Abstract
Formycin is a naturally occurring biologically responsive C-nucleoside. In pursuing the design and syntheses of novel C-nucleosides, convenient access to carbocyclic C-nucleosides based on the formycin framework was a goal. One such target was carbocyclic 4′-epiformycin (4). This compound is reported via a procedure based on an asymmetric aldol/ring closure methathesis strategy. To provide a preliminary glimpse into the biological characterization of 4 an antiviral assay was conducted. Target 4 was found to be inactive and to lack cytotoxicity to the host cells.
Keywords: cyclopentyl; C-nucleoside; pyrazolo[4,3-d]pyrimidine; diastereomeric aldol
Introduction
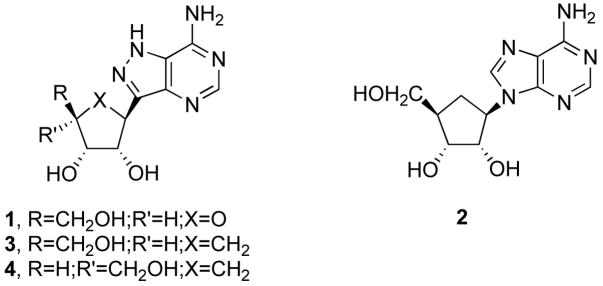
Formycin A (1) is the C-nucleoside isomer of adenosine, which has achieved a place of potential that has not been fully developed for its value in medicinal agent discovery.1 Similarly, but more thoroughly studied, is the carbocyclic adenine derivative aristeromycin (2).2 Little attention3 has been devoted to combining these two structural features into carbocyclic C-nucleosides (for example, carbocyclic formycin A, 3) as a source for a new chemotherapeutic library. Our goal is to gain access to representatives of this latter group of compounds. The 4′-epimer of 3 (that is, 4), which is an analog of α-L-lyxoadenosine,4 is a member of that series and it is described herein.
Chemistry
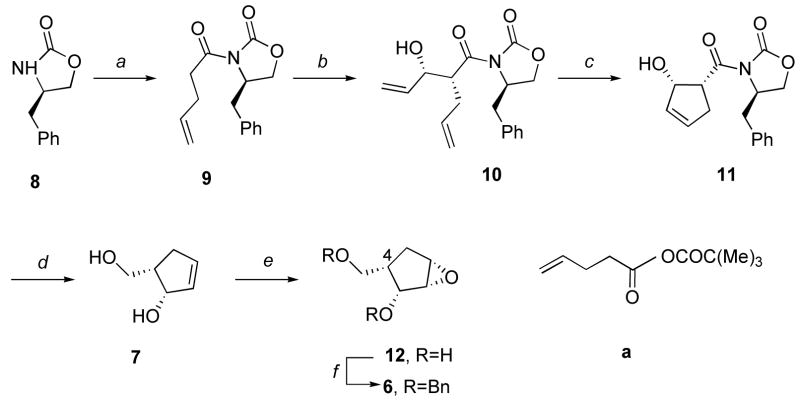
The plan to 4 required the availability of the cyclopentyl alkyne 5 as the starting material for construction of the requisite pyrazolo[4,3-d]pyrimidine base unit.3d,3e,5a–c,e Because of the numerous examples of stereoselective epoxide ring openings as synthetic sequence components,6 5 was seen as being accessible from the chiral 6 and an alkynyl organoaluminum reagent.7 To gain access to 6, we adapted literature conditions8–10 beginning with (R)-4-benzyl-2-oxazolidinone (8) (Scheme 1) and called on asymmetric aldol/ring closure metathesis steps.
Scheme 1a.
aReaction conditions: a, (i) n-BuLi/hexanes, THF; (ii) a, TBME, 100% for 2 steps; b, (i) n-Bu2BOTf, i-Pr2NEt, CH2Cl2, −78 °C; (ii) acrolein, −78 °C, CH2Cl2, 92% for 2 steps; c, 1st gen. Grubbs catalyst, CH2Cl2, 59%; d, LiBH4, MeOH, THF, 0 °C, 66%; e, m-CPBA, CH2Cl2; f, NaH, BnBr, TBNI, THF, 72% (for steps e and f).
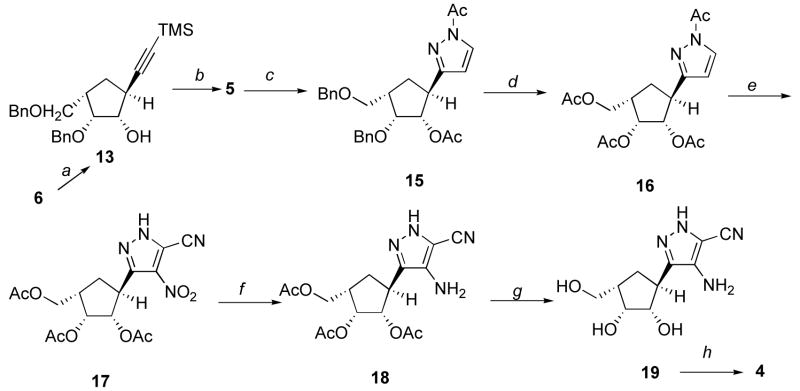
With 6 in hand, its reaction with diethyl[(trimethylsilyl)ethynyl]aluminum resulted in 13 (Scheme 2) and a minor side-product assigned as 14.11 Desilylation of 13 yielded 5, whose X-ray crystallographic analysis (Figure 3) confirmed that the hydroxy, benzyloxy, benzyloxymethyl were on same face of cyclopentyl ring and opposite to the alkynyl unit.
Scheme 2a.
aReaction conditions: a, Me3SiCCH, BuLi, −78 °C, N2 followed by Et2AlCl, 0 °C, 62%; b, TBAF, THF, 92%; c, (i) n-BuLi/hexanes, TBME followed by DMF; (ii) hydrazine monohydrate, AcOH; (iii) Ac2O, pyridine, DMAP, 85% for 3 steps, d, (i) BBr3, CH2Cl2, (ii) Ac2O, Et3N, DMAP, CH2Cl2,76%; e, (i) ammonium nitrate, TFA, TFAA; (ii) KCN, EtOH/EtOAc, 88% for 2 steps; f, H2, Pd/C, MeOH, 92%; g, NH3, MeOH, 88%; h, formamidine acetate, EtOH, 60%.
Figure 3.
X-ray structure for compound 5
Formylation 5 and subsequent reaction of the resultant substituted propargylic aldehyde with hydrazine and then acetylation provided 15.3d,3e Debenzylation (boron tribromide was superior to catalytic hydrogenolysis) of 15 then acetylation (to 16) was followed by nitration and cine-cyano substitution3d,3e,5c–5e to yield 17. Hydrogenation of 17 gave 18. Deacetylation of 18 (to 19) and subsequent treatment with formamidine3d,3e,5c,5e produced the fused pyrimidine target 4.
Antiviral Analysis
To gain preliminary insight into the biological potential of 4, it was subjected to antiviral screening versus herpes simplex-1, herpes simplex-2, herpes simplex-1 (TK−), vaccinia, vesicular stomatitis, coxsackie B4, respiratory syncytial, parainfluenza 3, reovirus-1, Sinbis, and Punta Toro.12 No activity was found. Also, no cytotoxicity arose in the cell lines used in the antiviral assays: human erythroleukemia (HEL), HeLa, and Vero.
Conclusion
A straightforward chiral synthesis of carbocyclic 4′-epiformycin has been achieved using practical means and avails a rare example of a 4′-epi carbocyclic nucleoside.13 The absence of antiviral activity with 4 may be the consequence of its failure to undergo conversion to the 5′-nucleotide derivative, an efficient intracellular process for the parent formycin,14 and/or its lack of inhibitory effect on S-adenosylhomocysteine hydrolase, a site of action of aristeromycin (3).2
Experimental
Materials and Methods
Melting points were recorded on a Meltemp II melting point apparatus and are uncorrected. 1H and 13C NMR spectra were recorded on a Bruker AV- 400 spectrometer (operated at 400 or 250 MHz, respectively). All 1H chemical shifts are reported in δ relative to internal standard tetramethylsilane (TMS, δ0.00). 13C chemical shifts are reported in δ relative to CDCl3 (center of triplet, δ 77.23). The spin multiplicities are indicated by the symbols s (singlet), d (doublet), t (triplet), q (quartet), m (multiplet) and br (broad), dd (doublet of doublet). Elemental analyses were performed by Atlantic Microlabs, Atlanta, Georgia. Reactions were monitored by thin layer chromatography (TLC) using 0.25 mm E. Merck silica gel 60-F254 precoated silica gel plates with visualization by irradiation with a Mineral light UVGL-25 lamp or exposure to iodine vapor. Column chromatography was performed on Whatman silica gel (average particle size 5–25 μm, 60 Å) and elution with the indicated solvent system. Yields refer to chromatographically and spectroscopically (1H and 13C NMR) homogeneous materials. The reactions were generally carried out in a N2 atmosphere under anhydrous conditions.
(4R)-Benzyl-3-(pent-4-enoyl)oxazolidin-2-one (9)
Triethylamine (15.3 mL, 0.11 mol) was added to a solution of 4-pentenoic acid (11.6 mL, 11.3 g, 0.11 mol) in t-butyl methyl ether (800 mL) cooled to −78 °C. The solution was stirred for 5 min, and pivaloyl chloride (13.5 mL, 0.11 mol) was added. After 15 min, the bath was removed and replaced by an ice H2O bath. The heterogeneous mixture (containing a) was mechanically stirred at 0 °C for 1 h. In a separate flask, a solution of (R)-4-benzyl-2-oxazolidinone (8, 19.2 g, 0.11 mol) in THF (200 mL) was cooled to −78 °C, whereupon n-BuLi (44.0 mL, 2.5 M in hexanes, 0.11 mol) was added slowly. This solution was stirred for 10 min at −78 °C. The flask containing the mixed anhydride was cooled to −78 °C, and the lithiated oxazolidinone transferred via cannula into the mixed anhydride a. After being stirred at −78 °C for 15 min, the reaction mixture was warmed to 0 °C and stirred for 30 min, then the reaction was quenched by addition of aqueous, saturated NH4Cl. After extraction twice with CH2Cl2, the combined organic phases were dried (anhyd. Na2SO4), filtered and the filtrate concentrated. Purification by flash silica gel column chromatography (hexanes/EtOAc, 3:1) gave 9 (28.5 g, 100%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 2.42 (m, 2H), 2.76 (dd, 1H, J = 9.4, 13.4 Hz), 3.03 (m, 2H), 3.24 (dd, 1H, J = 10.1, 13.4 Hz,), 4.12 (m, 2H), 4.64 (m, 1H), 5.04 (m, 2H), 5.86 (m, 1H), 7.28 (m, 5H). 13C NMR (100 MHz, CDCl3) δ 28.6, 35.2, 38.2, 55.5, 66.6, 116.0, 127.7, 129.3, 129.8, 135.8, 137.2, 153.8, 172.8. Anal. Calcd for C15H17NO3: C, 69.48; H, 6.61; N, 5.40. Found: C, 69.44; H, 6.65; N, 5.31.
[3(2R,3S),4R]-3-(2-Allyl-3-hydroxypent-4-enoyl)-4-benzyloxazolidin-2-one (10)
To a solution of 9 (26 g, 0.10 mol) in CH2Cl2 (50 mL) at −78 °C under N2 atmosphere was added diisopropylethylamine (39 mL, 0.22 mol) followed by dibutylboron triflate (200 mL, 0.20 mol, 1.0M solution in CH2Cl2). The reaction mixture was allowed to stir for 1 h at room temperature. After re-cooling to −78°C, a solution of acrolein (16 mL, 0.22 mol) in CH2Cl2 (100 mL) was added dropwise. The mixture was stirred for 2 h at −78°C. Then, the reaction mixture was slowly warmed to room temperature and allowed to stir overnight. It was quenched with saturated NaHCO3 solution and extracted with CH2Cl2. The organic phase was washed with brine, dried (anhyd. Na2SO4), filtered, and the filtrate evaporated under reduced pressure. Purification of the residue via silica gel flash chromatography (gradient elution from 10 % to 25 % EtOAc: hexanes) afforded 10 (29 g, 92 %) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 2.52 (m, 1H), 2.66 (m, 2H), 2.86 (br s, 1H), 3.31 (m, 1H), 4.18 (m, 2H), 4.29 (m, 1H), 4.47, (br s, 1H), 4.73 (m, 1H), 5.24 (m, 4H), 5.91 (m, 2 H), 7.32 (m, 5H). 13C NMR (100 MHz, CDCl3) δ 32.1, 38.1, 47.4, 55.7, 66.1, 73.4, 116.9, 117.4, 127.5, 129.1, 129.6, 135.3, 135.4, 137.4, 153.7, 174.5. Anal. Calcd for C18H21NO4: C, 68.55; H, 6.71; N, 4.44. Found: C, 68.20; H, 7.07; N, 4.11.
[3(1R,2S),4S]-4-Benzyl-3-(2-hydroxycyclopent-3-enecarbonyl)oxazolidin-2-one (11)
A solution of compound 10 (22.2 g, 70.4 mmol) in CH2Cl2 (300 mL) was degassed by passing through a stream of N2 for 25 min and 0.5 mol% of Grubbs’ catalyst (first generation) was then added under an atmosphere of N2. After stirring overnight, the reaction mixture was exposed to air for 1 h, and purified by flash silica gel column chromatography (hexanes/EtOAc, 1:1) to yield 11 (12 g, 59%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 2.43 (m, 1H), 2.76 (m, 1H), 2.86 (d, 1H, J = 7.5 Hz), 3.14 (m, 1H), 3.27 (m, 1H), 4.09 (m, 1H), 4.22 (m, 1H), 4.40 (m, 1H), 4.69, (m, 1H), 5.10 (br s, 1H), 5.72 (m, 1H), 5.99 (m, 1H), 7.26 (m, 5H). 13C NMR (100 MHz, CDCl3) δ 33.0, 37.9, 47.2, 55.4, 66.2, 76.9, 127.2, 128.8, 129.4, 130.9, 134.4, 135.5, 153.7, 172.0. Anal. Calcd for C16H17NO4: C, 66.89; H, 5.96; N, 4.88. Found: C, 66.69; H, 6.04; N, 4.71.
(1S,5S)-5-Hydroxymethyl-2-cyclopenten-1-ol (7)
A solution of LiBH4 (1.0 g, 43.0 mmol) in THF (55 mL) cooled at 0 °C was added into a solution of 11 (11.0 g, 38.3 mmol) in THF (150 mL) and MeOH (1.7 mL). The reaction mixture was stirred at 0 °C for 45 min and then allowed to warm to room temperature. After 1 h, 10% NaOH (61 mL) was added, and the stirring continued until both phases were clear. The mixture was extracted with Et2O. The organic phase was washed with brine, dried (anhyd. Na2SO4), filtered, and the filtrate concentrated in vacuo. Purification by silica gel flash chromatography (gradient elution from 50% to 70% EtOAc/hexanes) yielded 7 (2.9 g, 66.3%) as a colorless liquid, [α]D22.8 124.0° (c, 0.26, methanol) (lit.10 reported [α]D24 -125.1° (c, 0.47, CH2Cl2) for the enantiomer of 7). 1H NMR (400 MHz, CDCl3) δ 2.19 (m, 1H), 2.37 (m, 2H), 3.75 (br s, 2H), 4.24 (d, 2H, J=16.8 Hz), 4.85 (br s, 1H), 5.81 (m, 1H), 5.97 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 33.6, 42.7, 62.2, 76.9, 132.4, 134.7. Anal. Calcd for C6H10O2: C, 63.14; H, 8.83. Found: C, 63.04; H, 8.98.
(1S,2R,3S,4S)-1-Benzyloxymethyl-2-benzyloxy-3,4-epoxyclopentane (6)
A solution of 7 (2.1 g, 18.4 mmol), m-chloroperoxybenzoic acid (7.2 g) and CH2Cl2 (100 mL) was stirred at room temp overnight. The solvent was removed and the residue partitioned between Et2O and H2O. The solvent was then removed in vacuo to obtain 12 (2.2 g), which was used in the next step without further purification. 1H NMR (400 MHz, CDCl3) δ 2.02 (m, 1H), 2.13 (m, 1H), 2.43 (m, 1H), 3.40 (m, 2H), 3.52 (m, 1H), 3.61 (m, 1H), 3.69 (m, 2H), 4.60 (dd, 1H, J = 1.5, 9.0 Hz). 13C NMR (100 MHz, CDCl3) δ 31.4, 37.8, 56.2, 61.5, 64.0, 75.2.
To a solution of 12 (2.2 g) in anhydrous THF (50 ml) at 0 °C, NaH (2 g, 50 mmol, 60% dispersion in mineral oil) was added in several portions. After 30 min, benzyl bromide (6.1 ml, 51.3 mmol) and a catalytic amount of tetrabutylammonium iodide were added. The reaction mixture was then allowed to stir at room temp overnight. After the starting material was no longer present (TLC), ice H2O was added to quench the reaction. The resultant mixture was extracted with CH2Cl2. The combined extracts were washed with brine, dried (anhyd. Na2SO4), filtered and the filtrate concentrated in vacuo. The residue was purified by silica gel flash column chromatography (hexanes/EtOAc, 4:1) afforded 6 (4.1 g, 72% over 2 steps) as an oil. 1H NMR (400 MHz, CDCl3) δ 1.92 (m, 1H), 2.32 (d, 1H, J = 14.9 Hz), 2.53 (m, 1H), 3.48 (m, 1H), 3.58 (m, 2H), 3.90 (dd, 1H, J = 4.7, 9.2 Hz), 4.23 (dd, 1H, J = 1.2, 8.5 Hz), 4.56 (s, 2H), 4.70 (q, 2H, J = 12.0 Hz), 7.41 (m, 10H). 13C NMR (100 MHz, CDCl3) δ 30.2, 35.7, 54.9, 57.8, 72.5, 72.6, 73.7, 80.9, 127.6, 127.7, 127.9, 128.0, 128.5, 128.6, 138.3, 138.9. Anal. Calcd for C20H22O3: C, 77.39; H, 7.14. Found: C, 77.42; H, 7.10.
(1S,2R,3S,5S)-2-Benzyloxy-3-benzyloxymethyl-5-(trimethylsilylethynyl)-cyclopentanol (13)
To a solution of trimethylsilylacetylene (15.3 mL, 108.2 mmol) in anhydrous toluene (390 mL) with stirring at −78 °C under N2 was added n-butyllithium (43.3 mL, 108.3 mmol, 2.5 M solution in hexanes), and the reaction mixture was stirred at the same temperature for 30 min. Then, a solution of diethylaluminum chloride (60.1 mL, 108.2 mmol, 1.8 M solution in toluene) was added at 0 °C. Upon the addition a slow precipitation of LiCl was observed. The reaction mixture was stirred for 5 h at 10 °C. A solution of epoxide 6 (6.6 g, 21.3 mmol) in toluene (20 mL) was then added immediately. The reaction mixture was stirred at 0 °C for 45 min and 3 h at room temperature, monitored by TLC analysis, and quenched with MeOH (CAUTION: gas evolution). After adding H2O (4.2 mL), 15% NaOH solution (4.2 mL), and H2O (12.6 mL), the reaction mixture was filtered, washed with CH2Cl2, and the organic layer then washed with brine, and dried (anhyd. Na2SO4). The solvent was evaporated, and the crude residue was purified by silica gel column chromatography (hexanes/EtOAc, 6:1) to give 13 (5.4 g, 62%) as a white solid, mp 47–48 °C. 1H NMR (250 MHz, CDCl3) δ 0.24 (s, 9H), 1.98 (m, 2H), 2.64 (m, 1H), 2.91 (m, 1H), 3.33 (m, 1H), 3.62 (m, 2H), 4.21 (m, 2H), 4.61 (s, 2H), 4.74 (d, 2H, J=2.8 Hz), 7.41 (m, 10H). 13C NMR (62.5 MHz, CDCl3) δ 0.46, 31.8, 36.7, 39.8, 69.4, 73.6, 78.4, 80.9, 85.9, 109.2, 128.0 (2C), 128.1(2C), 128.5 (2C), 128.7 (2C), 138.6. Anal. Calcd for C25H32O3Si: C, 73.49; H, 7.89. Found: C, 73.36; H, 7.93.
Further elution of the column gave 14 (1.8 g, 24.4%) as a colorless oil. 1H NMR (400 MHz, CDCl3) δ 2.01 (m, 1H), 2.31 (m, 1H), 2.65 (m, 1H), 3.48 (dd, 1H, J=5.7, 9.1 Hz), 3.56 (dd, 1H, J=4.3, 9.1 Hz), 3.75 (m, 1H), 4.15 (m, 2H), 4.30 (dd, 1H, J=4.1, 7.7 Hz), 4.51 (s, 2H), 4.60 (dd, 2H, J=11.4, 36.9 Hz), 7.31 (m, 10H). 13C NMR (100 MHz, CDCl3) δ 34.7, 38.7, 62.2, 68.6, 73.2, 73.5, 78.3, 79.3, 127.8 (2C), 128.0 (2C), 128.6 (2C), 137.7, 138.2. Anal. Calcd for C20H23ClO3: C, 69.26; H, 6.68. Found: C, 69.17; H, 6.73.
(1S,2R,3S,5S)-2-Benzyloxy-3-benzyloxymethyl-5-ethynylcyclopentanol (5)
To a solution of 13 (3.2 g, 7.8 mmol) in THF (60 mL) under N2 was added of tetrabutylammonium fluoride (7.8 mL, 7.8 mmol, 1 M solution in THF) by injection at 0 °C. The mixture was stirred for overnight at room temperature. The solution was absorbed on silica gel, and the crude product purified by silica gel flash column chromatography (hexanes/EtOAc, 4:1) to give 5 (2.4 g, 92%) as a white solid, mp 66–67 °C. 1H NMR (400 MHz, CDCl3) δ 1.84 (m, 1H), 1.96 (m, 1H), 2.11 (d, 1H, J=2.5 Hz), 2.54 (m, 1H), 2.78 (m, 1H), 3.19 (d, 1H, J=8.7 Hz), 3.53 (m, 2H), 4.12 (m, 2H), 4.51 (m, 2H), 4.64 (dd, 2H, J=11.5, 13.7 Hz), 7.30 (m, 10H). 13C NMR (100 MHz, CDCl3) δ 31.4, 35.4, 39.7, 69.3, 69.9, 73.5, 73.6, 78.3, 80.9, 86.6, 127.9 (2C), 128.6 (2C), 128.7 (2C), 138.1, 138.5. Anal. Calcd for C22H24O3 C, 78.54; H, 7.19. Found: C, 78.56; H, 7.22.
(1S,2R,3S,5S)-1-Acetyl-5-(1-acetyl-1H-pyrazol-3-yl)-2-benzyloxy3-(benzyloxymethyl)cyclopentane (15)
To a solution of 5 (2.4 g, 7.1 mmol) in anhydrous t-butyl methyl ether (90 mL) with stirring at −78 °C under N2 was added n-butyllithium (7.1 mL, 17.8 mmol, 2.5 M solution in hexanes), and the reaction mixture stirred at the same temperature for 30 min. An excess of DMF (2.7 mL, 35.0 mmol) was added in one portion and the cold bath removed. The reaction mixture was allowed to warm to room temperature and aged for 30 min. The TBME solution was poured into a vigorously stirred, biphasic solution prepared from a 10% aqueous solution of KH2PO4 (45 mL) and TBME (45 mL) cooled over ice to ca. +5 °C. The resulting layers were separated and the organic extract was washed with H2O. The combined aqueous layers were back extracted with TBME. The combined organic layers were dried (anhyd. Na2SO4), filtered and the filtrate concentrated to give the crude acetylenic aldehyde as an oil. The crude product thus isolated was committed to the next step without further purification.
To a solution of crude aldehyde (from the previous process) in glacial AcOH (78 mL) was added a solution of hydrazine monohydrate (3.7 g, 34.9 mmol) in glacial AcOH (38 mL). The resulting solution was heated at reflux for 8 h and then concentrated in vacuo to afford a dark brown oil. This crude product was dissolved in pyridine (20 mL) and Ac2O (9.3 mL, 98.4 mmol) and DMAP were added. The resulting solution was stirred for 16 h at room temperature. The solvent was removed in vacuo, and the crude residue dissolved in EtOAc (800 mL), washed with 10% HCl and brine, dried (anhyd. Na2SO4), concentrated, and purified by silica gel column chromatography to afford 15 (2.8 g, 85% over 3 steps) as a light yellow oil. 1H NMR (400 MHz, CDCl3) δ 1.99 (s, 3H), 2.00 (m, 2H), 2.63 (s, 3H), 2.95 (m, 1H), 3.48 (dd, 1H, J=6.4, 9.0 Hz), 3.60 (m, 1H), 3.72 (t, 1H, J=8.7 Hz), 4.29 (t, 1H, J=4.0 Hz), 4.50 (dd, 2H, J=11.9, 13.6 Hz), 4.60 (q, 2H, J=11.6 Hz), 5.20 (dd, 1H, J=3.7 9.4 Hz), 6.28 (d, 1H, J=2.8 Hz), 7.29 (m, 10H), 8.14 (d, 1H, J=2.8 Hz). 13C NMR (100 MHz, CDCl3) δ 21.1, 21.8, 29.9, 39.3, 40.4, 69.8, 73.3, 74.1, 79.6, 80.5, 108.7, 127.7, 127.7, 127.8, 127.8, 128.5, 128.5, 129.1, 138.5, 138.9, 159.0, 169.6, 170.8. Anal. Calcd for C27H30N2O5: C, 70.11; H, 6.54; N, 6.06. Found: C, 70.10; H, 6.48; N, 6.08.
(1S,2R,3S,5S)-1,2-Diacetoxy-5-acetoxymethyl-3-(1-acetyl-1H-pyrazol-3-yl)-cyclopentane (16)
To a stirred solution of 15 (2.0 g, 4.3 mmol) in anhydrous CH2Cl2 (180 mL) was added boron tribromide (43.2 mL, 43.2 mmol, 1.0 M solution in CH2Cl2) at −78 °C and the reaction mixture stirred at the same temperature for 1 h. To this mixture was added MeOH (400 mL) and the reaction mixture neutralized with Ag2CO3 and filtered through a pad of Celite. The filtrate was evaporated and the crude product dissolved in CH2Cl2 (300 mL). To this triethylamine (4.8 mL), Ac2O (1.9 mL) and a catalytic amount of DMAP were added, and the resulting solution stirred overnight. The mixture was then washed with brine, dried (anhyd. Na2SO4), concentrated, and the residue chromatographed (silica gel, hexanes/EtOAc, 3:1) to afford 16 (1.2 g, 76% over 2 steps) as a syrup. 1H NMR (400 MHz, CDCl3) δ 2.07 (m, 11H), 2.66 (m, 3H), 3.56 (m, 1H), 4.11 (m, 3H), 5.35 (m, 1H), 5.55 (m, 1H), 6.30 (m, 1H), 8.18 (d, 1H, J=2.5 Hz). 13C NMR (100 MHz, CDCl3) δ 20.9, 21.0, 21.1, 21.9, 29.8, 37.9, 39.3, 62.9, 73.3, 78.1, 108.6, 129.5, 157.8, 170.3 (2C), 170.4, 171.2. Anal. Calcd for C17H22N2O7: C, 55.73; H, 6.05; N, 7.05. Found: C, 55.85; H, 6.19; N, 7.67.
(1S,2R,3S,5S)-1,2-Diacetoxy-5-acetoxymethyl-3-(5-cyano-4-nitro1H-pyrazol-3-yl)cyclopentane (17)
Trifluoroacetic anhydride (2.1 mL) was added dropwise to a stirred solution of 16 (0.99 g, 2.7 mmol) and ammonium nitrate (1.9 g) in TFA (30 mL) at 0 °C. The resulting solution was allowed to warm to room temperature and stirred overnight. The solvent was evaporated in vacuo at room temperature and then diluted with CH2Cl2, washed with H2O, sat. aqueous NaHCO3 solution and brine, dried (anhyd. Na2SO4), and the organic phase concentrated in vacuo to give the 1,4-dinitro pyrazole derivative (1.1 g) as a syrup. The crude product thus isolated was committed to the next step without further purification.
At room temperature, a solution of the 1,4-dinitro compound in EtOH (9.3 mL) and EtOAc (9.3 mL) was added dropwise over 5 min to a stirred solution of KCN (1.3 g, 19.5 mmol) in EtOH (23.0 mL) and H2O (5.5 mL). Following an additional 5 min at room temp, the reaction mixture was neutralized with AcOH (2.0 mL). After evaporation of the solvent in vacuo, the residue was diluted with EtOAc (110 mL), washed with H2O and brine, dried (anhyd. Na2SO4) and concentrated in vacuo to a residue that was subjected to chromatographic purification (silica gel, CH2Cl2/MeOH, 20:1) to afford 17 (0.94 g, 88% over 2 steps) as a light yellow syrup. 1H NMR (400 MHz, CDCl3) δ 2.00 (s, 3H), 2.09 (s, 3H), 2.11 (m, 2H), 2.21 (s, 3H), 2.36 (m, 1H), 2.96 (m, 1H), 4.21 (m, 3H), 5.58 (m, 1H), 5.64 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 20.6, 20.7, 20.8, 30.0, 36.9, 37.8, 62.5, 72.9, 76.4, 110.7, 122.6, 133.9, 145.0, 170.6, 171.4, 171.8. Anal. Calcd for C16H18N4O8: C, 48.73; H, 4.60; N, 14.21. Found: C, 48.29; H, 4.59; N, 13.90.
(1S,2R,3S,5S)-1,2-Diacetoxy-3-acetoxymethyl-5-(4-amino-5-cyano-1H-pyrazol-3-yl)cyclopentane (18)
A catalytic amount of Pd/C was added to a solution of 17 (0.83 g, 2.1 mmol) in MeOH (30 mL). The resulting mixture was shaken under 30 psi of H2 overnight. After the reaction was complete, the solvent was evaporated in vacuo and the product purified by silica gel chromatography (CH2Cl2/EtOAc/MeOH, 8:1:0.5) to afford 18 (0.7 g, 92%) as a syrup. 1H NMR (400 MHz, CDCl3) δ 2.07 (m, 14H), 2.72 (m, 1H), 3.51 (m, 1H), 4.12 (m, 2H), 5.28 (m, 1H), 5.50 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 20.9 (2C), 21.1, 28.7, 36.4, 37.8, 63.1, 73.1, 78.0, 110.1, 112.6, 133.8, 135.7, 170.4, 171.0, 171.4. Anal. Calcd for C16H20N4O6: C, 52.74; H, 5.53; N, 15.38. Found: C, 52.81; H, 5.89; N, 15.62.
(1S,2R,3S,5S)-1,2-Dihydroxy-3-hydroxymethyl-5-(4-amino-5-cyano-1H-pyrazol-3-yl)cyclopentane (19)
Anhydrous NH3 was introduced to a solution of compound 18 (0.59 g, 1.6 mmol) in MeOH (80 mL) at 0 °C. The reaction mixture was stirred at room temperature. After the starting material was no longer present (TLC), the solvent was removed in vacuo and the residue purified by silica gel chromatography (CH2Cl2/MeOH, 6:1) to afford 19 (0.33g, 88%) as a light yellow solid, mp 174–176 °C. 1H NMR (400 MHz, CDCl3) δ 1.73 (m, 2H), 2.08 (m, 1H), 3.18 (m, 1H), 3.37 (m, 1H), 3.60 (m, 1H), 3.89 (m, 2H), 4.34 (m, 3H), 4.52 (m, 1H), 4.75 (m, 1H), 13.17 (s, 1H). 13C NMR (100 MHz, CDCl3) δ 29.2, 37.1, 41.9, 61.0, 72.7, 79.1, 114.1, 115.4, 131.7, 132.9. Anal. Calcd for C10H14N4O3: C, 50.41; H, 5.92; N, 23.52. Found: C, 50.39; H, 6.03; N, 23.35.
(1S,2R,3S,5S)-1,2-Dihydroxy-3-hydroxymethyl-5-(7-amino-1H-pyrazolo[4,3-d]pyrimidin-3-yl)cyclopentane (4)
A solution of 19 (0.25 g, 1.0 mmol) in EtOH (30 mL) was stirred with formamidine acetate (0.16 g, 1.5 mmol) under reflux for 50 min. The resulting white precipitate was isolated by filtration, washed with EtOH, and dried to afford analytically pure 4 (0.16 g, 60%) as a white solid, mp 269–270 °C (dec.): [α]D22.5: −53.5° (c, 0.26, DMSO). 1H NMR (400 MHz, CDCl3) δ 1.83 (m, 2H), 2.28 (m, 1H), 3.41 (m, 2H), 3.62 (m, 1H), 3.98 (m, 1H), 4.34 (m, 3H), 4.94 (s, 1H), 7.24 (br s, 2H), 8.13 (s, 1H), 12.37 (m, 1H). 13C NMR (100 MHz, CDCl3) δ 30.8, 40.1, 42.8, 61.3, 73.3, 79.0, 122.2, 139.8, 148.2, 150.8, 151.2. Anal. Calcd for C11H15N5O3: C, 49.81; H, 5.70; N, 26.40. Found: C, 50.00; H, 5.83; N, 26.30.
X-ray data for compound 5
Crystallographic data (excluding structure factors) for 5 has been deposited with Cambridge Crystallographic Data Centre as supplementary publication number CCDC 642212. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK [fax: +44(0)1223-336033 or e mail: deposit@ccdc.cam.ac.uk].
Figure 1.
Figure 2.
Acknowledgments
This research has been supported by funds from the Department of Health and Human Services (Al 56540), which is appreciated. We are also indebted to the following individuals for providing the antiviral data12 following their standard protocols: Dr. Erik De Clercq, the Rega Institute, Leuven Belgium; Dr. Earl Kern, University of Alabama at Birmingham, Birmingham, AL; Dr. Robert Sidwell, Utah State University, Logan, UT; Dr. Brent Korba, Georgetown, University, Rockville, MD; and, Dr. John Huggins, the US Army Medical Research Institute of Infectious Diseases, Ft. Detrick, MD 21702.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Simons C. Nucleoside Mimetics: Their Chemistry and Biological Properties. Gordon and Breach; Amsterdam: 2001. pp. 127–129. [Google Scholar]
- 2.(a) Schneller SW. Current Topics in Med Chem. 2002;2:1087–1092. doi: 10.2174/1568026023393228. [DOI] [PubMed] [Google Scholar]; (b) Herdewijn P, Balzarini J, De Clercq E, Vanderhaeghe H. J Med Chem. 1985;28:1385–1836. doi: 10.1021/jm00148a002. [DOI] [PubMed] [Google Scholar]; (c) De Clercq E. Biochem Pharmacol. 1987;36:2567–2575. doi: 10.1016/0006-2952(87)90533-8. [DOI] [PubMed] [Google Scholar]; (d) Cools M, De Clercq E. Biochem Pharmacol. 1989;38:1061–1067. doi: 10.1016/0006-2952(89)90249-9. [DOI] [PubMed] [Google Scholar]; (e) Hori M, Ito E, Takita T, Koyama G, Takeuchi T, Umezawa H. J Antibiot. 1964;17A:96–99. [PubMed] [Google Scholar]; (f) Koyama G, Umezawa H. J Antibiot. 1965;18A:175–177. [PubMed] [Google Scholar]
- 3.(a) Boyer SJ, Leahy JW. J Org Chem. 1997;62:3976–3980. [Google Scholar]; (b) Chun BK, Song GY, Chu CK. J Org Chem. 2001;66:4852–4858. doi: 10.1021/jo010224f. and references cited therein. [DOI] [PubMed] [Google Scholar]; (c) Tuncbilek M, Schneller SW. Bioorg Med Chem. 2003;11:3331–3334. doi: 10.1016/s0968-0896(03)00268-2. [DOI] [PubMed] [Google Scholar]; (d) Zhou J, Yang M, Schneller SW. Tetrahedron Lett. 2004;45:8233–8234. [Google Scholar]; (e) Zhou J, Yang M, Akdag A, Schneller SW. Tetrahedron. 2006;62:7009–7013. [Google Scholar]
- 4.Ugarkar BG, Castellino AJ, DaRe JS, Ramirez-Weinhouse M, Kopcho JJ, Rosengren S, Erion MD. J Med Chem. 2003;46:4750–4760. doi: 10.1021/jm030230z. [DOI] [PubMed] [Google Scholar]
- 5.(a) Rycroft AD, Singh G, Wightman RH. J Chem Soc, Perkin Trans 1. 1995:2667–2668. [Google Scholar]; (b) Buchanan JG, Quijano ML, Wightman RHJ. Chem Soc, Perkin Trans 1. 1992:1573–1576. [Google Scholar]; (c) Evans GB, Furneaux RH, Gainsford GJ, Hanson JC, Kicska GA, Sauve AA, Schramm VL, Tyler PC. J Med Chem. 2003;46:155–160. doi: 10.1021/jm0203332. [DOI] [PubMed] [Google Scholar]; (d) Buchanan JG, Jumaah AO, Kerr G, Talekar RR, Wightman RH. J Chem Soc, Perkin Trans 1. 1991:1077–1083. [Google Scholar]; (e) Buchanan JG, Stobie A, Wightman RH. Can J Chem. 1980;58:2624–2627. [Google Scholar]
- 6.For reviews, see: Posner GH. Org React. 1975;22:253–400.Lipshutz BH, Sengupta S. Org React. 1992;41:135–631.Bonini C, Righi G. Synthesis. 1994:225–238.Trost BM, Pattenden G, editors. Carbon-Carbon –bond Formation. Vol. 3. Pergamon Press; Oxford: 1991. Comprehensive Organic Synthesis.
- 7.(a) Shanmugam P, Miyashita M. Org Lett. 2003;5:3265–3268. doi: 10.1021/ol035075x. [DOI] [PubMed] [Google Scholar]; (b) Stichler-Bonaparte J, Bernet B, Vasella A. Helv Chim Acta. 2002;85:2235–2257. [Google Scholar]; (c) Zhou HY, Campbell EJ, Nguyen ST. Org Lett. 2001;3:2229–2231. doi: 10.1021/ol0161110. [DOI] [PubMed] [Google Scholar]; (d) Sasaki M, Tanino K, Miyashita M. J Org Chem. 2001;66:5388–5394. doi: 10.1021/jo010240c. [DOI] [PubMed] [Google Scholar]; (e) Tanino K, Honda Y, Miyashita M. Tetrahedron Lett. 2000;41:9281–9285. [Google Scholar]; (f) Schneider C, Brauner J. Tetrahedron Lett. 2000;41:3043–3046. [Google Scholar]; (g) Sasaki M, Miyazawa M, Tanino K, Miyashita M. Tetrahedron Lett. 1999;40:9267–9270. [Google Scholar]; (h) Ishibashi N, Miyazawa M, Miyashita M. Tetrahedron Lett. 1998;39:3775–3778. [Google Scholar]; (i) Inghardt T, Frejd T. Tetrahedron. 1991;47:6483–6492. [Google Scholar]; (j) Akita H, Matsukura H, Oishi T. Tetrahedron Lett. 1986;27:5397–5400. [Google Scholar]; (k) Roush WR, Adam MA, Peseckis SM. Tetrahedron Lett. 1983;24:1377–1380. [Google Scholar]; (l) Suzuki T, Saimoto H, Tomioka H, Oshima K, Nozaki H. Tetrahedron Lett. 1982;23:3597–3600. [Google Scholar]
- 8.Kuang R, Ganguly AK, Chan TM, Pramanik BN, Blythin DJ, McPhail AT, Saksena AK. Tetrahedron Lett. 2000;41:9575–9579. [Google Scholar]
- 9.Gage JR, Evans DA. Org Synth. 1990;68:83–87. [Google Scholar]
- 10.Crimmins MT, King BW, Zuercher WJ, Choy AL. J Org Chem. 2000;65:8499–8509. doi: 10.1021/jo005535p. [DOI] [PubMed] [Google Scholar]
- 11.Bartlett PA, Tanzella DJ, Barstow JF. J Org Chem. 1982;47:3941–3945. [Google Scholar]
- 12.For leading references for the antiviral and cytotoxicity procedures used Rajappan VP, Schneller SW, Williams SL, Kern ER. Bioorg Med Chem. 2002;10:883–886. doi: 10.1016/s0968-0896(01)00344-3.Siddiqi SM, Chen X, Schneller SW, Ikeda S, Snoeck R, Andrei G, Balzarini J, De Clercq E. J Med Chem. 1994;37:551–554. doi: 10.1021/jm00030a014.Chu CK, Jin YH, Baker RO, Huggins J. Bioorg Med Chem Lett. 2003;13:9–12. doi: 10.1016/S0960-894X(02)00841-7. [August 27, 2007]; http://www.usu.edu/iar/Brochure/brochure.html.
- 13.Wolfe MS, Lee Y, Bartlett WJ, Borcherding DR, Borchardt RT. J Med Chem. 1992;35:1782–1791. doi: 10.1021/jm00088a013. [DOI] [PubMed] [Google Scholar]
- 14.(a) Secrist JA, III, Shortnacy AT, Montgomery JA. J Med Chem. 1985;28:1740–1742. doi: 10.1021/jm00149a033. [DOI] [PubMed] [Google Scholar]; (b) Dye FJ, Rossomando EF. Biosci Rept. 1982;2:229–234. doi: 10.1007/BF01136721. [DOI] [PubMed] [Google Scholar]