patient who was older at the time of treatment cognitive difficulties may not seem particularly evident to caregivers until a few years after treatment, a time when many will be attending high-school. High school curriculum brings increasing academic demands. Coupled with a decline in the ability to learn at a rate comparable to healthy peers, academic performance may drop sharply. Intervention in the form of special education services, individual education plans, and educating teachers regarding challenges the survivor faces may be necessary to ensure academic success.
For the patient who was younger at the time of treatment, challenges in school may be immediately evident upon returning to regular classroom activities in the elementary years and these challenges may continue to negatively impact academic achievement throughout their middle school years and beyond. By the time the patient reaches adolescence, special services or individual education plans may have already been needed and should be reassessed and transferred to the high school environment. These supports may be even more critical for those who were treated as a high risk patient, receiving higher doses of irradiation than their standard risk counterparts.
Academic failure has been described as secondary to changes in the more core abilities of processing speed, memory and attention (Mulhern and Palmer, 2003), significant processes by which new learning occurs (Dennis et al., 1998). However, difficulties with these core abilities can be subtle in their presentation. For the adolescent cancer survivor, these early but relatively subtle changes in behavior could be interpreted by teachers as fatigue, lack of motivation, inattention, or even disinterest, and should not be dismissed. Rather than overt behavior such as hyperactivity or impulsivity, cancer survivors are often described as having a “sluggish cognitive tempo” similar to what is known as predominantly inattentive subtype of ADHD (Reeves et al., 2007). Neurocognitive functions of otherwise healthy children diagnosed with ADHD demonstrated that those diagnosed with predominately inattentive subtype showed slower processing speed than children without ADHD (Solanto et al., 2007). Among a similar group of children, processing speed deficits have been related to comorbid reading disabilities (Shanahan et al., 2006).
Difficulty with cognitive function has also been associated with behavioral and social outcomes among adolescent survivors. The term “executive functioning” is used to describe several related cognitive functions such as selective- and sustained-attention, working memory, and organization (Mulhern and Palmer, 2003, Polderman et al., 2007). A study of a large group of pediatric cancer survivors (N=7147) revealed that adolescent and young adult survivors with executive function limitations were less likely to graduate from high school, be employed, have household incomes greater than $20,000, be married or report living as married. It was these conditions that were also linked to reports of poor emotional health, which in turn were also associated with reports of poor health related quality of life (Ness et al., 2008). Adolescent survivors of CNS tumors have also been identified as at risk for symptoms of depression, anxiety, and diminished social competence (Mabbott et al., 2005, Schultz et al., 2007) making an already exigent time in the social lives of adolescents even more challenging.
Risk-based Management Model
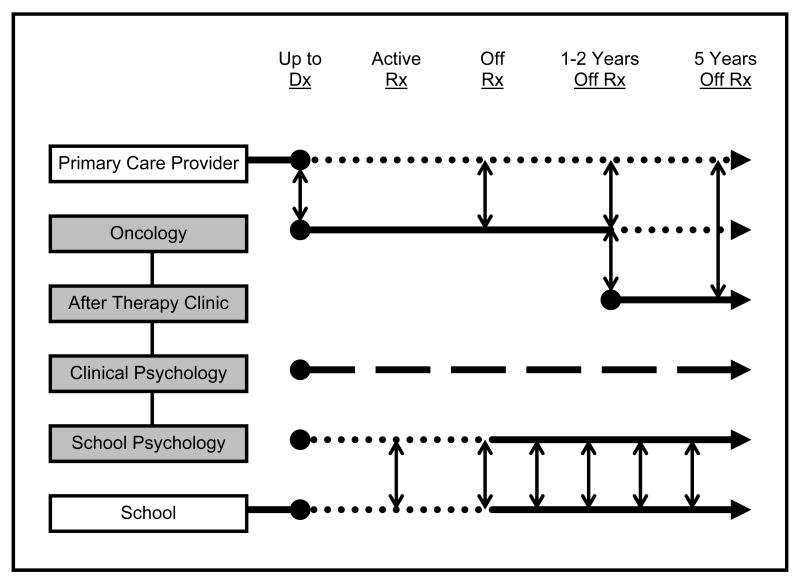
It is clear that adolescent and young adults who are survivors of pediatric posterior fossa tumors experience a number of challenges in a wide variety of functional areas (Bull et al., 2007, Maddrey et al., 2005, Ness et al., 2008, Schultz et al., 2007). Disease and treatment have been found to impact neurocognitive function, academic performance, emotional and social behavior well into adulthood. Without regular and rigorous follow-up, early changes, that can be subtle in nature but amenable with early diagnosis and treatment, may go undetected (Oeffinger and Robison, 2007). Several models of survivorship care have been proposed in the literature (Children's Oncology Group, 2006, Oeffinger and McCabe, 2006). Expanding on the last model put forth by Oeffinger and McCabe (2006), the present model attempts to describe the roles of psychology professionals in more detail as they relate to caring for survivors of a posterior fossa tumor within the cancer treatment center environment (Figure 1).
Figure 1.
Multi-disciplinary approach to long-term risk-based care for survivors of pediatric posterior fossa tumors. Shaded boxes represent primary oncology team personnel. School – patient's home community education providers. Solid lines represent primary role during designated phase while dotted lines represent supportive role. Dashed line (clinical psychology) represents consistent role as primary psychological evaluator, with results communicated to the rest of the team on regular basis.
Up to the point of tumor diagnosis the patient is usually monitored by a primary care physician in their home community. Upon diagnosis of a posterior fossa tumor the role of principal care provider is assumed by the oncologist, communicating patient progress to the PCP as treatment progresses (vertical arrows; Figure 1). Within a cancer center environment, the oncologist leads several professionals constituting a multi-disciplinary care team, including but certainly not limited to clinical psychologists and school psychologists, each with a complimentary but specialty focus contributing to the patient's overall treatment and well being (shaded boxes; Figure 1). This team meets regularly to share information to develop and act upon team recommendations. Outside a cancer center, this type of communication, important for the continuity of patient care, may be less formal and depend on the individual care providers to initiate.
Prospective Evaluation
Recommendations and guidelines for monitoring pediatric cancer survivors have been developed (Children's Oncology Group, 2006, Landier et al., 2004, Skinner et al., 2005). These guidelines make it clear that prospective evaluation must be completed based on known or suspected risk. Clinical psychology fulfills this important need with their expertise in assessment of cognitive function.
In the proposed model (Figure 1) clinical psychology first conducts psychological evaluation as soon as the patient is physically and cognitively able to do so. In many cases this early assessment serves as a benchmark to compare future performance. Therefore, it is important for the assessment battery to be risk-based; evaluating areas of ability known to be vulnerable to decline following treatment. The assessment should include evaluation of both broad spectrum abilities, such as general intelligence and areas of academic performance, as well as the core cognitive skills, including at minimum information processing speed, attention and memory processes. With favorable survivorship rates, the patient may be assessed by clinical psychology well into adulthood. Assessment instruments that are suitable for a wide age range, without compromising quality, are therefore favorable. This eliminates problems with changing test versions as the patient ages and improves the accuracy of longitudinal monitoring.
Continued and regular prospective evaluation following end of therapy is also critical to the overall quality of survival. While patient questionnaires and surveys have provided important insight into the survivorship experience, it is also important to directly test and examine. Rather than relying solely on patient self-report of performance, research has indicated that cognitive function should also be objectively evaluated. In a study of pediatric cancer survivors 10 years following treatment, self-reports of quality of life and self-perception of cognitive abilities were compared to actual testing results (Maddrey et al., 2005). Interestingly, both survivors and caregivers overestimated the survivor's neurocognitive abilities. Actual test scores were significantly lower on measures of overall cognitive ability, attention, memory and problem solving. However, perceived academic function did not significantly differ from test scores obtained, with both self-report and direct testing indicating low-average ability. This is most likely due to the fact that the survivor and their caregivers receive feedback on academic function in the form of report cards, grades, or assignment marks, improving the accuracy of their self-report. Therefore in the proposed model, patients return to see clinical psychology for regular assessment of cognitive function. Results are recurrently shared with the team and recommendations, areas of concern, and description of strengths communicated.
Depending on patient needs, the setting and team, clinical psychology can also include the provision of family based services in the form of crisis intervention, support groups, individual therapy, and referral to psychiatric service.
Academic Support
In the proposed model, professionals within school psychology offer an expertise that combines a number of fields to offer valuable service to the cancer center, the patient's community school, as well as the family. Ideally this person or persons would be established within the cancer center and become specialized in working with those treated for pediatric cancer. However this is not always possible and often clinical psychologists fulfill the roles of both cognitive evaluator and those described below as school psychology.
School psychology supports the transformation of the clinical evaluation into action within the patient's community school system. Using their understanding of psychological evaluation, as well as the processes, laws and rights of the patient to obtain the support needed, school psychology can bring a heightened level of understanding, insight, and accomplishment that may not otherwise be achieved once the patient leaves the treatment center to return to their own communities.
Shortly after diagnosis, school psychology makes contact with the family to obtain information regarding the patient's education history, setting, curriculum and any pre-existing academic concerns. As the liaison between the patient's family, school and cancer treatment team, school psychology is the primary contact for issues related to cognitive function and academic performance. During the active treatment phase, if the patient is medically able, school psychology will assist the family in initiating homebound educational services through their local school system.
Initially fulfilling an important supportive role (dotted line; Figure 1), school psychology assumes a more prominent role when the patient is off treatment (solid line; Figure 1). Survivors of pediatric cancer comprise a growing but still small percentage of overall population. Therefore many professionals, including teachers and other education specialists, may not be aware of the risks survivors face. In the proposed model, school psychology becomes a patient advocate regarding education and academic issues. School psychology makes a critical impact by discussing a written summary of the patient's specific diagnosis, dates of treatment, type of treatment received, and a description of the evidence-based treatment-related risks the survivor faces. These discussions can take place with both the family and the patient's education providers. By informing those who are involved, school psychology improves the ability to recognize risks, facilitate proactive health care and prospectively monitor the patient.
Integrating information obtained from multiple sources, including concerns raised by teachers, family, and results from direct evaluation by clinical psychology, school psychology also initiates Individualized Education Plans or 504 Plans as necessary (in the United States as per IDEIA 2004 and the Rehabilitation Act of 1973, respectively). By documenting, quantifying, and discussing the health of the pediatric cancer survivor with their teachers, school psychology plays a critical role in improving the academic success of the patient and completing our understanding of the long-term effects as the patient ages. Communication between school psychology and the patient's home community school continues regularly (vertical arrows, Figure 1). Constant re-evaluation of accommodations and security of support services, including necessary interventions that may benefit the academic progress of the survivor, is critical.
Intervention
While some researchers have begun to learn from methods used to intervene with other populations with similar deficits, much remains to be accomplished. Interventions found beneficial for children with attention deficit, reading disabilities, and working memory impairments offer direction. Intervention programs can include pharmacotherapy, cognitive therapy, experimental interventions designed relative to specific deficits or the utilization of programs already commercially available (Palmer et al., 2007). Utilizing an accommodation approach, intervention can also take the form of improvements to the survivor's instructional environment at home or school. Interventions can be implemented by those within school psychology, clinical psychology, or other specialties.
Attention deficit hyperactivity disorder (ADHD) is the most commonly diagnosed behavioral disorder of childhood (National Institute of Health, 1998) with stimulant medications such as methylphenidate (MPH) being widely prescribed as pharmacological treatment. Survivors of malignant posterior fossa tumors exhibit deficits in attention similar to those children diagnosed with ADHD inattentive subtype. Therefore, it is believed that treatment with MPH may be beneficial to the cognitive rehabilitation among survivors (Butler and Mulhern, 2005). In 2001, a study was published that compared immediate cognitive function of 15 pediatric cancer survivors randomized to receive MPH to 17 children randomized to placebo (Thompson et al., 2001). The patients who had received MPH showed significantly greater improvements on a sustained attention task. Expanding on this study the same group then conducted a 3 week double-blind cross-over study with 83 pediatric cancer survivors (Mulhern et al., 2004). Both parents and teachers rated the child as having better attention skills while the child was receiving MPH. In 2007 a follow-up study was published on the acute effectiveness of MPH among 122 pediatric cancer survivors using several neurocognitive measures (Conklin et al., 2007). There was evidence to suggest benefits for a selective attention task but other tasks failed to show significant improvements following MPH. A scholarly literature review stated that while preliminary support for the efficacy and safety of the stimulants is promising, more research is needed concerning the long-term effects of the stimulants among cancer survivors (Daly and Brown, 2007).
While pharmacological intervention offers one alternative, there is a large proportion of caregivers who desire a non-pharmacological approach. The study of MPH with pediatric cancer survivors identified 192 eligible participants (Conklin et al., 2007). However, the parents of over 34% (n=66) of those eligible declined participation citing concern for placing their child on a stimulant medication among the primary reasons for refusal. Researchers must respond by developing and testing non-pharmacological interventions, such as cognitive behavioral methods, aimed at reducing the treatment related late-effects. One such effort was put forth with the development of a cognitive remediation program combining methods used by three disciplines: brain injury rehabilitation, special education and clinical psychology (Butler, 1998, Butler and Copeland, 2002). The program consisted of approximately 50 hours of instruction over a period of 6 months. Those who received the remediation program demonstrated significant improvement on tests of attention, short-term memory and sentence memory, while those who did not receive the program showed no significant improvement over the same time period. Neither group showed improvement on math achievement. The authors recognize that the program was time consuming and costly to provide but feel the potential benefits outweigh these expenditures, and continue to investigate methods to intervene.
Since pediatric cancer survivors have been found to experience particular declines in reading ability, interventions that have been developed specifically for those diagnosed with reading disabilities warrant exploration. Reading disabilities are the most common and most studied of the academic deficits with research showing unremitting persistence of difficulties unless intervention is obtained (Shaywitz et al., 2008). Children do not simply grow out of reading difficulties. While children with and without reading difficulties will both improve in their ability over time, a gap between the two groups remains (Shaywitz et al., 2008). It is believed that a weakness in phonological awareness, the ability to recognize and manipulate phonemes within language, is the basis of many reading disabilities. Programs that focus on phonological awareness, phonics and meaning of text have demonstrated significant improvement in core reading skills, even among the weakest readers (see Fletcher et al., 2007 for review). One such intervention emphasizing phonological awareness has also shown functional changes in an fMRI study among children diagnosed with dyslexia when activity patterns within reading-related areas of the brain increased to levels comparable to non-impaired control subjects (Temple et al., 2003). As phonological awareness improves, the idea is that word reading becomes more automatic requiring less effortful attention to the act of reading. Therefore more focus can be given to the meaning of the text. However, direct transfer of improved phonological awareness to gains in reading fluency and comprehension is not always automatic (National Reading Panel, 2000). Studies with adolescents have shown that the rate at which they read may be the most useful clinical marker to differentiate poor from average readers. Using untimed tests of reading may not identify those in need for support, where tests emphasizing the rate of reading would provide improved sensitivity (Shaywitz et al., 2008).
At the middle and high school level reading fluency becomes more important given the large amounts of written information the curriculum requires. Relying on contextual clues to derive meaning will not prove to be an efficient strategy. Students with phonological weakness will get delayed in their struggle with the individual words such that comprehending the whole passage becomes very difficult. Utilizing books on tape could aid in their ability to acquire the required information, reduce reading demand, and improve comprehension. Testing could also be done with a teacher presenting questions orally while allowing the survivor to provide answers in the same format. Gains in oral reading fluency have also been seen using a parent tutoring intervention program (Gortmaker et al., 2007).
Working memory ability is critical for learning and deficits may lead to eventual failure in the classroom (Gathercole and Alloway, 2006). Middle and high school frequently require the student to take notes from lectures. This skill may be particularly problematic for the survivor with working memory deficits as they may not be able to keep the information in memory long enough to organize and record accurately. This is also true for material that will need to be copied from the board or is presented on PowerPoint slides. To improve retention of material, teachers could provide outlines of the content that is to be covered during the lecture or provide copies of the slides for the student to take home. A partner student could provide notes or lectures may be tape recorded. Mathematical problems that have sequential steps, such as long division, algebra and chemistry equations may also pose a great deal of difficulty. Written directions broken down into brief and simple steps could be used for easy referral during class time and while working on assignments independently. The number of equations or volume of work could also be reduced. Assignments or tests requiring the survivor to write an essay, paragraph or short answer may also seem complicated as problems with executive function would impair ability to organize information, with timed situations adding to the already high demand. It is imperative for the survivor's success that they be given extended time for assignments and tests. Multiple choice formats could be used for tests rather than requiring the survivor to write out their answers. For creative writing assignments the instructions may have to be broken down into smaller more manageable steps such as create topic, list 3 main points, outline content, and summarize. This helps the survivor feel less overwhelmed by the task and provides focus to their efforts.
Survivors that are experiencing deficits in processing speed require additional accommodation. Their homework could easily take them 2-3× longer than it takes other students. Coupled with the motivation to do well, they may spend all their evening and weekend time working on homework or class projects. These students can become discouraged by this constant high amount of work to maintain a certain grade or grade point average. At some point they will become frustrated and overwhelmed by the volume of work and loose enthusiasm. Use of a computer for word processing instead of writing by hand may be especially useful in completing assignments and tests in a timely manner and giving only every second question as homework could help to reduce volume. Curriculum demands need to be reduced and communication between the caregivers and teachers remains regular and open. Through emails and conferences, parents and teachers could monitor classroom performance as well as homework loads and adjust demands accordingly.
Future Directions
A wait-to-fail approach is not considered desirable within the risk-based management model proposed. Proactive, long-term care, based on known risks of cognitive decline is critical to the success of the patient and quality of survivorship. Active examination and implementation of prophylactic intervention, with emphasis on prevention of negative outcomes, is critical to improving the lives of posterior fossa tumor survivors. Interventions could be initiated during or immediately following treatment with the aim to strengthen those skills and abilities known to be vulnerable to decline following treatment (Moore, 2005). Unfortunately, the development of empirically validated interventions for cancer survivors has not been broadly carried out and no prophylactic intervention has yet to be validated.
There is an already growing population of adolescent and young adult survivors of pediatric posterior fossa tumors. A large number of survivors are experiencing cognitive difficulties leading to academic failure, behavioral challenges and social difficulties. These survivors need efficacious interventions to help strengthen their weaknesses and highlight their strengths.
The model proposed should be considered dynamic and a work in progress. Modifications are needed for those providing follow-up care in environments other than a cancer treatment center. As interventions are tested and better data become available, the proposed model can be refined and edited to specifically include the timing of intervention application. Additional team members, with their specific roles described, may also enhance the proposed model. A multi-disciplinary team approach, each bringing expertise and insight from a specialized field, is essential to the success of the patient and their optimal quality of survivorship.
Acknowledgments
This research is supported by the American Lebanese Syrian Associated Charities (ALSAC) and Cancer Center Support (CORE) grant P30CA21765.
References
- Bull KS, Spoudeas HA, Yadegarfar G, Kennedy CR. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: a follow-up study in PNET 3 trial survivors on behalf of the CCLG (formerly UKCCSG) J Clin Oncol. 2007;25(27):4239–4245. doi: 10.1200/JCO.2006.08.7684. [DOI] [PubMed] [Google Scholar]
- Butler RW. Attentional processes and their remediation in childhood cancer. Med Pediatr Oncol. 1998 1:75–78. doi: 10.1002/(sici)1096-911x(1998)30:1+<75::aid-mpo11>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- Butler RW, Copeland DR. Attentional processes and their remediation in children treated for cancer: a literature review and the development of a therapeutic approach. J Int Neuropsychol Soc. 2002;8(1):115–124. [PubMed] [Google Scholar]
- Butler RW, Mulhern RK. Neurocognitive interventions for children and adolescents surviving cancer. J Pediatr Psychol. 2005;30(1):65–78. doi: 10.1093/jpepsy/jsi017. [DOI] [PubMed] [Google Scholar]
- Children's Oncology Group. Long-term follow-up guidelines for survivors of childhood, adolescent, and young adult cancers 2006 [Google Scholar]
- Conklin HM, Khan RB, Reddick WE, Helton S, Brown R, Howard SC, Bonner M, Christensen R, Wu S, Xiong X, Mulhern RK. Acute neurocognitive response to methylphenidate among survivors of childhood cancer: a randomized, double-blind, cross-over trial. J Pediatr Psychol. 2007;32(9):1127–1139. doi: 10.1093/jpepsy/jsm045. [DOI] [PubMed] [Google Scholar]
- Copeland DR, deMoor C, Moore BD, 3rd, Ater JL. Neurocognitive development of children after a cerebellar tumor in infancy: A longitudinal study. J Clin Oncol. 1999;17(11):3476–3486. doi: 10.1200/JCO.1999.17.11.3476. [DOI] [PubMed] [Google Scholar]
- Daly BP, Brown RT. Scholarly literature review: management of neurocognitive late effects with stimulant medication. J Pediatr Psychol. 2007;32(9):1111–1126. doi: 10.1093/jpepsy/jsm012. [DOI] [PubMed] [Google Scholar]
- Dennis M, Hetherington CR, Spiegler BJ. Memory and attention after childhood brain tumors. Med Pediatr Oncol. 1998 1:25–33. doi: 10.1002/(sici)1096-911x(1998)30:1+<25::aid-mpo4>3.0.co;2-a. [DOI] [PubMed] [Google Scholar]
- Dennis M, Spiegler BJ, Hetherington CR, Greenberg ML. Neuropsychological sequelae of the treatment of children with medulloblastoma. J Neurooncol. 1996;29(1):91–101. doi: 10.1007/BF00165522. [DOI] [PubMed] [Google Scholar]
- Fletcher JM, Lyon GR, Fuchs L, Barnes M. Learning disabilities: From identification to intervention. Guildford; New York: 2007. [Google Scholar]
- Frank B, Schoch B, Richter S, Frings M, Karnath H, Timmann D. Cerebellar lesion studies of cognitive function in children and adolescents - limitations and negative findings. Cerebellum. 2007;6(3):242–253. doi: 10.1080/14734220701297432. [DOI] [PubMed] [Google Scholar]
- Fry AF, Hale S. Relationships among processing speed, working memory, and fluid intelligence in children. Biol Psychol. 2000;54(13):1–34. doi: 10.1016/s0301-0511(00)00051-x. [DOI] [PubMed] [Google Scholar]
- Gajjar A, Chintagumpala M, Ashley D, Kellie S, Kun LE, Merchant TE, Woo S, Wheeler G, Ahern V, Krasin MJ, Fouladi M, Broniscer A, Krance R, Hale GA, Stewart CF, Dauser R, Sanford RA, Fuller C, Lau C, Boyett JM, Wallace D, Gilbertson RJ. Risk-adapted craniospinal radiotherapy followed by high-dose chemotherapy and stem-cell rescue in children with newly diagnosed medulloblastoma (St Jude Medulloblastoma-96): long-term results from a prospective, multicentre trial. Lancet Oncol. 2006;7(10):813–820. doi: 10.1016/S1470-2045(06)70867-1. [DOI] [PubMed] [Google Scholar]
- Gathercole SE, Alloway TP. Practitioner review: short-term and working memory impairments in neurodevelopmental disorders: diagnosis and remedial support. J Child Psychol Psychiatry. 2006;47(1):4–15. doi: 10.1111/j.1469-7610.2005.01446.x. [DOI] [PubMed] [Google Scholar]
- Gortmaker VJ, Daly EJ, 3rd, McCurdy M, Persampieri MJ, Hergenrader M. Improving reading outcomes for children with learning disabilities: using brief experimental analysis to develop parent-tutoring interventions. J Appl Behav Anal. 2007;40(2):203–221. doi: 10.1901/jaba.2007.105-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottardo NG, Gajjar A. Current Therapy for Medulloblastoma. Curr Treat Options Neurol. 2006;8(4):319–334. doi: 10.1007/s11940-006-0022-x. [DOI] [PubMed] [Google Scholar]
- Kieffer-Renaux V, Bulteau C, Grill J, Kalifa C, Viguier D, Jambaque I. Patterns of neuropsychological deficits in children with medulloblastoma according to craniospatial irradiation doses. Developmental Medicine and Child Neurology. 2000;42:741–745. doi: 10.1017/s0012162200001377. [DOI] [PubMed] [Google Scholar]
- Landier W, Bhatia S, Eshelman DA, Forte KJ, Sweeney T, Hester AL, Darling J, Armstrong FD, Blatt J, Constine LS, Freeman CR, Friedman DL, Green DM, Marina N, Meadows AT, Neglia JP, Oeffinger KC, Robison LL, Ruccione KS, Sklar CA, Hudson MM. Development of risk-based guidelines for pediatric cancer survivors: the Children's Oncology Group Long-Term Follow-Up Guidelines from the Children's Oncology Group Late Effects Committee and Nursing Discipline. J Clin Oncol. 2004;22(24):4979–4990. doi: 10.1200/JCO.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Penkman L, Witol A, Strother D, Bouffet E. Core neurocognitive functions in children treated for posterior fossa tumors. Neuropsychology. 2008;22(2):159–168. doi: 10.1037/0894-4105.22.2.159. [DOI] [PubMed] [Google Scholar]
- Mabbott DJ, Spiegler BJ, Greenberg ML, Rutka JT, Hyder DJ, Bouffet E. Serial evaluation of academic and behavioral outcome after treatment with cranial radiation in childhood. J Clin Oncol. 2005;23(10):2256–2263. doi: 10.1200/JCO.2005.01.158. [DOI] [PubMed] [Google Scholar]
- Maddrey AM, Bergeron JA, Lombardo ER, McDonald NK, Mulne AF, Barenberg PD, Bowers DC. Neuropsychological performance and quality of life of 10 year survivors of childhood medulloblastoma. J Neurooncol. 2005;72(3):245–253. doi: 10.1007/s11060-004-3009-z. [DOI] [PubMed] [Google Scholar]
- Merchant TE, Kiehna EN, Kun LE, Mulhern RK, Li C, Xiong X, Boop FA, Sanford RA. Phase II trial of conformal radiation therapy for pediatric patients with craniopharyngioma and correlation of surgical factors and radiation dosimetry with change in cognitive function. J Neurosurg. 2006;104(2 Suppl):94–102. doi: 10.3171/ped.2006.104.2.5. [DOI] [PubMed] [Google Scholar]
- Mitby PA, Robison LL, Whitton JA, Zevon MA, Gibbs IC, Tersak JM, Meadows AT, Stovall M, Zeltzer LK, Mertens AC. Utilization of special education services and educational attainment among long-term survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Cancer. 2003;97(4):1115–1126. doi: 10.1002/cncr.11117. [DOI] [PubMed] [Google Scholar]
- Moore BD., 3rd Neurocognitive outcomes in survivors of childhood cancer. J Pediatr Psychol. 2005;30(1):51–63. doi: 10.1093/jpepsy/jsi016. [DOI] [PubMed] [Google Scholar]
- Mostow EN, Byrne J, Connelly RR, Mulvihill JJ. Quality of life in long-term survivors of CNS tumors of childhood and adolescence. J Clin Oncol. 1991;9(4):592–599. doi: 10.1200/JCO.1991.9.4.592. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Hancock J, Fairclough D, Kun L. Neuropsychological status of children treated for brain tumors: a critical review and integrative analysis. Med Pediatr Oncol. 1992;20(3):181–191. doi: 10.1002/mpo.2950200302. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Kepner JL, Thomas PR, Armstrong FD, Friedman HS, Kun LE. Neuropsychologic functioning of survivors of childhood medulloblastoma randomized to receive conventional or reduced-dose craniospinal irradiation: a Pediatric Oncology Group study. J Clin Oncol. 1998;16(5):1723–1728. doi: 10.1200/JCO.1998.16.5.1723. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Merchant TE, Gajjar A, Reddick WE, Kun LE. Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol. 2004;5(7):399–408. doi: 10.1016/S1470-2045(04)01507-4. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL. Neurocognitive late effects in pediatric cancer. Curr Probl Cancer. 2003;27(4):177–197. doi: 10.1016/s0147-0272(03)00026-6. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Merchant TE, Wallace D, Kocak M, Brouwers P, Krull K, Chintagumpala M, Stargatt R, Ashley DM, Tyc VL, Kun L, Boyett J, Gajjar A. Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol. 2005;23(24):5511–5519. doi: 10.1200/JCO.2005.00.703. [DOI] [PubMed] [Google Scholar]
- Mulhern RK, Palmer SL, Reddick WE, Glass JO, Kun LE, Taylor J, Langston J, Gajjar A. Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol. 2001;19(2):472–479. doi: 10.1200/JCO.2001.19.2.472. [DOI] [PubMed] [Google Scholar]
- Nagel BJ, Delis DC, Palmer SL, Reeves C, Gajjar A, Mulhern RK. Early patterns of verbal memory impairment in children treated for medulloblastoma. Neuropsychology. 2006;20(1):105–112. doi: 10.1037/0894-4105.20.1.105. [DOI] [PubMed] [Google Scholar]
- National Institute of Health. NIH Consensus statement - Diagnosis and Treatment of Attention Deficit Hyperactivity Disorder. National Institute of Health. 1998:1–37. [PubMed] [Google Scholar]
- National Reading Panel. Teaching children to read: An evidence based assessment of the scientific research literature on reading and its implications for reading instruction. U.S. Department of Health Human Services, Public Health Service, National Institute of Health, National Institute of Child Health and Human Development; Washington, D.C.: 2000. [Google Scholar]
- Ness KK, Gurney JG, Zeltzer LK, Leisenring W, Mulrooney DA, Nathan PC, Robison LL, Mertens AC. The impact of limitations in physical, executive, and emotional function on health-related quality of life among adult survivors of childhood cancer: a report from the Childhood Cancer Survivor Study. Arch Phys Med Rehabil. 2008;89(1):128–136. doi: 10.1016/j.apmr.2007.08.123. [DOI] [PubMed] [Google Scholar]
- Oeffinger K, McCabe M. Models for delivering survivorship care. Journal of Clinical Oncology. 2006;24(32):5117–5124. doi: 10.1200/JCO.2006.07.0474. [DOI] [PubMed] [Google Scholar]
- Oeffinger KC, Robison LL. Childhood cancer survivors, late effects, and a new model for understanding survivorship. JAMA. 2007;297(24):2762–2764. doi: 10.1001/jama.297.24.2762. [DOI] [PubMed] [Google Scholar]
- Packer R. Reduction of health status 7 years after addition of chemotherapy to craniospinal irradiation for medulloblastoma: a follow-up study on PNET-3 trial survivors. Current neurology and neuroscience reports. 2008;8(2):111–113. doi: 10.1007/s11910-008-0018-x. [DOI] [PubMed] [Google Scholar]
- Packer R, Goldwein J, Nicholson HS, Vezina LG, Allen JC, Ris MD, Muraszko K, Rorke LB, Wara WM, Cohen BH, Boyett JM. Treatment of children with medulloblastomas with reduced-dose craniospinal radiation therapy and adjuvant chemotherapy: A Children's Cancer Group Study. J Clin Oncol. 1999;17(7):2127–2136. doi: 10.1200/JCO.1999.17.7.2127. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Gajjar A, Reddick WE, Glass JO, Kun LE, Wu S, Xiong X, Mulhern RK. Predicting intellectual outcome among children treated with 35-40 Gy craniospinal irradiation for medulloblastoma. Neuropsychology. 2003;17(4):548–555. doi: 10.1037/0894-4105.17.4.548. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Goloubeva O, Reddick WE, Glass JO, Gajjar A, Kun L, Merchant TE, Mulhern RK. Patterns of intellectual development among survivors of pediatric medulloblastoma: a longitudinal analysis. J Clin Oncol. 2001;19(8):2302–2308. doi: 10.1200/JCO.2001.19.8.2302. [DOI] [PubMed] [Google Scholar]
- Palmer SL, Reddick WE, Gajjar A. Understanding the cognitive impact on children who are treated for medulloblastoma. J Pediatr Psychol. 2007;32(9):1040–1049. doi: 10.1093/jpepsy/jsl056. [DOI] [PubMed] [Google Scholar]
- Polderman TJ, Posthuma D, De Sonneville LM, Stins JF, Verhulst FC, Boomsma DI. Genetic analyses of the stability of executive functioning during childhood. Biol Psychol. 2007;76(12):11–20. doi: 10.1016/j.biopsycho.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Reeves CB, Palmer S, Gross AM, Simonian SJ, Taylor L, Willingham E, Mulhern RK. Brief report: sluggish cognitive tempo among pediatric survivors of acute lymphoblastic leukemia. J Pediatr Psychol. 2007;32(9):1050–1054. doi: 10.1093/jpepsy/jsm063. [DOI] [PubMed] [Google Scholar]
- Ris MD, Packer R, Goldwein J, Jones-Wallace D, Boyett JM. Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's Cancer Group study. J Clin Oncol. 2001;19(15):3470–3476. doi: 10.1200/JCO.2001.19.15.3470. [DOI] [PubMed] [Google Scholar]
- Rutkowski S, Bode U, Deinlein F, Ottensmeier H, Warmuth-Metz M, Soerensen N, Graf N, Emser A, Pietsch T, Wolff J, Kortmann R, Kuehl J. Treatment of early childhood medulloblastoma by postoperative chemotherapy alone. New England Journal of Medicine. 2005;352(10):978–986. doi: 10.1056/NEJMoa042176. [DOI] [PubMed] [Google Scholar]
- Schatz J, Kramer JH, Ablin A, Matthay KK. Processing speed, working memory, and IQ: a developmental model of cognitive deficits following cranial radiation therapy. Neuropsychology. 2000;14(2):189–200. doi: 10.1037//0894-4105.14.2.189. [DOI] [PubMed] [Google Scholar]
- Schultz KA, Ness KK, Whitton J, Recklitis C, Zebrack B, Robison LL, Zeltzer L, Mertens AC. Behavioral and social outcomes in adolescent survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2007;25(24):3649–3656. doi: 10.1200/JCO.2006.09.2486. [DOI] [PubMed] [Google Scholar]
- Shanahan MA, Pennington BF, Yerys BE, Scott A, Boada R, Willcutt EG, Olson RK, DeFries JC. Processing speed deficits in attention deficit/hyperactivity disorder and reading disability. J Abnorm Child Psychol. 2006;34(5):585–602. doi: 10.1007/s10802-006-9037-8. [DOI] [PubMed] [Google Scholar]
- Shaywitz SE, Morris R, Shaywitz BA. The Education of Dyslexic Children from Childhood to Young Adulthood. Annu Rev Psychol. 2008;59:451–475. doi: 10.1146/annurev.psych.59.103006.093633. [DOI] [PubMed] [Google Scholar]
- Skinner R, Wallace WH, Levitt G. Therapy based long term follow up: a practice statement. United Kingdom Children's Cancer Study Group, Late Effects Group; 2005. [Google Scholar]
- Solanto MV, Gilbert SN, Raj A, Zhu J, Pope-Boyd S, Stepak B, Vail L, Newcorn JH. Neurocognitive functioning in AD/HD, predominantly inattentive and combined subtypes. J Abnorm Child Psychol. 2007;35(5):729–744. doi: 10.1007/s10802-007-9123-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinlin M. The cerebellum in cognitive processes: supporting studies in children. Cerebellum. 2007;6(3):237–241. doi: 10.1080/14734220701344507. [DOI] [PubMed] [Google Scholar]
- Steinlin M, Imfeld S, Zulauf P, Boltshauser E, Lovblad K, Ridolfi-Luthy A, Perrig W, Kaufmann F. Neuropsychological long-term sequelae after posterior fossa tumor resection during childhood. Brain. 2003;126(9):1998–2008. doi: 10.1093/brain/awg195. [DOI] [PubMed] [Google Scholar]
- Temple E, Deutsch GK, Poldrack RA, Miller SL, Tallal P, Merzenich MM, Gabrieli JD. Neural deficits in children with dyslexia ameliorated by behavioral remediation: evidence from functional MRI. Proc Natl Acad Sci U S A. 2003;100(5):2860–2865. doi: 10.1073/pnas.0030098100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas PR, Deutsch M, Kepner JL, Boyett JM, Krischer J, Aronin P, Albright L, Allen JC, Packer RJ, Linggood R, Mulhern R, Stehbens JA, Langston J, Stanley P, Duffner P, Rorke L, Cherlow J, Friedman HS, Finlay JL, Vietti TJ, Kun LE. Low-stage medulloblastoma: final analysis of trial comparing standard-dose with reduced-dose neuraxis irradiation. J Clin Oncol. 2000;18(16):3004–3011. doi: 10.1200/JCO.2000.18.16.3004. [DOI] [PubMed] [Google Scholar]
- Thompson SJ, Leigh L, Christensen R, Xiong X, Kun LE, Heideman RL, Reddick WE, Gajjar A, Merchant T, Pui CH, Hudson MM, Mulhern RK. Immediate neurocognitive effects of methylphenidate on learning-impaired survivors of childhood cancer. J Clin Oncol. 2001;19(6):1802–1808. doi: 10.1200/JCO.2001.19.6.1802. [DOI] [PubMed] [Google Scholar]
- Vaquero E, Gomez C, Quintero E, Gonzalez-Rosa J, Marquez J. Differential prefrontal-like deficit in children after cerebellar astrocytoma and medulloblastoma tumor. Behav Brain Funct. 2008;4(18) doi: 10.1186/1744-9081-1184-1118. [DOI] [PMC free article] [PubMed] [Google Scholar]