Abstract
Introduction
Smooth muscle apoptosis in the penis is common in prostatectomy patients and animal models of erectile dysfunction (ED). A critical regulator of smooth muscle apoptosis in the penis is the secreted protein Sonic hedgehog (SHH). Since SHH protein treatment of the penis prevents cavernous nerve (CN) injury induced apoptosis, SHH has the potential to treat post-prostatectomy apoptosis. However little is known about how SHH signaling is regulated in the adult penis.
Aim
The goal of this review is to examine what is known about SHH signaling in the penis, to offer insight as to how SHH inhibition induces apoptosis in penile smooth muscle, and to define the role of the SHH pathway in maintaining CN integrity.
Methods
Information presented in this review was derived from a literature search using the National Library of Medicine PubMed Services. Search terms included SHH, apoptosis, smooth muscle, penis, ED, pelvic ganglia, corpora cavernosa, CN, regeneration, Schwann cell, neural activity and transport.
Results
In this review we have discussed the role of the CN in regulation of SHH abundance and apoptosis induction in the penis and have examined the function and localization of SHH signaling in the CN.
Conclusion
There is substantial potential to develop SHH for delivery to the penis of prostatectomy patients at the time of surgery in order to prevent apoptosis induction and long term ED development. Studies are in progress which will identify if SHH may be used as a regenerative therapy to speed CN regeneration.
Erectile Dysfunction
Forty to seventy percent of prostate cancer patients treated by radiotherapy1–2 and 30–87% of patients treated by radical prostatectomy experience ED2–5. Although potency improves with time post prostatectomy6, ED is common 5 years following surgery7. There is a greater concern for quality of life in prostatectomy populations due to improved treatment strategies and younger age of diagnosis. Survey studies of men electing treatment for localized prostate cancer reveal quality of life is a primary concern in 45% of participants8. Radical prostatectomy is performed on over 60,000 men each year and is the most common treatment for prostate cancer9–10. The current ten-year survival rate is estimated to be 92%, suggesting that men will experience the side effects of treatment for long periods of time10. Since oral therapy with PDE5 inhibitors are ineffective in 29–86% of prostatectomy patients who experience ED11–12, novel therapeutic approaches to treat ED are needed.
Smooth muscle apoptosis is a cause of ED
Significantly increased apoptosis of penile smooth muscle is a common etiology in both animal models and human patients with ED13–20 and in patients that do not respond to phosphodiesterase type 5 (PDE5) inhibitors, smooth muscle cell atrophy is abundant21. These results suggest that abundant apoptosis observed in penile smooth muscle when the CN is cut is a major contributing factor to ED development and that innervation of the penis by the CN plays an important role in maintaining normal penile morphology necessary for erection to occur. When SHH function is inhibited in normal Sprague Dawley rats, smooth muscle apoptosis and ED occur, confirming that smooth muscle apoptosis in the penis is a cause of ED22,15. Current treatment strategies for ED are designed to increase smooth muscle relaxation; however, these strategies become ineffective once smooth muscle apoptosis occurs due to lack of penile innervation23. If penile apoptosis could be prevented following prostatectomy while the CN regenerates, then resumption of normal erectile function would occur more quickly, and fibrosis would be prevented as would long term ED development. Thus in order to prevent penile apoptosis after CN injury/prostatectomy, it is critical to develop therapies that will aid/speed the process of CN regeneration and thus prevent smooth muscle apoptosis.
SHH pathway
Sonic hedgehog (SHH) is a secreted protein that is expressed at sites of mesenchymal-epithelial interaction in many organs. SHH orchestrates tissue sculpting by regulating cellular proliferation, apoptosis, differentiation and cell survival, via manipulation of its targets transcription. SHH can either act directly or by induction of secondary signals including, its receptor patched (PTCH1), smoothened (SMO), hedgehog-interacting-protein (HIP), HOX, bone morphogenetic protein-4 (BMP4), vascular endothelial growth factor (VEGF), nitric oxide synthase (NOS), fibroblast growth factor (FGF), and WNT.
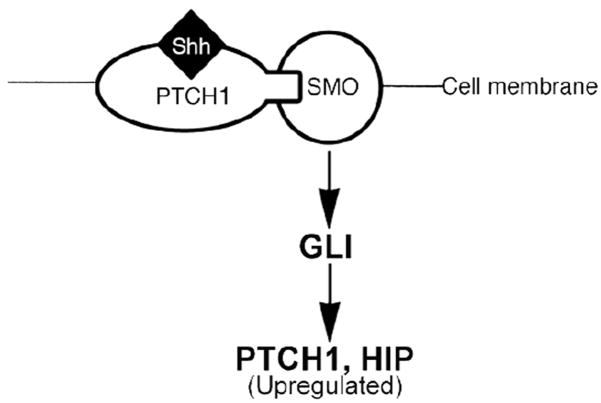
Little is known about SHH signal transduction in the adult penis and pelvic ganglia, however SHH signaling has been well established in other organs during embryogenesis. The SHH signal is transduced through the interplay between PTCH1 and SMO. PTCH1 is a 12-transmembrane protein24–27 that functions as a receptor for SHH. PTCH1 binds to SHH but does not transduce the intracellular signal. SMO, a 7-transmembrane protein that forms a receptor complex with PTCH127, does not bind to SHH but transduces the SHH signal through activation of the GLI family of transcription factors. In the absence of SHH protein bound to PTCH1, PTCH1 represses down stream targets of SHH signaling by inhibiting the activity of SMO at the substoichiometrical level28. When SHH protein binds to PTCH1, this relieves the repression of PTCH1 on SMO and allows transcription of SHH’s down stream targets29–34 (Figure 1). A transcriptional target of SHH signaling is PTCH1 itself. When SHH binds to PTCH1, PTCH1 expression is increased35. By increasing the level of PTCH1 protein in responding cells, SHH signaling attenuates its own activity in a negative feedback loop36. Thus when SHH protein is abundant so is PTCH1 and when SHH protein is decreased, PTCH1 protein is similarly decreased. We have shown in animal models of neuropathy and ED that Ptch1 signaling is altered in parallel with Shh expression37, 15.
Figure 1.
Diagram of the pathway of SHH signal transduction.
SHH regulates penile development and postnatal differentiation
During embryogenesis, SHH is expressed in the urogenital sinus, the tissue from which the penis and prostate derive38. Shh expression is necessary for both genital tubercle outgrowth and differentiation39 and external genitalia are absent in mice with a targeted deletion of Shh40. Shh is expressed through out the entire period of postnatal differentiation of the penis, with increased expression occurring at puberty and the highest expression evident in the adult penis, suggesting continued function in the adult. SHH is localized primarily in the smooth muscle lining the corpora cavernosal sinuses, but is also present in a proliferative region under the tunica, the nerves and urethra. It has been shown in SHH inhibition studies in the penis that SHH is essential to establish and maintain the sinusoid morphology of the penis22.
Why is SHH important in the adult penis?
SHH is a critical regulator of penile smooth muscle. When SHH function is inhibited in adult rat penis using the 5E1 inhibitor (disrupts binding of SHH to PTCH141–42), a 12-fold increase in apoptosis occurs. Electron microscopy and dual TUNEL analysis/α-actin immunohistochemical analysis show that apoptosis occurs primarily in penile smooth muscle15. The morphological changes induced by SHH inhibition are so severe that ED occurs, as measured by a 4-fold decrease in ICP/blood pressure (p-value=0.009)22. The morphology changes caused by SHH inhibition are reversible when SHH inhibition is removed15. These results show that inhibition of SHH function in vivo causes ED due to extensive smooth muscle apoptosis.
SHH protein treatment of the corpora cavernosa suppresses CN injury induced apoptosis15. When SHH protein is delivered to the penis via Affi-Gel beads at the same time as bilateral CN injury is performed in adult Sprague Dawley rats, apoptosis was suppressed 1.25-fold after 4 days of SHH treatment (p-value=0.02)15. When double the concentration of SHH protein was applied, apoptosis was suppressed in a larger region surrounding the bead vehicles and was further reduced 2.5-fold (p-value=3.39E−05). At 8 days post CN injury, apoptosis was suppressed 3-fold in the presence of SHH protein (p-value=9.03E−05). These results show that SHH has significant potential for clinical application to suppress apoptosis post prostatectomy. Thus is would be highly beneficial to have a better understanding of the mechanisms which regulate SHH abundance in the penis.
How is SHH signaling regulated in the adult penis?
Although SHH regulation of apoptosis in the penis is well established15, how SHH itself is regulated is not well defined. Since SHH protein is decreased at the same time as apoptosis is increased in both the BB/WOR diabetic37 and CN injured Sprague Dawley15 rat models of neuropathy, this suggests that neural input plays a role in regulation of SHH abundance in the penis. There are three potential mechanisms of how neural input may regulate SHH abundance and apoptosis induction in the penis. These are: 1.) Neural activity may regulate SHH abundance, 2.) Transport of a trophic factor from the pelvic ganglia to the penis may regulate SHH in the penis, and 3.) SHH protein may be transported from the pelvic ganglia to the penis via the CN. We have examined each of these potential mechanisms by using gel foam to deliver lidocaine or colchicine to the CN and examine SHH abundance and apoptosis in the penis and by performing bilateral CN tie and examining the CN for potential buildup of SHH protein near the tie. We found that inhibition of neural activity and transport with 2% lidocaine for two days (an inhibitor of nerve conduction and axonal transport43–44) in adult Sprague Dawley rats caused a 1.3-fold decrease in SHH protein (p-value=0.01) and a 1.6-fold increase in apoptosis (p-value=0.049) in the penis45. Similar experiments performed using 5 mM colchicine treatment for one day (inhibits the polymerization of tubulin and prevents biological processes where microtubules are involved, including blockade of anterograde and retrograde transport46–50) caused a 1.2-fold decrease in SHH protein (p-value=0.006) and a 1.8-fold increase in apoptosis (p-value=0.015) in the penis45. These findings are significant because they show that both neural activity and transport are critical for regulation of SHH abundance and apoptosis induction in the penis.
In order to test if SHH protein undergoes anterograde transport from the pelvic ganglia to the penis via the CN, a silk tie was placed around the CN bilaterally in adult Sprague-Dawley rats. Immunohistochemical analysis of the CN and pelvic ganglia assayed for SHH protein show that SHH protein does not build up on either side of the tie, indicating that SHH is not transported by the CN45. However, neuronal nitric oxide synthase (NOSI) was observed to build up on the ganglia side of the tie, indicating that protein build up would be visualized if present. Thus SHH protein is not transported from the CN to the penis. SHH was abundant in Schwann cells of the CN, which were recruited to the site of tie placement to aid in the process of axonal repair45. Both myelinating and non-myelinating Schwann cells exist in peripheral nerves such as the CN51–53. Upon injury, they help to phagocytize the damaged end of an axon and then proliferate rapidly to form a scaffold to guide regenerating axons. Thus the presence of SHH in Schwann cells suggests a role for the SHH pathway in maintaining CN integrity. This is supported by previous observations in which SHH inhibition in the pelvic ganglia decreased SHH protein abundance and increased apoptosis in the penis despite SHH protein not being transported by the CN45. These studies suggest that SHH plays a role in regulating CN integrity and that CN activity and a trophic factor from the CN are at least partially responsible for maintaining SHH abundance and normal morphology in the penis.
The SHH pathway and CN regeneration
After CN injury, profound changes in neurotrophic factor signaling occur in the CN. Schwann cells crucially contribute to successful regeneration by mechanical and paracrine influences in the injured nerve. Schwann cells have been successfully used to promote CN regeneration54 and Schwann cell seeded nerve guidance tubes have been used to restore erections and promote regeneration after bilateral CN cut in rats55. The addition of neurotrophic factors, extra cellular matrix components and Schwann cells have been shown to promote regeneration56–59. Several molecules have been described to have neurotrophic effects in the penis including VEGF60–61, which is a target of SHH signaling62. In normal adult rats, SHH is abundant in NOSI positive neurons of the pelvic ganglia that innervate the penis and in Schwann cells of the CN45. These results suggest that SHH may be a factor of the Schwann cells that is required for CN regeneration and that SHH may play a regulatory role in regeneration after CN injury.
Conclusions
Since SHH is a critical regulator of smooth muscle apoptosis in the penis that is able to prevent CN injury induced apoptosis in rats, there is substantial potential to develop SHH for delivery in a localized manner to the penis of prostatectomy patients at the time of surgery in order to prevent apoptosis induction and long term ED development as the CN regenerates. Studies are in progress which will identify whether SHH may have application as a regenerative therapy to speed the process of peripheral nerve regeneration.
Acknowledgments
Grant Sponsor-National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases, Grant numbers: DK068507.
Footnotes
Conflict of Interest: None
References
- 1.Korfage IJ, Essink-Bot ML, Borsboom GJ, Madalinska JB, Kirkels WJ, Habbema JD, Schroder FH, de Koning HJ. Five-year Follow-up of Health-related Quality of Life After Primary Treatment of Localized Prostate Cancer. Int J Cancer. 2005;116:291–6. doi: 10.1002/ijc.21043. [DOI] [PubMed] [Google Scholar]
- 2.Katz A. What happened? Sexual Consequences of Prostate Cancer and Its Treatment. Can Fam Physician. 2005;51:977–82. [PMC free article] [PubMed] [Google Scholar]
- 3.Alivizatos G, Skolarikos A. Incontinence and Erectile Dysfunction Following Radical Prostatectomy: a Review. Scientific World Journal. 2005;5:747–58. doi: 10.1100/tsw.2005.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendirci M, Hellstrom WJ. Current Concepts in the Management of Erectile Dysfunction in Men With Prostate Cancer. Clin Prostate Cancer. 2004;3:87–92. doi: 10.3816/cgc.2004.n.017. [DOI] [PubMed] [Google Scholar]
- 5.Vale J. Erectile Dysfunction Following Radical Therapy for Prostate Cancer. Radiother Oncol. 2000;57:301–305. doi: 10.1016/s0167-8140(00)00293-0. [DOI] [PubMed] [Google Scholar]
- 6.Carroll PR. Achieving Optimal Outcomes After Radical Prostatectomy. Urol Oncol. 2005;23:461. doi: 10.1200/JCO.2005.12.922. [DOI] [PubMed] [Google Scholar]
- 7.Penson DF, McLerran D, Feng Z, Li L, Albertsen PC, Gilliland FD, Hamilton A, Hoffman RM, Stephenson RA, Potosky AL, Stanford JL. 5-year Urinary and Sexual Outcomes After Radical Prostatectomy: Results From the Prostate Cancer Outcomes Study. J Urol. 2005;173:1701–1705. doi: 10.1097/01.ju.0000154637.38262.3a. [DOI] [PubMed] [Google Scholar]
- 8.Crawford ED, et al. Comparison of Perspectives on Prostate Cancer: Analyses of Survey Data. Urology. 1997;50(3):366–372. doi: 10.1016/s0090-4295(97)00254-9. [DOI] [PubMed] [Google Scholar]
- 9.Burkhardt JH, Litwin MS, Rose CM, Correa RJ, Sunshine JH, Hogan C, Hayman JA. Comparing the Costs of Radiation Therapy and Radical Prostatectomy for the Initial Treatment of Early-stage Prostate Cancer. J Clin Oncol. 2002;20:2869–2875. doi: 10.1200/JCO.2002.11.136. [DOI] [PubMed] [Google Scholar]
- 10.McCullough AR. Rehabilitation of Erectile Function Following Radical Prostatectomy. Asian J Androl. 2008;10:61–74. doi: 10.1111/j.1745-7262.2008.00366.x. [DOI] [PubMed] [Google Scholar]
- 11.Raina R, Agarwal A, Zippe CD. Management of Erectile Dysfunction After Radical Prostatectomy. Urology. 2005;66:923–929. doi: 10.1016/j.urology.2005.05.044. [DOI] [PubMed] [Google Scholar]
- 12.Perimenis P, Markou S, Gyftopoulos K, Athanasopoulos A, Giannitsas K, Barbalias G. Switching from Long-term Treatment with Self-injections to Oral Sildenafil in Diabetic Patients with Severe Erectile Dysfunction. Eur Urol. 2002;41:387–91. doi: 10.1016/s0302-2838(02)00032-5. [DOI] [PubMed] [Google Scholar]
- 13.Klein LT, Miller MI, Buttyan R, Raffo AJ, Burchard M, Devris G, Cao YC, Olsson C, Shabsigh R. Apoptosis in the Rat Penis After Penile Denervation. J Urology. 1997;158:626–630. [PubMed] [Google Scholar]
- 14.User HM, Hairston JH, Zelner DJ, McKenna KE, McVary KT. Penile Weight and Cell Subtype Specific Changes in a Post-radical Prostatectomy Model of Erectile Dysfunction. J Urol. 2003;169:1175–1179. doi: 10.1097/01.ju.0000048974.47461.50. [DOI] [PubMed] [Google Scholar]
- 15.Podlasek CA, Meroz CL, Tang Y, McKenna KE, McVary KT. Regulation of Cavernous Nerve Injury Induced Apoptosis by Sonic Hedgehog. Biol Reprod. 2007;76:19–28. doi: 10.1095/biolreprod.106.053926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lysiak JJ, Yang S-K, Klausner AP, Son H, Tuttle JB, Steers WD. Tadalafil Increases Akt and Extracellular Signal-regulated Kinase, Activation, and Prevents Apoptotic Cell Death in the Penis Following Denervation. Journal of Urology. 2008;179:779–785. doi: 10.1016/j.juro.2007.09.021. [DOI] [PubMed] [Google Scholar]
- 17.Iacono F, Giannella R, Somma P, et al. Histological Alterations in Cavernous Tissue After Radical Prostatectomy. J Urol. 2005;173:1673–1676. doi: 10.1097/01.ju.0000154356.76027.4f. [DOI] [PubMed] [Google Scholar]
- 18.Schwartz EJ, Wong P, Graydon RJ. Sildenafil Preserves Intracorporeal Smooth Muscle after Radical Retropubic Prostatectomy. J Urol. 2004;171:771. doi: 10.1097/01.ju.0000106970.97082.61. [DOI] [PubMed] [Google Scholar]
- 19.Yaman O, Yilmaz E, Bozlu M, Anafarta K. Alterations of Intracorporal Structures in Patients with Erectile Dysfunction. Urol Int. 2003;71:87–90. doi: 10.1159/000071101. [DOI] [PubMed] [Google Scholar]
- 20.Fraiman MC, Lepor H, McCullough AR. Changes in Penile Morphometrics in Men With Erectile Dysfunction After Nerve-sparing Radical Retropublic Prostatectomy. Mol Urol. 1999;3:109–115. [PubMed] [Google Scholar]
- 21.Wespes E, Rammal A, Garbar C. Sildenafil Non-responders: Haemodynamic and Morphometric Studies. Eur Urol. 2005;48:1061–1062. doi: 10.1016/j.eururo.2005.03.030. [DOI] [PubMed] [Google Scholar]
- 22.Podlasek CA, Zelner DJ, Jiang HB, Tang Y, Houston J, McKenna KE, McVary KT. Shh Cascade is Required for Penile Postnatal Morphogenesis, Differentiation and Adult Homeostasis. Biol Reprod. 2003;68:423–438. doi: 10.1095/biolreprod.102.006643. [DOI] [PubMed] [Google Scholar]
- 23.Steers W. Pharmacologic Treatment of Erectile Dysfunction. Rev Urol. 2002;4 (suppl 3):S17–S25. [PMC free article] [PubMed] [Google Scholar]
- 24.Hooper JE, Scott MP. The Drosophila Patched Gene Encodes a Putative Membrane Protein Required for Segmental Patterning. Cell. 1989;59:751–765. doi: 10.1016/0092-8674(89)90021-4. [DOI] [PubMed] [Google Scholar]
- 25.Nakano Y, Guerrero I, Hidalgo A, Taylor A, Whittle JRS, Ingham PW. A Protein with Several Possible Membrane-spanning Domains Encoded by the Drosophila Segment Polarity Gene . Patched Nature. 1989;341:508–513. doi: 10.1038/341508a0. [DOI] [PubMed] [Google Scholar]
- 26.Goodrich LV, Johnson RL, Milenkovic L, McMahon JA, Scott MP. Conservation of the Hedgehog/patched Signaling Pathway From Flies to Mice: Induction of a Mouse Patched Gene by Hedgehog. Genes Dev. 1996;10:301–312. doi: 10.1101/gad.10.3.301. [DOI] [PubMed] [Google Scholar]
- 27.Stone DM, Hynes M, Armanini M, Swanson TA, Gu Q, Johnson RL, Scott MP, Pennica D, Goddard A, Phillips H, et al. The Tumor-suppressor Gene Patched Encodes a Candidate Receptor for Sonic Hedgehog. Nature. 1996;14:129–134. doi: 10.1038/384129a0. [DOI] [PubMed] [Google Scholar]
- 28.Taipale J, Cooper MK, Maiti T, Beachy PA. Patched Acts Catalytically to Suppress the Activity of Smoothened. Nature. 2002;418:892–897. doi: 10.1038/nature00989. [DOI] [PubMed] [Google Scholar]
- 29.Hidalgo A, Ingham P. Cell Patterning in the Drosophila Segment: Spatial Regulation of the Segment Polarity Gene . Patched Development. 1990;110:291–301. doi: 10.1242/dev.110.1.291. [DOI] [PubMed] [Google Scholar]
- 30.Ingham PW, Taylor AM, Nakano Y. Role of the Drosophila Patched Gene in Positional Signaling. Nature. 1991;353:184–187. doi: 10.1038/353184a0. [DOI] [PubMed] [Google Scholar]
- 31.Capdevila J, Estrada MP, Sanchez-Herrero E, Guerrero I. The Drosophila Segment Polarity Gene Patched Interacts with Decapentaplegic in Wing Development. EMBO J. 1994;13:71–82. doi: 10.1002/j.1460-2075.1994.tb06236.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Alexandre C, Jacinto A, Ingham PW. Transcriptional Activation of Hedgehog Target Genes in Drosophila is Mediated Directly by the Cubitus Interruptus Protein, a Member of the GLI Family of Zinc Finger DNA-binding Proteins. Genes Dev. 1996;10:2003–2013. doi: 10.1101/gad.10.16.2003. [DOI] [PubMed] [Google Scholar]
- 33.Dominguez M, Brunner M, Hafen E, Basler K. Sending and Receiving the Hedgehog Signal: Control by the Drosophila Gli Protein Cubitus Interruptus. Science. 1996;272:1621–1625. doi: 10.1126/science.272.5268.1621. [DOI] [PubMed] [Google Scholar]
- 34.Hepker J, Wang I-T, Motzny CK, Holmgran R, Orenic TV. Drosophila Cubitus Interruptus Forms a Negative Feedback Loop with Patched and Regulates Expression of Hedgehog Target Genes. Development. 1997;124:549–558. doi: 10.1242/dev.124.2.549. [DOI] [PubMed] [Google Scholar]
- 35.Chen Y, Struhl G. Dual Roles for Patched in Sequestering and Transducing Hedgehog. Cell. 1996;87:553–563. doi: 10.1016/s0092-8674(00)81374-4. [DOI] [PubMed] [Google Scholar]
- 36.Karahira H, Ma NH, Tzanakakis ES, McMahon AP, Chuang P-T, Hebrok M. Combined Activities of Hedgehog Signaling Inhibitors Regulate Pancreas Development. Development. 2003;130:4871–4879. doi: 10.1242/dev.00653. [DOI] [PubMed] [Google Scholar]
- 37.Podlasek CA, Zelner DJ, Harris JD, Meroz CL, Tang Y, McKenna KE, McVary KT. Altered Sonic Hedgehog Signaling is Associated with Morphological Abnormalities in the BB/WOR Diabetic Penis. Biol Reprod. 2003;69:816–827. doi: 10.1095/biolreprod.102.013508. [DOI] [PubMed] [Google Scholar]
- 38.Podlasek CA, Barnett DH, Clemens !, Bak PM, Bushman W. Prostate Development Requires Sonic Hedgehog Expressed by the Urogenital Sinus Epithelium. Dev Biology. 1999;209:28–39. doi: 10.1006/dbio.1999.9229. [DOI] [PubMed] [Google Scholar]
- 39.Haraguchi R, Mo R, Hui C, Motoyama J, Makino S, Shiroishi T, Gaffield W, Yamada G. Unique Functions of Sonic Hedgehog Signaling During External Genitalia Development. Development. 2001;128:4241–50. doi: 10.1242/dev.128.21.4241. [DOI] [PubMed] [Google Scholar]
- 40.Perriton CL, Powles N, Chiang C, Maconochie MK, Cohn MJ. Sonic Hedgehog Signaling From the Urethral Epithelium Controls External Genital Development. Dev Biol. 2002;247:26–46. doi: 10.1006/dbio.2002.0668. [DOI] [PubMed] [Google Scholar]
- 41.Ericson J, Morton S, Kawakami A, Roelink H, Jessell TM. Two Critical Periods of Long-range Sonic Hedgehog Signaling Required for the Specification of Motor Neuron Identity. Cell. 1996;87:661–673. doi: 10.1016/s0092-8674(00)81386-0. [DOI] [PubMed] [Google Scholar]
- 42.Pepinsky RB, Rayhorn P, Day ES, Dergay A, Williams KP, Galdes A, Taylor FR, Boriack-Sjodin PA, Garber EA. Mapping Sonic Hedgehog-receptor Interactions by Steric Interference. J Biol Chem. 2000;275:10995–11001. doi: 10.1074/jbc.275.15.10995. [DOI] [PubMed] [Google Scholar]
- 43.Byers MR, O’Neill PC, Fink BR. Lidocaine (Without Epinephrine) Does Not Affect the Fine Structure or Microtubules of the Trigeminal Nerve In Vivo. Anesthesiology. 1979;51:55–57. doi: 10.1097/00000542-197907000-00012. [DOI] [PubMed] [Google Scholar]
- 44.Fink BR, Kish SJ. Reversible Inhbition of Rapid Axonal Transport In Vivo By Lidocaine Hydrochloride. Anesthesiology. 1976;44:139–146. doi: 10.1097/00000542-197602000-00009. [DOI] [PubMed] [Google Scholar]
- 45.Bond C, Tang Y, Podlasek CA. Neural Regulation of Sonic Hedgehog and Apoptosis in the Penis. Biol Reprod. 2008;78:947–956. doi: 10.1095/biolreprod.107.064766. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Angevine JB., Jr Nerve Destruction by Colchicines in Mice and Golden Hamsters. J Exp Zool. 1957;136:363–391. doi: 10.1002/jez.1401360209. [DOI] [PubMed] [Google Scholar]
- 47.Ginn SR, Peterson GM. Studies Related to the Use of Colchicine as a Neurotoxin in the Septohippocampal Cholinergic System. Brain Res. 1992;590:144–152. doi: 10.1016/0006-8993(92)91090-2. [DOI] [PubMed] [Google Scholar]
- 48.Singer M, Steinberg MC. Wallarian Degeneration: A Reevaluation Based on Transected and Colchicine-poisoned Nerves in the Amphibiam, Triturus. Am J Anat. 1972;133:51–83. doi: 10.1002/aja.1001330105. [DOI] [PubMed] [Google Scholar]
- 49.Aquino JB, Musolino PL, Coronel MF, Villar MJ, Setton-Avruj CP. Nerve Degeneration is Prevented by a Single Intraneural Apotransferrin Injection into Colchicine-injured Sciatic Nerves in the Rat. Brain Res. 2006;1117:80–91. doi: 10.1016/j.brainres.2006.02.045. [DOI] [PubMed] [Google Scholar]
- 50.Lecci A, Patacchini R, de Giorgio R, Corinaldesi R, Theodorsson E, Giuliani S, Santicioli P, Maggi CA. Functional, Biochemical and Anatomical Changes in the Rat Urinary Bladder Induced by Perigangliar Injection of Colchicines. Neuroscience. 1996;71:285–296. doi: 10.1016/0306-4522(95)00422-x. [DOI] [PubMed] [Google Scholar]
- 51.Campana WM. Schwann cells: Activated Peripheral Glia and Their Role in Neuropathic Pain. Behavior and Immunity. 2007;21:522–527. doi: 10.1016/j.bbi.2006.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bhatheja K, Field J. Schwann Cells: Origins and Role in Axonal Maintenance and Regeneration. International Journal of Biochemistry & Cell Biology. 2006;38:1995–1999. doi: 10.1016/j.biocel.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 53.Mulhall JP, Muller A, Donohue JF, Mullerad M, Kobylarz K, Paduch DA, Tal R, Li PS, Cohen-Gould L, Scardino PT. The Functional and Structural Consequences of Cavernous Nerve Injury are Ameliorated by Sildenafil Citrate. J Sex Med. 2008;5 (5):1126–1136. doi: 10.1111/j.1743-6109.2008.00794.x. [DOI] [PubMed] [Google Scholar]
- 54.May F, Vroeman M, Matiasek K, Henke J, Brill T, Lehmer A, Apprich M, Erhardt W, Schoeler S, Paul R, Blesch A, Hartung R, Gansbacher B, Weidner N. Nerve Replacement Strategies for Cavernous Nerves. European Urology. 2005;48:372–378. doi: 10.1016/j.eururo.2005.04.025. [DOI] [PubMed] [Google Scholar]
- 55.May F, Weidner N, Matiasek K, Caspers C, Mrva T, Vroemen M, Henke J, Lehmer A, Schwaibold H, Erhardt W, Gansbacher B, Hartung R. Schwann Cell Seeded Guidance Tubes Restore Erectile Function After Ablation of Cavernous Nerves in Rats. J Urology. 2004;172:374–377. doi: 10.1097/01.ju.0000132357.05513.5f. [DOI] [PubMed] [Google Scholar]
- 56.Frostick SP, Yin Q, Kemp GJ. Schwann Cells, Neurotrophic Factors, and Peripheral Nerve Regeneration. Microsurgery. 1998;18:397. doi: 10.1002/(sici)1098-2752(1998)18:7<397::aid-micr2>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 57.Rodriguez FJ, Verdu E, Ceballos D, Navarro X. Nerve Guides Seeded with Autologous Schwann Cells Improve Nerve Regeneration. Exp Neurol. 2000;161:571. doi: 10.1006/exnr.1999.7315. [DOI] [PubMed] [Google Scholar]
- 58.Liu MH. Growth Factors and Extracellular Matrix in Peripheral Nerve Regeneration, Studies with a Nerve Chamber. J Periph Nerv Syst. 1996;2:97. [PubMed] [Google Scholar]
- 59.Dahlin LB, Lundborg G. Use of Tubes in Peripheral Nerve Repair. Neurosurg Clin North Am. 2001;12:341. [PubMed] [Google Scholar]
- 60.Hsieh PS, Bochinski DJ, Lin GT, Nunes L, Lin CS, Lue TF. The Effect of Vascular Endothelial Growth Factor and Brain-derived Neurotrophic Factor on Cavernosal Nerve Regeneration in a Nerve-crush Rat Model. BJU Int. 2003;92:470–475. doi: 10.1046/j.1464-410x.2003.04373.x. [DOI] [PubMed] [Google Scholar]
- 61.Bakircioglu ME, Lin CS, Fan P, Sievert KD, Kan YW, Lue TF. The Effect of Adenoassociated Virus Mediated Brain Derived Neurotrophic Factor in an Animal Model of Neurogenic Impotence. J Urol. 2001;165:2103–2109. doi: 10.1097/00005392-200106000-00078. [DOI] [PubMed] [Google Scholar]
- 62.Podlasek CA, Meroz CL, Korolis H, Tang Y, McKenna KE, McVary KT. Sonic Hedgehog, the Penis and Erectile Dysfunction: A Review of Sonic Hedgehog Signaling in the Penis. Current Pharmaceutical Design. 2005;11:4011–4027. doi: 10.2174/138161205774913408. [DOI] [PubMed] [Google Scholar]