Abstract
Transcriptional activators in prokaryotes have been shown to stimulate different steps in the initiation process including the initial binding of RNA polymerase (RNAP) to the promoter and a postbinding step known as the isomerization step. Evidence suggests that activators that affect initial binding can work by a cooperative binding mechanism by making energetically favorable contacts with RNAP, but the mechanism by which activators affect the isomerization step is unclear. A well-studied example of an activator that normally exerts its effect exclusively on the isomerization step is the bacteriophage λ cI protein (λcI), which has been shown genetically to interact with the C-terminal region of the σ70 subunit of RNAP. We show here that the interaction between λcI and σ can stimulate transcription even when the relevant portion of σ is transplanted to another subunit of RNAP. This activation depends on the ability of λcI to stabilize the binding of the transplanted σ moiety to an ectopic −35 element. Based on these and previous findings, we discuss a simple model that explains how an activator's ability to stabilize the binding of an RNAP subdomain to the DNA can account for its effect on either the initial binding of RNAP to a promoter or the isomerization step.
Many transcriptional activators in prokaryotes bind to specific sequences associated with the promoters they regulate and affect the initiation process through direct contacts with RNA polymerase (RNAP; subunit structure, α2ββ′σ) (1–3). The process of transcription initiation in Escherichia coli can be described by a simplified two-step model (4). First, RNAP binds to fully duplex promoter DNA to form what is called the closed complex. Formation of this complex is reversible and is described by an equilibrium binding constant KB. For transcription to initiate, the closed complex must then isomerize to form the transcriptionally active open complex in which the DNA is locally melted to expose the transcription start site. This isomerization step is usually irreversible and is described by a forward rate constant kf. The cAMP receptor protein (CRP) is a well-characterized example of an activator that can exert its effect exclusively on KB, whereas the bacteriophage λcI protein is an activator that normally exerts its effect exclusively on kf (4–6).
CRP activates transcription from the lac promoter by binding to a recognition site centered 61.5 bp upstream from the start point of transcription and contacting the α subunit of RNAP (7). The α subunit consists of two independently folded domains, an N-terminal domain (NTD) and a C-terminal domain (CTD), separated by a flexible linker region (8–10). Whereas the αNTD mediates formation of the α dimer and serves as a scaffold for the assembly of RNAP, the αCTD is a DNA-binding domain that also serves as the target for many transcriptional activators (1, 3, 11). When bound at the lac promoter, CRP has been shown to stabilize the binding of the αCTD to the DNA in the region between the CRP recognition site and the promoter −35 element (7). Thus, CRP appears to work by a simple cooperative binding mechanism (1–3), stabilizing the closed complex at the lac promoter (4, 6).
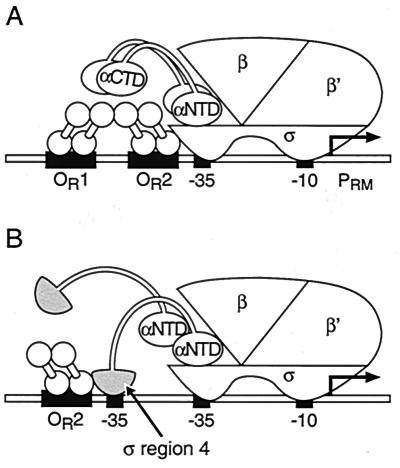
In contrast, λcI activates transcription from the λ promoter PRM when bound to an operator site centered 42 bp upstream from the start point of transcription and is thought to contact the σ subunit of RNAP (reviewed in ref. 12). λcI is a two-domain protein that binds as a dimer to its operator sites on the phage chromosome (13). Its NTD contains a helix-turn-helix DNA-binding motif, and its CTD mediates dimer formation as well as cooperative binding to pairs of operator sites (14). At the right operator region (OR), λcI dimers bind cooperatively to sites OR1 and OR2, and the dimer at OR2 activates transcription from promoter PRM (see Fig. 1A) (13). The isolation of λcI mutants specifically defective for activation (positive control mutants) led originally to the identification of a positive control surface located in the NTD of λcI (15–17). The suggestion that λcI uses this positive control surface to contact the σ subunit of RNAP is based on the isolation and analysis of σ mutants that affect λcI-stimulated transcription (18–20).
Figure 1.
(A) λcI binds cooperatively to operator sites OR1 and OR2 to activate transcription from PRM. Activation is mediated by the λcI dimer bound at OR2, which likely contacts the σ70 subunit of RNAP. (B) Genetic strategy for detecting the interaction between λcI and region 4 of σ70. Replacement of the RNAP αCTD with region 4 of σ70 permits interaction between the transplanted region of σ70 and a λcI dimer bound adjacent to an auxiliary −35 element. The artificial promoter derivative plac OR2–55/Cons-35 is shown; this bears the auxiliary −35 element (TTGACA) and the λ operator OR2, centered 45.5 bp and 55 bp, respectively, upstream of the transcriptional start site of the lac promoter.
The σ subunit of RNAP is responsible for recognition of specific promoter sequences; alternative σ factors combine with the enzymatic core (α2ββ′) to form alternative holoenzyme species (21). Promoter PRM is recognized by the σ70 form of RNAP (Eσ70), which directs transcription of the majority of E. coli genes. The σ70 subunit makes base-specific contacts with the promoter in both its −10 and −35 regions, using conserved regions 2 and 4, respectively, to do so (see ref. 21). λOR2 is centered just upstream of the −35 region of PRM (at position −42), and residues in region 4 of σ70, which contains a putative helix-turn-helix DNA-binding motif, have been implicated in the interaction with λcI (18, 19). Nevertheless, there has been no direct demonstration of an interaction between λcI and region 4 of σ70.
Here, we design an in vivo assay that permits the detection of an energetically favorable interaction between λcI and a σ fragment encompassing region 4. Specifically, we tether the relevant portion of σ to the α subunit of RNAP and then show that λcI can activate transcription from a suitably designed test promoter by stabilizing the binding of the transplanted σ moiety to an ectopic −35 element (Fig. 1B). We present a model that explains how the ability of λcI to stabilize the binding of region 4 of σ to the DNA can account for its effect on the rate of isomerization at PRM.
Materials and Methods
Plasmids and Strains.
Plasmid pACλcI harbors the wild-type cI gene under the control of the lacUV5 promoter (22). pACλcI(Sa109) is a derivative of pACλcI and encodes λcI(S35L, D38Y, K39N). pACλcI(Sa104) is a derivative of pACλcI and encodes λcI(S35L, D38Y, K39E). Plasmids pACλcI(Sa109) and pACλcI(Sa104) were constructed by cloning the appropriate NdeI–NsiI cut PCR products [made using plasmids pFB109 and pFB104 (see ref. 17) as templates] into an NdeI–NsiI cut derivative of pACλcI that contains an NdeI site at the start of the cI gene. pACλcI(Sa109-Y38N) is a derivative of pACλcI(Sa109) in which the Y38N change was introduced by the PCR. pACλcI(D38N) is a derivative of pACλcI that was made by cloning an NdeI–NsiI cut PCR product [made using plasmid p16 (see ref. 16) as template] into the NdeI–NsiI cut derivative of pACλcI. pACΔcI and pBRα have been described previously (22).
Plasmid pBRα-σ70 encodes residues 1–248 of the α subunit of E. coli RNAP fused to residues 528–613 of the σ70 subunit of E. coli RNAP under the control of tandem lpp and lacUV5 promoters. The hybrid α-σ70 gene was created using overlap PCR and cloned into EcoRI–BamHI-digested pBRα to make pBRα-σ70. Plasmid pBRα-σ70(R596H) was made in a similar manner and is identical to pBRα-σ70, except the σ moiety of the encoded chimera contains the R596H substitution. pBRα-σ70(R588H) is a derivative of pBRα-σ70 in which the R588H change in the σ moiety of the chimera was introduced by the PCR. Plasmid pBRα-σ38 encodes residues 1–248 of the α subunit of E. coli RNAP fused to residues 243–330 of the σ38 subunit of E. coli RNAP under the control of tandem lpp and lacUV5 promoters. pBRα-σ38 was made essentially the same way as pBRα-σ70.
Plasmid pFW11-OR2–55/Cons-35 was constructed by cloning an EcoRI–HindIII cut PCR product containing the lac promoter derivative plac OR2–55/Cons-35 into pFW11 (23) cut with EcoRI–HindIII. pFW11-OR2–55/Cons-35 was then transformed into strain CSH100, and the promoter-lacZ fusion was recombined onto an F′ episome and mated into strain FW102 to create reporter strain SF1 (see ref. 23). Plasmid pFW11-OR2–55/TTAACA was similarly constructed, and reporter strain SF2, which is identical to strain SF1 except for the sequence of the auxiliary −35 element of the test promoter (TTAACA instead of TTGACA), was made in the same manner as strain SF1. The PCR-amplified regions of all constructs were sequenced to confirm that no errors had been introduced as a result of the PCR process.
Experimental Procedures
For all experiments, cells were grown in LB supplemented with carbenicillin (50 μg ml−1), chloramphenicol (25 μg ml−1), and kanamycin (50 μg ml−1) together with isopropyl-β-D-thiogalactoside (IPTG) at the concentration indicated. SDS-CHCl3 permeabilized cells were assayed for β-galactosidase activity essentially as described (24). Assays were done at least three times in duplicate on separate occasions, with similar results. Values are the averages from one experiment; duplicate measurements differed by <10%. For all primer extension analyses, IPTG was added to the growth medium to a final concentration of 50 μM. RNA isolation, primer labeling, and primer extension assays were essentially as described previously (22).
Results
Design of the Experiment.
We devised a strategy for assaying the ability of λcI to interact with region 4 of σ in vivo. This strategy was based on our previous demonstration that transcription can be activated by any sufficiently strong contact between a DNA-bound protein and a protein domain fused to RNAP (refs. 22 and 24; see also refs. 2 and 25). In particular, we showed that protein domains fused to the α subunit of RNAP in place of the αCTD can mediate transcriptional activation by serving as artificial activation targets for DNA-bound proteins (22, 24). We also showed that transcription can be activated by a sufficiently strong protein–DNA interaction between a DNA-binding domain tethered to RNAP and a cognate recognition site positioned upstream of a test promoter (24). Accordingly, we reasoned that region 4 of σ70 might be able to activate transcription from a suitably designed test promoter (bearing an auxiliary −35 element) when tethered to the αNTD. We anticipated further that such a system should allow us to detect energetically favorable protein–protein interactions between the tethered σ moiety and adjacently bound proteins because such interactions would stabilize the binding of the σ moiety to the DNA and hence increase the magnitude of the activation.
Following this strategy, we replaced the αCTD with a C-terminal fragment of σ70 encompassing region 4 and constructed a test promoter bearing an auxiliary −35 element in the upstream region together with a flanking λ operator. This experimental setup enabled us to ask whether a DNA-bound λcI dimer would activate transcription from the test promoter by stabilizing the binding of the tethered σ moiety to the auxiliary −35 element (see Fig. 1B). The hybrid α−σ70 gene consisted of codons 1–248 of α fused to codons 528–613 of σ70 (the final 86 codons). The test promoter plac OR2–55/Cons-35 consisted of the lac core promoter, a second −35 hexamer centered at position −45.5, and the flanking λ operator (OR2) centered at position −55. The position of OR2 relative to the auxiliary −35 element is the same as at the λ PRM promoter.
λcI Proteins Activate Transcription from the Test Promoter in the Presence of the α-σ Chimera.
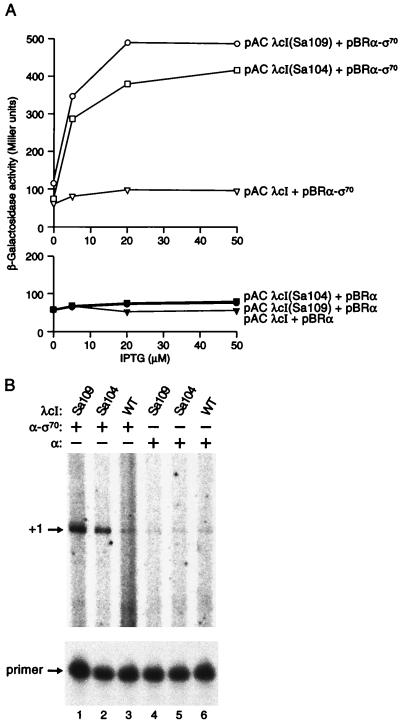
The test promoter (plac OR2–55/Cons-35) was fused to the lacZ gene and introduced into E. coli strain FW102 (23) in single copy on an F′ episome to create reporter strain SF1. We assayed the abilities of wild-type λcI and two superactivating variants (17) to activate transcription from the test promoter in the presence or absence of the α-σ70 chimera. Superactivators 104 and 109 activated transcription a maximum of ≈6-fold in the presence of the α-σ70 chimera, and wild-type λcI activated transcription weakly (<2-fold) (Fig. 2A). This difference in the stimulatory activities of wild-type λcI and the superactivators mirrors that previously observed when the proteins were assayed for their abilities to activate transcription from λ PRM (17). The λcI proteins only activated transcription from the test promoter in the presence of the α-σ70 chimera; no stimulation was detected in the presence of excess wild-type α (Fig. 2A) or excess wild-type σ70 (data not shown). Furthermore, the α-σ70 chimera did not mediate any stimulatory effect in the absence of λcI (data not shown). Primer extension analysis confirmed that the three λcI proteins stimulated the production of correctly initiated transcripts (Fig. 2B).
Figure 2.
Effects of wild-type λcI and λcI superactivators on transcription in the presence of the α-σ70 chimera. (A) SF1 cells harboring the indicated plasmids were assayed for β-galactosidase activity. pACYC-derived plasmids encoded λcI (pACλcI), λcISa109 [pACλcI(Sa109)], or λcISa104 [pACλcI(Sa104)]; pBR322-derived plasmids encoded either the α-σ70 chimera (pBRα-σ70) or wild-type α (pBRα). (B) Primer extension analysis of transcripts produced from plac OR2–55/Cons-35 with wild-type λcI or λcI superactivators in the presence of the α-σ70 chimera. Total RNA was isolated from SF1 cells harboring plasmids encoding the indicated proteins, and the primer extension analysis was done by using a primer complementary to the lacZ transcript produced by the plac OR2–55/Cons-35 promoter. Primer extension products produced by correctly initiated transcripts are indicated by +1. Excess unincorporated primer is shown in the lower panel.
Transcriptional Activation by λcI Proteins at the Test Promoter Depends on the Activating Region of λcI and on the Ability of the Tethered σ Moiety to Interact with the Auxiliary −35 Element.
The results shown in Fig. 2 suggest that λcI and the two superactivators are interacting with the tethered σ moiety and stabilizing its binding to the auxiliary −35 element. To test the hypothesis that the observed activation depends on the positive control surface of λcI, we introduced a positive control mutation into the genes encoding λcISa109 and λcISa104. The resulting λcI variants (bearing amino acid substitution Y38N) manifested a substantially decreased ability to activate transcription from the test promoter (Fig. 3B and data not shown). We confirmed that these activation defects were not attributable to DNA-binding defects of the altered superactivators by performing an in vivo repression assay using a test promoter bearing a single λ operator between its −10 and −35 regions (data not shown).
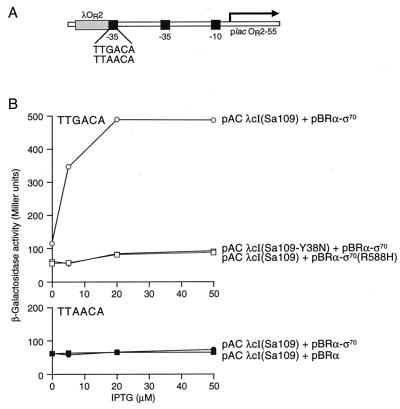
Figure 3.
Effects of mutations in the α-σ70 chimera or the additional −35 element on activation by λcI superactivator. (A) Schematic of test promoters used to determine the effect of mutating the additional −35 element on transcriptional activation by Sa109 in the presence of the α-σ70 chimera. The sequences of the additional −35 elements from the two test promoters are indicated (consensus = TTGACA). (B) SF1 and SF2 cells harboring the indicated plasmids were assayed for β-galactosidase activity. In each panel, the sequence of the additional −35 element from the relevant test promoter is given. pACYC-derived plasmids encoded either λcISa109 [pACλcI(Sa109)] or λcISa109-Y38N [pACλcI(Sa109-Y38N)]; pBR322-derived plasmids encoded the α-σ70 chimera (pBRα-σ70), the α-σ70 (R588H) chimera [pBRα-σ70 (R588H)], or wild-type α (pBRα).
We then tested the hypothesis that the observed activation depends on the ability of the tethered σ moiety to interact with the auxiliary −35 element positioned adjacent to the λ operator. To do this, we introduced mutations predicted to disrupt this interaction into either the −35 element (see Fig. 3A) or the gene encoding the α-σ70 chimera. When we weakened the auxiliary −35 element present on the reporter template by introducing a G to A substitution at the third position, λcISa109 failed to activate transcription from the test promoter (Fig. 3B). Similarly, λcISa109 failed to activate transcription in the presence of a mutant α-σ70 chimera bearing an amino acid substitution in the σ moiety (R588H) predicted to disrupt DNA binding (26) (Fig. 3B).
Activation with a Mutant–Suppressor Pair.
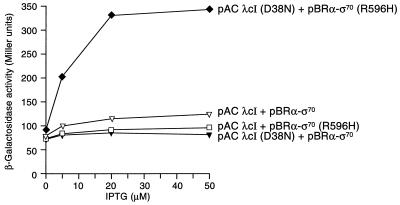
Our results demonstrate that wild-type λcI and two superactivating variants can stabilize the sequence-specific binding of a C-terminal fragment of σ70 to DNA. For wild-type λcI, this effect is close to the threshold of detection in our in vivo assay, and for the superactivating variants the effects are larger, as predicted based on their activities at PRM (17). Because these superactivating variants of λcI have not been subjected to kinetic analysis at PRM, we examined the effect of another λcI mutant that has been analyzed kinetically and was found to stimulate the rate of isomerization more efficiently than wild-type λcI. Mutant λcI-D38N is a positive control mutant (16), the activation defect of which can be suppressed by a mutant form of σ bearing the substitution R596H (σ-R596H) (18). In vitro experiments done with reconstituted mutant RNAP (Eσ-R596H) revealed that λcI-D38N exerts its effect exclusively on the isomerization step, producing a 27-fold increase in the isomerization rate constant (kf) as compared with a 17-fold increase produced by wild-type λcI working on wild-type RNAP (20).
To test whether our artificial system could detect an interaction between λcI-D38N and σ-R596H, we compared the abilities of λcI-D38N to activate transcription from the artificial promoter in the presence of the α-σ70 chimera with or without the R596H substitution in the σ moiety. λcI-D38N failed to activate transcription in the presence of the unmodified form of the α-σ70 chimera but activated transcription ≈4-fold when the σ moiety of the chimera bore the R596H substitution (Fig. 4). Primer extension analysis confirmed that this activation reflected an increase in correctly initiated transcripts (data not shown). Like the unmodified form of the α-σ70 chimera, the α-σ70 (R596H) variant did not activate transcription from the test promoter in the absence of λcI-D38N (data not shown).
Figure 4.
Effects of wild-type and mutant λcI on transcription in the presence of α-σ70 chimeras. SF1 cells harboring the indicated plasmids were assayed for β-galactosidase activity. pACYC-derived plasmids encoded either λcI (pACλcI) or λcI(D38N) [pACλcI(D38N)]; pBR322-derived plasmids encoded either the α-σ70 chimera (pBRα-σ70) or the α-σ70 (R596H) chimera [pBRα-σ70 (R596H)].
Superactivating Variants of λcI Activate Transcription from the Test Promoter in the Presence of an α-σ38 Chimera.
The stationary phase σ factor, σ38, is very similar to σ70 in the DNA-binding regions, particularly throughout region 4.2, and recognizes the same −35 consensus sequence as does σ70 (refs. 27 and 28; T. Gaal & R. L. Gourse, personal communication). We replaced the σ70 moiety of the α-σ70 chimera with the corresponding region of σ38 (residues 243 to 330) and tested whether the resulting α-σ38 chimera could mediate transcriptional activation from our artificial test promoter. The experiment of Fig. 5A shows that both λcISa109 and λcISa104 stimulated transcription efficiently in the presence of the α-σ38 chimera.
Figure 5.
(A) Effects of wild-type λcI and λcI superactivators on transcription in the presence of the α-σ38 chimera. SF1 cells harboring the indicated plasmids were assayed for β-galactosidase activity. pACYC-derived plasmids encoded λcI (pACλcI), λcISa109 [pACλcI(Sa109)], or λcISa104 [pACλcI(Sa104)]; the pBR322-derived plasmid encoded the α-σ38 chimera (pBRα-σ38). (B) Interaction between σ38 region 4 and the DNA mediates transcriptional activation. SF1 and SF2 cells harboring the indicated plasmids were assayed for β-galactosidase activity. In each panel, the sequence of the additional −35 element from the relevant test promoter is given. The pACYC-derived plasmid encoded no λcI (pACΔcI); pBR322-derived plasmids encoded either the α-σ38 chimera (pBRα-σ38) or wild-type α (pBRα).
Wild-type λcI appeared to exert a slight stimulatory effect on transcription in the presence of the α-σ38 chimera (Fig. 5A); however, the interpretation of this effect is complicated by the results obtained in the absence of λcI (see Discussion). Fig. 5B shows that in the absence of any form of λcI, the α-σ38 chimera activated transcription from the test promoter bearing the consensus (TTGACA) ectopic −35 element ≈6-fold. This activation evidently depends on the ability of the tethered σ38 moiety to bind to the ectopic −35 element since replacement of the consensus element with a mutated element (TTAACA) significantly reduced the magnitude of the activation (Fig. 5B). Primer extension analysis confirmed that the activation mediated by the α-σ38 chimera either in the absence or the presence of a λcI variant reflected an increase in correctly initiated transcripts (data not shown).
Discussion
Genetic Evidence That the Interaction Between λcI and the Tethered σ Moiety Is the Same Interaction That Activates Transcription at PRM.
Our demonstration that λcI can stabilize the binding of region 4 of σ70 to a −35 element provides strong support for the idea that there is an energetically favorable interaction between the activating region of λcI and a complementary surface of σ. Importantly, we observed a close correlation between the effects of amino acid substitutions in both λcI and region 4 of σ70 on transcriptional activation at PRM (16–18) and at our artificial promoter. First, we showed that two superactivating variants of λcI (Sa104 and Sa109), so designated based on their behavior at PRM (17), also activated transcription more strongly than wild-type λcI at the artificial promoter. Second, we showed that activation by wild-type λcI and the superactivating variants at the artificial promoter was dependent on their having functional activating regions as defined by their abilities to activate transcription from PRM. Finally, we tested the effect of introducing into the σ moiety of the α-σ chimera an amino acid substitution (R596H) that suppresses the activation defect of a λcI positive control mutant (λcI-D38N) at PRM (18); in the context of the α-σ chimera, this amino acid substitution specifically enhanced the ability of λcI-D38N to activate transcription from the artificial promoter.
Mechanism by Which λcI Influences the Isomerization Step at PRM.
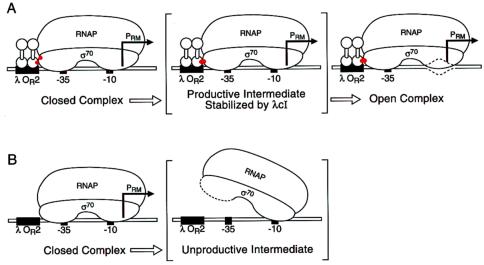
We have shown that an activator (either wild-type λcI or λcI-D38N), which is known to function by accelerating the rate of isomerization at PRM, can activate transcription from our artificial promoter by stabilizing the binding of a tethered σ moiety encompassing region 4 to an ectopic −35 element. The simplest interpretation of these findings is that both wild-type λcI and λcI-D38N similarly stabilize the binding of intact σ70 to the −35 element when they activate transcription from PRM. How can this proposal be reconciled with the observed kinetics of the activation process? Any detailed mechanistic model for the action of λcI at PRM must account both for its stimulatory effect on kf and for its lack of an effect on the initial binding step (described by an equilibrium constant KB for the formation of the closed complex). We suggest that when Eσ70 (or Eσ70-R596H) forms a closed complex at PRM, the activating region of λcI (or λcI-D38N) and its target surface on σ are improperly aligned so that no energetically significant interaction can occur (Fig. 6A). We propose further that during the transition from the closed to the transcriptionally active open (melted) complex, the activating region of λcI and its target on σ come into alignment, thus permitting an energetically significant interaction to occur (Fig. 6A). To explain the stimulatory effect of λcI on the rate of isomerization, we postulate that λcI stabilizes an intermediate along the pathway from the closed to the open complex, the formation of which limits the rate of initiation. More particularly, we suggest that during the isomerization process, there may be a tendency for region 4 of σ to disengage from the −35 element, which limits open complex formation (Fig. 6B). DNA-bound λcI would function to counteract this tendency, thus stabilizing a productive intermediate along the pathway to open complex formation (Fig. 6A). The ability of λcI to stabilize the binding of the tethered σ moiety to the DNA at our artificial promoter but not when RNAP forms a closed complex at PRM suggests that region 4 of σ may be more constrained in its natural context in the holoenzyme than when it is tethered to the αNTD by a flexible linker region.
Figure 6.
Model for mechanism of action of λcI at PRM. (A) Activating region and its target (red patches) are misaligned in the closed complex but come into alignment subsequently during the process of open complex formation. Depicted in brackets is a hypothetical productive intermediate that is stabilized by λcI. (B) In the absence of λcI, formation of an unproductive intermediate limits open complex formation at PRM.
We think it likely that the interaction between λcI and the σ moiety of the α-σ chimera functions to stabilize the closed complex (affects KB) at our artificial test promoter. Using the same core promoter, we have previously shown that transcription can be activated by any sufficiently strong contact between a DNA-bound protein and a protein domain tethered to a subunit of RNAP or between a DNA-binding domain tethered to RNAP and a cognate recognition site positioned upstream of the core promoter (22, 24). Furthermore, our results established a correlation between the strength of the protein–protein (or the protein–DNA) interaction and the magnitude of the activation (ref. 22; S.L.D & A.H., unpublished results). The simplest interpretation of our findings is that these arbitrarily selected protein–protein or protein–DNA interactions stabilize the binding of RNAP to the promoter. In the experimental setup used here, transcriptional activation results from the combined effects of a relatively weak protein–protein interaction (between λcI and the tethered σ moiety) and a relatively weak protein–DNA interaction (between the tethered σ moiety and the auxiliary −35 element). We note that the role of λcI at this artificial promoter is formally analogous to the role of CRP at the natural lac promoter; that is, CRP interacts with the αCTD and stabilizes its association with the DNA in the region between the CRP recognition site and the promoter −35 element (1, 7). In this case, CRP has been shown to exert its effect exclusively on KB (6).
Regardless of the kinetic effect of λcI on transcription at our artificial test promoter, two principal findings, namely that λcI can (i) interact productively with its target when that target is transplanted from the σ subunit to the α subunit and (ii) stabilize the binding of the transplanted σ fragment to an ectopic −35 element, suggest that the functional significance of the λcI-σ interaction is simply that contacts between σ region 4 and the −35 element of PRM are stabilized.
A Common Mechanism for the Effects of Activators That Work at Different Steps in the Initiation Process.
An implication of our results is that there need not be any fundamental difference between an activator that affects KB and an activator that affects kf, both merely requiring a surface that can interact with an accessible complementary surface on RNAP (2, 3, 29). The kinetic effect of a particular activator working at a particular promoter may instead depend on when during the initiation process the appropriate surfaces can interact (29). If the interaction can take place while RNAP is in the closed complex, an effect on KB would be expected, whereas if the interaction can take place only after the closed to open transition has begun, then an effect on kf would be expected. Thus, the same protein–protein interaction between an activator and RNAP might, in principle, produce an effect on KB, kf, or both, depending on spatial constraints imposed by the promoter itself and on the arrangement of the activator-binding site(s).
This picture of the activation process provides an explanation for an unexpected effect uncovered by the kinetic analysis of the λcI-D38N/Eσ70-R596H mutant/suppressor pair, namely that wild-type λcI at PRM stimulates closed complex formation (i.e., exerts its effect predominantly on KB) when assayed with Eσ70-R596H (20). We suggest that this apparent change in activation mechanism may simply reflect a subtle change in the geometry of the interaction so that λcI can interact productively with region 4 of σ70 when Eσ70-R596H forms a closed complex (29).
It is possible that other σ70-dependent activators that exert their effects on the rate of isomerization may in some cases do so by stabilizing the interaction of region 4 of σ with the −35 element. In the case we have described, this stabilization apparently occurs by a simple cooperative binding mechanism: the activator contacts region 4 of σ directly. In principle, however, direct contact with this DNA-binding domain of σ would not necessarily be required. Instead, contact with another region or subunit of RNAP might function indirectly to stabilize the interaction between σ region 4 and the −35 element. Several σ70-dependent activators have been shown to affect the rate of isomerization (5, 20, 30, 31). A particularly well-characterized example is provided by CRP, which possesses at least two distinct activating regions (AR1 and AR2). When bound at a so-called class II promoter, which bears a CRP recognition site that overlaps the promoter −35 region, CRP uses AR1 to contact the αCTD and AR2 to contact the αNTD, the former contact mediating an effect on KB and the latter an effect on kf (31). Moreover, it has been shown that AR2 of CRP participates in an energetically favorable interaction with RNAP (31).
Activators of another class have been shown to exert their effects on the isomerization step; these activators work on promoters recognized by the alternative σ factor, σ54, which appears to be unrelated to the σ70 class of σ factors (27). Unlike most σ70-dependent activators, the σ54-dependent activators generally bind well upstream of their target promoters and interact with RNAP with concomitant formation of a DNA loop (see ref. 32); furthermore, ATP hydrolysis is required for this activation. Although the mechanism of action of the σ54-dependent activators is likely to be complex, it is possible that stabilization of appropriate σ54–DNA contacts is a component of the activation process. It should be noted, however, that any such stabilization evidently does not occur by a tethering mechanism because σ54-dependent activators can, when present at high concentrations, work directly from solution (33). In eukaryotes, as well, transcriptional activators have been implicated in postbinding steps in the initiation process (34–37), and our findings could be relevant to the understanding of how eukaryotic activators can exert these effects.
A General Assay for the Interaction of DNA-Bound Regulators with σ Factors.
Many prokaryotic activators that bind to the DNA upstream of the promoters they regulate have binding sites that are centered roughly 40 bp upstream from the transcription start site, and some of these have been shown genetically to interact with region 4 of σ70 (38–43). Our in vivo cooperative binding assay should be useful in determining whether any of these activators can stabilize the binding of region 4 to a −35 element. For those that can, our assay should also facilitate the genetic dissection of the protein–protein interaction between the activator and the tethered σ moiety. A potential benefit of our system is that it permits the isolation of amino acid substitutions in the σ moiety of an inessential α-σ chimera, the transcriptional effects of which should be limited to the test promoter. Thus, mutations affecting an essential σ factor that might otherwise be pleiotropic or even lethal can be isolated and studied.
We have used our experimental system to detect interactions of both σ70 and σ38. Interestingly, the α-σ38 chimera, unlike the α-σ70 chimera, activated transcription on its own from our artificial test promoter (i.e., in the absence of an adjacently bound λcI molecule). The reason for this difference is, as yet, unknown. We note that this activation-based assay may provide an especially convenient system for carrying out a genetic analysis of the protein–DNA interaction between region 4 of σ38 and the −35 element. Since our in vivo assays were performed with cells that contain both plasmid-encoded α-σ chimera and chromosomally encoded wild-type α, we do not know whether the activation mediated by the α-σ38 chimera in the absence of λcI results from homodimeric or heterodimeric RNAP complexes. One σ38 moiety is evidently bound to the auxiliary −35 element, and either a second σ38 moiety or the αCTD may be bound nonspecifically to the DNA upstream of this −35 element (within the λ operator). This hypothesis could account for the inhibitory effect of wild-type λcI on α-σ38-dependent activation, as λcI might displace either a second, nonspecifically bound σ38 moiety or the αCTD from the DNA, thereby reducing the magnitude of the activation. Further experiments will be required to distinguish between these and other possibilities.
Conclusions
In summary, our findings with the α-σ70 chimera define a minimal target (86 amino acids) of σ70 that can interact with the activating region of λcI. Furthermore, we have shown that λcI can stabilize the binding of this region of σ70 to a −35 element. We propose that the ability of λcI to stabilize the binding of region 4 of σ to a −35 element can account for its stimulatory effect on transcription from PRM, and we discuss a model that can reconcile this finding with the apparently paradoxical observation that λcI does not stabilize the initial binding of RNAP to PRM but rather stimulates the isomerization step. Finally, our findings validate the use of a novel genetic system that should facilitate the detection and analysis of interactions between other transcriptional regulatory proteins and various σ factors from E. coli or other bacteria.
Acknowledgments
We thank M. Ptashne and A. Gann for discussion, R. Kolter for plasmid pDEB2 encoding σ38, and Yan Ye Xia for excellent technical assistance. This work was supported by National Institutes of Health Grant GM44025, an established investigatorship from the American Heart Association (to A.H.), and a Charles A. King Trust postdoctoral fellowship (to S.L.D.).
Abbreviations
- OR
operator region
- RNAP
RNA polymerase
- CRP
cAMP receptor protein
- NTD
N-terminal domain
- CTD
C-terminal domain
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Busby S, Ebright R H. Cell. 1994;79:743–746. doi: 10.1016/0092-8674(94)90063-9. [DOI] [PubMed] [Google Scholar]
- 2.Ptashne M, Gann A. Nature (London) 1997;386:569–577. doi: 10.1038/386569a0. [DOI] [PubMed] [Google Scholar]
- 3.Hochschild A, Dove S L. Cell. 1998;92:597–600. doi: 10.1016/s0092-8674(00)81126-5. [DOI] [PubMed] [Google Scholar]
- 4.McClure W R. Annu Rev Biochem. 1985;54:171–204. doi: 10.1146/annurev.bi.54.070185.001131. [DOI] [PubMed] [Google Scholar]
- 5.Hawley D K, McClure W R. J Mol Biol. 1982;157:493–525. doi: 10.1016/0022-2836(82)90473-9. [DOI] [PubMed] [Google Scholar]
- 6.Malan T P, Kolb A, Buc H, McClure W R. J Mol Biol. 1984;180:881–909. doi: 10.1016/0022-2836(84)90262-6. [DOI] [PubMed] [Google Scholar]
- 7.Busby S, Ebright R H. J Mol Biol. 1999;293:199–213. doi: 10.1006/jmbi.1999.3161. [DOI] [PubMed] [Google Scholar]
- 8.Blatter E, Ross W, Tang H, Gourse R L, Ebright R H. Cell. 1994;78:889–896. doi: 10.1016/s0092-8674(94)90682-3. [DOI] [PubMed] [Google Scholar]
- 9.Negishi T, Fujita N, Ishihama A. J Mol Biol. 1995;248:723–728. doi: 10.1006/jmbi.1995.0254. [DOI] [PubMed] [Google Scholar]
- 10.Jeon Y H, Yamazaki T, Otomo T, Ishihama A, Kyogoku Y. J Mol Biol. 1997;267:953–962. doi: 10.1006/jmbi.1997.0902. [DOI] [PubMed] [Google Scholar]
- 11.Ross W, Gosink K K, Salomon J, Igarashi K, Zou C, Ishihama A, Severinov K, Gourse R L. Science. 1993;262:1407–1413. doi: 10.1126/science.8248780. [DOI] [PubMed] [Google Scholar]
- 12.Hochschild A. Curr Biol. 1994;4:440–442. doi: 10.1016/s0960-9822(00)00097-x. [DOI] [PubMed] [Google Scholar]
- 13.Ptashne M. A Genetic Switch: Phage λ and Higher Organisms. 2nd Ed. Cambridge, MA: Blackwell; 1992. [Google Scholar]
- 14.Sauer R T, Jordan S R, Pabo C O. Adv Protein Chem. 1990;40:1–61. doi: 10.1016/s0065-3233(08)60286-7. [DOI] [PubMed] [Google Scholar]
- 15.Guarente L, Nye J S, Hochschild A, Ptashne M. Proc Natl Acad Sci USA. 1982;79:2236–2239. doi: 10.1073/pnas.79.7.2236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hochschild A, Irwin N, Ptashne M. Cell. 1983;32:319–325. doi: 10.1016/0092-8674(83)90451-8. [DOI] [PubMed] [Google Scholar]
- 17.Bushman F D, Shang C, Ptashne M. Cell. 1989;58:1163–1171. doi: 10.1016/0092-8674(89)90514-x. [DOI] [PubMed] [Google Scholar]
- 18.Li M, Moyle H, Susskind M M. Science. 1994;263:75–77. doi: 10.1126/science.8272867. [DOI] [PubMed] [Google Scholar]
- 19.Kuldell N, Hochschild A. J Bacteriol. 1994;176:2991–2998. doi: 10.1128/jb.176.10.2991-2998.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li M, McClure W R, Susskind M M. Proc Natl Acad Sci USA. 1997;94:3691–3696. doi: 10.1073/pnas.94.8.3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Gross C A, Chan C, Dombroski A, Gruber T, Sharp M, Tupy J, Young B. Cold Spring Harbor Symp Quant Biol. 1998;63:141–155. doi: 10.1101/sqb.1998.63.141. [DOI] [PubMed] [Google Scholar]
- 22.Dove S L, Joung J K, Hochschild A. Nature (London) 1997;386:627–630. doi: 10.1038/386627a0. [DOI] [PubMed] [Google Scholar]
- 23.Whipple F W. Nucleic Acids Res. 1998;26:3700–3706. doi: 10.1093/nar/26.16.3700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dove S L, Hochschild A. Genes & Dev. 1998;12:745–754. doi: 10.1101/gad.12.5.745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Farrell S, Simkovich N, Wu Y, Barberis A, Ptashne M. Genes & Dev. 1996;10:2359–2367. doi: 10.1101/gad.10.18.2359. [DOI] [PubMed] [Google Scholar]
- 26.Gardella T, Moyle H, Susskind M M. J Mol Biol. 1989;206:579–590. doi: 10.1016/0022-2836(89)90567-6. [DOI] [PubMed] [Google Scholar]
- 27.Lonetto M, Gribskov M, Gross C A. J Bacteriol. 1992;174:3843–3849. doi: 10.1128/jb.174.12.3843-3849.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Nguyen L H, Burgess R R. Biochemistry. 1997;36:1748–1754. doi: 10.1021/bi961175h. [DOI] [PubMed] [Google Scholar]
- 29.Roy S, Garges S, Adhya S. J Biol Chem. 1998;273:14059–14062. doi: 10.1074/jbc.273.23.14059. [DOI] [PubMed] [Google Scholar]
- 30.Shih M-C, Gussin G N. J Mol Biol. 1984;172:489–506. doi: 10.1016/s0022-2836(84)80019-4. [DOI] [PubMed] [Google Scholar]
- 31.Niu W, Kim Y, Tau G, Heyduk T, Ebright R H. Cell. 1996;87:1123–1134. doi: 10.1016/s0092-8674(00)81806-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rombel I, North A, Hwang I, Wyman C, Kustu S. Cold Spring Harb Symp Quant Biol. 1998;63:157–166. doi: 10.1101/sqb.1998.63.157. [DOI] [PubMed] [Google Scholar]
- 33.North A K, Kustu S. J Mol Biol. 1997;267:17–36. doi: 10.1006/jmbi.1996.0838. [DOI] [PubMed] [Google Scholar]
- 34.Kingston R E, Green M R. Curr Biol. 1994;4:325–332. doi: 10.1016/s0960-9822(00)00071-3. [DOI] [PubMed] [Google Scholar]
- 35.Chi T, Carey M. Genes & Dev. 1996;10:2540–2550. doi: 10.1101/gad.10.20.2540. [DOI] [PubMed] [Google Scholar]
- 36.Holstege F C, Fiedler U, Timmers H T. EMBO J. 1997;16:7468–7480. doi: 10.1093/emboj/16.24.7468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kassavetis G A, Kumar A, Letts G A, Geiduschek E P. Proc Natl Acad Sci USA. 1998;95:9196–9201. doi: 10.1073/pnas.95.16.9196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Artsimovitch I, Murakami K, Ishihama A, Howe M M. J Biol Chem. 1996;271:32343–32348. doi: 10.1074/jbc.271.50.32343. [DOI] [PubMed] [Google Scholar]
- 39.Lonetto M A, Rhodius V, Lamberg K, Kiley P, Busby S, Gross C. J Mol Biol. 1998;284:1353–1365. doi: 10.1006/jmbi.1998.2268. [DOI] [PubMed] [Google Scholar]
- 40.Landini P, Busby S J. J Bacteriol. 1999;181:1524–1529. doi: 10.1128/jb.181.5.1524-1529.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Rhodius V A, Busby S J. J Mol Biol. 2000;299:311–324. doi: 10.1006/jmbi.2000.3737. [DOI] [PubMed] [Google Scholar]
- 42.Kim S K, Makino K, Amemura M, Nakata A, Shinagawa H. Mol Gen Genet. 1995;248:1–8. doi: 10.1007/BF02456607. [DOI] [PubMed] [Google Scholar]
- 43.Hu J C, Gross C A. Mol Gen Genet. 1985;199:7–13. doi: 10.1007/BF00327502. [DOI] [PubMed] [Google Scholar]