Abstract
NZB/W female mice spontaneously develop systemic lupus, an autoantibody mediated disease associated with immune complex glomerulonephritis. Natural Killer (NK) T cells augment anti-dsDNA antibody secretion by NZB/W B cells in vitro, and blocking NKT cell activation in vivo with anti-CD1 mAb ameliorates lupus disease activity. In the current study, we show that β-galactosylceramide reduces the in vivo induction of serum IFN-γ and/or IL-4 by the potent NKT cell agonist α-galactosylceramide and reduces NKT cell helper activity for IgG secretion. Treatment of NZB/W mice with the β-galactosylceramide ameliorated lupus disease activity as judged by improvement in proteinuria, renal histopathology, IgG anti-dsDNA antibody formation, and survival. In conclusion, β-galactosylceramide, a glycolipid that reduces the cytokine secretion induced by a potent NK T cell agonist ameliorates lupus in NZB/W mice.
Keywords: Innate Immunity, NK T Cells, T Cells, Systemic Lupus Erythematosus
Introduction
NZB/W female mice spontaneously develop systemic lupus erythematosus with immune complex glomerulonephritis appearing at about 6 months of age[1, 2]. The onset of kidney disease is associated with the IgM to IgG isotype switching of autoantibodies to a variety of nucleic acids and associated proteins[1, 2]. T cells play an important role in augmenting autoantibody secretion of B cells and in facilitating switching to the IgG2a pathogenic isotype [3, 4, 5]. The marked amelioration of disease activity after treatment of NZB/W mice with anti-CD4 monoclonal antibody (mAb) suggested an important contribution of CD4+ T cells to the development of lupus in vitro[3]. In addition, CD4+ T cells have been shown to augment spontaneous secretion of IgM, IgG, and autoantibodies by B cells in vitro[4, 5]. CD1d reactive natural killer (NK) T cells have been shown to contribute to the development of lupus in NZB/W mice by helping B cells to secrete autoantibodies including IgG anti-dsDNA antibodies in vitro [6, 7]. Interestingly, conventional CD4+ T cells failed to show helper activity in vitro under the culture conditions that were used[7]. The large majority of these NKT cells express CD4 and can recognize endogenous ligands associated with the CD1d antigen presenting molecule on B cells [7]. BALB/c mouse T cells with a CD1d TCR transgene induced lupus in nu/nu BALB/c mice after adoptive transfer [8].
Although some CD1d binding glycolipids such as α-galactosyl ceramide are potent activators of NK T cells and induce robust secretion of IL-4 and IFN-γ in the serum, a single parenteral injection of β-galactosylceramide (β-GalCer) with a 12 carbon acyl chain (C12) has been reported to cause a rapid reduction of the percentage of NKT cells in the liver and spleen without the appearance of IL-4 and IFN-γ in the serum of normal C57BL/6 mice [9]. In the current study, we determined whether β-GalCer (C12) was able to reduce the NKT cell cytokine secretion induced by the potent NKT cell activator α-GalCer (C26), and whether β-GalCer (C12) treatment of NZB/W mice can ameliorate lupus.
Materials and Methods
Mice
NZB/W and C57BL/6 female mice were purchased from the Jackson Laboratories (Bar Harbor, ME), and were maintained in the Stanford University Department of Comparative Animal Medicine until they were used for experiments at 10-12 weeks of age, unless otherwise stated in the text. All animal protocols were reviewed and approved by the Stanford Administrative Panels on Laboratory Animal Care.
Reagents and monoclonal antibodies (mAbs)
Anti-mouse mAbs including FITC-anti-CD5 (53-7.3), FITC-anti-CD21 (7G6), PE-anti-CD23 (B3B4), APC-anti-B220 (RA3-6B2), APC-anti-TCRβ (H57-597), and mouse CD1d-dimers were purchased from BD Biosciences (San Diego, CA). PE-conjugated PBS-57 glycolipid-loaded CD1d tetramers were obtained from the NIH Tetramer Facility, Rockville MD. αGalCer (C26) and αGalCer (C8) were kindly provided from Dr. Paul B. Savage (Brigham Young University, UT). Biochemical synthesis of these compounds has been described in detail previously [10]. β-GalCer (C12) was purchased from the Avanti Polar Lipid Company (Alabaster, AL). The procedure for loading of CD1d-dimers with glycolipids has been reported [6].
Administration of glycolipids
Lyophilized glycolipids were diluted in distilled water to make stock solutions of 200 μg/ml, and then further diluted in phosphate buffered saline to achieve the desired dose in 0.5ml for intraperitoneal injections. Administration of β-GalCer by gavage was performed thrice a week at the dose of 800 μg/ml in 0.5 ml of phosphate buffered saline.
Cell preparation and immunofluorescent staining
Single-cell suspensions were prepared from livers, filtered through a fine nitex membrane, overlaid on lympholyte-M (Cedarlane, Ontario, Canada), separated by density centrifugation, and then washed with MACS buffer. Stainings were performed in the presence of anti-CD16/32 mAb (2.4G2, Pharmingen) at saturation to block FcRII/III receptors. Propidium iodide (Sigma Chemicals, St Louis, MO) was added to staining reagents to exclude dead cells. Staining for CD1 on liver and spleen cells used biotinylated anti-CD1 antibodies and PE-streptavidin for counterstaining. Single-cell suspensions from spleens were washed twice, and filtered through a fine nitex membrane. Cells were incubated with various combinations of mAbs, and two- to three-color immunostaining and flow cytometry were performed using standard techniques and equipment (LSR, BD Biosciences, San Jose, CA) with FlowJo software (TreeStar, Ashland, OR) for data analysis [6, 7]. Four mice were used per experiment.
ELISA assays
A standard sandwich ELISA was used to measure concentrations of IgM, total IgG, IgG1, and IgG2a in serum and cell culture supernatant as described previously [6, 7, 8]. Reagents for the ELISA were purchased from BD Bioscience. Measurement of IL-4 or IFNγ-ing an ELISA kit according to the manufacture's protocol (BD Biosciences). IgM or IgG anti-dsDNA antibodies were captured using deproteinized calf thymus DNA, and isotype specific anti-globulin reagents were used in an ELISA as described in detail [6, 7, 8]. Anti-dsDNA antibodies titers are expressed in units per milliliter, using a reference-positive standard of pooled serum from 9-month-old NZB/W mice. A 1:100 dilution of this standard serum was arbitrarily assigned a value of 100 U/ml as described before [6, 7, 8].
In vitro cultures of NKT cells and B-1 B cells
NKT cells were sorted by flow cytometry using CD1d-tetramers for staining, and B-1 B cells were sorted on the basis of the CD19+CD5+CD23- phenotype as described in detail previously[7]. The purified B-1 B cells were either cultured alone (1×105 cells/well) or together with purified NKT cells (5×104 cells/well) in 96-well round bottom plates for 10 days in complete medium[7]. Supernatants were harvested at the end of the culture period from triplicate wells, and the concentrations of IgM, IgG1, and IgG2a were determined by ELISA [7].
Monitoring Lupus Disease Activity
Proteinuria of female NZB/W mice was measured weekly on a scale of 1- 4+ using a colorimetric assay for albumin (Albustix; Miles Inc., Elkhart, IN). Mice were considered to have proteinuria if at least two consecutive urine samples were greater than 2+, according to the scale (100 mg/dl). Blood samples were harvested monthly starting at 10 weeks of age and serum concentrations of total IgG and IgG-anti-dsDNA antibodies were determined by ELISA as described above. Survival of mice was monitored daily, and moribund mice were euthanized according to NIH guidelines. A control group of 15 NZB/W female mice was injected with phosphate buffered saline twice weekly starting at 10 weeks of age. An additional control group of 10 NZB/W female mice was injected twice weekly starting at 22 weeks of age. The two control groups were combined because there were no significant differences in proteinuria and survival (p>0.1). Kidney histopathologic changes were examined using paraffin tissue sections stained with hematoxylin and eosin or para-aminosalacylic acid (PAS). Photomicrographs were made using an Eclipse E 1000M microscope (Nikon, Melville, N.Y.) with a SPOT RT digital camera. Image processing was performed with PhotoshopCS (Adobe Systems, San Jose, CA) software.
Statistical analyses
Differences in mean immunoglobulin and cytokine concentrations were analyzed using the two-tailed Student's t test. A value of p < 0.05 was considered statistically significant. Statistical analyses of differences in the survival and kinetics of proteinuria of control and experimental groups of NZB/W mice were performed using the log-rank test.
Results
CD1d dimers loaded with α and β galactosylceramides bind to the invariant NKT cell TCR
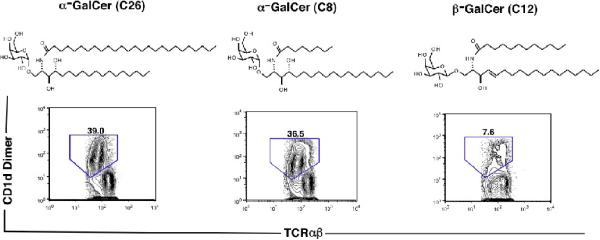
The chemical structures of the three galactosylceramides used in the current study are shown in Figure 1. α-GalCer with an acyl chain containing 26 carbons (C26) has been studied extensively, and is a potent activator of invariant NKT cells [11, 12]. α-GalCer with an acyl chain containing 8 carbons (C8) with the same phytosphingosine chain has been reported previously to activate invariant NKT cells and induce a Th2 biased cytokine patterns as compared to α-GalCer (C26) [10]. β-GalCer with an acyl chain containing 12 carbons (C12) differs from the α-GalCers with regard to the β anomeric oxygen linkage to the galactose moiety, and the presence of a sphingosine chain (one hydroxyl group) instead of a phytosphingosine chain (two hydroxyl groups). β-GalCer (C12) has been reported to induce minimal cytokine secretion in the serum [9].
Figure 1.
Chemical structures of α and β-galactosylceramide and staining of liver NKT cells after loading of galactosylceramides onto CD1d dimers. The structures include α-GalCer (C26) with a 26 carbon acyl chain, α-GalCer (C8) with an 8 carbon acyl chain, and β-GalCer with a 12 carbon acyl chain. The phytosphingosine chains of the α-anomers differ from the sphingosine chain of the β-anomer by the presence of a second hydroxyl group. Below each structure is the two color flow cytometric pattern of representative stainings of NZB/W liver mononuclear cells with CD1d dimers loaded with each galactosylceramide versus staining for TCRαβ. Mononuclear cells were gated first on the TCRαβ+ cells. The CD1d dimer+TCRαβ+ cells are enclosed in the boxes, and the percentage of TCRαβ+ T cells within the boxes are shown.
CD1d dimers were loaded with each of the three glycolipids at the same final concentration (50 ng/ml), and then used to stain liver mononuclear cells from 12 week old NZB/W female mice and 12 week old C57BL/6 female mice. Figure 1 shows the staining patterns of CD1d dimer versus TCRab after gating on TCRαβ+ liver mononuclear cells from NZB/W mice. Dimers loaded with α-GalCer (C26) and with α-GalCer (C8) stained about 36 to 39% of TCRαβ+ T cells whereas dimers loaded with β-GalCer (C12) stained only about 8% of TCRαβ+ T cells (Fig. 1). Staining of C57BL/6 liver mononuclear cells showed a similar pattern using the dimers loaded with α-GalCer (C26) and (C8), and only 2 to 5% of liver T cells were stained with dimers loaded with β-GalCer (C12) (data not shown). Control stainings of NZB/W and C57BL/6 liver mononuclear cells with unloaded dimers uniformly resulted in staining of less than 1% of TCRαβ+ T cells, and an increase in concentration of α-GalCer (C26) for loading dimers resulted in a gradual increase in the percentage of tetramer + stained cells until a plateau was achieved (data not shown).
Comparison of α and β-GalCer induction of NKT cell TCR down regulation and serum IL-4 and IFN-γ secretion
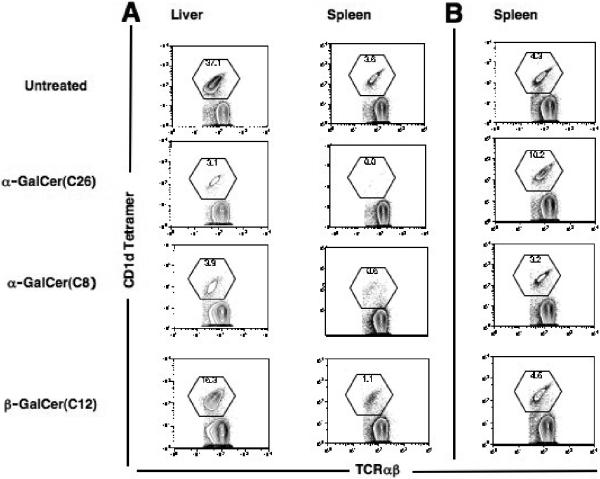
α-GalCer (C26), α-GalCer (C8), and β-GalCer (C12) were injected intraperitoneally into NZB/W and C57BL/6 mice, and 24 hours later the percentage of NKT cells among all T cells in the liver was determined by staining with CD1d tetramer versus TCRαβ. In preliminary studies, dose response curves were developed to determine the minimum dose of each glycolipid that resulted in the nadir of NKT cell reduction (data not shown). Figure 2A shows representative examples of CD1d tetramer versus TCRαβ staining on gated spleen and liver TCRαβ+ T cells in NZB/W mice for the nadir doses of the three glycolipids. Whereas injection of 0.1 μg of α-GalCer (C26) and 20 μg of α-GalCer (C8) resulted in about a 90% reduction in NKT cells, injection of 50μg of β-GalCer (C12) resulted in about a 60% reduction. The reduced percentage of NKT cells in the spleen at 24 hours returned to the untreated range or above at 72 hours (Fig. 2B), and a similar result was observed in the liver (data not shown). The transient decrease in NKT cells is consistent with down regulation of the NKT cell TCR as reported previously [13, 14].
Figure 2.
Downregulation of the NKT cell TCR 24 hours after intraperitoneal injection of a single dose of galactosylceramides into NZB/W mice. (A) shows the two color stainings of liver mononuclear cells and spleen cells of untreated mice, and of mice given single injection of 0.1μg α-GalCer (C26), 20μg α-GalCer (C8), and 50μg β-GalCer (C12). Cells were harvested 24 hours after injections, and gated TCRαβ+ cells were analyzed for staining with CD1d tetramers loaded with glycolipid PBS-57 (NIH Tetramer Facility) versus TCRαβ. Boxes enclose CD1d tetramer+ TCRαβ+ T cells. (B) shows spleen cells from mice 72 hours after injection of galactosyl ceramides or from an untreated mouse stained on the same day.
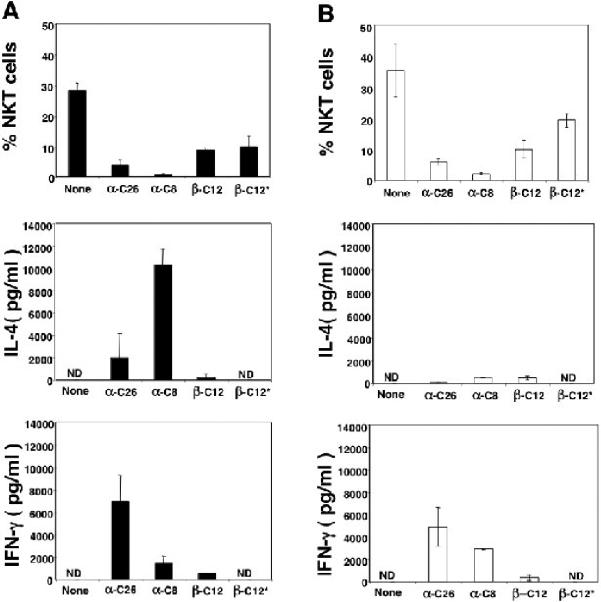
Figure 3A and B show that a single i.p. injection of 0.1μg α-GalCer (C26) reduced the mean percentage of NKT cells in the liver of C57BL/6 and NZB/W mice from about 28 and 35% respectively to about 5% or less in both strains (p<0.001). Similar reductions (p<0.001) in the mean percentage of NKT cells were achieved with 20μg of α-GalCer (C8) (Fig. 3A and B). Injection of 50μg β-GalCer (C12) reduced the mean percentage of NKT cells to about 10 to 12% (p<0.05) (Fig. 3A and B).
Figure 3.
Reductions in the percentages of liver NKT cells (CD1d tetramer+TCRαβ+) and increases in the concentrations of serum IL-4 and IFN-γ after administration of single doses of galactosylceramides into NZB/W or C57BL/6 mice. Bars in upper panels show the mean (±SD) percentages of NKT cells in untreated control mice or 24 hours after injections in experimental mice. Bars in lower panels show mean serum concentrations of IL-4 and IFN-γ 2 and 24 hours after injections respectively. There were 4 to 6 mice in each group for each dose. ND-not detectable. A, shows results in C57BL/6 mice and B shows results in NZB/W mice at age 12 weeks. None-untreated mice, α-C26-0.1μg, i.p., α-GalCer (C26); α-C8-20μg, i.p., α-GalCer (C8); β-C12-50μg, i.p., β-GalCer (C12); β-C12*-800μg, oral, β-GalCer (C12).
Figure 3A shows that α-GalCer (C26) induced mean concentrations of serum IL-4 of about 2,000 pg/ml, and of about 7,000 pg/ml IFN-γ at 2 and 24 hours respectively in C57BL/6 mice. The latter values represented peak concentrations as determined by measuring serum samples at 2, 6, 18, and 24 hours after glycolipid administration (data not shown). The ratio of the peak IFN-γ: IL-4 concentrations was about 3.5:1. The mean serum IL-4 concentration was less than 200 pg/ml using α-GalCer (C26) in NZB/W mice (Fig 3B). However, the mean concentration of serum IFN-γ was 5,000 pg/ml and the ratio of IFN-γ: IL-4 was about 25:1 (Fig. 3B). The result is consistent with the Th1 skewing of NZB/W NKT cell cytokine secretion reported previously [6, 7].
α-GalCer (C8) induced mean concentrations of serum IL-4 and IFN-γ of about 10,000 pg/ml and 2,000 pg/ml respectively in C57BL/6 mice (Fig. 3A). This resulted in a Th2 cytokine skewing as shown before with a ratio of IFN-γ: IL-4 of about 1:5 [15]. Injections of α-GalCer (C8) into NZB/W mice induced mean serum IL-4 and IFN-γ levels in the range of about 1,000 and 2,500 pg/ml respectively, and the ratio of IFN-γ: IL-4 was about 2.5:1. β-GalCer (C12) induced mean concentrations of serum IL-4 or IFN-γ below 500 pg/ml in both C57BL/6 and NZB/W mice (Figs. 3A and B), and was a weak agonist as compared to α-GalCer (C8) or α-GalCer (C26). The dichotomy of significant reductions of NKT cells in liver and minimal increases in IL-4 and IFN-γ in the serum after intraperitoneal injection of β-GalCer (C12) was reported previously by Ortaldo et. al. [9]. Neither α-GalCer (C26) or α-GalCer (C12) induced detectable levels of IL-4 or IFN-γ in the serum of Jα18-/-C57 BL6 mice (data not shown).
In further experiments, single doses of β-GalCer (C12) were administered orally (gavage), and dose response curves were developed to determine the minimal dose that induced the nadir of NKT cell reduction in the liver (data not shown). The nadir oral dose (800μg) was administered to C57BL/6 and NZB/W mice, and the mean percentage of NKT cells in the liver was determined 24 hours later. As shown in Figures 3A and B, oral administration of β-GalCer (C12) reduced the mean percentage of liver NKT cells to about 10%. Thus, a 16 fold higher dose of β-GalCer had to be administered orally to achieve the reduction observed with intraperitoneal administration. The mean concentrations of serum IL-4 and IFN-γ were not detectable (<20 pg/ml) at 2 and 24 hours respectively after the oral administration of β-GalCer (Figs. 3A and B).
β-GalCer (C12) but not α -GalCer (C26) reduces serum cytokine levels induced by a subsequent injection α-GalCer (C26)
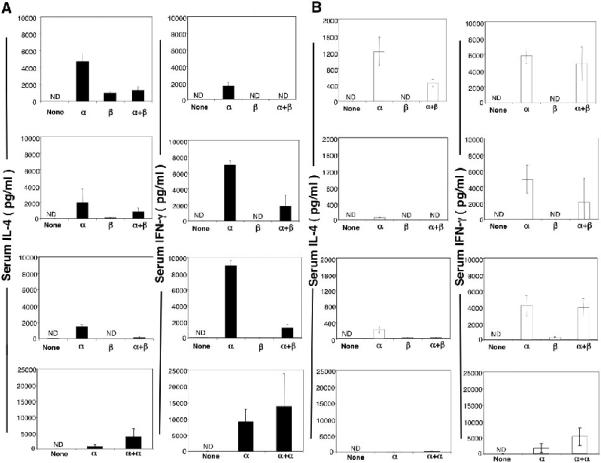
Since intraperitoneal injection of 50μg of β-GalCer (C12) induced significant decreases in the expression of the TCR of NKT cells in the liver and spleen with minimal increases in serum IL-4 and IFN-γ in C57BL/6 and NZB/W mice, we determined whether β-GalCer (C12) can reduce the considerably higher serum cytokine elevations induced by intraperitoneal injection of 0.1 μg of α-GalCer (C26). Figure 4A shows results of experiments with C57BL/6 mice in which a single dose of β-GalCer (C12) alone, α-GalCer (C26) alone, or β-GalCer (C12) followed 4 hours later by α-GalCer (C26) were injected intraperitoneally. The top row of panels indicate that the mean serum IL-4 concentration induced by α-GalCer (C26) alone (~ 5,000 pg/ml) was significantly attenuated when β-GalCer (C12) was injected 4 hours earlier (p< 0.004). β-GalCer (C12) alone induced a serum IL-4 concentration of about 1,000 pg/ml. A similar pattern was observed for IFN-γ in which the mean level induced by α-GalCer (C26) alone (~ 2,000 pg/ml) was markedly reduced by the earlier injection of β-GalCer (C12) (p< 0.0001).
Figure 4.
β-GalCer (C12) reduces secretion of IL-4 and IFN-γ induced by α-GalCer (C26) in NZB/W and C57BL/6 mice. (A) top row of panels, show the mean serum concentrations of IL-4 and IFN-γ 2 and 24 hours respectively in C57BL/6 mice after a single intraperitoneal injection of 0.1μg α-GalCer (C26) alone (α), a single injection of 50 μg β-GalCer (C12) alone, or an injection of 50μg β-GalCer (C12) followed by an injection 0.1μg α-GalCer (C26) 4 hours later. Untreated control NZB/W mice are shown also. Second row of panels show mean concentrations after a single intraperitoneal injection of 0.1μg α-GalCer,a single oral dose of 800μg β-GalCer, or after an oral dose at 800μg β-GalCer followed by an injection of α-GalCer 4 hours later. Third row of panels show mean concentrations after a single injection of 0.1μg α-GalCer, after 3 intraperitoneal injections of 50 μg β-GalCer,(48 hour intervals between injections) or after 3 injections of 50 μg β-GalCer followed 4 hours later by an injection of 0.1 μg α-GalCer. Bottom row of panels show mean concentrations after a single intraperitoneal injection of 0.1μg α-GalCer or after 2 injections of 0.1 μg α-GalCer separated by 4 hours. (B) shows the same analysis of mean serum concentrations of IL-4 and IFN-γ in NZB/W mice. There were 4 to 6 mice in each group. Note reduced scale of concentrations for IL-4 for NZB/W mice. ND- not detectable.
When β-GalCer (C12) was administered to C57BL/6 mice as a single oral dose of 800μg, the mean serum IL-4 and IFN-γ concentration were minimal (<100 pg/ml) as compared to the intraperitoneal injection of 0.1μg α-GalCer (C26) alone (Fig. 4A second row of panels). Oral administration of β-GalCer (C12) 4 hours before α-GalCer (C26) significantly reduced the mean concentration of IFN-γ (p<0.002) but not IL-4 (p>0.2) as compared to that observed with α-GalCer (C26) alone. In another series of experiments, β-GalCer (C12) was injected intraperitoneally (50μg each) into C57BL/6 mice every other day for a total of three doses, and the last dose was followed 4 hours later by an injection of α-GalCer (C26) (0.1μg). Figure 4A, third row of panels, shows that the multiple injections of β-GalCer (C12) significantly reduced (p< 0.0001) the levels of IL-4 and IFN-γ observed with α-GalCer (C26) alone. Since it was possible that any glycolipid that causes downregulation of the NKT cell TCR can inhibit cytokine secretion induced by α-GalCer (C26), C57BL/6 mice were given 0.1μg α-GalCer (C26) i.p. followed 4 hours later by a second injection of 0.1μg intrapertoneally. As shown in the bottom row of panels of Figure 4A, two injections of α-GalCer (C26) increased the concentrations of IL-4 and IFN-γ in the serum as compared to a single injection.
In Figure 4B, the experiments performed with C57BL/6 mice were repeated using instead NZB/ W mice. Again the administration of a single intraperitoneal injection of β-GalCer (C12) significantly reduced the serum IL-4 concentrations induced by α-GalCer (C26) alone (p<0.002). In contrast to the results with C57BL/6 mice, the β-GalCer (C12) did not significantly attenuate (p>0.05) the increase in serum IFN-γ induced by α-GalCer (C26) alone. Similar significant reductions of serum levels of IL-4 were obtained with oral or multiple injected β-GalCer, but the levels of IFN-γ were not significantly changed (p>0.05) (Fig. 4B). Two i.p. injections of α-GalCer resulted in increased serum levels of IL-4 and IFN-γ as compared to one injection (Fig. 4B). It should be noted that the administration of α-GalCer (C26) alone induced highly variable increases in the mean serum IL-4 and IFN-γ concentration in C57BL/6 mice, and in the mean serum IL-4 concentrations in NZB/W mice. For this reason all experimental groups used for comparison were challenged with the glycolipids alone or in combination on the same day, and were assayed for cytokine levels on the same day.
Administration of β-GalCer C(12) reduces spontaneous IgG secretion of NZB/W NKT and B cell cultures
Our previous studies showed that purified NZB/W NKT cells help purified NZB/W total B cells or B-1 B cells to spontaneously secrete IgM, IgG1, IgG2a, and IgM and IgG anti-dsDNA antibodies in vitro [6, 7]. Cultures contained no polyclonal activators of T or B cells, and conventional CD4+ T cells failed to help antibody secretion under the culture conditions that were studied[6, 7]. In order to determine whether in vivo administration of β-GalCer (C12) to NZB/W mice reduces spontaneous immunoglobulin secretion of cultures of NKT cells and B-1 B cells, 12 week old NZB/W female mice were given two intraperitoneal injections per week of 50 μg each of β-GalCer (C12) over a period of 2 weeks. Controls were given injections of phosphate buffered saline. One day after the fourth injection, spleen cells were harvested and purified splenic NKT cells and purified splenic B-1 B cells were cultured for 10 days. Supernatants were collected at the end of the culture period, and assayed for the concentrations of IgM, IgG1, and IgG2a. Figures 5A, B, and C show that cultures of B cells alone from control or treated mice had mean concentrations of less than 3 μg/ml IgM, and less than 10 μg/ml of IgG1 or IgG2a. Addition of control NKT cells to the control B cells enhanced secretion of IgM, IgG1, and IgG2a at least ten fold (Fig. 5). Treated mice showed a similar level of enhancement of IgM production when NKT cells and B cells were cultured together as compared to control mice (Fig. 5A). However, IgG1 and IgG2a mean concentrations in cultures from treated mice were about two to four fold lower than those from control mice, but the differences did not achieve statistical significance using 6 cultures with cells from a total of 8 mice (Figs. 5B and C) (p=0.06 for IgG1 and p=0.07 for IgG2 as judged by the two tailed student t test of independent means).
Figure 5.
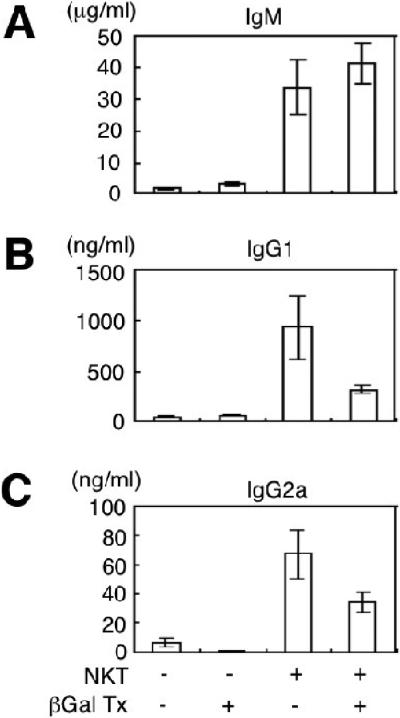
Injections of β-GalCer (C12) reduces spontaneous secretion of IgG1 and IgG2a in cultures of NZB/W NKT cells and B-1 B cells. NZB/W female mice, age 12 weeks, were injected intraperitoneally with phosphate buffered saline or 50 μg of β-GalCer (C12) 4 times over 2 weeks. Spleen cells were harvested 1 day after the last injection, and purified NKT cells and B-1 B cells, or B-1 B cells alone were cultured for 10 days. (A) compares the mean concentrations of IgM in cultures from control mice or β-GalCer treated mice (βGalTx) with B-1 B cells alone or with B-1 B cells and NKT cells. (B) and (C) show mean concentrations of IG1 and IG2a respectively. Brackets show standard errors. There were 6 wells in each group from pools of 4 mice in 2 replicate experiments.
Administration of β-GalCer C(12) ameliorates lupus in NZB/W mice
We compared the efficacy of treatment of NZB/W female mice using α-GalCer (C8), a glycolipid agonist that skewed the serum cytokine secretion toward a Th2 pattern and β-GalCer (C12), a glycolipid that reduced the serum cytokine secretion induced by the agonist α-GalCer (C26), and that reduced spontaneous IgG secretion of NKT and B cell cultures as noted above. In these experiments a control group of NZB/W mice was injected intraperitoneally twice weekly with phosphate buffered saline, the vehicle used for diluting the glycolipids.
In the first series of experiments the kinetics of proteinuria and survival in the vehicle treated control group was compared to groups given twice weekly intraperitoneal injections (20μg each) of α-GalCer (C8) or twice weekly injections (50μg) of β-GalCer (C12) starting at 10 weeks of age. As shown in Figures 6A and C, about 50% of the control mice developed proteinuria by about 36 weeks, and 50% died by about 48 weeks. In the experimental group treated with α-GalCer (C8) 50% of mice developed proteinuria by 30 weeks. The difference in kinetics of proteinuria between the control and α-GalCer (C8) group was not significant (p>0.1). In addition, the α-GalCer (C8) treatment worsened survival as compared to the control group, and 50% of the mice in the former group died by 36 weeks. Again, the difference was not significant (p>0.1) (Fig. 6C).
Figure 6.
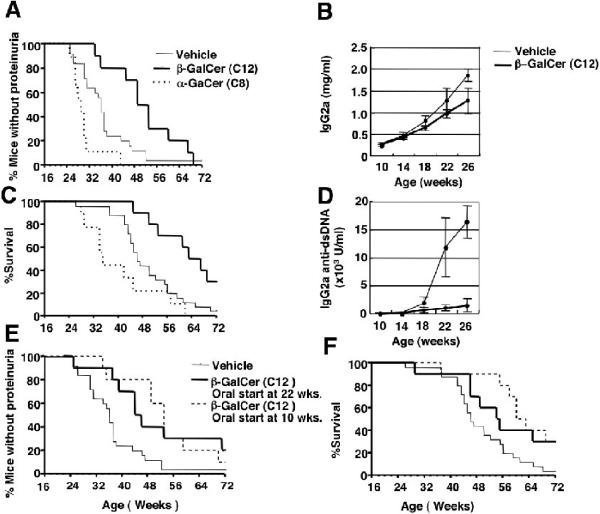
Injections of β-GalCer (C12) improve lupus and injections of α-GalCer (C8) worsen lupus in NZB/W mice. (A) shows the percentage of NZB/W female mice without proteinuria and (B) shows the percentage surviving versus age of mice (in weeks). Mice were given intraperitoneal injections twice per week starting at 10 weeks of age until death using either vehicle (PBS), 50 μg β-GalCer (C12) each, or 20μg α-GalCer (C8) each. There were 25, 10, and 9 and mice in the vehicle, β-GalCer, and α-GalCer groups. (C) shows the total serum IgG2a concentrations of the vehicle and β-GalCer treated groups between 10 to 26 weeks of age. Points represent means and brackets represent standard errors. (D) shows serum concentrations of IgG2a anti-dsDNA antibodies of the vehicle and β-GalCer treated groups. There were 10 samples in each group. In (E) and (F) NZB/W female mice were given 800 μg β-GalCer (C12) orally 3 times per week starting at age 22 weeks (5.5 months). Panels show percentage of mice without proteinuria, and percentage of mice surviving versus age. Control mice received twice weekly intraperitoneal injections of vehicle. There were 25 mice in the control group, and 10 mice in the experimental group.
In contrast, the β-GalCer (C12) treated group had a significantly delayed onset of proteinuria (p<0.02), and a significantly improved survival (p<0.007) as compared to the control group. (Figs. 6A and C). Whereas 50% of mice in the treated group developed proteinuria by 54 weeks, 50% of the control group had developed proteinuria about 18 weeks earlier. The point at which 50% of mice died was delayed about 18 weeks in the treated as compared to the control group, and 30% of treated mice survived at least 70 weeks as compared to less than 5% in the control group. The differences in the onset of proteinuria between the α-GalCer (C8) and β-GalCer (C12) treated groups were highly significant (p<0.0001) as were the differences in survival (p<0.0006) (Figs. 6B and C).
Weekly serum samples were examined for total IgG and IgG anti-dsDNA antibodies from all groups from the start of treatment at 10 weeks to the first onset of proteinuria at 26 weeks. We did not examine these serum parameters beyond 26 weeks, since IgG can be lost in urine and can result in low serum concentrations. Figures 6B and D show that both total serum IgG2a and the concentration of IgG2a anti-dsDNA antibodies rose progressively during the 10 to 26 week interval in the control group. Although β-GalCer (C12) treatment decreased the concentrations of total IgG2a in the serum at 18, 22 and 26 weeks of age as compared to the control group, the differences did not achieve statistical significance (p>0.05) (Fig. 6B). Nevertheless, there was a highly significant (p<0.001) attenuation of the rise in the IgG2a anti-dsDNA antibody levels in the serum as compared to the control group (Figure 6D). Kinetics of the rise in IgG and IgG2a anti-dsDNA antibodies were not significantly different when the control and α-GalCer (C8) groups were compared (p>0.1) (data not shown). We did not measure the levels of IgG anti-dsDNA serum antibodies beyond 26 weeks due to urinary loss of IgG as a confounding factor after that time.
Oral administration of β-GalCer (C12) improves lupus kidney disease activity in old NZB/W female mice
In another series of experiments, β-GalCer (C12) was administered by gavage on alternate days (total of 3 doses of 800μg each per week) to a group of NZB/W female mice starting at age 10 weeks. The oral treatment significantly delayed proteinuria (p<0.01) and significantly improved survival (p<0.01) as compared to the control group given the vehicle twice per week. (Figs. 6E and F). Subsequently, we administered the thrice weekly oral treatment to a group of NZB/W mice starting at age 22 weeks. Anti-dsDNA antibodies are detected in the serum of control mice at this time point (Fig. 6D). The oral β-GalCer (C12) treated group had significantly slowed kinetics of proteinuria (p<0.02) as compared to the vehicle treated group (Fig. 6E). The age at which 50% of the β-GalCer treated mice developed proteinuria was 10 weeks later than that of the control group. About 30% of the β-GalCer treated group had not developed proteinuria by 70 weeks, whereas less than 5% of the controls were without proteinuria at that point. More importantly, about 30% of the oral treatment group survived more than 70 weeks (18 months), whereas 96% of mice in the control group died by 68 weeks (Fig. 6F). Differences in the kinetics of survival of the control and treated groups were significant (p<0.01).
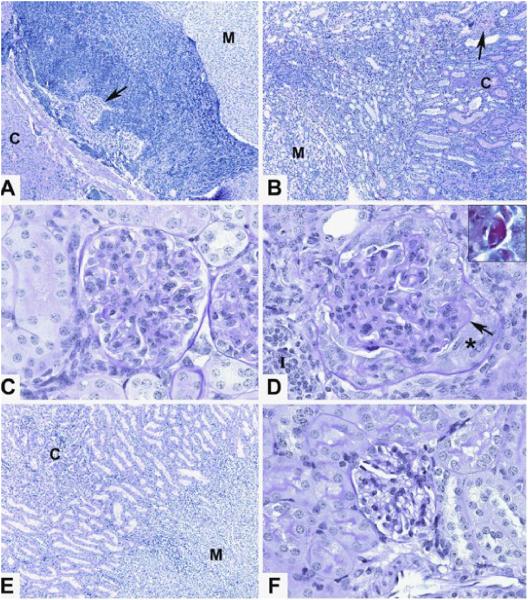
In order to determine the effect of oral β-GalCer (C12) treatment starting at 22 weeks on the histopathologic changes in the kidneys of the control and treated groups at age 49 weeks, NZB/W mice were treated or given vehicle in addition to those shown in Figures 6E and F. About 50% of the additional control mice died by 49 weeks, and all survivors were euthanized. Figure 7 shows representative histopathologic analysis of kidney tissue sections. Whereas the vehicle treated mice had a dense lymphocytic infiltrate in the corticomedullary junction that extended to the glomeruli, the β-GalCer (C12) treated mice had a markedly reduced infiltrate in the junction (Figs. 7A and B). Glomeruli in the vehicle treated group showed severe changes of injury including marked basement membrane thickening of capillary loops, and crescent formation (Fig. 7C). Intense staining of basement membranes with PAS was consistent with immune complex deposition (Fig. 7C). Glomeruli in the β-GalCer (C12) treated group showed less membrane thickening and little or no crescent formation (Fig. 7D). The corticomedullary junction and glomeruli of a kidney from an 8 week old NZB/W female mouse without proteinuria had a normal appearance (Figs. 7E and F).
Figure 7.
Reduced severity of glomerular injury and lymphocytic infiltration after treatment of NZB/W mice with β-GalCer. Control NZB/W mice given phosphate buffered saline or oral β-GalCer starting at age 22 weeks were euthanized at 49 weeks, and tissue sections of the kidneys were obtained. Sections were stained with hematoxylin and eosin or with PAS. (A) shows a representative section including the corticomedullary function (cortex-C, medulla-M) with a dense lymphocytic infiltrate. Arrow shows a glomerulus surrounded by infiltrate. 100x magnification. (B) shows the corticomedullary junction from a treated β-GalCer mouse with little infiltrate. Arrow shows a blood vessel in the cortex. 100x magnification. (C) shows a representative glomerulus from a β-GalCer treated mouse with mild capillary loop thickening and no crescent formation, 600x magnification. (D) shows a glomerulus from a control mouse with markedly thickened capillary loops. Arrow shows area of thickened basement membrane and insert shows intense PAS staining of that area. Asterix marks glomerular crescent. 600x magnification. (E) shows the corticomedullary junction of a kidney from an untreated 8 week old NZB/W female mouse without proteinuria for comparison. 100x magnification. (F) shows a normal appearing glomerulus from the 8 week old mouse, 600x magnification. There were 3-4 mice examined in the β-GalCer treated group and in the PBS treated group.
Discussion
The roles of NKT cells in murine autoimmune and allergic diseases are complex, and related not only to the specific disease entities but also to genetic background of the strains that are under investigation. Whereas the potent NKT cell activator, α-GalCer (C26), ameliorated lupus kidney disease in pristane treated BALB/c mice and skin disease in MRL/lpr mice, α-GalCer (C26) worsened lupus kidney disease in pristane treated B10.PL mice [16, 17]. Deficiency of NKT cells in CD1d-/- BALB/c mice worsened lupus nephritis as compared to wild-type mice after injection of pristane [18]. In contrast, deficiency of NKT cells in CD1d-/- MRL/lpr mice did not affect skin or kidney disease [19]. Very old (>24 months of age) wild-type BALB/c and C57BL/6 mice develop a spontaneous lupus-like glomerulonephritis that is significantly worse in CD1d-/- mice [20].
NZB/W NKT cells have an abnormal Th1 bias, and are likely to contribute to the pathogenesis of spontaneous lupus in NZB/W female mice [6]. Blocking of NKT cell activation with anti-CD1d mAb ameliorated lupus, and activation of NKT cells by administration of α-GalCer (C26) induced robust IFN-γ secretion and worsened lupus [6]. Purified NZB/W NKT cells augmented the spontaneous IgM secretion of purified NZB/W B cells as well as the secretion of IgM anti-dsDNA antibodies [6], and IgG anti-dsDNA antibodies in vitro [7]. These in vitro studies show that NKT cells can help B cells directly under the culture conditions tested. In addition, NKT cells and innate immune B-1 and marginal zone B cells are expanded in the lymphoid tissues, kidney, and peritoneum of NZB/W mice in an age dependent fashion [15, 21]. It is likely that the expansion and somatic hypermutation in B cells that result in high affinity autoantibody formation occurs in the germinal centers under the influence of conventional T helper cells that recognize autoantigens [1, 2]. The role of NKT cells in this process is unclear.
The goal of the current study was to determine whether a glycolipid, β-GalCer (C12), reported to reduce the number of NKT cells in the liver and lymphoid tissues while inducing minimal cytokine secretion in the serum [9], can reduce cytokine secretion induced by the NKT cell agonist, α-GalCer (C26), and ameliorate lupus. We confirmed that β-GalCer (C12) given intraperitoneally and orally resulted in a significant reduction of the percentage of NKT cells among all T cells in the liver and spleen of C57BL/6 and NZB/W mice 24 hours later as judged by both staining for NK1.1 (data not shown) and the NKT cell TCR. The reduction in NKT cells was transient, and levels returned to or above normal values at 72 hours. The results are best explained by the transient downregulation of the NK1.1 and T cell antigen receptors after glycolipid administration as reported previously [13, 14, 22, 23].
When β-GalCer (C12) was administered either intraperitoneally or orally 4 hours before the α-GalCer (C26), then the ability of the α-GalCer (C26) to induce both serum IL-4 and IFN-γ was significantly reduced in the C57BL/6 mice. In NZB/W mice, prior β-GalCer (C12) treatment significantly reduced the levels of IL-4 in the serum induced by α-GalCer (C26). Although the levels of IFN-γ were also reduced, the reduction was not significant. We evaluated the changes in the level of IL-17 and IL-10 also, we found no detectable levels before or after α-GalCer (C26) administration (data not shown). A possible explanation of the inhibitory activity of the β-GalCer (C12) is that the latter compound downregulated the NKT cell TCR with minimal cytokine secretion such that the NKT cell cytokine response to the subsequent injection of α-GalCer (C26) was blunted. TCR downregulation per se by any glycolipid that activates NK T cells is unlikely to explain the reduction, since administration of two injections of α-GalCer 4 hours apart increased cytokine secretion as compared to a single injection. In addition, a 2 week course of intraperitoneal injections of β-GalCer reduced the spontaneous IgG1 and IG2a secretion of cultures of purified NZB/W NKT cells and purified B-1 B cells. However, the reduction did not achieve statistical significance (p=0.06 and 0.07).
In further experiments, we treated NZB/W female mice with β-GalCer (C12) and α-GalCer (C8) with intraperitoneal injections twice per week starting at age 10 weeks. We hypothesized that β-GalCer (C12) would improve lupus disease activity as judged by proteinuria, serum antidsDNA antibody levels, and survival by altering NKT cell responses to endogenous glycolipids such as isoglobloside 3 [24]. We expected also that the Th2 biased response induced by α-GalCer (C8) could be beneficial in lupus, since the Th2 biased glycolipid OCH had been reported to be more effective in the treatment of experimental allergic encephalomyelitis than α-GalCer (C26) [25].
The experimental results showed that the β-GalCer (C12) treatment significantly slowed the onset of proteinuria and the rise in anti-dsDNA antibodies, and improved the survival of the mice as compared to vehicle or α-GalCer (C8) treated groups. In contrast, treatment with α-GalCer (C8) worsened proteinuria and survival as compared to the vehicle treated controls, but the differences were not significant. Although the α-GalCer (C8) induced a marked Th2 bias in C57BL/6 mice, it failed to induce a Th2 bias in NZB/W mice, and instead induced a robust increase in serum IFN-γ levels that was about 5 fold higher than that of IL-4. The ability of β-GalCer (C12) serum to reduce IL-4 but not IFN-γ induced by α-GalCer (C26) in NZB/W mice suggests that IL-4 secretion by NZB/W NK T cells contributes to lupus disease activity along with IFN-γ. In further experiments, oral treatment with β-GalCer (C12) was administered to NZB/W female mice starting at 22 weeks (5.5 months) of age. At the latter time, IgG anti-dsDNA antibody levels in the serum were rising rapidly in the control mice. The β-GalCer (C12) treated mice had a significant improvement in proteinuria and survival as compared to the vehicle treated mice. Importantly, the onset of proteinuria was delayed until 70 weeks and survival was prolonged beyond 80 weeks (18 months) in 30% of β-GalCer (C12) treated mice. The β-GalCer (C12) treated mice had a marked reduction in the dense lymphocytic infiltrate in the corticomedullary junction as compared to that of 49 week old vehicle treated mice. Although NKT cells have been reported to expand in the kidneys of old NZB/W mice as judged by flow cytometric analysis of kidney mononuclear cells [15], our preliminary studies indicate that the large majority of the lymphocytes in old NZB/W kidneys are conventional T cells (Morshed, S., unpublished observations). Thus, the lymphocytic infiltrate is NKT cell dependent, but not made up of predominantly NKT cells. It is not clear whether NKT cells in NZB/W mice recognize any lipid containing autoantigens such as cardiolipin, since almost all NKT cell TCR ligands that have been studied are glycosylceramides[10, 11, 12, 26].
The amelioration of NZB/W lupus by intraperitoneal injections of 50 μg of β-GalCer (C12) and the worsening of lupus by injections of α-GalCer is opposite to the results reported for the treatment of EAE in B6 mice [26]. Injections of 50 μg of β-GalCer worsened EAE, and injections of α-GalCer improved EAE [26]. However, the differences may be due to the effect of IL-4 on the underlying disease, since induction of robust IL-4 secretion may improve EAE but worsen lupus. In addition, the Th2 bias of CD1d reactive T cells in some strains of mice may contribute to amelioration of autoimmune diseases by α-GalCer treatment [27, 28, 29, 30, 31, 32], and Th1 bias of CD1d reactive T cells in other strains may contribute to worsening of autoimmune disease including lupus using the same treatment [7, 8]. Inactivation or depletion of Th2 biased NKT cell appears to have a harmful effect on some autoimmune diseases [16, 17, 18, 20]. In the case of NZB/W mice, inactivation of the CD1d gene and the associated depletion of invariant NKT cells resulted in exacerbation of lupus [33].The latter result may indicate a favorable regulatory role of NKT cells in young NZB/W mice, since the ability of NKT cells to help NZB/W B cells secrete autoantibodies in vitro and to develop a Th1 bias was observed only in old mice[7]. However, NK1.1 + TCR+ T cells remained in the NZB/W mice with the inactivated CD1d gene[33].
In conclusion, treatment with β-GalCer (C12) reduced the IL-4 or IFN-γ secretion induced by the subsequent injection of the potent NKT cell agonist α-GalCer (C26), whereas treatment with α-GalCer (C26) enhanced cytokine secretion in C57BL/6 and NZB/W mice. Administration of multiple injections of α-GalCer (C26) worsened lupus disease activity in NZB/NZW female mice [6]. β-GalCer (C12) treatment given either intraperitoneally or orally significantly improved lupus disease activity in NZB/W female mice even when it was started after the appearance of IgG antidsDNA antibodies in the serum.
Acknowledgments
We thank Glenna Letsinger for assistance in the submission of the manuscript. We thank the NIH tetramer facility, Rockville Md., for providing CD1d-tetramer.
Grant Support: This work was supported by grants from the National Institutes of Health NIAID RO1 AI-40093, NIH NIAMSD RO1 AR-051748
Abbreviations
- NK
natural killer
- dsDNA
double stranded DNA
- α-GalCer
α-galactosylceramide
- β-GalCer
β-galactosylceramide
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosures The authors have no commercial or financial conflict of interest.
References
- [1].Kotzin BL. Systemic lupus erythematosus. Cell. 1996;85:303–6. doi: 10.1016/s0092-8674(00)81108-3. [DOI] [PubMed] [Google Scholar]
- [2].Hahn BH. Antibodies to DNA. N Engl J Med. 1998;338:1359–68. doi: 10.1056/NEJM199805073381906. [DOI] [PubMed] [Google Scholar]
- [3].Wofsy D, Seaman WE. Successful treatment of autoimmunity in NZB/NZW F1 mice with monoclonal antibody to L3T4. J Exp Med. 1985;161:378–91. doi: 10.1084/jem.161.2.378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Datta SK, Patel H, Berry D. Induction of a cationic shift in IgG anti-DNA autoantibodies. Role of T helper cells with classical and novel phenotypes in three murine models of lupus nephritis. J Exp Med. 1987;165:1252–68. doi: 10.1084/jem.165.5.1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Ando DG, Sercarz EE, Hahn BH. Mechanisms of T and B cell collaboration in the in vitro production of anti-DNA antibodies in the NZB/NZW F1 murine SLE model. J Immunol. 1987;138:3185–90. [PubMed] [Google Scholar]
- [6].Zeng D, Liu Y, Sidobre S, Kronenberg M, Strober S. Activation of natural killer T cells in NZB/W mice induces Th1-type immune responses exacerbating lupus. J Clin Invest. 2003;112:1211–22. doi: 10.1172/JCI17165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Takahashi T, Strober S. Natural killer T cells and innate immune B cells from lupus-prone NZB/W mice interact to generate IgM and IgG autoantibodies. Eur J Immunol. 2008;38:156–65. doi: 10.1002/eji.200737656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Zeng D, Dick M, Cheng L, Amano M, Dejbakhsh-Jones S, Huie P, Sibley R, Strober S. Subsets of transgenic T cells that recognize CD1 induce or prevent murine lupus: role of cytokines. J Exp Med. 1998;187:525–36. doi: 10.1084/jem.187.4.525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [9].Ortaldo JR, Young HA, Winkler-Pickett RT, Bere EW, Jr., Murphy WJ, Wiltrout RH. Dissociation of NKT stimulation, cytokine induction, and NK activation in vivo by the use of distinct TCR-binding ceramides. J Immunol. 2004;172:943–53. doi: 10.4049/jimmunol.172.2.943. [DOI] [PubMed] [Google Scholar]
- [10].Goff RD, Gao Y, Mattner J, Zhou D, Yin N, Cantu C, 3rd, Teyton L, Bendelac A, Savage PB. Effects of lipid chain lengths in alpha-galactosylceramides on cytokine release by natural killer T cells. J Am Chem Soc. 2004;126:13602–3. doi: 10.1021/ja045385q. [DOI] [PubMed] [Google Scholar]
- [11].Kawano T, Cui J, Koezuka Y, Toura I, Kaneko Y, Motoki K, Ueno H, Nakagawa R, Sato H, Kondo E, Koseki H, Taniguchi M. CD1d-restricted and TCR-mediated activation of valpha14 NKT cells by glycosylceramides. Science. 1997;278:1626–9. doi: 10.1126/science.278.5343.1626. [DOI] [PubMed] [Google Scholar]
- [12].Kronenberg M. Toward an understanding of NKT cell biology: progress and paradoxes. Annu Rev Immunol. 2005;23:877–900. doi: 10.1146/annurev.immunol.23.021704.115742. [DOI] [PubMed] [Google Scholar]
- [13].Wilson MT, Johansson C, Olivares-Villagomez D, Singh AK, Stanic AK, Wang CR, Joyce S, Wick MJ, Van Kaer L. The response of natural killer T cells to glycolipid antigens is characterized by surface receptor down-modulation and expansion. Proc Natl Acad Sci U S A. 2003;100:10913–8. doi: 10.1073/pnas.1833166100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Harada M, Seino K, Wakao H, Sakata S, Ishizuka Y, Ito T, Kojo S, Nakayama T, Taniguchi M. Down-regulation of the invariant Valpha14 antigen receptor in NKT cells upon activation. Int Immunol. 2004;16:241–7. doi: 10.1093/intimm/dxh023. [DOI] [PubMed] [Google Scholar]
- [15].Forestier C, Molano A, Im JS, Dutronc Y, Diamond B, Davidson A, Illarionov PA, Besra GS, Porcelli SA. Expansion and hyperactivity of CD1d-restricted NKT cells during the progression of systemic lupus erythematosus in (New Zealand Black x New Zealand White)F1 mice. J Immunol. 2005;175:763–70. doi: 10.4049/jimmunol.175.2.763. [DOI] [PubMed] [Google Scholar]
- [16].Singh AK, Yang JQ, Parekh VV, Wei J, Wang CR, Joyce S, Singh RR, Van Kaer L. The natural killer T cell ligand alpha-galactosylceramide prevents or promotes pristaneinduced lupus in mice. Eur J Immunol. 2005;35:1143–54. doi: 10.1002/eji.200425861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].Yang JQ, Saxena V, Xu H, Van Kaer L, Wang CR, Singh RR. Repeated alpha-galactosylceramide administration results in expansion of NK T cells and alleviates inflammatory dermatitis in MRL-lpr/lpr mice. J Immunol. 2003;171:4439–46. doi: 10.4049/jimmunol.171.8.4439. [DOI] [PubMed] [Google Scholar]
- [18].Yang JQ, Singh AK, Wilson MT, Satoh M, Stanic AK, Park JJ, Hong S, Gadola SD, Mizutani A, Kakumanu SR, Reeves WH, Cerundolo V, Joyce S, Van Kaer L, Singh RR. Immunoregulatory role of CD1d in the hydrocarbon oil-induced model of lupus nephritis. J Immunol. 2003;171:2142–53. doi: 10.4049/jimmunol.171.4.2142. [DOI] [PubMed] [Google Scholar]
- [19].Chan OT, Paliwal V, McNiff JM, Park SH, Bendelac A, Shlomchik MJ. Deficiency in beta(2)-microglobulin, but not CD1, accelerates spontaneous lupus skin disease while inhibiting nephritis in MRL-Fas(lpr) nice: an example of disease regulation at the organ level. J Immunol. 2001;167:2985–90. doi: 10.4049/jimmunol.167.5.2985. [DOI] [PubMed] [Google Scholar]
- [20].Sireci G, Russo D, Dieli F, Porcelli SA, Taniguchi M, La Manna MP, Di Liberto D, Scarpa F, Salerno A. Immunoregulatory role of Jalpha281 T cells in aged mice developing lupus-like nephritis. Eur J Immunol. 2007;37:425–33. doi: 10.1002/eji.200636695. [DOI] [PubMed] [Google Scholar]
- [21].Morshed SR, Mannoor K, Halder RC, Kawamura H, Bannai M, Sekikawa H, Watanabe H, Abo T. Tissue-specific expansion of NKT and CD5+B cells at the onset of autoimmune disease in (NZBxNZW)F1 mice. Eur J Immunol. 2002;32:2551–61. doi: 10.1002/1521-4141(200209)32:9<2551::AID-IMMU2551>3.0.CO;2-C. [DOI] [PubMed] [Google Scholar]
- [22].Parekh VV, Wilson MT, Olivares-Villagomez D, Singh AK, Wu L, Wang CR, Joyce S, Van Kaer L. Glycolipid antigen induces long-term natural killer T cell anergy in mice. J Clin Invest. 2005;115:2572–83. doi: 10.1172/JCI24762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Uldrich AP, Crowe NY, Kyparissoudis K, Pellicci DG, Zhan Y, Lew AM, Bouillet P, Strasser A, Smyth MJ, Godfrey DI. NKT cell stimulation with glycolipid antigen in vivo: costimulation-dependent expansion, Bim-dependent contraction, and hyporesponsiveness to further antigenic challenge. J Immunol. 2005;175:3092–101. doi: 10.4049/jimmunol.175.5.3092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Zhou D, Mattner J, Cantu C, 3rd, Schrantz N, Yin N, Gao Y, Sagiv Y, Hudspeth K, Wu YP, Yamashita T, Teneberg S, Wang D, Proia RL, Levery SB, Savage PB, Teyton L, Bendelac A. Lysosomal glycosphingolipid recognition by NKT cells. Science. 2004;306:1786–9. doi: 10.1126/science.1103440. [DOI] [PubMed] [Google Scholar]
- [25].Miyamoto K, Miyake S, Yamamura T. A synthetic glycolipid prevents autoimmune encephalomyelitis by inducing TH2 bias of natural killer T cells. Nature. 2001;413:531–4. doi: 10.1038/35097097. [DOI] [PubMed] [Google Scholar]
- [26].Parekh VV, Singh AK, Wilson MT, Olivares-Villagomez D, Bezbradica JS, Inazawa H, Ehara H, Sakai T, Serizawa I, Wu L, Wang CR, Joyce S, Van Kaer L. Quantitative and qualitative differences in the in vivo response of NKT cells to distinct alpha- and beta-anomeric glycolipids. J Immunol. 2004;173:3693–706. doi: 10.4049/jimmunol.173.6.3693. [DOI] [PubMed] [Google Scholar]
- [27].Hong S, Wilson MT, Serizawa I, Wu L, Singh N, Naidenko OV, Miura T, Haba T, Scherer DC, Wei J, Kronenberg M, Koezuka Y, Van Kaer L. The natural killer T-cell ligand alpha-galactosylceramide prevents autoimmune diabetes in non-obese diabetic mice. Nat Med. 2001;7:1052–6. doi: 10.1038/nm0901-1052. [DOI] [PubMed] [Google Scholar]
- [28].Sharif S, Arreaza GA, Zucker P, Mi QS, Sondhi J, Naidenko OV, Kronenberg M, Koezuka Y, Delovitch TL, Gombert JM, Leite-De-Moraes M, Gouarin C, Zhu R, Hameg A, Nakayama T, Taniguchi M, Lepault F, Lehuen A, Bach JF, Herbelin A. Activation of natural killer T cells by alpha-galactosylceramide treatment prevents the onset and recurrence of autoimmune Type 1 diabetes. Nat Med. 2001;7:1057–62. doi: 10.1038/nm0901-1057. [DOI] [PubMed] [Google Scholar]
- [29].Wang B, Geng YB, Wang CR. CD1-restricted NK T cells protect nonobese diabetic mice from developing diabetes. J Exp Med. 2001;194:313–20. doi: 10.1084/jem.194.3.313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Hammond KJ, Poulton LD, Palmisano LJ, Silveira PA, Godfrey DI, Baxter AG. alpha/beta-T cell receptor (TCR)+CD4-CD8- (NKT) thymocytes prevent insulin-dependent diabetes mellitus in nonobese diabetic (NOD)/Lt mice by the influence of interleukin (IL)-4 and/or IL-10. J Exp Med. 1998;187:1047–56. doi: 10.1084/jem.187.7.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Lehuen A, Lantz O, Beaudoin L, Laloux V, Carnaud C, Bendelac A, Bach JF, Monteiro RC. Overexpression of natural killer T cells protects Valpha14- Jalpha281 transgenic nonobese diabetic mice against diabetes. J Exp Med. 1998;188:1831–9. doi: 10.1084/jem.188.10.1831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Jahng AW, Maricic I, Pedersen B, Burdin N, Naidenko O, Kronenberg M, Koezuka Y, Kumar V. Activation of natural killer T cells potentiates or prevents experimental autoimmune encephalomyelitis. J Exp Med. 2001;194:1789–99. doi: 10.1084/jem.194.12.1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Yang JQ, Wen X, Liu H, Folayan G, Dong X, Zhou M, Van Kaer L, Singh RR. Examining the role of CD1d and natural killer T cells in the development of nephritis in a genetically susceptible lupus model. Arthritis Rheum. 2007;56:1219–33. doi: 10.1002/art.22490. [DOI] [PMC free article] [PubMed] [Google Scholar]