Introduction
Developments over the last 10 years in ceramic materials science for dental applications have led to a class of high strength materials (e.g., alumina and zirconia-based ceramics) that potentially provide better fracture resistance and long-term durability than traditional porcelain and other ceramic alternatives. Although superior in terms of mechanical performance (strength, toughness, fatigue resistance), there is an inherent limitation associated with high strength ceramic materials. Bonding of resins to these materials is more difficult than it is for silica-based ceramics. Fortunately, high strength ceramic restorations do not always require adhesive bonding to tooth structure and can be placed using conventional cements that rely only on micromechanical retention. However, conventional cementation techniques do not provide sufficient bond strength for some clinical applications. Good adhesion is important for high retention [1], prevention of microleakage, and increased fracture and fatigue resistance, and is mediated by the use of resin-based cements. Strong resin bonding relies on micromechanical interlocking and adhesive chemical bonding to the ceramic surface [1], and requires a combination of surface roughening and chemical functionalization for effective attachment. A non-destructive, simple method for treating the ceramic surfaces would be very useful in such cases, especially if it would be compatible with existing adhesive bonding techniques (silane + resin cement).
Bonding to traditional silica-based ceramics, generally employing both mechanical and adhesive retention, has been well researched and bond strengths are predictable. A strong resin bond relies on micromechanical interlocking created by surface roughening and on chemical adhesion between the cement and ceramic (by way of silane chemistry). Current techniques are: (1) grinding, (2) abrasion with diamond rotary instruments, (3) surface abrasion with alumina particles, (4) acid-etching (typically hydrofluoric acid [HF]), and (5) a combination of these techniques.
Unfortunately, the chemistry and microstructure of high strength ceramics (specifically alumina and zirconia) differ from those of conventional silica-based materials. These specific ceramics are not easily etched or chemically functionalized using conventional treatments, and require very aggressive mechanical abrasion methods to increase surface roughness, possibly creating strength-reducing surface flaws as a result. Therefore, in order to achieve acceptable cementation, alternative attachment methods are required for alumina and zirconia ceramics.
Because the majority of dental practitioners use silane-based treatments for organic resin-based cementation, the authors sought to develop a chemical treatment compatible with silane chemistry. Silanes are organic compounds that contain silicon (Si) atoms, are similar to orthoesters in structure, and display dual reactivity. Their uses in clinical dentistry and effects on adhesive bonding have been described in detail in the scientific literature [1-10]. One end of the molecule is organically functional (e.g., with vinyl groups –CH=CH2), and can polymerize with an organic matrix (e.g., methacrylate). Silanes are commonly used in dentistry to coat inorganic filler particles in polymer matrix composites and to achieve adhesive bonding to resin luting cements of porcelain (or other silica-containing ceramics) for restorative applications. Traditional silane chemistry is not truly effective with high strength ceramics such as alumina and zirconia, as the ceramics are more chemically stable than silica-containing glasses and ceramics and not as easily hydrolyzed.
Bonding to zirconia has become a topic of great interest in recent years [11-26]. As mentioned, traditional adhesive chemistry is ineffective on zirconia surfaces. Also, acid etchants such as HF does not selectively etch the surface for simple micromechanical attachment. Surface abrasion with alumina particles and application of a tribochemical silica coating allows for chemical bonds to a silane coupling agent and to resin cement [27]. This is a somewhat complicated procedure that does not produce bond strengths as high as those reported for silane-bonded porcelain [1, 25]. In addition, there is some speculation that air particle abrasion should not be used, particularly with zirconia ceramics, because it might cause micro-fractures that would lead to premature, catastrophic failure.
The use of phosphoric acid primers or phosphate-modified resin cements has been shown to produce silane-like adhesion, through similar types of hydrolyzation-driven chemistry. However, bond strength values reported in the literature through use of these agents are generally lower that the values reported for tribochemical silica coating, coupling with silane and resin cement [24]. More recently, Aboushelib et. al. reported on increased bond strength utilizing selective infiltration etching and novel silane-based zirconia primers [26]. The available approaches for adhesive bonding of zirconia ceramics are not adequate for all clinical applications and their long-term efficacy is currently unknown.
One potential method for improving adhesion to zirconia is a unique vapor-phase deposition technique whereby chloro-silane is combined with water vapor to form a more reactive, SixOy -functionalized surface. The process utilizes a molecular vapor deposition (MVD) tool, developed specifically to deposit conformal, thin films to serve as hydrophobic, hydrophilic, biocompatible, protective, ordering, or otherwise reactive coatings [28]. This flexible system allows deposition of numerous materials from simple liquid precursors. Examples of materials include fluoropolymers for hydrophobicity, silanes for polymer and metal adhesion or hydrophillicity, and PEG-based coatings for biocompatibility. Deposition conditions and precursor chemistry can also be modified to produce a range of surface characteristics. This process is a very simple and fast method for producing thin, high quality, conformal coatings of almost any organic material with a boiling point below 150°C [29].
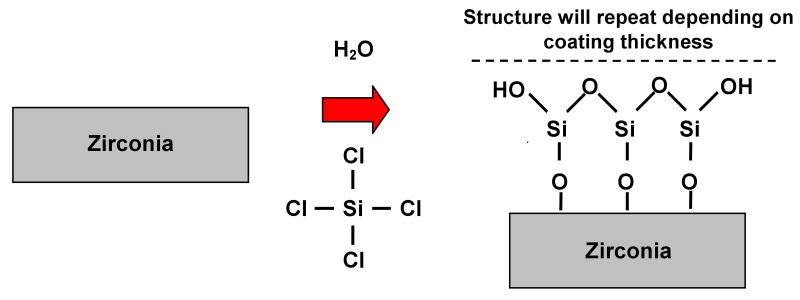
Typical treatment for a metal, polymer, and/or metal-oxide material to enable the surface to be silanated is shown in Figure 1. As the silicon tetrachloride (SiCl4) and water react with the substrate surface, active hydroxyl groups are formed on the surface subsequently forming a silicon oxide layer on the substrate surface. This treatment serves as a primer step for subsequent reactions with organo-silanes, used as adhesion promoters in conventional resin bonding applications. It is thus hypothesized that the chloro-silane pretreatment process will allow for conventional silanation and resin bonding techniques to be a viable option for high strength ceramics.
Figure 1.
Chemical reaction representative of introducing water and silicon tetrachloride to activate non-silica-based materials (i.e. zirconia), for subsequent silanation treatment [28].
In the present study, molecular vapor deposition was used to modify the surface of zirconia specimens to enhance silanation using traditional techniques. The aim was to develop and evaluate a practical method of chemical surface modification of high strength dental ceramics (i.e. zirconia) to facilitate viable adhesive bonding using commercially available silanes and resin cements. Adhesion was measured using microtensile bond strength testing.
Materials and Methods
Blocks of pre-sintered zirconia and leucite-filled porcelain (ZirCAD and ProCad, Ivoclar Vivadent, Schaan, Liechtenstein) measuring 14 × 12 × 20 mm were obtained from the manufacturer. Composite blocks (AELITE All-Purpose Body, Bisco, Inc., Schaumburg, IL) were fabricated by condensing the material into a Teflon mold (14 × 12 × 20 mm) in 2 mm increments, light-activating each increment for 40 seconds at 500mW/cm2. Surfaces of each material were polished through 1200-grit polishing paper to ensure starting surface roughness. After polishing, the surfaces were air-abraded (50 μm alumina abrasive, 0.29 MPa, 20 sec) prior to chemical surface treatments or bonding procedures. Specimens were placed in an ultra-sonic DI bath for five minutes.
The zirconia surface functionalization was performed using SiCl4 (Gelest SIT7085.0) reaction with H2O[gas] by vapor deposition in a commercial tool (MVD-100, Applied Microstructures, San Jose, CA). The specimens are exposed for approximately 15 minutes to a vapor-phase mixture of H2O and SiCl4, resulting in the deposition of the SixOy seed layer with an HCl gas byproduct. The film thickness was controlled primarily by deposition time, and specimens were pre-calibrated on silicon wafers followed by measurement of the SixOy seed layer thickness via ellipsometry. Two film thicknesses were used for this study: 2.6 nm and 23 nm and they were obtained by increasing the number of deposition cycles. After the zirconia specimens were cleaned, they were placed in the MVD tool for chloro-silane pretreatment. Prior to deposition the surfaces were exposed to 10-min oxygen plasma, which cleans any organic contaminants and ensures surface hydroxylation. The details of the reaction process are described in detail by Kobrin et. al. [28].
The following different types of specimen were prepared for microtensile tests:
Group 1 (control): Porcelain was acid-etched using 9.5% HF gel (Porcelain Etchant, Bisco, Inc., Schaumburg, IL) for 60 seconds, rinsed and dried, and treated with silane (Porcelain Primer, Bisco, Inc., Schaumburg, IL). Resin adhesive was brushed on (air-dried and light-cured for 10 seconds) both porcelain and composite mating surfaces (One-Step Plus, Bisco, Inc., Schaumburg, IL). The porcelain and composite blocks were bonded together using a resin luting cement (C&B Cement, Bisco, Inc., Schaumburg, IL) under a fixed load of 20 N.
Group 2: Zirconia (no surface treatment) was treated with silane and bonded to composite blocks (unless otherwise noted, all adhesive materials are the same for each group).
Group 3: Zirconia (no surface treatment) was silica-coated using 30 μm alumina particles modified with salicylic acid (CoJet, 3M-ESPE, St. Paul, MN – 0.28 MPa, 5-10 mm working distance, 15 sec), treated with silane and bonded to composite blocks.
Group 4: Zirconia (SixOy seed layer thickness approximately 2.6 nm) was treated with silane and bonded to composite blocks.
Group 5: Zirconia (SixOy seed layer thickness approximately 23 nm silica layer) was treated with silane and bonded to composite blocks.
Group 6: Composite bars were also constructed and tested to calculate the “bulk” strength limit of the composite.
After storage at room temperature for a period of 24 h, microtensile bar specimens were cut into plates using a low-speed diamond-coated saw (Isomet 1000, Buehler, Lake Bluff, IL). The plates were then subsequently cut into bars (approximately 1.5 mm × 1.5 mm) using a high precision cutting saw (DAD 341, Disco Corp., Tokyo, Japan). Specimens were then attached to a microtensile fixture with cyanoacrylate adhesive (Permabond, Pottstown, PA). All specimens were subjected to a tensile force at a crosshead speed of 1 mm/min in an EZ-Test testing device (Shimadzu, Kyoto, Japan). Bond strengths were calculated by dividing fracture peak load by the cross-sectional area of the specimen. Single-factor analysis of variance (ANOVA) at a 5% confidence level was performed for the bonding strength data.
Scanning electron microscopy (SEM) was used to evaluate the mating surfaces of the fractured specimens to determine failure mode, either composite cohesive failure (partial or complete cohesive failure within composite) or adhesive failure (partial or complete adhesive failure).
Results
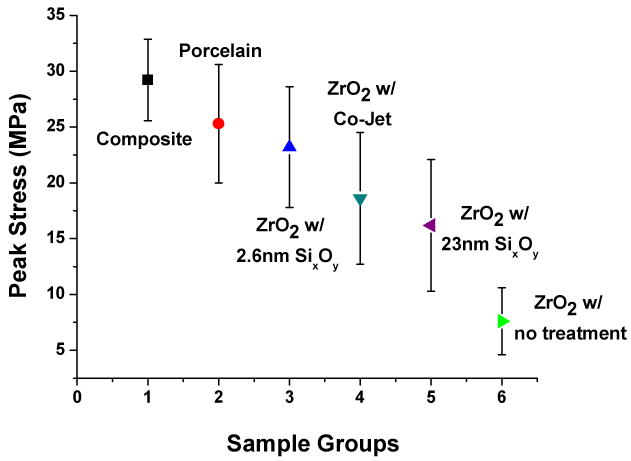
The mean values and standard deviations of the microtensile bond strength testing for bonded specimens are shown in Figure 2. ANOVA revealed a significant difference in mean microtensile bond strengths. Composite microtensile bars were fabricated to create a control strength value (29.2 MPa) and used as a strength gauge of tested groups. The untreated zirconia specimens had the lowest strength values and were the only group to show failure during cutting (approximately 40% failed in this manner). The specimens with surface treatments exhibited higher bond strengths and none failed during cutting. The mean peak stress values from specimens with surface treatment have higher standard deviation than the controls because of variations in failure modes. These groups included cases of cohesive failure (highest bond strength) and combination of cohesive and adhesive failure which created a large spread in bond strength values. Values for peak stress with standard deviations and percent breakdown of failure mode are shown in Table 1.
Figure 2.
Microtensile testing peak stress values for all groups tested. Values are plotted with standard deviation error bars.
Table 1.
Microtensile peak stress (MPa) with standard deviation of the different test groups. Numbers of specific failure types are represented in percents.
| Group | Peak Stress (SD) (MPa) |
Adhesive (%) |
Mixed (%) |
Cohesive (%) |
|---|---|---|---|---|
| Composite (bars only)a | 29.2 (3.7) | control | ||
| Porcelainb – (control) | 25.3 (5.3) | 16 | 35 | 49 |
| Zirconia (SixOy - 2.6nm thickness)b | 23.2 (5.4) | 15 | 40 | 45 |
| Zirconia (Co-Jet™ treatment)c | 18.6 (5.9) | 32 | 52 | 16 |
| Zirconia (SixOy - 23nm thickness)c | 16.2 (5.9) | 50 | 43 | 7 |
| Zirconia (no treatment)d | 7.6 (3.0) | 72 | 28 | - |
SEM analysis of the fractured microtensile bars revealed several types of failure modes: (1) adhesive failure, (2) mixed mode of adhesive and cohesive failure, and (3) cohesive failure. Figure 3 illustrates a typical adhesive failure where the bond interfacial surfaces are exposed. Here, the zirconia surface can be discerned with areas of resin cement. Figures 4 and 5 illustrate two further different failure modes, respectively: a mixed mode (failure surface displays areas of exposed bonding surface and remnants of composite failure) and cohesive failure (the fracture is purely in the composite).
Figure 3.
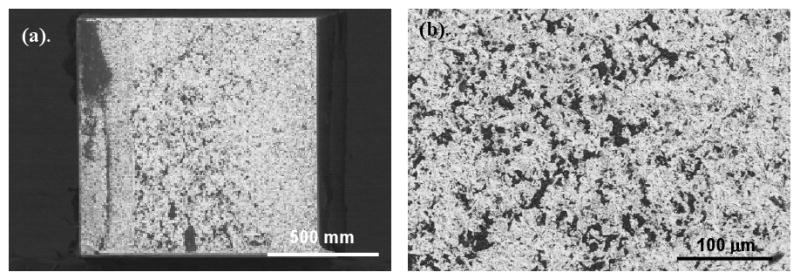
SEM micrograph of zirconia specimen, tested without a surface treatment. This is representative of a primarily adhesive failure which exposes a majority of the zirconia surface. The white areas are the zirconia surface and small dark regions show areas of residual resin cement.
Figure 4.
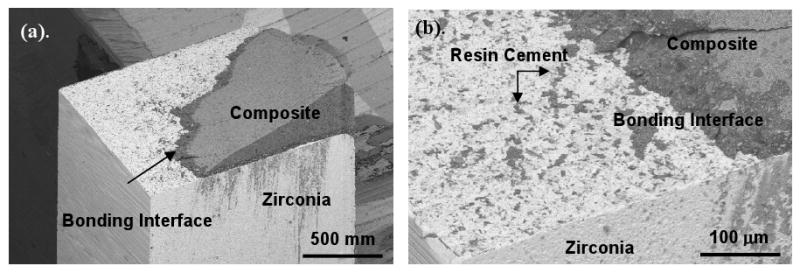
SEM of the zirconia portion with a silica seed-layer (23nm) treated surface. As shown in the micrographs, the failure was determined to be both adhesive and cohesive failure due to some composite still bonded the zirconia surface.
Figure 5.
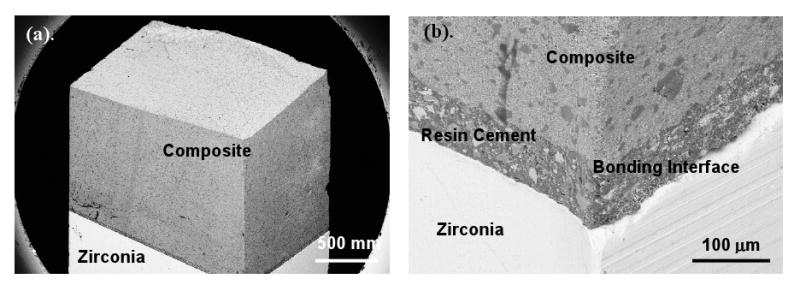
SEM image of a silica seed layer (2.3nm) treated specimen, failure mode is shown to be cohesive failure (completely within the composite). (b) Displays the post-fracture interface which still appears to be undisturbed.
Discussion
Shear bond and microtensile testing are the standard methods for evaluating bond strengths in dental materials. Typically, failure modes are directly influenced by the incorporation of surface flaws; internal flaws in the substrates; and/or adhesive layer failure (i.e., resin cement) [30]. Another critical aspect of testing has been the distribution of stress throughout the bonding layer. Pashley et al. reported that not only the distributions of stress gradients, but also defect concentrations are greatly reduced by the small dimensions of microtensile specimens [31].
A strong chemical bond with zirconia has proven very difficult to obtain, due in-part to the non-reactive nature of its surface. Studies have investigated multiple treatment techniques, ranging from physical roughening (to increase the bonding surface area) to the application of reactive monomers (to promote chemical attachment to the polymer adhesive). As reported in other studies, zirconia, with surface abrasion only, and no chemical functionalization, displayed relatively low bond strength and ultimately resulted in adhesive failures. This supports the hypothesis that some type of chemical modification to the surface, which binds to the resin cement, is required to obtain optimum bonding strength.
Surface abrasion with a tribochemical silica-coated alumina has been shown to be an effective bonding promoter treatment for zirconia by physically roughening the surface while also leaving behind both physically and chemically bound silica [1]. The bound silica particles serve as reactive sites for conventional organo-silane monomer primers, however residual bond strengths tend to be lower then those obtained on conventional dental porcelain surfaces. This was further supported in the present study, where specimens tested with a tribochemical technique had higher bond strengths than the un-treated controls, however they were lower then the best silica seed layer-treated surface. These data suggest a benefit from both roughening and chemical functionalization of the surface; however, the improved chemical reactivity of the silica seed-treated surfaces appears to be superior to that of the tribochemical technique. This is confirmed by the SEM evaluation of the specimen failures, the majority of which had remaining composite material still adhered to the zirconia.
The most promising results showed that the thinner seed layer (2.3 nm) treatment had bond strengths that were statistically the same as the bonded porcelain specimens. Approximately 85% of the failures, for both groups, were shown to be mixed mode (adhesive/cohesive) or cohesive failure. Interestingly, the bond strength for the silica seed layer-treated specimens seems to be a function of treatment thickness. It is possible that the thicker seed layers have a level of chemisorbed SixOy near the zirconia surface with stacking of additional layers being partially physisorbed. These additional layers that are not chemically linked provide reduce bonding to silane hydrocarbons. Therefore, increase of the surface-treated zirconia-composite bond strength may derive from optimization of the silica seed-layer thickness and surface roughness combination, enabling robust bonding of these high-strength dental ceramics in a whole new range of applications.
Conclusion
The overall goal of this research is to develop a practical method to chemically modify the surface of high strength dental ceramics, such as zirconia, to facilitate viable, robust adhesive bonding using commercially available organo-silanes and resin luting cements. By using a chloro-silane-based vapor-phase pretreatment, a silica-like surface layer was created on zirconia and used to increase the binding sites for the subsequent organo-silane primer for conventional dental adhesive applications. The mechanical data from this study support the concept that this approach of chemical surface modification can improve resin adhesion to zirconia using traditional silanation and bonding techniques.
Acknowledgments
Research was supported in-part by NIH/NIDCR DE013511-09, an Internal Research and Development Grant (provided by RTI International), and materials donated by Ivoclar-Vivadent.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Blatz MB, Sadan A, Kern M. Resin-ceramic bonding: a review of the literature. J Prosthet Dent. 2003;89(2):268–274. doi: 10.1067/mpr.2003.50. [DOI] [PubMed] [Google Scholar]
- 2.Stangel I, Nathanson D, Hsu CS. Shear strength of the composite bond to etched porcelain. J Dent Res. 1987;66(9):1460–1465. doi: 10.1177/00220345870660091001. [DOI] [PubMed] [Google Scholar]
- 3.Bailey JH. Porcelain-to-composite bond strengths using four organosilane materials. J Prosthet Dent. 1989;61(1):174–177. doi: 10.1016/0022-3913(89)90368-5. [DOI] [PubMed] [Google Scholar]
- 4.Soderholm KJM, Shang SW. Molecular orientation of silane at the surface of colloidal silica. J Dent Res. 1993;72(6):1050–1054. doi: 10.1177/00220345930720061001. [DOI] [PubMed] [Google Scholar]
- 5.Thurmond JW, Barkmeier WW, Wilwerding TM. Effect of porcelain surface treatments on bond strengths of composite resin bonded to porcelain. J Prosthet Dent. 1994;72(3):355–359. doi: 10.1016/0022-3913(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 6.Aida M, Hayakawa T, Mizukawa K. Adhesion of composite to porcelain with various surface conditions. J Prosthet Dent. 1995;73(4):464–470. doi: 10.1016/s0022-3913(05)80076-9. [DOI] [PubMed] [Google Scholar]
- 7.Hooshmand T, Van Noort R, Keshvad A. Bond durability of the resin-bonded and silane treated ceramic surface. Dent Mat. 2002;18:179–188. doi: 10.1016/s0109-5641(01)00047-1. [DOI] [PubMed] [Google Scholar]
- 8.Debnath S, Wunder SL, McCool JI, Baran GR. Silane treatment effects on glass/resin interfacial shear strengths. Dent Mat. 2003;19:441–448. doi: 10.1016/s0109-5641(02)00089-1. [DOI] [PubMed] [Google Scholar]
- 9.Shen C, Oh WS, Williams JR. Effect of post-silanization drying on the bond strength of composite to ceramic. J Prosthet Dent. 2004;91(4):453–458. doi: 10.1016/S0022391304001301. [DOI] [PubMed] [Google Scholar]
- 10.Matinlinna JP, Lassila LVJ, Ozcan M, Yli-Urpo A, Vallittu P. An introduction to silanes and their clinical applications in dentistry. Int J Prosthod. 2004;17:155–164. [PubMed] [Google Scholar]
- 11.Amaral R, Ozcan M, Bottino MA, Valandro LF. Microtensile bond strength of a resin cement to glass infiltrated zirconia-reinforced ceramic: The effect of surface conditioning. Dent Mat. 2006;22:283–290. doi: 10.1016/j.dental.2005.04.021. [DOI] [PubMed] [Google Scholar]
- 12.Derand T, Molin M, Kvam K. Bond strength of composite luting cement to zirconia ceramic surfaces. Dent Mat. 2005;21:1158–1162. doi: 10.1016/j.dental.2005.02.005. [DOI] [PubMed] [Google Scholar]
- 13.Kern M, Wegner SM. Bonding to zirconia ceramic: Adhesion methods and their durability. Dent Mat. 1998;14:64–71. doi: 10.1016/s0109-5641(98)00011-6. [DOI] [PubMed] [Google Scholar]
- 14.Matilinna JP, Lassila LVJ, Vallittu PK. The effect of a novel saline blend system on resin bond strength to silica-coated Ti substrate. J of Dent. 2006;34:436–443. doi: 10.1016/j.jdent.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Matinlinna JP, Lassila LVJ, Ozcan M, Yli-Urpo A, Vallittu P. An introduction to silanes and their clinical applications in dentistry. Int J Prosthod. 2004;17:155–164. [PubMed] [Google Scholar]
- 16.Peumans M, Hikita K, Munck JDe, Van Landuyt K, Poitevin A, Lambrechts P, Van Meerbeek B. Effects of ceramic treatments on the bond strength of an adhesive luting agent to CAD-CAM ceramic. J of Dent. 2007;35:282–288. doi: 10.1016/j.jdent.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 17.Saygili G, Sahmali S. Effect of ceramic surface treatment on the shear bond strengths of two resin luting agents to all-ceramic materials. J Oral Rehab. 2003;30:758–764. doi: 10.1046/j.1365-2842.2003.01027.x. [DOI] [PubMed] [Google Scholar]
- 18.Stsu SS, Kilicarslan MA, Kucuk HC, Aka PS. Effect of zirconium-oxide ceramic surface treatments on the bond strength to adhesive resin. J Prosthet Dent. 2006;95(4):430–436. doi: 10.1016/j.prosdent.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 19.Blatz MB, Sadan A, Martin J, Lang B. In vitro evaluation of shear bond strengths of resin to densely-sintered high-purity zirconium-oxide ceramic after long-term storage and thermal cycling. J Prosthet Dent. 2004;91:356–362. doi: 10.1016/j.prosdent.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 20.Bottino MA, Valandro LF, Scotti R, Buso L. Effect of surface treatments on the resin bond to zirconium-based ceramic. Int J Prosthodont. 2005;18:60–65. [PubMed] [Google Scholar]
- 21.Hummel M, Kern M. Durability of the resin bond strength to the alumina ceramic Procera. Dent Mat. 2004;20:498–508. doi: 10.1016/j.dental.2003.10.014. [DOI] [PubMed] [Google Scholar]
- 22.Kumbuloglu O, Lassila LVJ, User A, Vallittu PK. Bonding of resin composite sureface treatments on the tensile bond strength of composite resin to all-ceramic coping materials. Oper Dent. 2006;31-2:248–255. doi: 10.2341/05-22. [DOI] [PubMed] [Google Scholar]
- 23.Lüthy J, Loeffel O, Hammerle CHF. Effect of thermocycling on bond strength of luting cements to zirconia ceramic. Dent Mat. 2006;22:195–200. doi: 10.1016/j.dental.2005.04.016. [DOI] [PubMed] [Google Scholar]
- 24.Yoshida K, Tsuo Y, Atsuta M. Bonding of dual-cured resin cement to zirconia ceramic using phosphate acid ester monomer and zirconate coupler. J Biomed Mat Res B. 2006;77(1):28–33. doi: 10.1002/jbm.b.30424. [DOI] [PubMed] [Google Scholar]
- 25.Valandro LF, Özcan M, Bottino MA, Bottina MC, Scotti R, Della Bona A. Bond strength of a resin cement to high-alumina and zirconia-reinforced ceramics: The effect of surface conditioning. J Adhes Dent. 2006;8:175–181. [PubMed] [Google Scholar]
- 26.Aboushelib MN, Matinlinna JP, Salameh Z, Ounsi H. Innovations in bonding zirconia-based materials: Part I. Dent Mat. 2008;24:1268–1272. doi: 10.1016/j.dental.2008.02.010. [DOI] [PubMed] [Google Scholar]
- 27.Ozcan M, Vallittu PK. Effect of surface conditioning methods on the bond strength of luting cement to ceramics. Dental Materials. 2003;19(8):725–731. doi: 10.1016/s0109-5641(03)00019-8. [DOI] [PubMed] [Google Scholar]
- 28.Kobrin B, Chinn J, Ashurst RW. Durable anti stiction coatings by molecular vapor deposition (MVD) NSTI Nanotech. 2005;2:247–350. [Google Scholar]
- 29.US Patent 2006/0251765 A1. Applied MicroStrutures. Inc.; [Google Scholar]
- 30.Della Bona A, van Noort R. Shear versus tensile bond strength of resin composite bonded to ceramic. J Dent Res. 1995;74:1591–1596. doi: 10.1177/00220345950740091401. [DOI] [PubMed] [Google Scholar]
- 31.Pashley DH, Carvalho RM, Samo H, Nakajima M, Yodhiyama M, Shono Y, Fernandes CA, Tay F. The microtensile bond test: A review. J Adhes Dent. 1999;1:299–309. [PubMed] [Google Scholar]