Abstract
The free radical theory of aging states that reactive oxygen species (ROS) play a key role in age-related accumulation of cellular damage, and consequently influence aging and longevity. Therefore, variation in genes encoding proteins protecting against ROS could be expected to influence variation in aging and life span. The rs4880 and rs1050450 SNPs in the manganese superoxide dismutase (MnSOD) and glutathione peroxidase 1 (GPX1) genes, respectively, are associated with age-related diseases and appear to affect the activities of the encoded variant proteins.
In this study we genotyped these SNPs in 1650 individuals from the Danish 1905 cohort (follow-up time: 1998–2008, age at intake: 92–93 years, number of deaths: 1589 (96.3%)) and investigated the association with aging and longevity. We found decreased mortality of individuals holding either the MnSOD rs4880 C or the GPX1 rs1050450 T alleles (HR (MnSOD(CC/CT)) = 0.91, p = 0.002), HR (GPX1(TT/TC)) = 0.93, p = 0.008)). Furthermore, a synergetic effect of the alleles was observed (HR = 0.76, p = 0.001). Finally, moderate positive associations with good self rated health, decreased disability and increased cognitive capacity were observed. Our results thus indicate that genetic variation in MnSOD and GPX1 may be associated with aging and longevity.
Keywords: Longevity, aging, single nucleotide polymorphisms, Mn-superoxide dismutase, glutathione peroxidase 1
1. INTRODUCTION
Genetic factors contribute to the variation in life span by approximately 25% (Herskind et al. 1996), a contribution believed to be minimal before age 60 years and most profound from age 85 years and onwards (Hjelmborg et al. 2006). The candidate genes encode proteins involved in several biological processes including the protection against oxidative stress (Christensen et al. 2006). Reactive oxygen species (ROS) are produced because approximately 2–3% of the oxygen atoms taken up by the mitochondria are reduced insufficiently (Valko et al. 2004). ROS can oxidize and damage nucleic acids, proteins and lipids hereby altering their stability and function. Thus, protein modifications (such as protein carbonylation and nitration) and the formation of lipid peroxidation adducts (e.g. 4-hydroxynonenal (HNE)) can be the results of ROS damage. HNE might subsequently give rise to the exocyclic etheno adducts of adenine, cytosine and guanine in DNA. Moreover, exposure of nucleic acids to ROS can generate a wide variety of modified bases, 8-oxo-7,8-dihydroguanine (8-oxoguanine), which induces base-mispairing, being one of the most abundant. Furthermore, ROS exposure may also result in imidazol ring-opened derivatives of pyrimidines (FaPy products) and in single-strand breaks in DNA. Over time the sum of these alterations contribute to deleterious cellular changes leading to dysfunction of mitochondria, cells and ultimately of the organism, that is, the damage induced alterations contribute to aging and longevity (Harman1973; Harman1991). In addition, the damage induced alterations have been associated with certain cancers and cardiovascular and neurodegenerative disorders of old age (Antonella1999; Vijg2000).
Among the antioxidant enzymes involved in protecting against ROS, the manganese superoxide dismutase (MnSOD) and the glutathione peroxidase 1 (GPX1) play primary roles via the dismutation of the superoxide anion (O2.−) to hydrogen peroxide (H2O2) and the reduction of H2O2 to H2O, respectively. MnSOD is localized in the mitochondria (Slot et al. 1986), while the GPX1 localizes both to the mitochondria and in the cytoplasm (Esworthy et al. 1997).
An influence of MnSOD on aging and life span has been observed in several model organisms. Over expression of Sod2 (the MnSOD homologue) leads to increased life span in Drosophila melanogaster (Sun et al. 2002) and in Mus musculus (Hu et al. 2007), while Saccharomyces cerevisiae and Drosophila melanogaster Sod2 null mutants show a decreased life span (Duttaroy et al. 2003;Fabrizio et al. 2003). Sod2 knock out mice display neonatal lethality (Lebovitz et al. 1996;Huang et al. 2002), while mice expressing 50% of the normal Sod2 level have increased susceptibility to oxidative stress, severe mitochondrial dysfunction, cardiomyopathy and degeneration of central nervous system neurons (Van Remmen et al. 2003;Hinerfeld et al. 2004). Gpx1 (the mouse GPX1 homologue) knock out mice on the other hand develop normally, yet show increased sensitivity to oxidative stress-inducing agents and have increased lethality when exposed to high doses (de Haan et al. 1998). In addition, mouse Gpx1−/−cells in culture show senescence-like changes (such as reduced proliferation and DNA synthesis) as compared to Gpx1+/+ cells (de Haan et al. 2004)
The human gene encoding MnSOD is designated SOD2 (www.genenames.org/data/hgnc_data.php?hgnc_id=11180) and contains a c.47T>C single nucleotide polymorphism (SNP) (also referred to as rs4880 (www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=4880)). The SNP results in an valine>alanine substitution and is located within the mitochondrial targeting sequence, correspondingly at position 16 of the precursor protein and position -9 of the processed mature (active) protein (Rosenblum et al. 1996). Hence, the SNP is in the literature also referred to as p.V16A and p.-9V/-9A. The SNP possibly poses an effect on the localization and activity of the variant proteins (Shimoda-Matsubayashi et al. 1996;Sutton et al. 2003). In this paper we will refer to the SNP as the rs4880 MnSOD SNP. The rs4880 MnSOD SNP has been found to be associated with several diseases: the T allele with cardiomyopathy (Hiroi et al. 1999), atherosclerosis (Kakko et al. 2003), and lung cancer (Wang et al. 2001), while the C allele has been associated with breast, prostate and colorectal cancers (Ambrosone et al. 1999; Stoehlmacher et al. 2002;Woodson et al. 2003), hypertension (Hsueh et al. 2005), sporadic motor neuron disease (Van Landeghem et al. 1999), Parkinson’s disease (Shimoda-Matsubayashi et al. 1996), and Alzheimer’s disease (Wiener et al. 2007).
The human gene encoding GPX1 is designated GPX1 (www.genenames.org/data/hgnc_data.php?hgnc_id4553). GPX1 contains a c.599C>T SNP (previously referred to as c.593C>T), which is also referred to as rs1050450 (www.ncbi.nlm.nih.gov/SNP/snp_ref.cgi?rs=1050450)). The SNP leads to a proline>leucine substitution, hence the SNP is also referred to as p.P200L (however, previously it was referred to as Pro198Leu (see e.g. (Moscow et al. 1994)). The SNP likely affects the activity of the variant proteins (Ravn-Haren et al. 2006). In this paper we will refer to the SNP as the rs1050450 GPX1 SNP. The T allele of the rs1050450 GPX1 SNP has been associated with bladder, lung and breast cancers (Ratnasinghe et al. 2000;Hu and Diamond 2003;Ichimura et al. 2004), diabetic associated atherosclerosis (Hamanishi et al. 2004), intracerebral hemorrhage (Pera et al. 2008), metabolic syndrome (Kuzuya et al. 2008), and coronary artery disease (Tang et al. 2008).
To our knowledge, no studies have been published regarding an association of the rs1050450 GPX1 SNP with human survival. Three cross sectional studies have investigated the association of the rs4880 MnSOD SNP with human survival yet with inconsistent results (De Benedictis et al. 1998;Stessman et al. 2005;Taufer et al. 2005).
In the present study, we evaluated the association of the rs4880 MnSOD and the rs1050450 GPX1 SNPs with longevity in a longitudinal study of the long lived Danish 1905 cohort. Compared to a cross sectional set-up, a longitudinal set-up reduces the bias arising from cohort specific variations in environment. In addition, we investigated the associations of the SNPs with the functional and cognitive abilities of the elderly, since these phenotypes were previously shown to be important mortality risk factors in oldest old (Nybo et al. 2003).
2. MATERIALS AND METHODS
2.1. Subjects
Study participants were the Danish 1905 Cohort, which includes all Danes born in 1905 (Nybo et al. 2001a). The cohort members were assessed for the first time in 1998 and subsequently assessed every second year. Participants were followed until death or the 29th of January 2008, which ever came first. The cohort is now nearly extinct. The survey included multidimensional face-to-face interviews, assessment of functional and cognitive abilities and DNA sampling. DNA was available from a total number of 1650 participants. Due to national civil registers there is 100% follow-up regarding mortality. For evaluation of the functional ability of the elderly, self rated health and Activity of Daily Living (ADL) scores were used. Self reported health was determined by asking the participants to rate their present health as “excellent/good/acceptable” or “poor/very poor”, while the 11 item ADL strength score was based on the ability to walk, go upstairs and carry weights (Christensen et al. 2000;Nybo et al. 2001b). The scale of the ADL strength score was 1 to 4 (1 = cannot do, 2 = can do with aid or major difficulty, 3 = can do with fatigue or minor difficulties and 4 = can do without fatigue). For evaluation of the cognitive functioning of the elderly the Mini Mental State Examination (MMSE) (Folstein et al. 1975) scores were used, ranging from 0 to 30 (30 being maximal cognitive function). For all these three phenotypes, values from intake (baseline) were used. Permission to collect blood samples and usage of register based information on the oldest old was granted by The Danish National Committee on Biomedical Research Ethics.
2.2. Genotyping
DNA was isolated from cheek swabs or blood spots using the QIAamp DNA Mini Kit (Qiagen). The Danish 1905 Cohort was genotyped for the rs4880 MnSOD SNP and for the rs1050450 GPX1 SNP using Taqman technology. rs4880 MnSOD SNP primers: 5′-GGCTGTGCTTTCTCGTCTTCA-3′ (forward) and 5′-TCTGCCTGGAGCCCAGATAC-3′ (reverse) and rs1050450 GPX1 SNP primers: 5′-CATCGAAGCCCTGCTGTCT-3′ (forward) and 5′-CACTGCAACTGCCAAGCA-3′ (reverse) were purchased from DNA Technology A/S (Denmark), while rs4880 MnSOD SNP probes: 5′-Vic-CGAGGCCGAAACCC-3′ and 5′-Fam-ACCGAGGCCAAAACC-3′ and rs1050450 GPX1 SNP probes: 5′-Vic-ACAGCTGGGCCCTT-3′ and 5′-Fam-ACAGCTGAGCCCTT-3′ were purchased from Applied Biosystems (UK). DNA was amplified in a total volume of 10 μl containing 5 μl Taqman Universal Master Mix (Applied Biosystems), 900 nM of each primer, 200 nM of each probe and 10 ng template DNA. PCR was performed in the ABI Prism® 7700 Sequence Detector (Applied Biosystems) using the conditions recommended by the manufacturer and the PCR data was analyzed using the Sequence Detector software version 1.7 (Applied Biosystems). 37 of the 1650 cohort members could not be genotyped with certainty for the rs1050450 GPX1 SNP due to poor quality of the DNA sample.
2.3. Statistics
The STATA 10.0 statistical program (Stata Corporation, College Station, TX, USA) was used for statistical analysis. The χ2 test was used for comparing genotype frequencies and for Hardy-Weinberg equilibrium testing. For comparing the functional and cognitive abilities between groups linear regressing was applied. Mortality risks/hazard ratios (HR) were estimated using the Cox proportional hazard model. Statistical significance was set as P < 0.05.
3. RESULTS
3.1. Genotype frequencies
The genotype frequencies of the rs4880 MnSOD SNP and of the rs1050450 GPX1 SNP in the Danish 1905 cohort are summarized in table 1. The allele frequencies for the rs4880 MnSOD SNP were 49.9% for C and 50.1% for T, while for the rs1050450 GPX1 SNP they were 69.1% for C and 30.9% for T, which correspond with previous reports in Caucasians (Ambrosone et al. 1999; Van Landeghem et al 1999, Ravn-Haren et al. 2006). The p-values for Hardy-Weinberg disequilibrium were 0.88 for the rs4880 MnSOD SNP and 0.005 for the rs1050450 GPX1 SNP. Compared to a previous study of the rs1050450 GPX1 SNP genotype frequencies in 798 middle aged Danes (Raaschou-Nielsen et al. 2007), the frequencies of CC and CT individuals in our 1905 cohort were not significantly different (p-value (P) = 0.66), yet we did observe a slightly lower frequency of TT individuals. Genotype frequencies were not different between males and females (P (rs4880) = 0.15, P (rs1050450) = 0.23), therefore the combined frequencies were used for the subsequent survival analyses and association studies of the age-related phenotypes.
Table 1. The rs4880 MnSOD SNP and rs1050450 GPX1 SNP genotype frequencies in the 1905 cohort: the Danish 1905 cohort.
1905 cohort: The Danish 1905 Cohort
| The rs4880 MnSOD SNP in the 1905 cohort (N=1650) | The rs4050450 GPXI SNP in the 1905 cohort (N=1613) | ||||||
|---|---|---|---|---|---|---|---|
| Female | Males | Total | Female | Males | Total | ||
| Participants | 1185 | 465 | 1650 | Participants | 1160 | 453 | 1613 |
| Females : Males | 71.8% | 28.2% | 100% | Females: Males | 71.9% | 28.1% | 100% |
| Number of deaths | 1140 (96.2%) | 449 (96.6%) | 1589 (96.3%) | Number of deaths | 1115 (96.1%) | 437 (96.5%) | 1552 (96.2%) |
| Genotype | Genotype | ||||||
| CC | 284 (24%) | 128 (27.5%) | 412 (25%) | CC | 539 (46.5%) | 207 (45.7%) | 746 (46.2%) |
| CT | 589 (49.7%) | 233 (50.1%) | 822(49.8%) | CT | 536 (46.2%) | 201 (44.4%) | 737 (45.7%) |
| TT | 312 (26 3%) | 104 (22. 4%) | 416 (25.2%) | TT | 85 (73%) | 45 (99%) | 130 (8.1%) |
| Allele frequency C:T | 48.8%: 51.2% | 52.6% : 47.4% | 49.9% : 50.1% | Allele frequency C:T | 69.6% : 30.4% | 67.9% : 32.1% | 69.1%: 30.9% |
3.2. Longevity
In the 10-year follow-up 1589 individuals out of the 1650 initial individuals died. The sex adjusted mortality risks were calculated using the Cox proportional hazard model with the most frequent homozygote genotype as the reference group (TT for the rs4880 MnSOD SNP and CC for the rs1050450 GPX1 SNP). The sex adjusted mortality risks according to the rs4880 MnSOD SNP showed a significantly decreased mortality of CT and CC genotype individuals (Hazard ratio (HR) (CT) = 0.82; HR (CC) = 0.87), while the sex adjusted mortality risks according to the rs1050450 GPX1 SNP showed decreased mortality of CT and TT individuals (HR (CT) = 0.87; HR (TT) (HR) = 0.88, not being statistical significant for TT individuals).
The estimates for the rs4880 MnSOD SNP suggest a dominant effect of the C allele (a survival advantage of CC and CT individuals). Combining the CC and CT genotypes (and comparing to the TT genotype) shows a HR of 0.91 (P = 0.002).
With respect to the rs1050450 GPX1 SNP, there seems to be a heterozygote advantage, since there are less homozygotes (both CC and TT individuals) in the 1905 cohort than expected from the allele frequencies (using the Hardy-Weinberg equation). However, deviations from expected frequencies have been reported before for the oldest old (Bathum et al. 1998;Geesaman et al. 2003). In addition, the survival estimates obtained here have the same effect size and indicate a survival advantage of CT and TT individuals in old age, although not significant for the TT genotype. Combining the CC and TT individuals shows a HR of 0.93 (P = 0.008). The survival data is summarized in table 2.
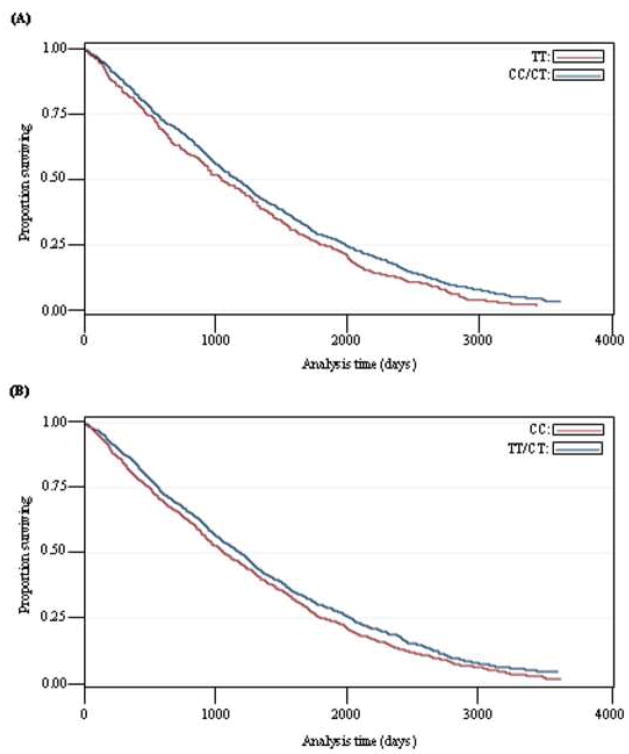
When fitting these sex adjusted mortality risk estimates to their respective Kaplan-Meier Survival estimates, there was good correspondence (data not shown), validating the use of the Cox proportional hazard model. The Kaplan-Meier Survival estimates when combining carriers of the minor allele are shown in figure 1.
Figure 1. Kaplan-Meier Survival estimate for the 1905 cohort by (A) rs4880 MnSOD SNP genotype or by (B) rs1050450 GPX1 SNP genotype.
N (rs4880 MnSOD SNP) = 1650, N (rs1050450 GPX1 SNP) = 1613.
These survival data indicate that both the rs4880 MnSOD SNP and the rs1050450 GPX1 SNP may be mortality risk factors.
3.3. Gender related difference in survival
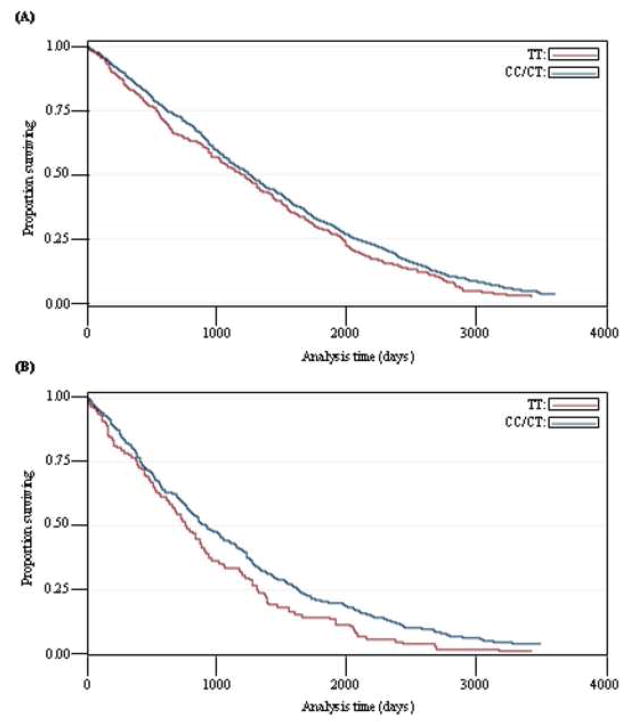
When looking at males and females separately, it was observed that the effects of the advantageous rs4880 MnSOD and rs1050450 GPX1 SNP genotypes on mortality seemed to be most pronounced for males (data not shown). Figure 2 shows an example of this difference in survival: the Kaplan-Meier Survival estimates for the rs4880 MnSOD SNP CC/CT and TT genotypes for females (figure 2A) and males (figure 2B) separately. When calculating the HR for the CC/CT genotype it was 0.86 for males (P = 0.011, 95% Confidence interval (CI): [0.775–0.967], N = 465), while it was 0.93 for females (P = 0.045, 95%CI: [0.875–0.998], N = 1185).
Figure 2. Kaplan-Meier Survival Estimates for the 1905 cohort by sex and rs4880 MnSOD SNP genotype.
(A) Females and (B) Males. N (females) = 1185, N (males) = 465
3.4. Synergetic effect of the MnSOD and GPX1 SNPs
Since the MnSOD and GPX1 proteins are involved in the same biological pathway we wanted to investigate a possible synergetic effect of the two SNPs. Hence, the association of the combinations of the rs4880 MnSOD and rs1050450 GPX1 SNP genotypes with survival was investigated. Individuals holding at least one minor allele in both SNPs had a significantly decreased mortality as compared to individuals being homozygous for both the major alleles. The HR was 0.76 (P = 0.001, 95% CI: [0.647–0.894], N = 1613), indicating that there might be a more than additive effect of holding both the “best survival” alleles as compared to holding both the “worst survival” alleles.
3.5. Moderate effects on aging related phenotypes
The possible associations of the two SNPs to the functional ability and to the cognitive function of the elderly were also investigated.
With respect to self reported health, a lower frequency of CC/CT rs4880 MnSOD individuals reported a “poor/very poor” health (9.02% (CC/CT) versus 13.75% (TT), N = 1586, P = 0.007). For the rs1050450 GPX1 SNP genotype 11.31% (CC), 9.31% (CT) and 11.11% (TT), (combined CT/TT: 9.58%) reported a “poor/very poor” health (N = 1551, P = 0.446) When applying the Activity of Daily Living (ADL) strength score, the rs4880 MnSOD SNP CC/CT genotype showed a positive (although not statistical significant) sex adjusted coefficient of 0.0359 (P = 0.296, 95%CI = [−0.0189–0.0622], N = 1633). With respect to the rs1050450 GPX SNP, a positive statistical significant sex adjusted coefficient of 0.0783 (P = 0.039, 95%CI = [0.0039–0.1528], N (CT) = 726) was found for the CT genotype, while a positive but non-significant coefficient of 0.0359 (P = 0.605, 95%CI = [−0.1001–0.1719], N (TT) = 129) was found for the TT genotype. The combined CT/TT group showed a statistical significant coefficient of 0.0216 (P = 0.049, 96%CI = [0.0002–0.0717], N = 1596). Still, a moderate increased ADL score may indicate that the individuals holding the genotypes associated with decreased mortality tended to be less disabled, which corresponds well with a tendency of a better self reported health.
Finally, the MMSE criteria showed a positive coefficient for the rs4880 MnSOD SNP CC/CT group; sex adjusted coefficient CC/CT = 0.404 (P = 0.015, 95%CI = [0.0790–0.7296], N = 1582). With respect to the rs1050450 GPX1 SNP, a coefficient of 0.307 (P = 0.309, 95%CI = [−0.2855–0.9001], N (CT) = 708) for the CT genotype and a coefficient of −0.267 (P = 0.627, 95%CI = [−1.3484–0.8131], N (TT) = 126) for the TT genotype were found. Nevertheless, a moderate increased MMSE for the rs4880 MnSOD SNP genotype individuals indicates a moderate increased cognitive function of the individuals holding the genotypes associated with decreased mortality.
4. DISCUSSION
In this paper we show associations of the rs4880 MnSOD SNP and of the rs1050450 GPX1 SNP with longevity, indicating that variation in the genes encoding MnSOD and GPX1 may influence the variation in human life span. Due to minimal immigration into the Danish 1905 cohort, population stratification is minimized, hence, the cohort must be considered to be genetically homogenous. Previously, three cross sectional studies have been published on the role of the rs4880 MnSOD SNP in human survival or longevity. Taufer et al. 2005 did not find an association with survival from newborn (N = 65), to age 21–79 years (N = 296) or to age 80–105 years (N = 75) in individuals of mixed European Caucasian and native South American origin (Taufer et al. 2005). De Benedictis et al. did not find an association with longevity in 167 centenarians and 186 controls (age 10–85 years) of Italian origin (De Benedictis et al. 1998). However, based on their geographical origin (northern or southern Italy) the centenarians and controls in the latter study were divided in two groups (62 + 80 individuals and 105 + 106 individuals, respectively) and in the former study the sample size of the 80–105 age group was 75 individuals. Hence, these observations may simply be due to lack of power to detect a moderate effect on longevity (as observed in our study) as a result of the relatively small sample sizes. Furthermore, the study groups in Taufer et al.’s study were of mixed European Caucasian and native South American origin, which could give rise to population stratification. However, Taufer et al. do argue that this is not the case, since mitochondrial DNA lineage studies previously showed homogeneous miscegenation (Parra et al. 2003). Finally, since the two studies are cross sectional, it cannot be excluded that cohort specific variation in environment may introduce bias.
One study indicated an association of the T allele with survival from age 22 (N = 441) to age 75 (N = 224) (Stessman et al. 2005). An increase in T allele frequency (from 33% to 51%) and an increase of TT homozygotes (from 25% to 45.5%) were here observed in an Israeli (Ashkenazi) population. These results are somehow contradictory to our results, yet there are several possible explanations for this inconsistency. Firstly, an association of the rs4880 MnSOD SNP with survival may differ between populations (Israeli versus Danes), secondly the role of rs4880 MnSOD genotype (and hence possibly activity (see below)) may be of different importance at different ages (22–75 years versus 92–100+) and finally, the difference in study design (cross sectional versus longitudinal) may be of importance.
When looking at the possible functional basis of the observed association of the rs4880 MnSOD SNP genotype with longevity, several experiments support such an association. The SNP is located in the mitochondrial targeting signal leading to disruption of its helical structure in the 16Valine(T) variant protein (Shimoda-Matsubayashi et al. 1996). By using purified rat liver mitochondria, Sutton et al. showed that this disruption hampers the transport of the protein into the mitochondrial matrix; about 35% of (recombinant) 16Valine(T) proteins are arrested in the inner mitochondrial membrane, as compared to only 8% of the 16Alanine(C) proteins (Sutton et al. 2003). In the matrix the mitochondrial targeting signal is cleaved off generating the active protein (Matsuda et al. 1990). This processing is hampered for the 16Valine(T) variant, since it generates 29% less processed protein than the 16Alanine(C) variant and the activity of the 16Valine(T) variant is 23% less than the 16Alanine(C) variant (Sutton et al. 2003). A hampered processing of the 16Valine(T) variant has also been found by Hiroi et al. (Hiroi et al. 1999). Sutton et al. later confirmed their data in a human in vivo system (hepatoma cells transfected with construct carrying either genotype); in 16Alanine(C) variant cells the levels of processed protein and the activity was four fold higher than in 16Valine(T) variant cells. Also, the O2.− level in 16Alanine(C) cells was 60% lower than in 16Valine(T) cells (Sutton et al. 2005).
These results indicate that the genetic variation in the rs4880 MnSOD SNP might lead to difference in MnSOD activity in the matrix and hence to difference in oxidant-antioxidant balance. CC (alanine-alanine) and CT (alanine-valine) individuals would likely hold a higher MnSOD activity (than TT (valine-valine) individuals) and hence have less O2.− accumulation in the matrix, which could lead to lower damage levels (as compared to TT (valine-valine) individuals). Such a difference in antioxidant activity and damage level could (according to the free radical theory of aging) be expected to affect the life span of the individual. This is indeed what we observe: the CC (alanine-alanine)/CT (alanine-valine) individuals hold reduced mortality as compared to TT (valine-valine) individuals.
However, there are a few studies which have not been able to find support for the correlation between genotype and activity as observed by Sutton et al. Two studies measuring the MnSOD activity in mitochondrial extracts of erythrocytes have been reported: Bastaki et al. observed a 33% reduced activity in CC extracts as compared to CT/TT extracts (Bastaki et al. 2006), while Elsakka et al. (Elsakka et al. 2007) detected no difference. The difference between these two studies might be explained by difference in mitochondrial purification, activity measurement procedures and in sample sizes (Bastaki et al.: 102 males and 129 females and Elsakka et al.: 20 individuals). Moreover, when measuring the MnSOD activity in mitochondrial extracts the genetic background of the individuals can influence the activity; hence the difference in results may simply be due to difference in genetic background of the study populations. In any case does the study by Bastaki et al. show the opposite as Sutton et al. However, Sutton et al. did use a cellular system only differing in the rs4880 MnSOD SNP, excluding influence from difference in genetic background. Yet, the in vivo data obtained by Sutton et al. remains to be confirmed in a separate study.
We report for the first time an association of the rs1050450 GPX1 SNP with longevity. The rs1050450 GPX1 SNP genotype frequencies in the 1905 cohort deviate from the once expected from the observed allele frequencies. However, as previously mentioned, such deviations have been reported before for SNP genotype frequencies in populations of the oldest old (Bathum et al. 1998;Geesaman et al. 2003). A deviation might indicate that there is a selection with respect to the rs1050450 GPX1 SNP genotypes up until old age (the 1905 cohort members were age 92–93 when they entered the survey, an age at which individuals can be said to be cases of extreme survival). Furthermore, the survival estimates obtained here have the same effect size and indicate a survival advantage of CT and TT individuals in old age, although not significant for the TT genotype. Compared to a cohort of middle aged Danes (Raaschou-Nielsen et al. 2007), we find a slightly lower frequency of TT individuals. This might indicate an antagonistic pleiotropic effect of the SNP in Danes; TT individuals might have an increased mortality from middle age to very old age (as compared to CC and CT individuals), whereas in very old age TT individuals have a survival advantage. On the other hand, the deviation in TT individuals may simple be a chance observation.
Three studies measuring the GPX1 activity (with respect to the rs1050450 GPX1 SNP) in human erythrocyte extracts have been reported; the activity of CT/TT extracts was found to be 9% lower than the activity in CC extracts (Ravn-Haren et al. 2006) and the activity was 13% lower in TT males as compared to CT/CC males (Bastaki et al. 2006), while Forsberg et al (Forsberg et al. 2000) detected no difference. Hence, it might be that the rs1050450 GPX1 SNP genotypes (CT and TT) we find to be associated with decreased mortality in the oldest old may be the variant holding slightly lower activity. This seems somehow contradictory to the idea that efficient antioxidant enzyme activity contributes to reduced mortality. However, oxidative stress is believed also to have beneficial effects; moderate levels of ROS or a temporary increase in ROS levels have positive effects on cellular homeostasis (Droge 2002; Radak et al. 2008). Hence, there is probably a delicate balance between the advantageous and disadvantageous effects of ROS, and the relation between ROS and life span is probably not as straight forward as low ROS levels (possibly ensured by efficient anti-oxidant enzymes) equaling increased life span. Finally, a slightly more active GPX1 could lead to increased OH− levels due to decreased levels of reduced glutathione. Therefore, it can not be excluded that a slight decrease in GPX1 activity (in the TT and CT rs1050450 GPX1 SNP genotypes) could have a positive effect on longevity.
We observe a difference between genders in the effects of genotypes on mortality; the effects are apparently more pronounced in males.Bastaki et al. (2006) observed a 16% decreased MnSOD activity in males as compared to females. Since the proposed hampering of the T variant is only partial and if women in general hold a slightly higher MnSOD activity, it can be speculated that the MnSOD activity in female TT individuals could reach a threshold of activity enabling them to overcome (at least in part) the oxidative stress, perhaps resulting in a less severe effect of the increased mortality in TT genotype individuals observed in our study. For GPX1 the activity in males also appear to be lower than in females; Bastaki et al. reported it to be 7.7% lower (Bastaki et al. 2006), whereas Blankenberg et al. measured a 5% difference (Blankenberg et al. 2003). Such a difference may explain the less severe effect of the CC rs1050450 GPX1 genotype in females.
We observe a synergy effect in individuals holding both the CC or CT rs4880 MnSOD genotypes and the TT or CT rs1050450 GPX1 genotypes, indicating that this genetic combination may influence human longevity in a positive direction. A synergy effect of the rs4880 MnSOD and rs1050450 GPX1 SNP genotypes has been reported once before; Cox et al. (2006) observed no association with breast cancer of the two SNPs independently, yet individuals holding both the rs4880 MnSOD CC and rs1050450 GPX1 TT genotypes showed increased breast cancer risk as compared to individuals holding both the rs4880 MnSOD TT and rs1050450 GPX1 CC genotypes. Finally, the sensitivity towards oxidative stress in Sod2+/−/Gpx1−/− mice has been found to be more than additive as compared to Sod2+/− or Gpx1−/− mice (Van Remmen et al. 2004), thus supporting a synergetic effect of MnSOD and GPX1 activities.
Finally, we investigated whether the two SNPs were associated with the functional and cognitive abilities of the elderly. Our results indicate that the SNPs may affect these age-related phenotypes in a modest manner.
Our data implies a moderate effect of the CC/CT rs4880 MnSOD SNP and TT/CT rs1050450 GPX1 SNP genotypes on good self rated health (yet, non-significant for rs1050450) and a moderate effect on increased ADL strength scores (yet, non-significant for rs4880). Looking at the CT and TT rs1050450 GPX SNP genotypes separately showed the same tendencies. These finding might indicate that the individuals holding the genotypes associated with decreased mortality might have had slightly better functional abilities.
The MMSE scores were used for evaluation of the cognitive functioning of the elderly. The values used were intake scores, since it has been shown previously that the MMSE scores at intake is heritable, while rate of change is not (McGue and Christensen 2002). We found a moderate effect of the rs4880 MnSOD SNP CC/CT genotype on increase in MMSE, indicating that the individuals holding the genotypes associated with decreased mortality also had slightly better cognitive abilities. As mentioned earlier, the rs4880 MnSOD SNP CC/CT genotype might be the genotype with the highest activity. Notably, centenarians having the highest Cu/ZnSOD activity have been found also to hold the highest cognitive capacity (Andersen et al. 1998). Our results indicate that this might also be the case for MnSOD.
Lastly, other SNPs may be in linkage to the SNPs investigated here, and other SNPs (in genes protecting against ROS) are expected to contribute to the variation in aging and longevity. Therefore, we continue our search of SNPs in such genes and their possible association with aging and longevity.
Table 2. Mortality risk according to the rs4880 MnSOD SNP genotypes or to the rs1050450 GPX1 SNP genotypes.
1905 cohort: The Danish 1905 Cohort. HR: hazard ratio. CI: confidence interval.
| rs4880 MnSOD SNP genotypes | HR | 95% CI | p value |
|---|---|---|---|
| TT | 1 | - | - |
| CT | 0.82 | 0.756–0 998 | 0.048 |
| CC | 0.87 | 0.727–0.925 | 0.001 |
| CC/CT | 0.91 | 0.864–0.967 | 0.002 |
| rs1050450 GPXI SNP genotypes | |||
| CC | 1 | - | - |
| CT | 0.87 | 0.787–0.968 | 0.010 |
| TT | 0.88 | 0.729–1.068 | 0.202 |
| TT/CT | 0.93 | 0.889–0.982 | 0.008 |
Acknowledgments
This study was financially supported by the National Institute on Aging (P01 AG08761-18). The Danish Aging Research Center is supported by a grant from the VELUX Foundation (95-103-11419).
Abbreviations
- SNP
single nucleotide polymorphism
- MnSOD
manganese superoxide dismutase
- GPX1
glutathione peroxidase 1
- ROS
reactive oxygen species
- HR
hazard ratio
- ADL
Activity of Daily Living
- MMSE
Mini Mental State Examination
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- Ambrosone CB, Freudenheim JL, Thompson PA, Bowman E, Vena JE, Marshall JR, Graham S, Laughlin R, Nemoto T, Shields PG. Manganese superoxide dismutase (MnSOD) genetic polymorphisms, dietary antioxidants, and risk of breast cancer. Cancer Res. 1999;59:602–606. [PubMed] [Google Scholar]
- Andersen HR, Jeune B, Nybo H, Nielsen JB, Andersen-Ranberg K, Grandjean P. Low activity of superoxide dismutase and high activity of glutathione reductase in erythrocytes from centenarians. Age Ageing. 1998;27:643–648. doi: 10.1093/ageing/27.5.643. [DOI] [PubMed] [Google Scholar]
- Antonella B. Oxidative Stress and Ventricular Dysfunction in Ischemic Heart Disease. In: Claudio C, Anna C, Palmira B, Salvatore C, Roberto F, editors. Heart Failure Reviews. 2. Vol. 4. 1999. pp. 147–156. [Google Scholar]
- Bastaki M, Huen K, Manzanillo P, Chande N, Chen C, Balmes JR, Tager IB, Holland N. Genotype-activity relationship for Mn-superoxide dismutase, glutathione peroxidase 1 and catalase in humans. Pharmacogenet Genomics. 2006;16:279–286. doi: 10.1097/01.fpc.0000199498.08725.9c. [DOI] [PubMed] [Google Scholar]
- Bathum L, Andersen-Ranberg K, Boldsen J, Brosen K, Jeune B. Genotypes for the cytochrome P450 enzymes CYP2D6 and CYP2C19 in human longevitY. Role of CYP2D6 and CYP2C19 in longevity. Eur J Clin Pharmacol. 1998;54:427–430. doi: 10.1007/s002280050487. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Torzewski M, Hafner G, Tiret L, Smieja M, Cambien F, Meyer J, Lackner KJ. Glutathione peroxidase 1 activity and cardiovascular events in patients with coronary artery disease. N Engl J Med. 2003;349:1605–1613. doi: 10.1056/NEJMoa030535. [DOI] [PubMed] [Google Scholar]
- Christensen K, Johnson TE, Vaupel JW. The quest for genetic determinants of human longevity: challenges and insights. Nat Rev Genet. 2006;7:436–448. doi: 10.1038/nrg1871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christensen K, McGue M, Yashin A, Iachine I, Holm NV, Vaupel JW. Genetic and environmental influences on functional abilities in Danish twins aged 75 years and older. J Gerontol A Biol Sci Med Sci. 2000;55:M446–M452. doi: 10.1093/gerona/55.8.m446. [DOI] [PubMed] [Google Scholar]
- Cox DG, Tamimi RM, Hunter DJ. Gene x Gene interaction between MnSOD and GPX-1 and breast cancer risk: a nested case-control study. BMC Cancer. 2006;6:217. doi: 10.1186/1471-2407-6-217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Benedictis G, Carotenuto L, Carrieri G, De LM, Falcone E, Rose G, Cavalcanti S, Corsonello F, Feraco E, Baggio G, Bertolini S, Mari D, Mattace R, Yashin AI, Bonafe M, Franceschi C. Gene/longevity association studies at four autosomal loci (REN, THO, PARP, SOD2) Eur J Hum Genet. 1998;6:534–541. doi: 10.1038/sj.ejhg.5200222. [DOI] [PubMed] [Google Scholar]
- de Haan JB, Bladier C, Griffiths P, Kelner M, O’Shea RD, Cheung NS, Bronson RT, Silvestro MJ, Wild S, Zheng SS, Beart PM, Hertzog PJ, Kola I. Mice with a homozygous null mutation for the most abundant glutathione peroxidase, Gpx1, show increased susceptibility to the oxidative stress-inducing agents paraquat and hydrogen peroxide. J Biol Chem. 1998;273:22528–22536. doi: 10.1074/jbc.273.35.22528. [DOI] [PubMed] [Google Scholar]
- de Haan JB, Bladier C, Lotfi-Miri M, Taylor J, Hutchinson P, Crack PJ, Hertzog P, Kola I. Fibroblasts derived from Gpx1 knockout mice display senescent-like features and are susceptible to H2O2-mediated cell death. Free Radic Biol Med. 2004;36:53–64. doi: 10.1016/j.freeradbiomed.2003.10.020. [DOI] [PubMed] [Google Scholar]
- Droge W. Free radicals in the physiological control of cell function. Physiol Rev. 2002;82:47–95. doi: 10.1152/physrev.00018.2001. [DOI] [PubMed] [Google Scholar]
- Duttaroy A, Paul A, Kundu M, Belton A. A Sod2 null mutation confers severely reduced adult life span in Drosophila. Genetics. 2003;165:2295–2299. doi: 10.1093/genetics/165.4.2295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elsakka NE, Webster NR, Galley HF. Polymorphism in the manganese superoxide dismutase gene. Free Radic Res. 2007;41:770–778. doi: 10.1080/10715760701338828. [DOI] [PubMed] [Google Scholar]
- Esworthy RS, Ho YS, Chu FF. The Gpx1 gene encodes mitochondrial glutathione peroxidase in the mouse liver. Arch Biochem Biophys. 1997;340:59–63. doi: 10.1006/abbi.1997.9901. [DOI] [PubMed] [Google Scholar]
- Fabrizio P, Liou LL, Moy VN, Diaspro A, Valentine JS, Gralla EB, Longo VD. SOD2 functions downstream of Sch9 to extend longevity in yeast. Genetics. 2003;163:35–46. doi: 10.1093/genetics/163.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folstein MF, Folstein SE, McHugh PR. “Mini-mental state” A practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12:189–198. doi: 10.1016/0022-3956(75)90026-6. [DOI] [PubMed] [Google Scholar]
- Forsberg L, de FU, Marklund SL, Andersson PM, Stegmayr B, Morgenstern R. Phenotype determination of a common Pro-Leu polymorphism in human glutathione peroxidase 1. Blood Cells Mol Dis. 2000;26:423–426. doi: 10.1006/bcmd.2000.0325. [DOI] [PubMed] [Google Scholar]
- Geesaman BJ, Benson E, Brewster SJ, Kunkel LM, Blanche H, Thomas G, Perls TT, Daly MJ, Puca AA. Haplotype-based identification of a microsomal transfer protein marker associated with the human lifespan. Proc Natl Acad Sci USA. 2003;100:14115–14120. doi: 10.1073/pnas.1936249100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamanishi T, Furuta H, Kato H, Doi A, Tamai M, Shimomura H, Sakagashira S, Nishi M, Sasaki H, Sanke T, Nanjo K. Functional variants in the glutathione peroxidase-1 (GPx-1) gene are associated with increased intima-media thickness of carotid arteries and risk of macrovascular diseases in japanese type 2 diabetic patients. Diabetes. 2004;53:2455–2460. doi: 10.2337/diabetes.53.9.2455. [DOI] [PubMed] [Google Scholar]
- Harman D. Free radical theory of aging. Triangle. 1973;12:153–158. [PubMed] [Google Scholar]
- Harman D. The aging process: major risk factor for disease and death. Proc Natl Acad Sci USA. 1991;88:5360–5363. doi: 10.1073/pnas.88.12.5360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herskind AM, McGue M, Holm NV, Sorensen TI, Harvald B, Vaupel JW. The heritability of human longevity: a population-based study of 2872 Danish twin pairs born 1870–1900. Hum Genet. 1996;97:319–323. doi: 10.1007/BF02185763. [DOI] [PubMed] [Google Scholar]
- Hinerfeld D, Traini MD, Weinberger RP, Cochran B, Doctrow SR, Harry J, Melov S. Endogenous mitochondrial oxidative stress: neurodegeneration, proteomic analysis, specific respiratory chain defects, and efficacious antioxidant therapy in superoxide dismutase 2 null mice. J Neurochem. 2004;88:657–667. doi: 10.1046/j.1471-4159.2003.02195.x. [DOI] [PubMed] [Google Scholar]
- Hiroi S, Harada H, Nishi H, Satoh M, Nagai R, Kimura A. Polymorphisms in the SOD2 and HLA-DRB1 genes are associated with nonfamilial idiopathic dilated cardiomyopathy in Japanese. Biochem Biophys Res Commun. 1999;261:332–339. doi: 10.1006/bbrc.1999.1036. [DOI] [PubMed] [Google Scholar]
- Hjelmborg JVb, Iachine I, Skytthe A, Vaupel JW, McGue M, Koskenvuo M, Kaprio J, Pedersen NL, Christensen K. Genetic influence on human lifespan and longevity. Human Genetics. 2006;119:312–321. doi: 10.1007/s00439-006-0144-y. [DOI] [PubMed] [Google Scholar]
- Hsueh YM, Lin P, Chen HW, Shiue HS, Chung CJ, Tsai CT, Huang YK, Chiou HY, Chen CJ. Genetic polymorphisms of oxidative and antioxidant enzymes and arsenic-related hypertension. J Toxicol Environ Health A. 2005;68:1471–1484. doi: 10.1080/15287390590967414. [DOI] [PubMed] [Google Scholar]
- Hu D, Cao P, Thiels E, Chu CT, Wu GY, Oury TD, Klann E. Hippocampal long-term potentiation, memory, and longevity in mice that overexpress mitochondrial superoxide dismutase. Neurobiol Learn Mem. 2007;87:372–384. doi: 10.1016/j.nlm.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu YJ, Diamond AM. Role of glutathione peroxidase 1 in breast cancer: loss of heterozygosity and allelic differences in the response to selenium. Cancer Res. 2003;63:3347–3351. [PubMed] [Google Scholar]
- Huang TT, Raineri I, Eggerding F, Epstein CJ. Transgenic and mutant mice for oxygen free radical studies. Methods Enzymol. 2002;349:191–213. doi: 10.1016/s0076-6879(02)49335-4. [DOI] [PubMed] [Google Scholar]
- Ichimura Y, Habuchi T, Tsuchiya N, Wang L, Oyama C, Sato K, Nishiyama H, Ogawa O, Kato T. Increased risk of bladder cancer associated with a glutathione peroxidase 1 codon 198 variant. J Urol. 2004;172:728–732. doi: 10.1097/01.ju.0000130942.40597.9d. [DOI] [PubMed] [Google Scholar]
- Kakko S, Paivansalo M, Koistinen P, Kesaniemi YA, Kinnula VL, Savolainen MJ. The signal sequence polymorphism of the MnSOD gene is associated with the degree of carotid atherosclerosis. Atherosclerosis. 2003;168:147–152. doi: 10.1016/s0021-9150(03)00091-1. [DOI] [PubMed] [Google Scholar]
- Kuzuya M, Ando F, Iguchi A, Shimokata H. Glutathione peroxidase 1 Pro198Leu variant contributes to the metabolic syndrome in men in a large Japanese cohort. Am J Clin Nutr. 2008;87:1939–1944. doi: 10.1093/ajcn/87.6.1939. [DOI] [PubMed] [Google Scholar]
- Lebovitz RM, Zhang H, Vogel H, Cartwright J, Jr, Dionne L, Lu N, Huang S, Matzuk MM. Neurodegeneration, myocardial injury, and perinatal death in mitochondrial superoxide dismutase-deficient mice. Proc Natl Acad Sci USA. 1996;93:9782–9787. doi: 10.1073/pnas.93.18.9782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuda Y, Higashiyama S, Kijima Y, Suzuki K, Kawano K, Akiyama M, Kawata S, Tarui S, Deutsch HF, Taniguchi N. Human liver manganese superoxide dismutase. Purification and crystallization, subunit association and sulfhydryl reactivity. Eur J Biochem. 1990;194:713–720. doi: 10.1111/j.1432-1033.1990.tb19461.x. [DOI] [PubMed] [Google Scholar]
- McGue M, Christensen K. The heritability of level and rate-of-change in cognitive functioning in Danish twins aged 70 years and older. Experimental Aging Research. 2002;28:435–451. doi: 10.1080/03610730290080416. [DOI] [PubMed] [Google Scholar]
- Moscow JA, Schmidt L, Ingram DT, Gnarra J, Johnson B, Cowan KH. Loss of heterozygosity of the human cytosolic glutathione peroxidase I gene in lung cancer. Carcinogenesis. 1994;15:2769–2773. doi: 10.1093/carcin/15.12.2769. [DOI] [PubMed] [Google Scholar]
- Nybo H, Gaist D, Jeune B, Bathum L, McGue M, Vaupel JW, Christensen K. The Danish 1905 cohort: a genetic-epidemiological nationwide survey. J Aging Health. 2001a;13:32–46. doi: 10.1177/089826430101300102. [DOI] [PubMed] [Google Scholar]
- Nybo H, Gaist D, Jeune B, McGue M, Vaupel JW, Christensen K. Functional status and self-rated health in 2,262 nonagenarians: the Danish 1905 Cohort Survey. J Am Geriatr Soc. 2001b;49:601–609. doi: 10.1046/j.1532-5415.2001.49121.x. [DOI] [PubMed] [Google Scholar]
- Nybo H, Petersen HC, Gaist D, Jeune B, Andersen K, McGue M, Vaupel JW, Christensen K. Predictors of mortality in 2,249 nonagenarians--the Danish 1905-Cohort Survey. J Am Geriatr Soc. 2003;51:1365–1373. doi: 10.1046/j.1532-5415.2003.51453.x. [DOI] [PubMed] [Google Scholar]
- Parra FC, Amado RC, Lambertucci JR, Rocha J, Antunes CM, Pena SD. Color and genomic ancestry in Brazilians. Proc Natl Acad Sci USA. 2003;100:177–182. doi: 10.1073/pnas.0126614100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pera J, Slowik A, Dziedzic T, Pulyk R, Wloch D, Szczudlik A. Glutathione Peroxidase 1 C593T Polymorphism Is Associated with Lobar Intracerebral Hemorrhage. Cerebrovasc Dis. 2008;25:445–449. doi: 10.1159/000126918. [DOI] [PubMed] [Google Scholar]
- Raaschou-Nielsen O, Sorensen M, Hansen RD, Frederiksen K, Tjonneland A, Overvad K, Vogel U. GPX1 Pro198Leu polymorphism, interactions with smoking and alcohol consumption, and risk for lung cancer. Cancer Lett. 2007;247:293–300. doi: 10.1016/j.canlet.2006.05.006. [DOI] [PubMed] [Google Scholar]
- Radak Z, Chung HY, Goto S. Systemic adaptation to oxidative challenge induced by regular exercise. Free Radic Biol Med. 2008;44:153–159. doi: 10.1016/j.freeradbiomed.2007.01.029. [DOI] [PubMed] [Google Scholar]
- Ratnasinghe D, Tangrea JA, Andersen MR, Barrett MJ, Virtamo J, Taylor PR, Albanes D. Glutathione peroxidase codon 198 polymorphism variant increases lung cancer risk. Cancer Res. 2000;60:6381–6383. [PubMed] [Google Scholar]
- Ravn-Haren G, Olsen A, Tjonneland A, Dragsted LO, Nexo BA, Wallin H, Overvad K, Raaschou-Nielsen O, Vogel U. Associations between GPX1 Pro198Leu polymorphism, erythrocyte GPX activity, alcohol consumption and breast cancer risk in a prospective cohort study. Carcinogenesis. 2006;27:820–825. doi: 10.1093/carcin/bgi267. [DOI] [PubMed] [Google Scholar]
- Rosenblum JS, Gilula NB, Lerner RA. On signal sequence polymorphisms and diseases of distribution. Proc Natl Acad Sci USA. 1996;93:4471–4473. doi: 10.1073/pnas.93.9.4471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoda-Matsubayashi S, Matsumine H, Kobayashi T, Nakagawa-Hattori Y, Shimizu Y, Mizuno Y. Structural dimorphism in the mitochondrial targeting sequence in the human manganese superoxide dismutase gene. A predictive evidence for conformational change to influence mitochondrial transport and a study of allelic association in Parkinson’s disease. Biochem Biophys Res Commun. 1996;226:561–565. doi: 10.1006/bbrc.1996.1394. [DOI] [PubMed] [Google Scholar]
- Slot JW, Geuze HJ, Freeman BA, Crapo JD. Intracellular localization of the copper-zinc and manganese superoxide dismutases in rat liver parenchymal cells. Lab Invest. 1986;55:363–371. [PubMed] [Google Scholar]
- Stessman J, Maaravi Y, Hammerman-Rozenberg R, Cohen A, Nemanov L, Gritsenko I, Gruberman N, Ebstein RP. Candidate genes associated with ageing and life expectancy in the Jerusalem longitudinal study. Mech Ageing Dev. 2005;126:333–339. doi: 10.1016/j.mad.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Stoehlmacher J, Ingles SA, Park DJ, Zhang W, Lenz HJ. The -9Ala/-9Val polymorphism in the mitochondrial targeting sequence of the manganese superoxide dismutase gene (MnSOD) is associated with age among Hispanics with colorectal carcinoma. Oncol Rep. 2002;9:235–238. [PubMed] [Google Scholar]
- Sun J, Folk D, Bradley TJ, Tower J. Induced overexpression of mitochondrial Mn-superoxide dismutase extends the life span of adult Drosophila melanogaster. Genetics. 2002;161:661–672. doi: 10.1093/genetics/161.2.661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutton A, Imbert A, Igoudjil A, Descatoire V, Cazanave S, Pessayre D, Degoul F. The manganese superoxide dismutase Ala16Val dimorphism modulates both mitochondrial import and mRNA stability. Pharmacogenet Genomics. 2005;15:311–319. doi: 10.1097/01213011-200505000-00006. [DOI] [PubMed] [Google Scholar]
- Sutton A, Khoury H, Prip-Buus C, Cepanec C, Pessayre D, Degoul F. The Ala16Val genetic dimorphism modulates the import of human manganese superoxide dismutase into rat liver mitochondria. Pharmacogenetics. 2003;13:145–157. doi: 10.1097/01.fpc.0000054067.64000.8f. [DOI] [PubMed] [Google Scholar]
- Tang NP, Wang LS, Yang L, Gu HJ, Sun QM, Cong RH, Zhou B, Zhu HJ, Wang B. Genetic variant in glutathione peroxidase 1 gene is associated with an increased risk of coronary artery disease in a Chinese population. Clin Chim Acta. 2008;395:89–93. doi: 10.1016/j.cca.2008.05.013. [DOI] [PubMed] [Google Scholar]
- Taufer M, Peres A, de AV, de OG, Sa G, do Canto ME, dos Santos AR, Bauer ME, da CI. Is the Val16Ala manganese superoxide dismutase polymorphism associated with the aging process? J Gerontol A Biol Sci Med Sci. 2005;60:432–438. doi: 10.1093/gerona/60.4.432. [DOI] [PubMed] [Google Scholar]
- Valko M, Izakovic M, Mazur M, Rhodes CJ, Telser J. Role of oxygen radicals in DNA damage and cancer incidence. Mol Cell Biochem. 2004;266:37–56. doi: 10.1023/b:mcbi.0000049134.69131.89. [DOI] [PubMed] [Google Scholar]
- Van Landeghem GF, Tabatabaie P, Beckman G, Beckman L, Andersen PM. Manganese-containing superoxide dismutase signal sequence polymorphism associated with sporadic motor neuron disease. Eur J Neurol. 1999;6:639–644. doi: 10.1046/j.1468-1331.1999.660639.x. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Ikeno Y, Hamilton M, Pahlavani M, Wolf N, Thorpe SR, Alderson NL, Baynes JW, Epstein CJ, Huang TT, Nelson J, Strong R, Richardson A. Life-long reduction in MnSOD activity results in increased DNA damage and higher incidence of cancer but does not accelerate aging. Physiol Genomics. 2003;16:29–37. doi: 10.1152/physiolgenomics.00122.2003. [DOI] [PubMed] [Google Scholar]
- Van Remmen H, Qi W, Sabia M, Freeman G, Estlack L, Yang H, Mao GZ, Huang TT, Strong R, Lee S, Epstein CJ, Richardson A. Multiple deficiencies in antioxidant enzymes in mice result in a compound increase in sensitivity to oxidative stress. Free Radic Biol Med. 2004;36:1625–1634. doi: 10.1016/j.freeradbiomed.2004.03.016. [DOI] [PubMed] [Google Scholar]
- Vijg J. Somatic mutations and aging: a re-evaluation. Mutat Res. 2000;447:117–135. doi: 10.1016/s0027-5107(99)00202-x. [DOI] [PubMed] [Google Scholar]
- Wang LI, Miller DP, Sai Y, Liu G, Su L, Wain JC, Lynch TJ, Christiani DC. Manganese superoxide dismutase alanine-to-valine polymorphism at codon 16 and lung cancer risk. J Natl Cancer Inst. 2001;93:1818–1821. doi: 10.1093/jnci/93.23.1818. [DOI] [PubMed] [Google Scholar]
- Wiener HW, Perry RT, Chen Z, Harrell LE, Go RC. A polymorphism in SOD2 is associated with development of Alzheimer’s disease. Genes Brain Behav. 2007;6:770–775. doi: 10.1111/j.1601-183X.2007.00308.x. [DOI] [PubMed] [Google Scholar]
- Woodson K, Tangrea JA, Lehman TA, Modali R, Taylor KM, Snyder K, Taylor PR, Virtamo J, Albanes D. Manganese superoxide dismutase (MnSOD) polymorphism, alpha-tocopherol supplementation and prostate cancer risk in the alpha-tocopherol, beta-carotene cancer prevention study (Finland) Cancer Causes Control. 2003;14:513–518. doi: 10.1023/a:1024840823328. [DOI] [PubMed] [Google Scholar]