Abstract
Background
The cholesterol content of LDL particles is variable, causing frequent discrepancies between concentrations of LDL cholesterol and LDL particle number. In managing patients at risk for cardiovascular disease (CVD) to LDL target levels, it is unclear whether LDL cholesterol provides the optimum measure of residual risk and adequacy of LDL lowering treatment.
Objective
To compare the ability of alternative measures of LDL to provide CVD risk discrimination at relatively low levels consistent with current therapeutic targets.
Methods
Concentrations of LDL cholesterol (LDL-C) and non-HDL cholesterol (non-HDL-C) were measured chemically and LDL particle number (LDL-P) and VLDL particle number (VLDL-P) were measured by nuclear magnetic resonance (NMR) in 3066 middle-aged white participants (53% women) without CVD in the Framingham Offspring cohort. The main outcome measure was incidence of first CVD event.
Results
At baseline, the cholesterol content per LDL particle was negatively associated with triglycerides and positively associated with LDL-C. On follow-up (median 14.8 yrs), 265 men and 266 women experienced a CVD event. In multivariable models adjusting for non-lipid CVD risk factors, LDL-P was related more strongly to future CVD in both sexes than LDL-C or non-HDL-C. Subjects with a low level of LDL-P (<25th percentile) had a lower CVD event rate (59 events per 1000 person-years) than those with an equivalently low level of LDL-C or non-HDL-C (81 and 74 events per 1000 person-years, respectively).
Conclusions
In a large community-based sample, LDL-P was a more sensitive indicator of low CVD risk than either LDL-C or non-HDL-C, suggesting a potential clinical role for LDL-P as a goal of LDL management.
INTRODUCTION
Low-density lipoprotein (LDL) measurements are complicated by the fact that LDL is not a single molecular species, but a multi-molecular particle aggregate composed of protein and thousands of molecules of cholesterol and other lipids. Quantification can thus be accomplished in different ways depending on the which molecular constituent of LDL is measured. Since cholesterol is the most abundant lipid in LDL and cholesterol assays have been available for many years, LDL concentrations in clinical practice are routinely expressed in terms of measured or estimated cholesterol content (LDL cholesterol or LDL-C) (1). An alternative is to measure plasma apolipoprotein B (apoB) to provide an estimate of LDL particle concentration, since all LDL and VLDL particles contain a single molecule of apoB protein and >90% of apoB is on LDL (2). Nuclear magnetic resonance (NMR) spectroscopy provides another means by which LDL particle concentrations (LDL-P) may be measured (3).
LDL measurements have two distinct clinical applications: risk assessment, with high LDL levels helping to identify patients with an elevated risk of cardiovascular disease (CVD), and risk management, with low LDL levels serving as treatment goals and indicators of the success of LDL-lowering therapies (4). When used for the risk assessment application, LDL measurements are always used in conjunction with other established risk factors as part of a multivariable risk stratification approach. Adult Treatment Panel III (ATPIII) guidelines recommend that elevated LDL-C along with age, gender, blood pressure, HDL cholesterol (HDL-C), diabetes, smoking, family history, and metabolic syndrome all be taken into account to determine the patient’s risk (4,5). The assigned risk category defines the corresponding LDL-C treatment goal needed to mitigate that risk. Recognizing the potential contribution to risk of other atherogenic lipoproteins besides LDL, such as VLDL remnants, ATP III designated LDL + VLDL cholesterol (non-HDL cholesterol or non-HDL-C) as “atherogenic cholesterol” and recommended its use as a secondary target of therapy in patients with elevated triglyceride levels (≥200 mg/dL) (4).
When used for the risk management application, LDL measurements are not employed in conjunction with other information, but as a stand-alone measure of progress towards a treatment goal. The LDL-C level (plus non-HDL-C) tells the clinician which patients have lowered their risk to acceptable levels (as inferred from their treatment goal having been reached) and which have not (indicating a need for more aggressive treatment). To function well for this clinical application, LDL-C levels in the low target range should have a strong correlation with CVD risk. From the documented non-linear (“curvilinear”) relationship of LDL-C with CVD risk, which is quite steep when LDL-C is elevated but more shallow when LDL-C levels are moderate to low (5), there is some reason to question whether low LDL-C values perform as well for the risk management application as do high LDL-C values for the risk assessment application.
Since risk management decision making relies so heavily on LDL measurement, it is important to know whether the two primary means of LDL quantification, LDL-C (the cholesterol in LDL) and LDL particle number, are equivalent in their relations with CVD. The amount of cholesterol carried by an LDL particle is not constant, but varies greatly between individuals (6–8). In a given patient, the cholesterol composition of LDL can also change in response to lipid altering treatments. There are thus frequent discrepancies between concentrations of LDL-C and LDL particle number, no matter whether the latter is estimated by apoB measurement (9–11) or NMR (6,7,12,13). To determine which LDL measure, LDL-C or LDL particle number, is related more strongly to future cardiovascular disease, particularly when levels are near target values, we examined their comparative relations with CVD risk in a large, population-based sample of men and women. Since apoB relations with CVD in the same study population were recently published (14), we report here only NMR-derived LDL particle number data. CVD relations with non-HDL-C were also examined to investigate the hypothesis that non-HDL-C predicts risk better than LDL-C because it accounts for other atherogenic lipoproteins besides LDL (4,15,16).
METHODS
Study Sample
The Framingham Offspring Study was initiated in 1971, and the design and selection criteria have been described previously (17). Participants who attended the fourth examination cycle (1987–1991) were eligible for the present study (n=4019). Participants were excluded for the following reasons: participants younger than 30 or older than 74 years of age (n=299), participants with prevalent cardiovascular disease at baseline (n=59), lack of follow-up data (n=17), serum triglycerides above 400 mg/dL or missing data on any lipid variable or other covariate (n=70). After these exclusions, 3066 individuals (mean age, 51 years; 53% women) were eligible, and constituted the study sample. The study protocol was approved by the Boston University Medical Center Institutional Review Board, and all participants provided written informed consent.
Lipid and Lipoprotein Particle Measurements
Twelve-hour fasting venous blood samples were collected in tubes containing 0.1% EDTA. Plasma was separated by centrifugation (2500 rpm, 4°C, 20 min), and plasma lipid concentrations (total cholesterol, triglycerides, and HDL-C) were measured as previously described (18). LDL-C concentrations were estimated using the Friedewald formula (1) and non-HDL-C was defined as the difference between total cholesterol and HDL-C. Participants with serum triglycerides above 400 mg/dL were excluded from the analyses.
Lipoprotein particle profiles were measured in 1995 on plasma samples stored at −70° by a commercially available NMR spectroscopic assay (LipoScience, Raleigh, NC) as described previously in detail (3,12,19). Briefly, the characteristic NMR signals broadcast by lipoprotein particles of different size serve as the basis for quantification of these lipoprotein subclasses in particle number concentration terms (moles of particles per liter). The concentrations of the VLDL and LDL subclasses were summed to provide total VLDL (VLDL-P) and LDL (LDL-P) particle concentrations (nmol/L). Some analyses examined the sum of LDL and VLDL particle numbers (LDL-P + VLDL-P) as a measure of total atherogenic particle concentration. Mean LDL particle size (nm diameter) was computed as the sum of the diameter of each subclass multiplied by its relative mass percentage as estimated from the amplitude of its NMR signal. Estimates of the cholesterol content of the LDL particles of individual subjects were obtained by dividing LDL-C (in mmol/L units, obtained by multiplying the mg/dL mass concentrations by 0.0259) by LDL-P (nmol/L). This ratio provides the approximate number of cholesterol molecules per LDL particle. The observed range of values corresponds closely to those determined independently by detailed lipid compositional analyses of isolated LDL samples of varying diameter (20). Inter-assay coefficients of variation for VLDL-P and LDL-P were <3%, and <0.5% for LDL size. For all biochemical and NMR analyses, samples were handled in a blinded fashion such that all investigators had no knowledge of participant status.
Follow-up and Outcome Events
The follow-up period for the present investigation was defined as from the baseline examination up to December 31, 2005. All study participants are under longitudinal surveillance for CVD occurrence, through periodic examinations at the Framingham Heart Study and through biennial health history updates between examinations. An endpoint adjudication committee consisting of three experienced investigators reviewed hospitalization and physician office visit records for all suspected CVD events. Incident CVD was defined as recognized or unrecognized myocardial infarction, angina pectoris, coronary insufficiency, coronary heart disease death, stroke, transient ischemic attack, intermittent claudication or congestive heart failure. Diagnosis criteria for these events have been described elsewhere (21).
Statistical Methods
We evaluated the distribution of lipid and lipoprotein particle measures and clinical covariates in men and women according to CVD status (those who did and did not experience CVD events during follow-up). Means and standard deviations are reported for continuous variables and proportions for categorical variables. Interrelations among the lipid and lipoprotein particle measures were estimated using Spearman rank correlation coefficients. We used multivariable Cox proportional hazards regression to investigate relations of the atherogenic lipid and lipoprotein particle measures to CVD incidence, adjusting for age, systolic and diastolic blood pressure, smoking, and lipid medication use. We examined the additional effects of diabetes and beta-blocker use, two lipid-altering variables, in separate models. Hazards ratios (HRs) and their 95% confidence intervals (CIs) and beta-coefficients (βs) and their standard errors (SEs) were determined for a one sex-specific standard deviation increment of each lipid or lipoprotein measure, thereby facilitating comparisons of the relative strengths of disease association of different measures of atherogenic lipoprotein levels. We additionally divided the population into sex-specific quartiles of LDL-C, non-HDL-C and LDL-P and determined age- and gender-adjusted event rates within each quartile. Crude Kaplan-Meier curves for event-free survival were also constructed for participants with LDL-C and LDL-P levels above or below the median. Cigarette smoking was defined by self-reported cigarette use within the year preceding the baseline examination. Diabetes was defined as a fasting blood glucose ≥ 126 mg/dL or use of insulin or oral hypoglycemic agents. The assumption of proportionality of hazards was confirmed by examining interactions of time-dependent covariates and survival time in Cox models. Two-sided p-values of <0.05 were considered statistically significant. All analyses were performed using SAS 9.1 (SAS Institute, Cary, NC).
RESULTS
During follow-up (median 14.8 years), there were 431 first CVD events (265 in men). The baseline characteristics of study participants who experienced events or remained free of events during follow-up are shown in Table 1. In women, all lipid and lipoprotein particle measures differed (p<0.0001) according to incident CVD status during follow-up. Somewhat weaker relations with CVD were seen in men. Notably, LDL-C levels differed little in men with and without CVD (138 vs 134 mg/dL; p=0.09), while LDL-P concentrations were significantly different (1641 vs 1509 nmol/L; p<0.0001).
Table 1.
Baseline Characteristics
| Men | Women | |||||
|---|---|---|---|---|---|---|
| No CVD During Follow-up (n=1175) | CVD During Follow-up (n=265) | p | No CVD During Follow-up (n=1460) | CVD During Follow-up (n=166) | p | |
| Clinical Features | ||||||
| Age, years | 50 (10) | 56 (9) | <.0001 | 50 (10) | 57 (9) | <.0001 |
| Systolic blood pressure, mmHg | 127 (16) | 136 (18) | <.0001 | 123 (19) | 135 (20) | <.0001 |
| Hypertension, % | 34 | 58 | <.0001 | 26 | 54 | <.0001 |
| Smoking, % | 22 | 29 | .02 | 23 | 31 | .02 |
| Diabetes, % | 3 | 14 | <.0001 | 2 | 8 | <.0001 |
| Lipid Measures | ||||||
| Total cholesterol, mg/dL | 203 (36) | 210 (36) | .005 | 203 (39) | 221 (41) | <.0001 |
| Triglycerides, mg/dL | 121 (70) | 150 (78) | <.0001 | 99 (56) | 133 (71) | <.0001 |
| HDL cholesterol, mg/dL | 45 (11) | 42 (13) | .001 | 57 (15) | 51 (15) | <.0001 |
| LDL cholesterol, mg/dL | 134 (33) | 138 (32) | .09 | 126 (36) | 143 (39) | <.0001 |
| Non-HDL cholesterol, mg/dL | 158 (38) | 168 (36) | .0002 | 146 (40) | 170 (44) | <.0001 |
| Atherogenic Particle Measures | ||||||
| VLDL-P, nmol/L | 77 (33) | 88 (35) | <.0001 | 68 (33) | 82 (39) | <.0001 |
| LDL-P, nmol/L | 1509 (403) | 1641 (428) | <.0001 | 1344 (421) | 1628 (473) | <.0001 |
| VLDL-P + LDL-P, nmol/L | 1586 (423) | 1729 (447) | <.0001 | 1412 (442) | 1710 (499) | <.0001 |
Values are means (SD) or percentages for the clinical features.
Abbreviations: VLDL-P, VLDL particle number; LDL-P, LDL particle number
Atherogenic Lipoprotein Measures and Variability of LDL Composition
There was a high correlation between different measures of atherogenic lipoprotein levels. LDL-C was correlated more strongly with non-HDL-C (r=0.94) than LDL-P (r=0.79). The correlation of LDL-P with non-HDL-C was 0.87. Adding VLDL particle number to LDL particle number (LDL-P + VLDL-P) only marginally strengthened the correlation with non-HDL-C (r=0.88). Triglyceride levels were more weakly associated with LDL-C (r=0.30) than with either non-HDL-C or LDL-P (r=0.57 for both) or LDL-P + VLDL-P (r=0.61).
Table 2 displays mean values of several parameters related to the size and lipid composition of LDL. 12 groups of subjects were examined, categorized by their triglyceride level (<100, 100–199, ≥200 mg/dL) and LDL-C level (<100, 100–129, 130–159, ≥160 mg/dL). Within a given LDL-C category, as expected, non-HDL-C levels rise substantially with increasing triglycerides since non-HDL-C is the sum of LDL-C plus VLDL-C (and VLDL-C is approximated by triglyceride concentration divided by 5) (1). Levels of LDL-P rise even more with increasing triglycerides than do levels of non-HDL-C. For example, in subjects with LDL-C between 100 and 129 mg/dL, LDL-P was 40% higher (1652 vs 1179 nmol/L) and non-HDL-C was 31% higher (168 vs 128 mg/dL) in those with triglycerides ≥200 mg/dL compared to <100 mg/dL. LDL-C levels were virtually identical in these 2 groups (115 vs 114 mg/dL).
Table 2.
LDL Concentrations, Size, and Composition in Framingham Subgroups (n=3066)
| LDL-C Subgroups (mg/dL) | Triglyceride Subgroups (mg/dL) | |||
|---|---|---|---|---|
| <100 | 100–199 | ≥200 | ||
| <100 | # subjects | 415 | 113 | 60 |
| Triglycerides (mg/dL) | 60 (18) | 134 (29) | 273 (57) | |
| LDL-C (mg/dL) | 85 (12) | 85 (13) | 84 (12) | |
| Non-HDL-C (mg/dL) | 97 (12) | 112 (14) | 139 (15) | |
| LDL-P (nmol/L) | 920 (201) | 1119 (235) | 1349 (278) | |
| LDL size (nm) | 21.1 (0.4) | 20.8 (0.5) | 20.3 (0.6) | |
| Cholesterol/LDL-P* | 2454 (424) | 2013 (309) | 1655 (303) | |
|
| ||||
| 100–129 | # subjects | 587 | 292 | 83 |
| Triglycerides (mg/dL) | 66 (17) | 141 (27) | 264 (53) | |
| LDL-C (mg/dL) | 114 (9) | 116 (8) | 115 (9) | |
| Non-HDL-C (mg/dL) | 128 (10) | 145 (9) | 168 (14) | |
| LDL-P (nmol/L) | 1179 (184) | 1395 (232) | 1652 (267) | |
| LDL size (nm) | 21.2 (0.4) | 20.8 (0.5) | 20.3 (0.5) | |
| Cholesterol/LDL-P | 2561 (357) | 2215 (367) | 1848 (285) | |
|
| ||||
| 130–159 | # subjects | 432 | 398 | 86 |
| Triglycerides (mg/dL) | 71 (17) | 139 (27) | 257 (50) | |
| LDL-C (mg/dL) | 144 (9) | 145 (9) | 145 (9) | |
| Non-HDL-C (mg/dL) | 158 (10) | 173 (10) | 196 (13) | |
| LDL-P (nmol/L) | 1457 (300) | 1656 (228) | 1929 (272) | |
| LDL size (nm) | 21.1 (0.3) | 20.8 (0.5) | 20.2 (0.5) | |
| Cholesterol/LDL-P | 2615 (412) | 2309 (299) | 1980 (301) | |
|
| ||||
| ≥160 | # subjects | 198 | 303 | 99 |
| Triglycerides (mg/dL) | 75 (16) | 139 (27) | 254 (43) | |
| LDL-C (mg/dL) | 179 (20) | 182 (21) | 190 (28) | |
| Non-HDL-C (mg/dL) | 194 (20) | 210 (22) | 241 (28) | |
| LDL-P (nmol/L) | 1726 (259) | 1955 (320) | 2281 (420) | |
| LDL size (nm) | 21.2 (0.4) | 20.8 (0.5) | 20.3 (0.5) | |
| Cholesterol/LDL-P | 2718 (341) | 2457 (400) | 2216 (453) | |
Values are means (SD).
Ratio of LDL cholesterol (LDL-C, mmol/L) to LDL particle number (LDL-P, nmol/L), giving the number of cholesterol molecules per LDL particle.
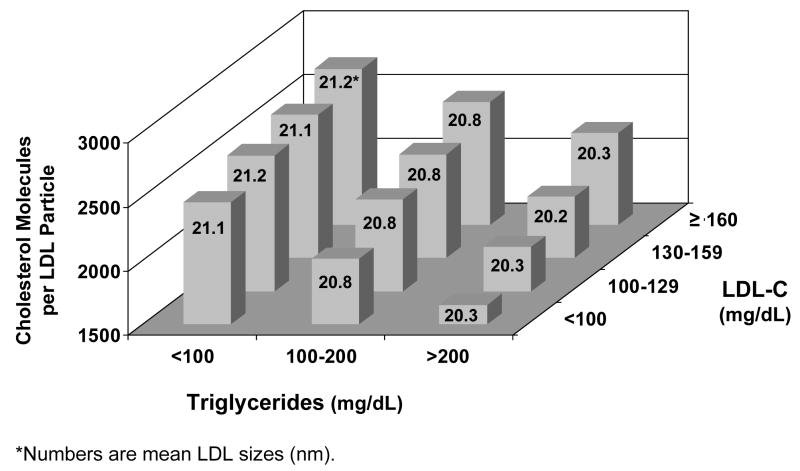
The higher LDL-P relative to LDL-C in the subjects with higher triglycerides is expected and consistent with the smaller size of the LDL particles in these individuals (Table 2), since smaller LDL particles carry less cholesterol than larger ones. An estimate of the number of cholesterol molecules per LDL particle can be obtained by calculating the ratio of LDL-C to LDL-P (when both are expressed in mol/L units). As shown in Table 2 and displayed graphically in Figure 1, the amount of cholesterol per LDL particle varied substantially in the study population not only as a function of triglyceride level, but also as a function of LDL concentration. Within each triglyceride subgroup, the lower the LDL level, the lower was the amount of cholesterol per particle. This progressive cholesterol compositional depletion of LDL particles at lower LDL concentrations was not associated with smaller LDL particle sizes. As shown in Table 2 and Figure 1, LDL size was invariant within each triglyceride subgroup as a function of LDL concentration.
Figure 1.
Cholesterol content of LDL particles in subgroups defined by levels of LDL cholesterol (LDL-C) and triglycerides (data from Table 2). Numbers indicate mean LDL size (nm) within each subgroup.
Cardiovascular Disease Incidence Associated with Alternative Measures of Atherogenic Lipoproteins
Table 3 shows the results of multivariable Cox regression analyses examining the hazards ratios and strengths of association, provided by the respective beta-coefficients, of alternative measures of atherogenic lipoproteins (LDL and VLDL) with CVD incidence in men and women, separately and combined. Because of the high degree of correlation among the lipid and lipoprotein particle variables, each variable was examined in a separate model that included the same non-lipid covariates. LDL-P was strongly associated with increased CVD risk in both men and women (p<0.0001), though less strongly in men. LDL-C was not associated with CVD in men (p=0.33) and LDL-C was only modestly associated with CVD risk in women (p=0.03). When data for men and women were combined, LDL-P was approximately twice as strongly related to CVD incidence as LDL-C (β-coefficient 0.24 for LDL-P vs 0.11 for LDL-C).
Table 3.
Relations of Alternative Measures of Atherogenic Lipoprotein Levels with Future Cardiovascular Disease
| Men (n=1440) | Women (n=1626) | Combined (n=3066) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| HR¶(95% CI) | β§ (SE) | p | HR (95% CI) | β(SE) | p | HR (95% CI) | β(SE) | p | |
| Lipid Measures | |||||||||
| LDL-C | 1.06 (0.94–1.20) | 0.06 (0.06) | 0.33 | 1.18 (1.02–1.37) | 0.16 (0.08) | 0.03 | 1.11 (1.01–1.22) | 0.11 (0.05) | 0.03 |
| Non-HDL-C | 1.17 (1.04–1.32) | 0.16 (0.06) | 0.01 | 1.27 (1.09–1.47) | 0.24 (0.08) | 0.002 | 1.21 (1.10–1.33) | 0.19 (0.05) | <0.0001 |
| Lipoprotein Measures | |||||||||
| LDL-P | 1.24 (1.10–1.39) | 0.21 (0.06) | 0.0005 | 1.33 (1.17–1.50) | 0.28 (0.06) | <0.0001 | 1.28 (1.17–1.39) | 0.24 (0.04) | <0.0001 |
| LDL-P + VLDL-P | 1.25 (1.1–.41) | 0.22 (0.06) | 0.0003 | 1.33 (1.17–1.51) | 0.29 (0.06) | <0.0001 | 1.28 (1.18–1.40) | 0.25 (0.04) | <0.0001 |
All estimates reported are from multivariable Cox regression analyses, adjusted for age, gender (for combined group), systolic and diastolic blood pressure, smoking, and lipid medication use.
Values are hazards ratios (95% confidence intervals) for a 1-SD increment of the lipid or lipoprotein measure.
Values are standardized beta coefficients (standard error), which give an estimate of the strength of association of a 1-SD increment of each lipid or lipoprotein variable with future CVD.
Non-HDL-C, which includes contributions from the cholesterol in VLDL as well as LDL, was more strongly associated with CVD than LDL-C in both men and women, but was less predictive of CVD events than LDL-P. Adding VLDL-P to LDL-P only very marginally strengthened CVD associations compared to LDL-P alone.
In further analyses, we explored whether results would be affected by inclusion of beta-blocker treatment and/or diabetes at baseline as covariates in the multivariable analyses, since these variables are known to have an effect on various lipid and lipoprotein parameters (22,23). Results were essentially unchanged upon inclusion of these additional covariates (data not shown).
In Table 4 are shown age-and gender-adjusted CVD event rates associated with increasing quartiles of LDL-C, non-HDL-C, and LDL-P. Comparing CVD risk in the bottom quartile of these targets and potential targets of LDL management showed that those with low levels of LDL-P (<25th percentile) had a lower rate of CVD (59 events per 1000 person-years) than those with equivalently low levels of LDL-C or non-HDL-C (81 and 74 events per 1000 person-years, respectively). Among the 764 individuals in the bottom quartile of LDL-C, 603 (79%) had concordantly low LDL-P (<25th percentile) while 161 (21%) had discordantly higher LDL-P (>25th percentile) (data not shown). The unadjusted event rate was more than double in the latter group; there were 46 events in the concordant group and 29 in the discordant group. After age and gender adjustment, event rates in these 2 groups were 65 and 85 events per 1000 person-years, respectively.
Table 4.
Age and Gender-Adjusted Incidence of CVD by Quartile of Alternative Measures of Atherogenic Lipoprotein Concentrations
| Quartile 1 | Quartile 2 | Quartile 3 | Quartile 4 | |
|---|---|---|---|---|
| LDL-C | ||||
| Median, mg/dL | 92 | 118 | 142 | 170 |
| Number of events | 75 | 100 | 114 | 142 |
| CVD event rate per 1000 person-years (95% CI) | 81 (57–103) | 86 (63–108) | 88 (66–110) | 119 (92–144) |
|
| ||||
| Non-HDL-C | ||||
| Median, mg/dL | 109 | 140 | 165 | 198 |
| Number of events | 65 | 95 | 115 | 156 |
| CVD event rate per 1000 person-years (95% CI) | 74 (51–96) | 79 (57–100) | 94 (71–116) | 123 (96–149) |
|
| ||||
| LDL-P | ||||
| Median, nmol/L | 967 | 1279 | 1548 | 1931 |
| Number of events | 55 | 109 | 101 | 166 |
| CVD event rate per 1000 person-years (95% CI) | 59 (38–79) | 89 (66–112) | 81 (60–102) | 139 (110–166) |
Quartiles were sex-specific. LDL-C cutpoints were 111, 134, and 156 mg/dL for men; 102, 124, and 150 mg/dL for women. Non-HDL-C cutpoint were 134, 159, and 183 mg/dL for men; 118, 144, and 174 mg/dL for women. LDL-P cutpoints were 1252, 1511, and 1785 nmol/L for men; 1061, 1313, and 1617 nmol/L for women.
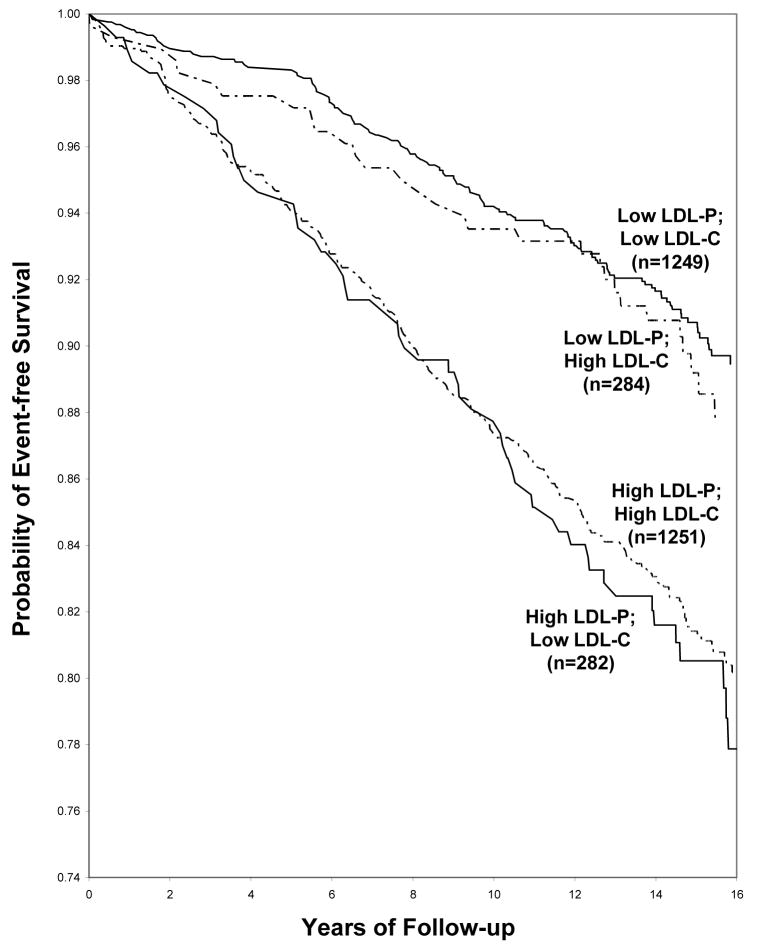
Event-free survival curves for participants with concordant or discordant LDL-C and LDL-P levels greater or less than the median are shown in Figure 2. Event-free survival was clearly worse for discordant individuals with low LDL-C and high LDL-P than for the group with high LDL-C and low LDL-P. Differences in LDL-C had little effect on event-free survival within both the high LDL-P and low LDL-P participants.
Figure 2.
Event-free survival among participants with LDL cholesterol (LDL-C) and LDL particle number (LDL-P) above or below the median. Median values were 131 mg/dL for LDL-C and 1414 nmol/L for LDL-P.
DISCUSSION
Principal Findings
In this large, community-based study of men and women, the cholesterol content of LDL particles from different individuals was highly variable, leading to frequent discrepancies between LDL-C and LDL particle number, two alternative measures of LDL concentration and LDL-associated CVD risk. LDL cholesterol levels under-represent the number of LDL particles in persons with relatively cholesterol-poor particles (6–12). As expected, because the particles are smaller, we found cholesterol-poor LDL among individuals with elevated triglycerides. But irrespective of triglyceride level and LDL size, individuals with low LDL concentration also have cholesterol-poor particles. This interesting finding suggests that simply having low LDL levels, either naturally or as a result of LDL-lowering therapy, can create a discrepancy between LDL-C and LDL particle number and contribute to the underestimation of both LDL and CVD risk by measured levels of LDL-C.
We compared the prediction of future CVD events by LDL-C and NMR-measured LDL particle number and, in agreement with previous NMR (24–27) and apoB (8,14,28) studies, found LDL particle number to be the stronger predictor. Supporting this conclusion are the results of Cox regression analyses in Table 3 and the higher and lower absolute event rates, respectively, of persons in the top and bottom quartiles of LDL-P compared to LDL-C in Table 4. Among individuals with low LDL-C (quartile 1), most had concordantly low LDL-P (quartile 1) and a low CVD risk. However, a substantial subset (21%) had higher LDL-P and these discordant individuals had a higher CVD event rate. The event-free survival curves in Figure 2 also demonstrate that when there is discordance between LDL-C and LDL-P (above or below the median), CVD risk tracks with LDL-P, not LDL-C. The relatively small study size and number of clinical events precluded any more detailed concordance/discordance subgroup analyses. Our results showing LDL-P to have stronger associations with future CVD than LDL-C are in complete accord with those reported recently for apoB in the same study (14).
LDL Particle Number as a Potential Treatment Target
LDL measurements are used clinically for both risk assessment and to monitor the progress of therapeutic interventions. A patient’s LDL level is only one of many factors taken into account to make an assessment of CVD risk, but once the risk estimate has been made, achieving the recommended LDL treatment goal becomes the primary focus of risk management (4,5). ATPIII guidelines did not consider other analytic measures of LDL, such as LDL particle number, as alternatives to LDL-C for the purpose of monitoring the adequacy of a patient’s LDL lowering therapy. Only LDL-C treatment goals were recommended: <130 mg/dL for moderately high-risk patients, <100 mg/dL for high-risk patients, and (optionally) <70 mg/dL for very high-risk patients. These goals were chosen somewhat arbitrarily since no clinical trials had been conducted in which patients were treated to different predetermined target levels to assess comparative benefit (28). Instead, they were based predominantly on results of statin trials that collectively demonstrated a log-linear relationship between LDL-C levels and CVD risk extending to very low LDL-C values (5).
By advocating specific LDL treatment goals for individual patients, there is at least the implicit assumption that LDL values in the vicinity of the target value can differentiate individuals whose risk is not yet adequately managed (indicating a need for more intensive LDL lowering) from those whose risk has been made acceptably low by the treatment. Although the present results are from an epidemiologic study, not a clinical intervention trial, they suggest that low LDL particle numbers may be a better indicator of low risk than equivalently low LDL cholesterol values. For Framingham participants with LDL-P or LDL-C below a population-equivalent cutpoint (<25th percentile), those with low LDL-P had a lower CVD event rate than those with low LDL-C (59 vs 81 events per 1000 person-years)
The recent impetus for consideration of LDL-C treatment goals lower than 100 mg/dL has come from two sources: 1) clinical trials showing that high-risk patients treated with statins to LDL-C goals continue to experience cardiovascular events at rates higher than desired (i.e., they have significant “residual risk”), and 2) clinical trial data showing that patient subgroups with LDL-C <100 mg/dL benefit from more aggressive LDL lowering (29–31). It has been postulated that the subset of individuals in these trials with low LDL-C and discordantly high numbers of LDL particles may be the ones with the greatest residual risk and the ones likely to be the main beneficiaries of additional LDL-lowering therapy (8,13). Our results showing that the subset of individuals with low LDL-C and higher LDL-P have higher CVD risk lend support to this hypothesis. Conversely, those with low LDL-C and correspondingly low LDL-P have lower CVD risk and thus might be expected to derive less benefit from any additional LDL lowering.
Non-HDL Cholesterol as a Treatment Target
It has long been recognized that patients with elevated triglyceride levels have CVD risk that appears to be incompletely accounted for by LDL-C, which is why non-HDL-C was recommended in ATPIII guidelines as a secondary treatment target for patients with triglyceride levels >200 mg/dL (4,5). The rationale was that triglyceride-rich lipoproteins (VLDL and remnant lipoproteins) are, like LDL, atherogenic and that by adding VLDL cholesterol to LDL cholesterol to give total “atherogenic cholesterol” (non-HDL-C), the risk from all of these atherogenic particles would be more completely accounted for (4,14,15). A similar argument was made for measurement of serum apo B, since both VLDL and LDL particles contain apo B as their major apolipoprotein (8,14). Studies comparing the strength of association of CVD risk with non-HDL-C, apoB, and LDL-C have consistently shown LDL-C to be the weaker predictor, supporting the apparent importance of measuring all atherogenic lipoproteins, not just LDL (8,14,27). The present data showing non-HDL-C to be more predictive of CVD events than LDL-C are consistent with these previous reports.
Our results, however, offer a different perspective on why non-HDL-C is a better risk predictor than LDL-C. The main reason appears to be that non-HDL-C functions as a surrogate marker for LDL particle number. Not only was non-HDL-C more weakly related to incident CVD than LDL-P, the risk prediction given by LDL-P was improved only slightly by taking into account the contribution of VLDL particles (Table 3). This latter finding is perhaps not surprising given that VLDL particles constitute only a small fraction (about 5%) of the total number of atherogenic VLDL + LDL particles (Table 1). Even when triglycerides are significantly elevated, VLDL particle numbers are only modestly higher because the excess triglyceride is carried predominantly by large VLDL particles that are relatively few in number (3,19). Furthermore, in terms of the percentage of total atherogenic particles, VLDL-P levels are not very different in persons with high triglycerides because these same individuals also typically have elevated numbers of LDL particles which are smaller than average.
Cholesterol Compositional Variability of LDL Particles
Variations in triglyceride levels are a well recognized cause of the cholesterol compositional variability of LDL particles (6,9–12,19). When triglycerides are elevated, small LDL particles predominate (33). Small LDL particles contain substantially less cholesterol than larger ones simply because of the smaller physical volume of the lipid core. Our data (Table 2, Figure 1) confirm these relationships by showing that as triglyceride levels increase, there is a progressive reduction in LDL particle size and a corresponding decrease in the number of cholesterol molecules per LDL particle.
Surprisingly, our data also indicate that LDL particles become progressively cholesterol-depleted as LDL concentrations decrease. This relationship, which we are unaware of having been noted previously, is independent of triglyceride level and is not associated with any change in LDL size. We speculate that the cause of this particle size-independent cholesterol compositional change is the lipid exchange reaction mediated by cholesterol ester transfer protein (CETP), in which a cholesterol ester molecule in the core of LDL is replaced by a triglyceride molecule from VLDL (34). Elevated triglycerides (VLDL) have generally been considered necessary to drive this reaction in the direction of making LDL more triglyceride-rich and cholesterol-poor. However, what is relevant is not the absolute VLDL concentration, but the relative difference between VLDL and LDL concentrations. Even with serum triglyceride (VLDL) levels that are not elevated, LDL particles can become cholesterol-depleted and triglyceride-enriched if LDL concentrations are low (Figure 1).
This finding has potentially important implications for the clinical management of LDL and LDL-related CVD risk because it suggests that LDL lowering treatment alone (with statins or other agents) may induce a disconnect between LDL-C and LDL particle numbers. Our data suggest that the magnitude of this disconnect would increase in proportion with the magnitude of LDL reduction, irrespective of triglyceride level (although the greatest disconnect would be seen in those with the highest triglyceride levels because the LDL particles would be both small and compositionally cholesterol-depleted and triglyceride-enriched). Direct evidence has been obtained that statin treatment changes the cholesterol and triglyceride content of LDL particles (35). After atorvastatin treatment, with no change in LDL particle size, isolated LDL particles contained a lower ratio of cholesterol ester to triglycerides. If LDL lowering does indeed give rise to cholesterol-depleted LDL particles, one would predict that the percentage decrease in LDL-C produced by statin treatment would exceed the percentage decrease in LDL particle number. Such differences ranging from 4 to 15% have been noted in NMR studies of LDL particle reduction by 3 different statins (36–39) and in studies using apoB to estimate LDL particle number decreases (32).
Conclusions
Among alternative measures of LDL in this large, community-based study, LDL particle number was more strongly related to incident CVD events than LDL-C. Of particular relevance to the use of specific LDL treatment targets as indicators of the adequacy of LDL lowering therapy was the finding that low LDL particle number was a better index of low CVD risk than low LDL-C. Non-HDL-C provided risk prediction intermediate between LDL particle number and LDL-C, with evidence suggesting that the better prediction relative to LDL-C was due less to non-HDL-C including atherogenic triglyceride-rich particles (VLDL and remnants) and more to its strong correlation with LDL particle number. Finally, our novel finding that LDL particles are more cholesterol-depleted when LDL concentrations are lower, independent of triglycerides or LDL particle size, helps to explain why patients with low LDL-C often have disproportionately higher numbers of LDL particles (7–13). Our data show that persons with this LDL disconnect have higher CVD risk. It is therefore reasonable to anticipate that such discordant individuals would derive clinical benefit from more intensive LDL lowering than would have been indicated by their LDL-C level. Data from statin intervention trials are needed to test this hypothesis.
Acknowledgments
This work was supported through contracts N01-HC-25195 and 2K24-HL-4334 by the National Heart, Lung, and Blood Institute of the National Institutes of Health. We wish to thank Dr. Irina Shalaurova and Dr. Elias Jeyarajah for helpful suggestions and discussion.
GLOSSARY OF ABBREVIATIONS
- ATP III
Adult Treatment Panel III
- CVD
Cardiovascular disease
- CETP
Cholesterol ester transfer protein
- HDL-C
HDL cholesterol
- LDL-C
LDL cholesterol
- LDL-P
LDL particle number
- NCEP
National Cholesterol Education Program
- non-HDL-C
Non-HDL cholesterol
- VLDL-P
VLDL particle number
Footnotes
Disclosures
Dr. Cromwell is a consultant for or has served on the speakers’ bureau for Abbott, AstraZeneca, Isis, LipoScience, Kos, Merck, Merck-Schering, Reliant, and Schering-Plough, in addition to receiving research or grant funding from Merck, Kos and Pfizer.
Dr. Otvos is an employee and stockholder of Liposcience.
Drs. Keyes, Pencina, Sullivan, Vasan, Wilson, and D’Agostino report no conflicts.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Friedewald WT, Levy RI, Fredrickson DS. Estimation of the concentration of low-density lipoprotein cholesterol in plasma, without use of the preparative ultracentrifuge. Clin Chem. 1972;18:499–502. [PubMed] [Google Scholar]
- 2.Sniderman A, Vu H, Cianflone K. Effect of moderate hypertriglyceridemia on the relation of plasma total and LDL apoB levels. Atherosclerosis. 1991;89:109–16. doi: 10.1016/0021-9150(91)90050-d. [DOI] [PubMed] [Google Scholar]
- 3.Jeyarajah EJ, Cromwell WC, Otvos JD. Lipoprotein particle analysis by nuclear magnetic resonance spectroscopy. Clin Lab Med. 2006;26:847–70. doi: 10.1016/j.cll.2006.07.006. [DOI] [PubMed] [Google Scholar]
- 4.Executive Summary of The Third Report of The National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 5.Grundy SM, Cleeman JI, Merz CN, Brewer HB, Jr, Clark LT, Hunninghake DB, Pasternak RC, Smith SC, Jr, Stone NJ. Implications of recent clinical trials for the National Cholesterol Education Program Adult Treatment Panel III guidelines. Circulation. 2004;110:227–39. doi: 10.1161/01.CIR.0000133317.49796.0E. [DOI] [PubMed] [Google Scholar]
- 6.Otvos JD, Jeyarajah EJ, Cromwell WC. Measurement issues related to lipoprotein heterogeneity. Amer J Cardiol. 2002;90(suppl):22i–29i. doi: 10.1016/s0002-9149(02)02632-2. [DOI] [PubMed] [Google Scholar]
- 7.Cromwell WC, Otvos JD. Low-density lipoprotein particle number and risk for cardiovascular disease. Curr Atheroscler Rep. 2004;6:381–7. doi: 10.1007/s11883-004-0050-5. [DOI] [PubMed] [Google Scholar]
- 8.Sniderman AD, Furberg CD, Keech A, Roeters van Lennep JE, Frohlich J, Jungner I, Walldius G. Apolipoproteins versus lipids as indices of coronary risk and as targets for statin therapy treatment. Lancet. 2003;361:777–80. doi: 10.1016/s0140-6736(03)12663-3. [DOI] [PubMed] [Google Scholar]
- 9.Sniderman AD, St-Pierre AC, Cantin B, Dagenais GR, Despres J-P, Lamarche B. Concordance/discordance between plasma apolipoprotein B levels and the cholesterol indexes of atherosclerotic risk. doi: 10.1016/s0002-9149(03)00262-5. [DOI] [PubMed] [Google Scholar]
- 10.Kim BJ, Hwang ST, Sung KC, Kim BS, Kang JH, Lee MH, Park JR. Comparison of the relationships between serum apolipoprotein B and serum lipid distributions. Clin Chem. 2005;51:2257–63. doi: 10.1373/clinchem.2005.052738. [DOI] [PubMed] [Google Scholar]
- 11.Stein EA, Sniderman A, Laskarzewski P. Assessment of reaching goal in patients with combined hyperlipidemia: low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, or apolipoprotein B. Am J Cardiol. 2005;96(suppl):36K–43K. doi: 10.1016/j.amjcard.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 12.Kathiresan S, Otvos JD, Sullivan LM, Keyes MJ, Schaefer EJ, Wilson PW, D’Agostino RB, Vasan RS, Robins SJ. Increased small low-density lipoprotein particle number: a prominent feature of the metabolic syndrome in the Framingham Heart Study. Circulation. 2006;113:20–9. doi: 10.1161/CIRCULATIONAHA.105.567107. [DOI] [PubMed] [Google Scholar]
- 13.Cromwell WC, Otvos JD. Heterogeneity of low density lipoprotein particle number in patients with type 2 diabetes mellitus and low density lipoprotein cholesterol <100 mg/dL. Am J Cardiol. 2006;98:1599–1602. doi: 10.1016/j.amjcard.2006.07.036. [DOI] [PubMed] [Google Scholar]
- 14.Ingelsson E, Schaefer EJ, Contois JH, McNamara JR, Sullivan L, Keyes MJ, Pencina MJ, Schoonmaker C, Wilson PWF, D’Agostino RB, Vasan RS. Clinical utility of different lipid measures for prediction of coronary heart disease in men and women. JAMA. 2007;298:776–85. doi: 10.1001/jama.298.7.776. [DOI] [PubMed] [Google Scholar]
- 15.Grundy SM. Low-density lipoprotein, non-high-density lipoprotein, and apolipoprotein B as targets of lipid-lowering therapy. Circulation. 2002;106:2526–2529. doi: 10.1161/01.cir.0000038419.53000.d6. [DOI] [PubMed] [Google Scholar]
- 16.Liu J, Sempos CT, Donahue RP, Dorn J, Trevisan M, Grundy SM. Non-high-density lipoprotein and very-low-density lipoprotein cholesterol and their risk predictive values in coronary heart disease. Am J Cardiol. 2006;98:1363–1368. doi: 10.1016/j.amjcard.2006.06.032. [DOI] [PubMed] [Google Scholar]
- 17.Kannel WB, Feinleib M, McNamara PM, Garrison RJ, Castelli WP. An investigation of coronary heart disease in families. The Framingham offspring study. Am J Epidemiol. 1979;110:281–90. doi: 10.1093/oxfordjournals.aje.a112813. [DOI] [PubMed] [Google Scholar]
- 18.McNamara JR, Schaefer EJ. Automated enzymatic standardized lipid analyses for plasma and lipoprotein fractions. Clin Chim Acta. 1987;166:1–8. doi: 10.1016/0009-8981(87)90188-4. [DOI] [PubMed] [Google Scholar]
- 19.Freedman DS, Otvos JD, Jeyarajah EJ, Shalaurova I, Cupples LA, Parise H, D’Agostino RB, Wilson PWF, Schaefer EJ. Sex and age differences in lipoprotein subclasses measured by nuclear magnetic resonance spectroscopy: The Framingham Study. Clin Chem. 2004;50:1189–1200. doi: 10.1373/clinchem.2004.032763. [DOI] [PubMed] [Google Scholar]
- 20.McNamara JR, Small DM, Li Z, Schaefer EJ. Differences in LDL subspecies involve alterations in lipid composition and conformational changes in apolipoprotein B. J Lipid Res. 1996;37:1924–35. [PubMed] [Google Scholar]
- 21.Framingham Heart Study: 30 Year Follow-Up. Bethesda, Md: US Department of Health and Human Services; 1987. Some risk factors related to the annual incidence of cardiovascular disease and death in pooled repeated biennial measurements. [Google Scholar]
- 22.Harvengt C, Heller FR, Martiat P, Van Nieuwenhuyze Y. Short-term effects of beta blockers atenolol, nadolol, pindolol, and propranolol on lipoprotein metabolism in normolipemic subjects. J Clin Pharmacol. 1987;27:475–80. doi: 10.1002/j.1552-4604.1987.tb03052.x. [DOI] [PubMed] [Google Scholar]
- 23.Festa A, Williams K, Hanley AJG, Otvos JD, Goff DC, Wagenknecht LE, Haffner SM. Nuclear magnetic resonance lipoprotein abnormalities in prediabetic subjects in the Insulin Resistance Atherosclerosis Study (IRAS) Circulation. 2005;111:3465–72. doi: 10.1161/CIRCULATIONAHA.104.512079. [DOI] [PubMed] [Google Scholar]
- 24.Kuller LH, Arnold A, Tracy R, Otvos J, Burke G, Psaty B, Siscovick D, Freedman DS, Kronmal R. Nuclear magnetic resonance spectroscopy of lipoproteins and risk of coronary heart disease in the Cardiovascular Health Study. Arterioscler Thromb Vasc Biol. 2002;22:1175–80. doi: 10.1161/01.atv.0000022015.97341.3a. [DOI] [PubMed] [Google Scholar]
- 25.Rosenson RS, Otvos JD, Freedman DS. Relations of lipoprotein subclass levels and low-density lipoprotein size to progression of coronary artery disease in the Pravastatin Limitation of Atherosclerosis in the Coronary Arteries (PLAC-I) Trial. Am J Cardiol. 2002;90:89–94. doi: 10.1016/s0002-9149(02)02427-x. [DOI] [PubMed] [Google Scholar]
- 26.Blake GJ, Otvos JD, Rifai N, Ridker PM. Low-density lipoprotein particle concentration and size as determined by nuclear magnetic resonance spectroscopy as predictors of cardiovascular disease in women. Circulation. 2002;106:1930–7. doi: 10.1161/01.cir.0000033222.75187.b9. [DOI] [PubMed] [Google Scholar]
- 27.Otvos JD, Collins D, Freedman DS, Shalaurova I, Schaefer EJ, McNamara JR, Bloomfield HE, Robins SJ. LDL and HDL particle subclasses predict coronary events and are changed favorably by gemfibrozil therapy in the Veterans Affairs HDL Intervention Trial (VA-HIT) Circulation. 2006;113:1556–63. doi: 10.1161/CIRCULATIONAHA.105.565135. [DOI] [PubMed] [Google Scholar]
- 28.Hayward RA, Hofer TP, Sandeep V. Lack of evidence for recommended low-density lipoprotein treatment targets: a solvable problem. Ann Intern Med. 2006;145:520–30. doi: 10.7326/0003-4819-145-7-200610030-00010. [DOI] [PubMed] [Google Scholar]
- 29.Davidson MH. Reducing residual risk for patients on statin therapy: the potential role of combination therapy. Am J Cardiol. 2005;96(suppl):3K–13K. doi: 10.1016/j.amjcard.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Heart Protection Study Collaborative Group. MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomized placebo-controlled trial. Lancet. 2002;360:7–22. [Google Scholar]
- 31.Deedwania P, Barter P, Carmena R, Fruchart J-C, Grundy SM, Haffner S, Kastelein JP, LaRosa JC, Schachneer H, Shepherd J, Waters DD. Reduction of low-density lipoprotein cholesterol in patients with coronary heart disease and metabolic syndrome: analysis of the Treating to New Targets study. Lancet. 2006;368:919–28. doi: 10.1016/S0140-6736(06)69292-1. [DOI] [PubMed] [Google Scholar]
- 32.Barter PJ, Ballantyne CM, Carmena R, Castro Cabezas M, Chapman MJ, Couture P, DeGraaf J, Durrington PN, Faergeman O, Frohlich J, Furberg CD, Gagne C, Haffner SM, Humphries SE, Jungner I, Krauss RM, Kwiterovich P, Marcovina S, Packard CJ, Pearson TA, Srinath Reddy K, Rosenson R, Sarrafzadegan N, Sniderman AD, Stalenhoef AF, Stein E, Talmud PJ, Tonkin AM, Walldius G, Williams KMS. Apo B versus cholesterol in estimating cardiovascular risk and in guiding therapy: report of the thirty-person/ten-country panel. J Intern Med. 2006;259:247–58. doi: 10.1111/j.1365-2796.2006.01616.x. [DOI] [PubMed] [Google Scholar]
- 33.Berneis KK, Krauss RM. Metabolic origins and clinical significance of LDL heterogeneity. J Lipid Res. 2002;43:1363–79. doi: 10.1194/jlr.r200004-jlr200. [DOI] [PubMed] [Google Scholar]
- 34.Barter PJ, Brewer HB, Jr, Chapman MJ, Hennekens CH, Rader DJ, Tall AR. Cholesteryl ester transfer protein: a novel target for raising HDL and inhibiting atherosclerosis. Arterioscler Thromb Vasc Biol. 2003;23:160–7. doi: 10.1161/01.atv.0000054658.91146.64. [DOI] [PubMed] [Google Scholar]
- 35.Guerin M, Lassel TS, Le Goff W, Farnier MA, Chapman MJ. Action of atorvastatin in combined hyperlipidemia: preferential reduction of cholesterol ester transfer from HDL to VLDL1 particles. Arterioscler Thromb Vasc Biol. 2000;20:189–97. doi: 10.1161/01.atv.20.1.189. [DOI] [PubMed] [Google Scholar]
- 36.Miller M, Dolinar C, Cromwell W, Otvos JD. Effectiveness of high doses of simvastatin as monotherapy in mixed hyperlipidemia. Amer J Cardiol. 2001;87:232–4. doi: 10.1016/s0002-9149(00)01327-8. [DOI] [PubMed] [Google Scholar]
- 37.Rosenson RS, Shalaurova I, Freedman DS, Otvos JD. Effects of pravastatin treatment on lipoprotein subclass profiles and particle size in the PLAC-I Trial. Atherosclerosis. 2002;160:41–8. doi: 10.1016/s0021-9150(01)00544-5. [DOI] [PubMed] [Google Scholar]
- 38.Blake GJ, Albert MA, Rifai N, Ridker PM. Effect of pravastatin on LDL particle concentration as determined by NMR spectroscopy: a substudy of a randomized placebo controlled trial. Eur Heart J. 2003;24:1843–7. doi: 10.1016/j.ehj.2003.07.008. [DOI] [PubMed] [Google Scholar]
- 39.Soedamah SS, Colhoun HM, Thomason MJ, Betteridge DJ, Durrington PN, Hitman GA, Fuller JH, Julier K, Mackness MI, Neil HAW The CARDS investigators. The effect of atorvastatin on serum lipids, lipoproteins and NMR spectroscopy defined lipoprotein subclasses in type 2 diabetic patients with ischemic heart disease. Atherosclerosis. 2003;167:243–55. doi: 10.1016/s0021-9150(02)00428-8. [DOI] [PubMed] [Google Scholar]