Abstract
Preservation of neural stem cells (NSCs) in the adult peripheral nervous system (PNS) has recently been confirmed. However, it is not clear whether peripheral NSCs possess predestined, bona fide phenotypes or a response to innate developmental cues. In this study, we first demonstrated the longevity, multipotency, and high fidelity of sensory features of postmigrating adult dorsal root ganglia (aDRG) stem cells. Derived from aDRG and after 4–5 years in culture without dissociating, the aDRG NSCs were found capable of proliferation, expressing neuroepithelial, neuronal, and glial markers. Remarkably, these aDRG NSCs expressed sensory neuronal markers vesicular glutamate transporter2 (VGluT2—glutamate terminals), transient receptor potential vanilloid1 (TrpV1—capsaicin sensitive), phosphorylated 200 kDa neurofilaments (pNF200—capsaicin insensitive, myelinated), and the serotonin transporter (5-HTT), which normally is transiently expressed in developing DRG. Furthermore, in response to neurotrophins, the aDRG NSCs enhanced TrpV1 expression upon exposure to nerve growth factor (NGF), but not to brain-derived neurotrophic factor (BDNF). On the contrary, BDNF increased the expression of NeuN. Third, the characterization of aDRG NSCs was demonstrated by transplantation of red fluorescent-expressing aDRG NSCs into injured spinal cord. These cells expressed nestin, Hu, and β-III-tubulin (immature neuronal markers), GFAP (astrocyte marker) as well as sensory neural marker TrpV1 (capsaicin sensitive) and pNF200 (mature, capsaicin insensitive, myelinated). Our results demonstrated that the postmigrating neural crest adult DRG stem cells not only preserved their multipotency but also were retentive in sensory potency despite the age and long-term ex vivo status.
Keywords: Long-term potency, Neural progenitor cells, Spinal cord injury, Sensory neurons, Brain-derived neurotrophic factor (BDNF), Nerve growth factor (NGF)
Introduction
It is now known that in the adult, pools of neuroepithelial stem cells are retained in the central nervous system (CNS) (e.g., hippocampus, subventricular zone, olfactory bulb, and spinal cord) (11,14,39). Neural crest-derived stem cells in the peripheral nervous system (PNS) have recently been isolated and characterized from sensory neurons in dorsal root ganglia (DRG) (25) and trigeminal ganglia (23). The neural stem cells (NSCs) in PNS are particular interesting in that, compared to CNS stem cells, those in the DRG remain devoid of trophic influences of a ventricular system. The conservation of stem cells in the PNS is less understood than that of the CNS. Furthermore, the NSCs in the CNS, disregarding embryonic or adult origin, once isolated from their local environment, adopt various differential pathways and lose their phenotype potentials. The typical example is that the embryonic stem cells screened from the substantia nigra largely lose their ability to express dopamine neurons (3,27). It is unclear how long the neural crest-derived stem cells can be preserved without loosing their stem cell potency and how much differential potency is preserved through numerous divisions. In this study, we characterized the NSCs derived from adult rat DRG, allowing in vitro propagation over 4–5 years. Three levels of characterization were investigated in these 4–5-year-old adult DRG NSCs in order to test the long-lasting potency of stem cells: (a) the proliferation and stem cell nature, (b) ability to express sensory features, and (c) the reliance on neurotrophins for differentiation.
Another compelling question is whether the adult DRG NSCs preserve plasticity comparable to DRG of embryonic origin. Comparisons between NSCs from embryonic(eDRG) and adult DRG (aDRG) were made in vitro. Furthermore, these stem cells were transplanted in the CNS (spinal cord) to investigate their multipotency in vivo.
Bromodeoxyuridine (BrdU) incorporation during DNA synthesis, expression of nestin (intermediate neurofilament expressed after differentiation of neuroepithelial and neural crest-derived NSCs), differentiation into astrocytes, and various stages of neurons were demonstrated. Sensory features including expression of vesicular glutamate transporter2 (VGluT2—glutamate terminals), transient receptor potential vanilloid1 (TrpV1—capsaicin-sensitive sensory neurons), and phosphorylated 200 kDa neurofilaments (pNF200—capsaicin-insensitive myelinated sensory neurons) were tested. A specific capacity of sensory neurons to temporarily express serotonin transporter (5-HTT) during early stages of development was analyzed. Expression of 5-HTT in neural crest-derived structures such as DRG has been previously reported (16,41). Serotonin (5-HT) is multifunctional during development, serving for guidance, trophic support, and neurotransmission, but 5-HT is not synthesized locally, rather 5-HTT facilitates with uptake into developing sensory neurons (12,41).
Further, we investigated the essential responsiveness of the aDRG NSC to fate-determining neurotrophins during normal early development including nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF). The effect of neurotrophins on modulating sensory differentiation, including the expression of the capsaicin receptor TrpV1 and neuronal maturity were evaluated. Finally, to investigate the potentiality in vivo, aDRG NSCs were transfected with red fluorescent protein (RFP) and transplanted into the injured spinal cord. Multipotency and their sensory phenotypes were similarly analyzed. The series of analyses together revealed that the peripheral sensory neuron is far more conserved in stem cell potency than the known stem cells preserved in the CNS.
Materials and Methods
Procurement and Maintenance of Dorsal Root Ganglia Neural Stem Cells
This study utilized DRG NSCs from embryonic day (E) 14–16 and adult rats that were previously established in our laboratory. In brief, DRGs were dissected and removed from adult or pregnant (at gestation 14–16 days) Sprague-Dawley rats (Harlan-Sprague Dawley, Inc., Indianapolis, IN) under deep anesthesia. DRG were washed twice in sterile phosphate buffer before being transferred to Hank's balanced salt solution (HBSS) for further dissection of DRG cells. DRG NSCs were isolated and screened using 10 ng/ml of epidermal growth factor (EGF, Harlan Bioproducts for Science, Indianapolis, IN) and basic fibroblast growth factor (bFGF, Pepro-Tech, Rocky Hill, NJ) in Dulbecco's modified Eagle medium/F-12 Nutrient Mix (DMEM/F-12) media containing N2 supplement (12 μl/ml, Invitrogen, Carlsbad, CA), and penicillin-streptomycin (12 μl/ml, Sigma, St. Louis, MO) and grown in a humidified incubator at 37°C and 5% CO2. The neurospheres were formed within 72 h and were characterized for their neural stem cell capacity with nestin and BrdU staining every 3–6 months. The long-term DRG NSC cultures were maintained in the above-mentioned medium in the presence of EGF and basic fibroblast growth factor (bFGF) (10 ng/ml each) to further screen for EGF/bFGF-responsive NSCs. In brief, DRG NSC were maintained in free-floating neurosphere culture, in the above medium (changed twice/week), continuously for 3–4 years; after this time samples from the main DRG NSC lines were periodically frozen. During the biweekly medium changes throughout the years, no dissociation (trypsin digestion and cell transferring) was performed. Although we have not performed karotyping to verify spontaneous transformation, we noted no signs of tumor formation in our in vivo or in vitro assay. To assure long-term in vivo use of these cells, karyotying should be performed to assure this potential nature.
Proliferation Rate in Response to EGF and bFGF
At the time of analysis, the degree of responsiveness to EGF and bFGF as indicated by proliferation rate, was determined by plating undissociated aDRG NSCs into a six-well culture dish (Nunc) containing DMEM/F-12 media plus random assignment to treatment, including EGF, bFGF, or EGF + bFGF (10 ng/ml); we did not use untreated control groups because NSCs are not known to survive without trophic factor supplementation. The number of neurospheres per well was counted and standardized to approximately 250 ± 50 neurospheres. Neurospheres were counted on day 7 and 14 using the criteria stated below.
Clonal Analysis
Clonal analysis was conducted to assess whether aDRG NSCs could form neurospheres following dissociating the cells. We dissociated aDRG NSCs using 0.25% trypsin at 37°C for 30 min. Following three centrifuge–wash cycles, cells were plated in a 48-well culture dish at a final density of 10 cells/ml, in DMEM/F-12 medium plus 10 ng/ml EGF/bFGF, similar to methods previously reported for clonal neurosphere assays (36). EGF/bFGF supplementation was used based on optimal response as determined from results of trophic factor dependence. The number of cells and neurospheres in each well were counted at 7, 10, 14, and 21 days after plating. Neurospheres were defined as spherical-shaped aggregates containing five or more cells.
Data presented here include neurospheres, not single cells, or cells encompassed in neurospheres.
Bromodeoxyuridine (BrdU) Labeling
In order to demonstrate proliferation of DRG NSCs, 5 μM BrdU was added to the medium for 48 h. The BrdU-containing medium was washed out and replaced with fresh Neurobasal medium devoid of BrdU, containing 10% FBS. Differentiation continued for another 5 days. Cultures were fixed with freshly made 4% formaldehyde.
Differentiation Studies
The potentiality of EGF/bFGF-responsive aDRG and eDRG NSCs was characterized by differentiation. The EGF + bFGF supplementation was withdrawn and neurospheres were subplated into 16-well chamber slides (Nunc, Rochester, NY) coated with poly-D-lysine (20 mg/ml, Sigma) and laminin (1 mg/ml, Sigma). Cells differentiated for 7–21 days. Neurobasal medium supplemented with 1.2% penicillin-streptomycin (Invitrogen) for differentiation induction (2% fetal bovine serum, FBS) for 2 days and was followed by maintenance medium (10% FBS) for the remainder of the experiment. DRG NSCs cultures differentiated in a humidified incubator at 37°C; medium was changed twice per week. At the end of the differentiation period, the cells were washed with 0.1 M PBS and fixed with 4% formaldehyde for immunocytochemistry. Fixation for VGluT2 immunostaining included the use of freshly made 4% formaldehyde + 0.4% picric acid.
Antibodies and Immunocytochemistry
In general, DRG NSCs were washed in 0.1 M phosphate-buffered saline (PBS) and treated with 3% H2O2 in PBS to quench the endogenous peroxidase activity. For VGluT2 immunostaining, cells were first incubated in 1% Triton X-100 (Tx-100) overnight at 4°C. For in vitro immunostaining, nonspecific binding was blocked using 4% normal serum from the secondary antibody host species, plus 0.1% Tx-100 in PBS (blocking buffer). Primary antibodies (detailed in Table 1) were incubated overnight at room temperature in the corresponding blocking buffer above. For immunofluorescent staining, DRG NSCs were washed with PBS and incubated with an Alexa 350, 488, or 635 fluorophor-conjugated secondary antibody (against the primary antibody species) at room temperature for 90 min. Immunoperoxidase staining utilized a biotinylated secondary antibody and avidin-biotin peroxidase kit (ABC Elite, Vector Laboratories, Burlingame, CA). Peroxidase activity was revealed by incubation in 0.1% diaminobenzidine tetra-hydrochloride (DAB) in 0.05 M Tris-HCl buffer, followed by 0.003% H2O2, allowing visualization of the chromagen. Slides were counterstained with methyl green.
Table 1.
Antibodies for Immunocytochemistry
| Antibody | Purpose | Species | Dilution | Source |
|---|---|---|---|---|
| Stem cell markers | ||||
| BrdU* | proliferation | mouse | 1:250 | Sigma, St. Louis, MO |
| Nestin | neuroepithelial origin | mouse | 1:400 | University of Iowa, Developmental Studies Hybridoma Bank |
| Neuronal markers | ||||
| β-III-Tubulin (TuJ1) | immature neurons | mouse | 1:250 | Sigma, St. Louis, MO |
| Hu C/D† | immature neurons | mouse | 1:250 | Invitrogen, Carlsbad, CA |
| MAP-2abc (clone HM-2)* | immature & mature neurons | mouse | 1:250 | Sigma, St. Louis, MO |
| Neu N* | mature neurons | mouse | 1:1,000 | Chemicon International, Temecula, CA |
| Glial markers | ||||
| GFAP* | astrocytes | rabbit | 1:400 | Sigma, St. Louis, MO |
| GFAP† | astrocytes | rabbit | 1:400 | Dako, Carpenteria, CA |
| Sensory markers | ||||
| VGluT2* | glutamatergic terminals | guinea pig | 1:1,500 | Chemicon International, Temecula, CA |
| Serotonin transporter* | immature sensory neurons | rabbit | 1:1,000 | Zhou Laboratory |
| TrpV1 | capsaicin-sensitive neurons | rabbit | 1:1,000 | Chemicon International, Temecula, CA |
| pNF200 (RT97) | capsaicin-insensitive neurons | mouse | 1:100 | University of Iowa, Developmental Studies Hybridoma Bank |
Used only for in vitro DRG NSC characterization studies.
Used only for in vivo characterization of aDRG NSC posttransplant.
For BrdU immunocytochemistry, DRG NSCs were permeablized with 1% Tx-100 in PBS for 30 min followed by 2 N HCl in 0.05 M PBS at 37°C and neutralization with 0.1 M borate buffer. The endogenous peroxidase activity was quenched with 3% H2O2 in 10% methanol. Nonspecific binding was blocked with 10% normal serum from the secondary antibody host species. Primary antibody was incubated in 0.5% Tween-20 + 2% normal sheep serum in PBS was applied overnight. Fluorescein-isothiocyanate (FITC)-conjugated secondary was used for fluorescent detection. Negative controls for immunostaining included omission of primary antibody and incubation with preimmune serum from the same species used to produce the secondary antibody. The immunostaining was performed in triplicate. The clonal analysis and responsiveness to EGF/bFGF experiments were performed in duplicates. Slides were viewed using a Leitz Orthoplan II microscope using bright field if DAB was the chromagen. Fluorescent labeling was viewed using the corresponding FITC, rhodamine, or UV filter. Photographs were taken with a Spot II camera and image acquisition software.
Sensory Neuron Differentiation
The dependence on or induction of DRG NSCs sensory differentiation by NGF or BDNF, which occurs normally during development of migrating neural crest cells differentiating into DRG neurons (2,29,35) was determined. Both eDRG- and aDRG NSCs were supplemented with exogenous NGF (10 ng/ml, BD Biosciences, Bedford, MA) or BDNF (10 ng/ml, Alomone Labs, Israel) immediately after subplating, continuing twice per week until the end of the experiment. Neurotrophin controls received an equal volume of medium. For quantitative analyses, aDRG NSCs were dissociated by incubating them at 37°C for 45 min in a digest medium containing 10 mg papain, 100 mg protease, 10 mg DNase1, 12.4 μl 1 M MgSO4 per 100 ml HBSS. The cells were centrifuged, washed three times, and plated at a concentration of 3,000–3,500 cells per well. The medium was the same as described above and treatment consisted of 10 ng/ml NGF or BDNF; controls received an equal volume of medium. After 28 days, cells were fixed with 4% formaldehyde for immunostaining for TrpV1 expression for subsequent counting (described below). Because BDNF influences neuronal maturation, we investigated whether the proportion of mature neurons, detected with an antibody against mature neuronal nuclear protein (NeuN), increased in BDNF-treated aDRG NSCs. All TrpV1-immunostained, NeuN-immunostained cells, and Nissl-stained cells were counted on a Leitz Orthoplan2 microscope under a 25× objective. Inclusion criteria were as follows: TrpV1-immunostained cells must have punctuate-like brown DAB staining on the plasma membrane and NeuN-immunostained cells contained nuclear staining, in some cases filling the cytoplasm.
In Vivo Analysis After Transplantation
We wanted to assess whether aDRG NSCs have continued multipotentiality and the propensity for sensory differentiation as demonstrated in situ. Adult female Sprague-Dawley (Harlan Sprague-Dawley, Inc., Indianapolis, IN, age 77 days old, 200–250 g) were used for this experiment. Animals were kept in the Laboratory Animal Resource Center (LARC) at Indiana University under standard laboratory conditions with food and water ad libitum on a 12-h light/dark cycle. Animals used in these procedures were maintained in facilities fully accredited by the Association for the Assessment and Accreditation of Laboratory Animal Care (AAALAC). All experimental procedures were approved by the Institutional Animal Care and Use Committee of Indiana University School of Medicine (Indianapolis, IN) in accordance with the guidelines of the Institutional Animal Care and Use Committee of the National Institutes of Health and the Guide for the Care and Use of Laboratory Animals (Institute of Laboratory Animal Resources, Commission on Life Sciences, 1996).
Surgical Procedures
Animals were anesthetized under 1% isofluorane with supplemental oxygen. Spinal cord laminectomy was performed at the 9th to 11th thoracic vertebrate levels (T9 to T11). Transection of the cord was done using a microsurgical knife (Fine Science Tools) by moving the knife tip against the osseous surface of the wall of central canal. Moving the blade in the reverse direction ensured complete transection of the spinal cord at T10. The muscles were closed in layers with 5-0 nonabsorbable sutures. The skin was closed with EZ-clips (Stoelting Co., Wood Dale, IL). During the surgery and postoperative recovery, rats were kept on a heating pad to maintain body temperature and were returned to their cages upon displaying adequate forelimb mobility. Following surgery, rats were given subcutaneous buprinorphine (analgesia, 0.03 mg/kg), sterile saline (supplemental hydration, 6 cc for 3 days), and cefazolin (antibiotic, 50 mg/kg, for 7 days). Manual bladder evacuation was performed twice daily for the first 2 weeks after surgery, when urinary reflex returned for most rats.
Transplantation of Neural Stem Cells
Prior to transplantation, aDRG NSCs were transfected with adeno-associated virus (AAV constructs courtesy of Drs. Brandon K. Harvey and Yun Wang, NIDA, Bethesda, MD) and were prepared as previously described (17) to label the cells with red fluorescent protein (RFP); virus titer was estimated at 4.1 × 1012 viral genomes/ml based on real-time PCR data. At the time of spinal cord injury, 4.3 × 104 RFP aDRG NSCs were transplanted directly into the injury site at T10. Surgical procedures, anesthesia, and wound closure were followed as detailed above.
Two weeks postinjury, rats were sacrificed by transcardial perfusion with 0.9% saline followed by 4% formaldehyde in 0.1 M PB (pH 7.0), under deep ketamine cocktail anesthesia (100 mg/kg ketamine, 2.2 mg/kg acepromazine, and 0.48 mg/kg atropine, diluted in 0.9% sterile saline). Three animals were assessed for expression of neuronal and sensory markers; data are reported as proportion of RFP NSCs expressing each marker (Table 3). The cord was segmentally cut at 1-cm increments: lesion site (L, centered at T10 transection site), 1 and 2 cm rostral, and 1 cm caudal to L, embedded together in gelatin and sectioned at 40 μm on a vibratome. Every sixth section was used for immunostaining. Primary antibodies for immunostaining are detailed in Table 1 and secondary antibodies are discussed above. In addition to use of preimmune serum controls for immunostaining, sections of heart, lung, liver, kidney, and muscle tissue were simultaneously stained in the same vial with the spinal cord sections.
Table 3.
Summary of Results From In Vivo Characterization
| Antigen | Endogenous | Colocalization With RFP+ aDRG NSCs | Purpose/Significance |
|---|---|---|---|
| Neural progenitor character | |||
| Nestin | + | ND | Endogenous NPC recruitment |
| Neural differentiation | |||
| β-III-Tubulin | + | 33% | Immature neurons |
| Hu | + | + | Immature neurons |
| Glial differentiation | |||
| GFAP | + | + | Mature astrocytes |
| Sensory character | |||
| TrpV1 | + | 25% | Capsaicin-sensitive nociceptive neurons |
| pNF200 | + | 10% | Capsaicin-insensitive myelinated proprioreceptive & mechanoreceptive neurons |
These results demonstrate the multipotent nature of aDRG NSCs following transplant into the injured spinal cord and their capacity for sensory differentiation. The data indicate the percent of RFP+ cells in the dorsal columns with immuno-staining for the given neuronal or sensory marker. Although no quantitation was performed, at least 33% of cells per section were β-III-tubulin/RFP+, 10% of cells were pNF200/RFP+, and about 25% per section were TrpV1/RFP+ for in vivo studies. ND, not detected.
Statistical Analysis
For the stem cell proliferation analysis, to examine the effect of EGF, bFGF, or combined EGF/bFGF treatment on proliferation (n = 3), ANOVA was used to compare the number of neurospheres between treatment groups. Significant results from ANOVA were analyzed by Fisher's Protected Least Significant Difference (PLSD) post hoc test for all pairwise comparisons. Unpaired Student's t-tests were used to compare the percent total TrpV1-immunostained cells between controls (n = 33) and NGF-treated (n = 35) cultures or to compare the percent total NeuN-immunostained cells between controls (n = 18) and BDNF-treated (n = 18). Statistical analysis was performed using Statview (SAS, Inc. Cary, NC).
Results
Stem Cell Proliferation Analyses
Neurosphere Formation
Adult and embryonic DRG NSCs were maintained under proliferating conditions in vitro for 4–5 years following procurement. Screening with EGF and bFGF allowed for isolation of NSCs, which aggregated to form neurospheres—spherical clusters of NSCs. The gross morphology in the phase contrast image of eDRG-NSCs (Fig. 1A) and aDRG NSCs (Fig. 1B) appeared similar, occurring as clustered as neurospheres (arrow), as shown nearly 3 weeks after procurement, or existing as individual cells. The trophic factor responsiveness, of 4–5 years' nondissociated aDRG NSCs, to EGF, bFGF, or a cocktail of EGF + bFGF was indicated by the continued formation of neurospheres (Fig. 1C). Significant differences in the number of neurospheres over time as well as day × treatment interactions (p < 0.05) were demonstrated. The neurosphere formation of eDRG and aDRG NSCs were similar despite the age difference of the animals at the start of NSC isolation.
Figure 1.
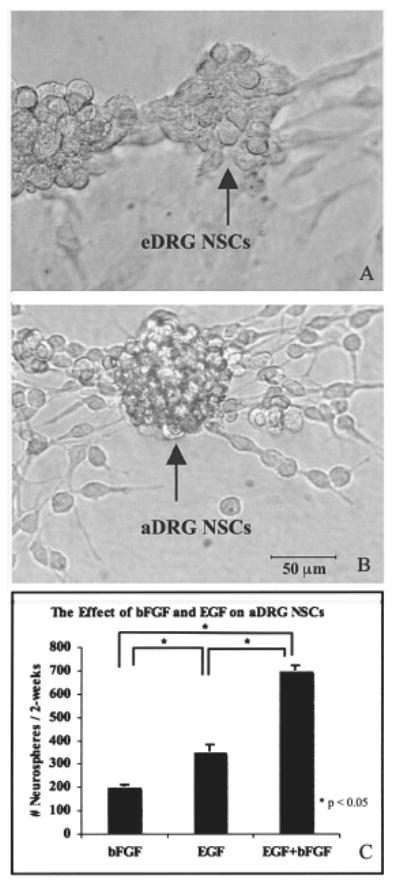
Embryonic DRG (eDRG) NSCs (A) and adult DRG (aDRG) NSCs (B) began to form neurospheres (arrow) after 20 days of treatment with EGF and bFGF. Responsiveness of aDRG NSCs, maintained in the progenitor cell state, to EGF, bFGF, and EGF + bFGF (10 ng/ml) revealed that a combination of EGF + bFGF resulted in the formation of significantly more neurospheres per 2 weeks compared to EGF or bFGF alone (p < 0.05) (C). Scale bar: 20 μm (A, B).
Clonal Analysis
The clonal analysis from dissociated cells indicted that clonal neurospheres were first evident after 7 days in culture; between days 14 and 21, 50% of the wells contained at least one neurosphere; in some wells, up to four neurospheres were found. After 21 days, 55% of wells contained neurospheres, which averaged 3 ± 1 (mean ± SEM) neurospheres formed per well. These results demonstrate that a small percentage of aDRG NSCs are capable of clonal neurosphere formation.
BrdU Incorporation
BrdU immunocytochemistry revealed that proliferation occurred during the 48 h of BrdU exposure. The robust BrdU immunostaining present in eDRG NSCs (Fig. 2A) is in contrast to relatively sparse BrdU immunostaining that was present in aDRG NSCs (Fig. 2B). Approximately 10 times as many cells had BrdU immunostaining in eDRG NSC cultures compared to their adult counterparts. Differentiation of early migrating cells began within hours after subplating. After 48 h, both neuronal- and glial-like morphology were seen in both eDRG and aDRG NSCs.
Figure 2.
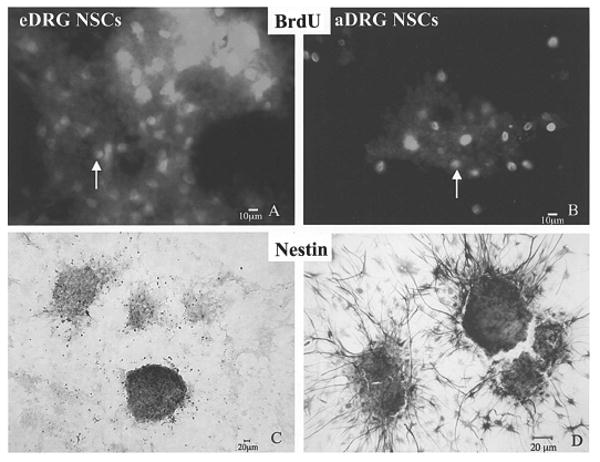
BrdU is incorporated in the DNA of eDRG NSCs (A) and aDRG NSCs (B). BrdU immunostaining (arrow) showed abundant BrdU-positive cells in the eDRG-NPCs shown in (A), as well as aDRG NSCs (B). The expression of intermediate neurofilament, nestin, is shown for eDRG NSCs (C) and aDRG NSCs (D), indicating their neuroepithelial nature. Scale bars: 10 μm (A, B); 20 μm (C, D).
Neural Differentiation
After 7–21 days of subculture, many of the aDRG and eDRG NSCs expressed the neuroepithelial marker, nestin, in the long, thin extending fibers (Fig. 2C and D for eDRG and aDRG NSCs, respectively). MAP-2abc staining on the soma, axon, and dendrites of neuronal-like cells (for both immature and mature neurons) was positive in differentiated eDRG NSC cultures (Fig. 3A) and was evident in differentiated aDRG NSCs (Fig. 3B). β-III-Tubulin immunostaining [immature and mature neuronal-like cells (28)] was seen in the soma and dendrites of neuronal-like cells derived from eDRG NSCs (Fig. 3C) and aDRG NSCs (Fig. 3D).
Figure 3.
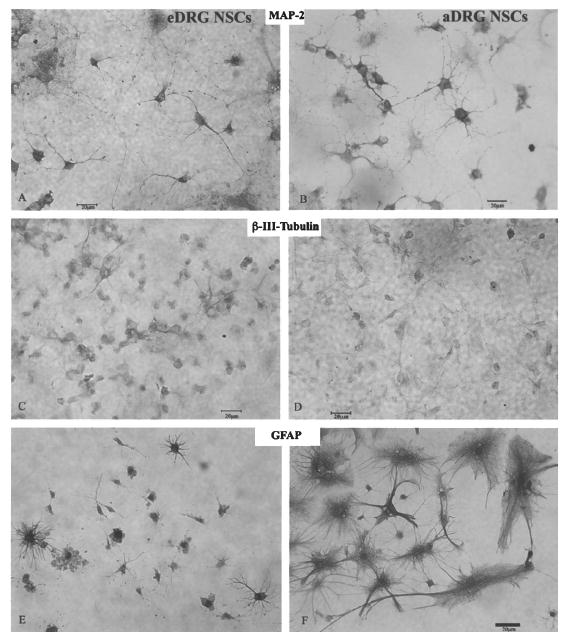
Neuron-like cells that differentiated from eDRG NSCs (A) and aDRG NSCs (B) stain positive for MAP-2abc. MAP-2abc immunostaining is seen in the soma, axon, and dendrite. β-III-Tubulin was also expressed in differentiated eDRG NSCs (C) and aDRG NSCs (D). GFAP immunostaining after differentiation shows that eDRG NSCs (E) and aDRG NSCs (F) differentiate into astrocyte-like cells. Both type 1 (protoplasmic, arrow) and type 2 (fibrous) astrocyte-like cells with their differential morphology of fibrous processes are seen among aDRG-derived cells. Scale bar: 20 μm.
GFAP immunostaining was found in astrocyte-like cells (Fig. 3E and F, respectively). Both type 1 (filamentous) astrocyte-like cells (Fig. 3F), with glial filaments lining the thin twig-like processes and type 2 (fibrous) astrocyte-like cells (arrowhead, Fig. 3F) containing thick branching processes, were present in both eDRG and aDRG NSCs.
Sensory Neuron Differentiation
We detected VGluT2 (as a glutamatergic neuron marker)-positive staining in both eDRG and aDRG NSCs (Fig. 4A, B). VGluT2 immunostaining was seen along the fibers and soma of aDRG and eDRG NSCs. Although we expected VGluT2 immunostaining to be localized in or near terminals, distribution in the soma, and along the processes suggests VGluT2 is transported to the terminal following synthesis. Transient receptor potential TrpV1 immunostaining (nociceptive, capsaicin-sensitive sensory neuron marker) (4,15,30,38) was found in differentiated eDRG and aDRG NSCs (Fig. 4C, D). Another population of eDRG and aDRG NSCs expressed pNF200 (marker for capsaicin-insensitive, myelinated DRG neurons) (Fig. 4E, F). These markers demonstrated multiple phenotypes of sensory neurons.
Figure 4.
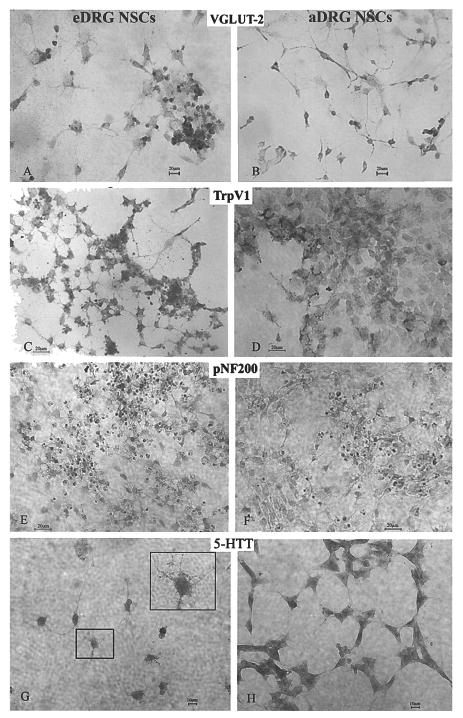
Sensory differentiation of DRG NSCs. Expression of the vesicular glutamate transporter-2 (VGluT2) for packaging neuronal glutamate into synaptic vesicles in glutamatergic neurons is evident on neuronal-like cells derived from both eDRG (A) and aDRG NSCs (B) 14 days after differentiation. TrpV1 (a marker for capsaicin-sensitive neurons) expression, indicated by immunostaining, is shown in eDRG NSCs (C) and aDRG NSCs (D) differentiated for 14 days in the absence of NGF treatment. Expression of phosphorylated 200 kDa neurofilament (pNF200), a marker for capsaicin-insensitive myelinated neurons is seen in the neuronal-like processes as well as in the soma of eDRG NSCs (E) and in aDRG NSCs (G). Expression of 5-HTT was found on cells derived from both eDRG NSCs (G) and aDRG NSCs (H) after 7 days of differentiation. The inset in (G) shows the 5-HTT immunostaining on the processes of eDRG NSCs. This provides evidence that after 4–5 years in the stem cell state, the aDRG and eDRG stem cells differentiated into neurons expressing sensory markers. Scale bar: 10 μm (A, B); 20 μm (C–H).
The transient expression of 5-HTT in the developing DRG (16,41) was also recapitulated in both eDRG and aDRG NSCs. Figure 4G and H show the presence of 5-HTT on neuronal-like cells derived from NSCs. The 5-HTT immunostaining was seen on differentiated NSCs along the processes decorated with spine- and varicosity-like protrusions. A detailed comparison of the multi-potentiality of aDRG and eDRG NSCs from our in vitro characterization is summarized in Table 2.
Table 2.
Summary of Results From In Vitro Characterization
| Antigen | eDRG NSCs* | aDRG NSCs* | Location | Purpose/Significance |
|---|---|---|---|---|
| Neural progenitor character | ||||
| BrdU | 3+/3 | 4+/4 | nucleus | Proliferation/DNA synthesis |
| Nestin | 4+/5 | 5+/5 | neurites | Neuroepithelial/neural crest origin |
| Neural differentiation | ||||
| β-III-Tubulin | 4+/5 | 5+/5 | somatodendritic | Immature neurons |
| MAP2abc | 5+/5 | 5+/5 | axon | Immature & mature neurons |
| Neu N | NT | 4+/4 | nucleus | Mature meurons |
| Glial differentiation | ||||
| GFAP | 5+/5 | 5+/5 | type I (filamentous) atrocytes | Glial differentiation |
| GFAP | 5+/5 | 5+/5 | type II (fibrous) astrocytes | Glial differentiation |
| Sensory character | ||||
| VGluT2 | 3+/3 | 3+/3 | synaptic vesicle-soma & axon terminal | Glutamatergic neurons/remote synthesis & transport |
| 5-HTT | 3+/3 | 2+/3 | plasma membrane/axon | Immature sensory neurons |
| TrpV1 | 5+/5 | 5+/5 | plasma membrane | Capsaicin-sensitive nociceptive neurons |
| pNF200 | 3+/3 | 3+/3 | Cpsaicin-insensitive proprioreceptive & mechanoreceptive neurons | |
| Neurotrophin responsive† | NA | aDRG NSCs responsiveness is similar to their developmental character |
The protein expression profiles of eDRG and aDRG NSCs provide evidence for and indicate the existence of a multipotent neural and sensory differentiating NSC derived from adult and embryonic DRG. The data are a summary of multiple cultures. The differentiation studies reveal that protein expression is remarkably similar between aDRG and eDRG-NSCs, despite the vast difference in temporal age of animals used for DRG-NSC procurement. NT, not tested; NA, not applicable.
The “x+/y” represents number (x) of positive replicates/total number (y) of replicate trials. Each replicate had eight or more wells. The “+” refers to replicates in which >80% wells are positive; the positive well refers to >10% of cells with positive phenotype marker in each well.
Quantitation detailed in Figure 5C and F.
Responsiveness of aDRG NSCs to NGF and BDNF
The responsiveness of DRG to innate neurotrophins for phenotype differentiation during early development was recapitulated in our long-term cultured aDRG NSCs in response to exogenous neurotrophins. The proportion of TrpV1 immunostaining in aDRG NSCs was significantly increased (p < 0.05) at the presence of NGF (Fig. 5A–C). In contrast, the percentage of TrpV1 immuno-staining in the BDNF-treated cultures was not significantly different compared to the controls (not shown). BDNF, however, increased the percentage of NeuN-positive cells (p < 0.0001) (Fig. 5D–F).
Figure 5.
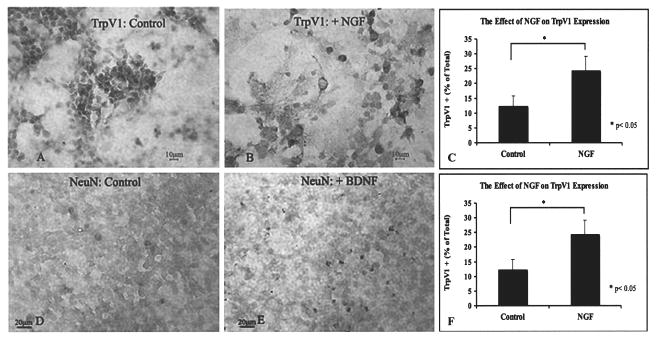
Neurotrophic factor responsiveness of aDRG NSCs. Immunostaining of TrpV1 in untreated controls (A) and NGF-treated cultures (B). Responsiveness of aDRG NSCs to NGF measured by expression of TrpV1 resulted in a significant increase in the proportion of TrpV1-positive cells (p < 0.05) (C). BDNF modulated neuronal maturation and expression of NeuN: controls (D) and BDNF treated (E). BDNF treatment resulted in significantly more NeuN-positive cells (p < 0.0001) as shown in (F). Scale bars: 20 μm (A, B, D, E).
In Vivo Assay: Differentiation of aDRG NSCs Following Transplantation
Two-weeks after transplantation, RFP+ aDRG NSCs were evident within the lesion area and up to 3 cm rostral and 1 cm caudal to the injury site. Most of the aDRG NSCs were found near the central canal or within the dorsal columns, specifically in the gracile fasciculus. A few aDRG NSCs scattered in the ventromedial funiculus in the white matter and within the gray matter. At the lesion site, many RFP+ aDRG NSCs were found but there was little colocalization with nestin (Fig. 6A–C). In the white matter, approximately one third of RFP+ aDRG NSCs in the dorsal columns and in the proprio-spinal and corticospinal tracts have neuronal-like morphology; the presence of β-III-tubulin/RFP+ cells (Fig. 6D–F) revealed that many differentiated into neural-like cells. β-III-Tubulin/RFP+ cells were located near the central canal, dorsal and ventral horn in the gray matter. RFP+ aDRG NSCs also expressed RNA binding protein Hu, but only a few were GFAP/RFP+ (Fig. 6G–I). Table 3 shows a summary of in vivo results. In examining sensory differentiation, we found a population of sensory neurons expressed pNF200, colocalized with RFP+ aDRG NSCs (Fig. 6J–L) in the dorsal columns rostral and caudal to injury. The pNF200/RFP+ aDRG NSCs accounted for 10% of RFP+ cells per section. A second population of aDRG NSCs expressed TrpV1 (Fig. 6M–O) in the dorsal columns and gray matter of the dorsal horn predominantly rostral to the injury site. One fourth of the RFP+ cells were TrpV1/RFP+ cells. The boxed area in Figure 6M, shown in detail in figure 6N, shows TrpV1 immunostaining of differentiated aDRG NSCs, indicating differentiation into capsaicin-sensitive sensory neurons.
Figure 6.
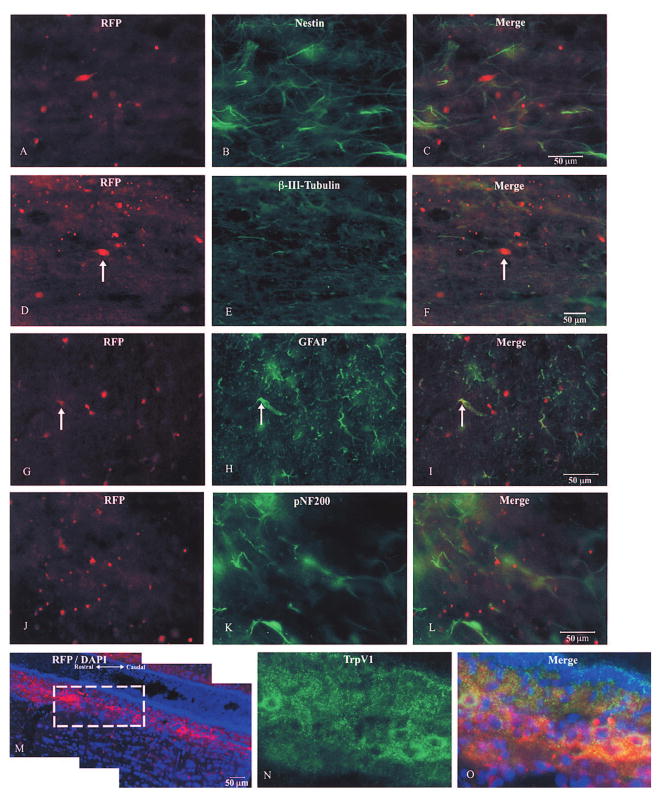
Multipotentiality of transplanted RFP-expressing aDRG NSCs. Immunostaining of neuroepithelial marker nestin (B) does not colocalize with nestin (C). Neural differentiating RFP aDRG NSCs (D) expressed β-III-tubulin (E), merged image in (F). Differentiated RFP aDRG NSCs (G) expressed GFAP, colocalization revealed in (H). Sensory differentiating RFP aDRG NSCs (I) expressed pNF200 (J—myelinated, capsaicin-insensitive sensory neurons); merged image shown in (L). RFP aDRG NSCs migrating (M, blue is DAPI counterstain) differentiated into TrpV1+ (N, same region as box in M) capsaicin-sensitive sensory neurons, merged image in O. Scale bars: 50 μm (C, F, L, M).
Discussion
Retentive Dorsal Root Ganglia Progenitor Cells
The issue of whether neurogenesis occurs in the adult mammalian DRG has been debated, suggesting that late migrating neural crest cells may enter DRG and then remain in an undifferentiated state, thus explaining a potential source of NSCs (6,13). Studies on age-related neurogenesis in adult DRG provide some evidence for the existence of latent NSCs. Several studies found an age-related increase in the number of DRG neurons (10,32). It has also been proposed that the number of DRG neurons decrease with age (1,13). Yet another possibility is that there is no change in the number of DRG neurons with increasing age (22,31). In DRG from post-natal and adult rats, a significant difference was found in the total number of neurons between neonatal and adult ages, yet bromodeoxyuridine (BrdU) labeling, an indicator of proliferation, was not evident (9). These studies argue that a quiescent NSCs population in DRG may continue neurogenesis through adulthood with declines in neuron number occurring in old age. Recently, we and others have demonstrated that a protracted maturation process persists in trigeminal and dorsal root ganglia and a population of undifferentiated neural crest cells remains into adulthood (23,25). Our current results further demonstrated that a population of cells with stem cell potential are not only preserved, but also after ex vivo can remain dormant for years after being awakened for differentiation of native phenotypes.
Progenitor and Sensory Features of Long-Term Cultured aDRG Neural Stem Cells
The 4–5-year-old aDRG NSCs not only preserve neuroepithelial features and can become neurons and astrocytes, but can also differentiate into sensory neurons as those of eDRG (Table 2). The aDRG NSCs were VGluT2 immunostained as the pan sensory neurons (20,26,37) and were also either TrpV1 or pNF200 immunostained. TrpV1 is known to localize to small and medium, mostly unmyelinated capsaicin-sensitive sensory neurons (4,15,30,38); while the pNF200 corresponds to myelinated, neurofilament-rich, capsaicin-insensitive proprioceptive and mechanoreceptive sensory neurons in DRG (19,24,34).
Furthermore, the preservation of transient 5-HTT expression was found in these long-term aDRG NSCs. The expression of serotonin is known to be important in embryonic and sensory system development. Transient expression of 5-HTT, responsible for uptake of serotonin, has been reported in developing neural crest tissues including DRG (5,16,19) and somatosensory cortex (41). We were expecting that eDRG NSCs, but not aDRG NSCs, would possess 5-HTT immunostaining. On the contrary, we found 5-HTT-im on both aDRG and eDRG NSCs.
Neurotrophic Modulation of Phenotypes
In contrast to normal DRG sensory development, which depends on a neurotrophin and its receptors for phenotypes, we found that the eDRG and aDRG NSCs were not entirely dependent on neurotrophins for sensory differentiation, as revealed by VGluT2 (sensory transmitter), TrpV1 (capsaicin receptor), and pNF200 (myelinated sensory fibers) immunostaining in control groups. This indicates that both eDRG NSC and aDRG NSC have been primed for their phenotypes at the time of procurement, and the epigenetic conditions were passed on to the daughter cells throughout the long-term culture. However, NGF did enhance the proportion of TrpV1-expressing sensory neurons; while BDNF enhanced neuronal maturation by increasing NeuN+ neurons, but did not increase the sensory differentiation. This is in agreement that BDNF accelerates the maturation of neurons in sensory ganglia during the developing period of neurotrophin independence (33,40). Neuro-trophins have been shown to regulate gene expression for NSC differentiation (18). It is likely that the differentiation of the phenotypes also depends on the expression of neurotrophin receptors such as p75 or Trk A, B, and C in the DRG NSCs (8).
Comparison of Potentiality In Vitro and In Vivo
Similar to DRG NSCs in vitro, neural differentiation was also evident in transplanted aDRG NSCs. Expression of Hu, an RNA binding protein, and β-III-tubulin were detected. We found that transplanted neural crest-derived DRG NSCs maintain multipotency when transplanted into the spinal cord. Sensory properties included expression of TrpV1 and pNF200 in the RFP+ aDRG NSCs after transplant. TrpV1 immunostaining/RFP+ and pNF200 immunostaining/RFP+ was found in the dorsal columns. Endogenous expression of TrpV1 was evident in the dorsal horn and pNF200 myelinated sensory fibers remained in the dorsal columns, ventrolateral white matter, and in DRG. Many studies have transplanted NSCs into the CNS. Thus, our results demonstrate that aDRG-derived NSCs are capable of sensory differentiation after transplantation in the injured spinal cord. Sensory differentiation of aDRG NSCs suggests a potential application in transplanting these NSCs for sensory deficits in addition to other known NSC transplant treatments for spinal cord injury (21). One of the known benefits for transplanting DRG cells into the injured spinal cord is their ability to overcome inhibitory proteoglycans and grow into and beyond the injury penumbra (7), suggesting another future application for DRG NSCs.
Comparison of aDRG and eDRG Neural Stem Cells
A remarkable finding in this report is that the age apparently did not make substantial change between the eDRG and aDRG in terms of stem cell preservation and their potential for sensory differentiation. We have found that aDRG and eDRG NSCs, following long-term maintenance without dissociating, displayed neural stem cell character (i.e., BrdU incorporation and nestin expression) and maintained multipotentiality—differentiating into glial, neuronal, and sensory-like cells. The preservation of aDRG NSCs response to NGF and BDNF treatments suggests that adult DRG is plastic and responsive to neurotrophic treatment for replenishment at least in the rat.
Conclusions
This study demonstrates that a subpopulation of post-migrating neural crest progenitor cells in the adult rat dorsal root ganglia not only preserve multipotent neural stem cells, but also after 4–5 years of culture displayed a retentive propensity to turn into neurons with sensory features. These neural crest progenitor cells in the adult dorsal root ganglia preserved as vigorous a propensity for multipotency as those from embryonic origins and respond to neurotrophin for their fate determination. In vivo transplantation into the injured spinal cord reveals continued capacity for differentiation into multiple phenotypes of sensory neurons, confirming retention of DRG-specific characteristics. This is the first evidence of such longevity of stem cells with sensory propensity. The long-term tenacity and stability are important features of understanding the stem cells in translational applications.
Acknowledgments
This work is partially supported by The Spinal Cord & Head Injury Research Center, Indiana University School of Medicine (F.C.Z. and P.N.); AA016698 (F.C.Z.); R.P.S. is partially supported by National Institute on Aging Fellowship.
References
- 1.Bergman E, Ulfhake B. Loss of primary sensory neurons in the very old rat: neuron number estimates using the disector method and confocal optical sectioning. J Comp Neurol. 1998;396(2):211–222. [PubMed] [Google Scholar]
- 2.Buchman VL, Davies AM. Different neurotrophins are expressed and act in a developmental sequence to promote the survival of embryonic sensory neurons. Development. 1993;118(3):989–1001. doi: 10.1242/dev.118.3.989. [DOI] [PubMed] [Google Scholar]
- 3.Caldwell MA, Svendsen CN. Heparin, but not other proteoglycans potentiates the mitogenic effects of FGF-2 on mesencephalic precursor cells. Exp Neurol. 1998;152(1):1–10. doi: 10.1006/exnr.1998.6815. [DOI] [PubMed] [Google Scholar]
- 4.Caterina MJ, Julius D. The vanilloid receptor: A molecular gateway to the pain pathway. Annu Rev Neurosci. 2001;24:487–517. doi: 10.1146/annurev.neuro.24.1.487. [DOI] [PubMed] [Google Scholar]
- 5.Chauvet N, Drian MJ, Privat A. Immunocytochemical study of phenotypic plasticity of cultured dorsal root ganglion neurons during development. Int J Dev Neurosci. 1995;13(7):673–683. doi: 10.1016/0736-5748(95)00066-6. [DOI] [PubMed] [Google Scholar]
- 6.Ciaroni S, Cecchini T, Cuppini R, Ferri P, Ambrogini P, Bruno C, Del Grande P. Are there proliferating neuronal precursors in adult rat dorsal root ganglia? Neurosci Lett. 2000;281(1):69–71. doi: 10.1016/s0304-3940(00)00785-0. [DOI] [PubMed] [Google Scholar]
- 7.Davies SJ, Goucher DR, Doller C, Silver J. Robust regeneration of adult sensory axons in degenerating white matter of the adult rat spinal cord. J Neurosci. 1999;19(14):5810–5822. doi: 10.1523/JNEUROSCI.19-14-05810.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Delfino-Machin M, Chipperfield TR, Rodrigues FS, Kelsh RN. The proliferating field of neural crest stem cells. Dev Dyn. 2007;236(12):3242–3254. doi: 10.1002/dvdy.21314. [DOI] [PubMed] [Google Scholar]
- 9.Farel PB. Late differentiation contributes to the apparent increase in sensory neuron number in juvenile rat. Brain Res Dev Brain Res. 2003;144(1):91–98. doi: 10.1016/s0165-3806(03)00163-9. [DOI] [PubMed] [Google Scholar]
- 10.Farel PB. Sensory neuron addition in juvenile rat: Time course and specificity. J Comp Neurol. 2002;449(2):158–165. doi: 10.1002/cne.10274. [DOI] [PubMed] [Google Scholar]
- 11.Gage FH, Kempermann G, Palmer TD, Peterson DA, Ray J. Multipotent progenitor cells in the adult dentate gyrus. J Neurobiol. 1998;36(2):249–266. doi: 10.1002/(sici)1097-4695(199808)36:2<249::aid-neu11>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 12.Gaspar P, Cases O, Maroteaux L. The developmental role of serotonin: News from mouse molecular genetics. Nat Rev Neurosci. 2003;4(12):1002–1012. doi: 10.1038/nrn1256. [DOI] [PubMed] [Google Scholar]
- 13.Geuna S, Borrione P, Fornaro M, Giacobini-Robecchi MG. Neurogenesis and stem cells in adult mammalian dorsal root ganglia. Anat Rec. 2000;261(4):139–140. doi: 10.1002/1097-0185(20000815)261:4<139::AID-AR2>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 14.Gritti A, Bonfanti L, Doetsch F, Caille I, Alvarez-Buylla A, Lim DA, Galli R, Verdugo JM, Herrera DG, Vescovi AL. Multipotent neural stem cells reside into the rostral extension and olfactory bulb of adult rodents. J Neurosci. 2002;22(2):437–445. doi: 10.1523/JNEUROSCI.22-02-00437.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guo A, Vulchanova L, Wang J, Li X, Elde R. Immunocytochemical localization of the vanilloid receptor 1 (VR1): Relationship to neuropeptides, the P2X3 purinoceptor and IB4 binding sites. Eur J Neurosci. 1999;11(3):946–958. doi: 10.1046/j.1460-9568.1999.00503.x. [DOI] [PubMed] [Google Scholar]
- 16.Hansson SR, Mezey E, Hoffman BJ. Serotonin transporter messenger RNA expression in neural crest-derived structures and sensory pathways of the developing rat embryo. Neuroscience. 1999;89(1):243–265. doi: 10.1016/s0306-4522(98)00281-4. [DOI] [PubMed] [Google Scholar]
- 17.Howard DB, Powers K, Wang Y, Harvey BK. Tropism and toxicity of adeno-associated viral vector sero-types 1, 2, 5, 6, 7, 8, and 9 in rat neurons and glia in vitro. Virology. 2008;372(1):24–34. doi: 10.1016/j.virol.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hsu YC, Lee DC, Chiu IM. Neural stem cells, neural progenitors, and neurotrophic factors. Cell Transplant. 2007;16(2):133–150. [PubMed] [Google Scholar]
- 19.Kai-Kai MA. Cytochemistry of the trigeminal and dorsal root ganglia and spinal cord of the rat. Comp Biochem Physiol A. 1989;93(1):183–193. doi: 10.1016/0300-9629(89)90206-5. [DOI] [PubMed] [Google Scholar]
- 20.Kaneko T, Fujiyama F, Hioki H. Immunohistochemical localization of candidates for vesicular glutamate transporters in the rat brain. J Comp Neurol. 2002;444(1):39–62. doi: 10.1002/cne.10129. [DOI] [PubMed] [Google Scholar]
- 21.Kim BG, Hwang DH, Lee SI, Kim EJ, Kim SU. Stem cell-based cell therapy for spinal cord injury. Cell Transplant. 2007;16(4):355–364. doi: 10.3727/000000007783464885. [DOI] [PubMed] [Google Scholar]
- 22.La Forte RA, Melville S, Chung K, Coggeshall RE. Absence of neurogenesis of adult rat dorsal root ganglion cells. Somatosens Mot Res. 1991;8(1):3–7. doi: 10.3109/08990229109144723. [DOI] [PubMed] [Google Scholar]
- 23.Lagares A, Li HY, Zhou XF, Avendano C. Primary sensory neuron addition in the adult rat trigeminal ganglion: Evidence for neural crest glio-neuronal precursor maturation. J Neurosci. 2007;27(30):7939–7953. doi: 10.1523/JNEUROSCI.1203-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lawson SN, Harper AA, Harper EI, Garson JA, Anderton BH. A monoclonal antibody against neurofilament protein specifically labels a subpopulation of rat sensory neurones. J Comp Neurol. 1984;228(2):263–272. doi: 10.1002/cne.902280211. [DOI] [PubMed] [Google Scholar]
- 25.Li HY, Say EH, Zhou XF. Isolation and characterization of neural crest progenitors from adult dorsal root ganglia. Stem Cells. 2007;25(8):2053–2065. doi: 10.1634/stemcells.2007-0080. [DOI] [PubMed] [Google Scholar]
- 26.Li JL, Fujiyama F, Kaneko T, Mizuno N. Expression of vesicular glutamate transporters, VGluT1 and VGluT2, in axon terminals of nociceptive primary afferent fibers in the superficial layers of the medullary and spinal dorsal horns of the rat. J Comp Neurol. 2003;457(3):236–249. doi: 10.1002/cne.10556. [DOI] [PubMed] [Google Scholar]
- 27.Ling ZD, Potter ED, Lipton JW, Carvey PM. Differentiation of mesencephalic progenitor cells into dopaminergic neurons by cytokines. Exp Neurol. 1998;149(2):411–423. doi: 10.1006/exnr.1998.6715. [DOI] [PubMed] [Google Scholar]
- 28.Memberg SP, Hall AK. Dividing neuron precursors express neuron-specific tubulin. J Neurobiol. 1995;27(1):26–43. doi: 10.1002/neu.480270104. [DOI] [PubMed] [Google Scholar]
- 29.Memberg SP, Hall AK. Proliferation, differentiation, and survival of rat sensory neuron precursors in vitro require specific trophic factors. Mol Cell Neurosci. 1995;6(4):323–335. doi: 10.1006/mcne.1995.1025. [DOI] [PubMed] [Google Scholar]
- 30.Michael GJ, Averill S, Nitkunan A, Rattray M, Bennett DL, Yan Q, Priestley JV. Nerve growth factor treatment increases brain-derived neurotrophic factor selectively in TrkA-expressing dorsal root ganglion cells and in their central terminations within the spinal cord. J Neurosci. 1997;17(21):8476–8490. doi: 10.1523/JNEUROSCI.17-21-08476.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mohammed HA, Santer RM. Total neuronal numbers of rat lumbosacral primary afferent neurons do not change with age. Neurosci Lett. 2001;304(3):149–152. doi: 10.1016/s0304-3940(01)01781-5. [DOI] [PubMed] [Google Scholar]
- 32.Popken GJ, Farel PB. Sensory neuron number in neonatal and adult rats estimated by means of stereologic and profile-based methods. J Comp Neurol. 1997;386(1):8–15. [PubMed] [Google Scholar]
- 33.Robinson M, Adu J, Davies AM. Timing and regulation of trkB and BDNF mRNA expression in placode-derived sensory neurons and their targets. Eur J Neurosci. 1996;8(11):2399–2406. doi: 10.1111/j.1460-9568.1996.tb01203.x. [DOI] [PubMed] [Google Scholar]
- 34.Sann H, McCarthy PW, Jancso G, Pierau FK. RT97: A marker for capsaicin-insensitive sensory endings in the rat skin. Cell Tissue Res. 1995;282(1):155–161. doi: 10.1007/BF00319142. [DOI] [PubMed] [Google Scholar]
- 35.Sieber-Blum M. Role of the neurotrophic factors BDNF and NGF in the commitment of pluripotent neural crest cells. Neuron. 1991;6(6):949–955. doi: 10.1016/0896-6273(91)90235-r. [DOI] [PubMed] [Google Scholar]
- 36.Singec I, Knoth R, Meyer RP, Maciaczyk J, Volk B, Nikkhah G, Frotscher M, Snyder EY. Defining the actual sensitivity and specificity of the neurosphere assay in stem cell biology. Nat Methods. 2006;3(10):801–806. doi: 10.1038/nmeth926. [DOI] [PubMed] [Google Scholar]
- 37.Todd AJ, Hughes DI, Polgar E, Nagy GG, Mackie M, Ottersen OP, Maxwell DJ. The expression of vesicular glutamate transporters VGLUT1 and VGLUT2 in neurochemically defined axonal populations in the rat spinal cord with emphasis on the dorsal horn. Eur J Neurosci. 2003;17(1):13–27. doi: 10.1046/j.1460-9568.2003.02406.x. [DOI] [PubMed] [Google Scholar]
- 38.Tominaga M, Caterina MJ, Malmberg AB, Rosen TA, Gilbert H, Skinner K, Raumann BE, Basbaum AI, Julius D. The cloned capsaicin receptor integrates multiple pain-producing stimuli. Neuron. 1998;21(3):531–543. doi: 10.1016/s0896-6273(00)80564-4. [DOI] [PubMed] [Google Scholar]
- 39.Weiss S, Dunne C, Hewson J, Wohl C, Wheatley M, Peterson AC, Reynolds BA. Multipotent CNS stem cells are present in the adult mammalian spinal cord and ventricular neuroaxis. J Neurosci. 1996;16(23):7599–7609. doi: 10.1523/JNEUROSCI.16-23-07599.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wright EM, Vogel KS, Davies AM. Neurotrophic factors promote the maturation of developing sensory neurons before they become dependent on these factors for survival. Neuron. 1992;9(1):139–150. doi: 10.1016/0896-6273(92)90229-7. [DOI] [PubMed] [Google Scholar]
- 41.Zhou FC, Sari Y, Zhang JK. Expression of serotonin transporter protein in developing rat brain. Brain Res Dev Brain Res. 2000;119(1):33–45. doi: 10.1016/s0165-3806(99)00152-2. [DOI] [PubMed] [Google Scholar]


