Abstract
Objective
Having demonstrated that prior history of prolapse was a risk factor for pelvic floor repair procedures after hysterectomy, the objective of this study was to assess medical risk factors for pelvic floor repair after hysterectomy.
Methods
Using the Rochester Epidemiology Project database of 8,220 Olmsted County, Minnesota women who had hysterectomy for benign indications in 1965-2002, we conducted a nested case-control study in 144 pairs, comparing women who underwent pelvic floor repair after hysterectomy (cases) to controls matched for known risk factors (ie, age, pelvic floor disorders at baseline, year and type of hysterectomy, and pelvic floor repair during hysterectomy).
Results
The median duration between hysterectomy and pelvic floor repair was 13 years. Chronic pulmonary disease (odds ratio [OR] 14.3; 95% CI 1.2 to 178) but not obstetric history, obesity, indication for hysterectomy, or chronic constipation was associated with an increased risk of pelvic floor repair after hysterectomy. Between the hysterectomy and subsequent pelvic floor repair, overall pelvic organ prolapse severity changed by 1 grade or less in 54 cases (38%, Group A) but increased by 2 or more grades in 72 cases (50%, Group B). In Group A, but not Group B, uterine prolapse (OR 25; 95% CI 2.1 to 300) and chronic pulmonary disease (OR 22; 95% CI 1.5 to 328) at baseline remained risk factors for pelvic floor repair after hysterectomy.
Conclusion
In this matched case-control study, chronic pulmonary disease was the only risk factor for pelvic floor repair after hysterectomy for benign indications, underscoring the need to address pulmonary status prior to surgery.
Introduction
Pelvic organ prolapse is a common problem that, when severe, is often managed surgically, and over 300,000 such procedures are performed annually in the United States.(1) A substantial proportion of women who undergo surgery for prolapse, up to 60% in one study,(2) have previously had pelvic surgery, i.e., either a pelvic floor repair procedure with or without hysterectomy (29%), a hysterectomy not for prolapse (24%), or a hysterectomy for an unknown indication (7%). Indeed, it has been suggested that hysterectomy may predispose to pelvic organ prolapse. Among women who had a hysterectomy, the risk of pelvic floor repair surgery increases over time, approaching 5% at 15 years in the Oxford Family Planning Study and 5% at 30 years in Olmsted County, Minnesota.(3, 4) However, rather than hysterectomy per se, it is the underlying indication for surgery (i.e., prolapse) which increases the risk for a future pelvic floor repair, particularly when the initial procedure is an isolated hysterectomy (i.e., not combined with pelvic floor repair).(3-5)
Studies suggest that advancing age, parity, a history of vaginal delivery, and sexual activity are risk factors for pelvic floor repair surgery.(5, 6) It has also been observed that women undergoing pelvic floor repair procedures are frequently overweight and smokers; nearly one-fifth had chronic pulmonary disease in one study.(2) However, there is either mixed or insufficient evidence for how these and other factors [e.g., constipation, hormonal disturbances (e.g., menopause), and other obstetric risk factors for pelvic floor injury (e.g., forceps deliveries)] may increase the risk of pelvic prolapse and pelvic floor repair surgery.(6, 7) Perhaps these conditions or events did not affect the risk of pelvic floor repair in previous studies because they were overshadowed by other risk factors (e.g., age, type of surgery, and uterine prolapse as the surgical indication). To overcome this limitation, we conducted a nested case-control study of the risk factors for pelvic floor repair after hysterectomy by matching cases and controls for the strongest known risk factors for pelvic floor repair after hysterectomy, i.e., age and pelvic floor disorders at baseline. Our hypotheses were that medical conditions (e.g., obesity, chronic pulmonary disease), obstetric risk factors for pelvic floor injury, and menopause increase the risk of pelvic floor repair after hysterectomy. This study was performed within a population-based cohort of 8,220 women undergoing hysterectomy. A complete understanding of the risk factors contributing to pelvic floor repair is necessary to reduce utilization of these procedures in the future.
Materials and Methods
Population
The study builds upon large population-based cohort studies on the epidemiology of hysterectomy and pelvic floor repair in Olmsted County.(4, 8) We utilized the resources of the Rochester Epidemiology Project, which links the records of the medical providers who serve Olmsted County, i.e., Mayo Medical Center (Mayo Clinic and its affiliated hospitals [Rochester Methodist and St. Marys]) and Olmsted Medical Center (Olmsted Medical Group and its affiliated Olmsted Community Hospital). Because medical care is virtually self-contained within this community and these institutions provide nearly all of the primary, secondary, and tertiary care for this population, the result is a unique resource for population-based epidemiologic research.(9) The linked inpatient and outpatient data are easily retrieved because the diagnoses and surgical procedures entered into these records are indexed and can be retrieved electronically.
Selection of Cases and Controls
Following approval by the Institutional Review Boards of the Mayo Clinic and Olmsted Medical Center, this database was utilized to identify all Olmsted County women who underwent hysterectomy, either as a single procedure or in combination with pelvic floor repair procedures, between January 1, 1965 and December 31, 2002.(8) Subsequently, these 8,220 women were followed forward in time for the occurrence of any pelvic floor repair procedure, emigration from the community or death through June 2006.(4) The present nested case-control study assessed risk factors in women who had a subsequent pelvic floor repair (“cases”) and a 1:1 matched sample of women who did not have a pelvic floor repair after hysterectomy (“controls”). Cases and controls were matched for age (± 5 years), year of initial registration (± 5 years), year of hysterectomy (± 10 years), and for features associated with an increased risk for pelvic floor repair in this cohort (i.e., pelvic floor disorders at baseline (characterized as present or absent), type of hysterectomy (i.e., vaginal or abdominal), and concomitant pelvic floor repair at baseline.(4) For the purpose of matching, pelvic floor disorders were considered present if the post-operative diagnoses following hysterectomy listed any of the following conditions: cystocele, urethrocele, uterovaginal prolapse, enterocele, stress incontinence, pelvic floor relaxation, or pelvic pressure based on surgical index coding. However, it was not feasible to match individual cases and controls for each of these conditions, or for their severity. Stress incontinence was included in this category since a specific type of pelvic floor repair (i.e., anterior colporrhaphy with Kelly bladder neck plication) was traditionally used to correct both relaxation of the anterior vaginal wall as well as mild to moderate degrees of stress urinary incontinence.
Two hundred and six cases and an equal number of controls from the original cohort were identified. We excluded one case who retracted authorization to her medical records for research purposes and her corresponding control. The procedure type and indications were identified electronically in the 205 remaining cases (4, 8) and confirmed by medical record review. Thereafter, 61 additional cases and their corresponding controls were excluded because they had a hysterectomy for malignancy (n=1), a pelvic floor repair prior to hysterectomy (n=3), incomplete medical records with no details of hysterectomy (n=1), procedures incorrectly coded as pelvic floor repairs (n=10) or a procedure for stress urinary incontinence only (i.e., retropubic colposuspension only, n=46).
Risk Factor Assessment
The complete inpatient and outpatient medical records for the remaining 144 cases and their 144 matched controls were reviewed by the same investigator (REB). Information was abstracted for medical conditions of interest [i.e., hormonal status, chronic pulmonary disease (i.e., bronchial asthma, bronchiectasis, chronic obstructive pulmonary disease [COPD], and interstitial lung disease), chronic constipation, obesity, coronary artery disease, hypertension, diabetes mellitus], smoking status, pertinent medications (hormone replacement therapy, chronic steroid use), history of abdominal wall hernia repairs, obstetric history (i.e., forceps delivery), and characteristics of the hysterectomy (i.e., post-operative diagnosis and adjunct procedures). With the exception of obstetric history, this information was recorded in the standardized medical evaluation, which is mandatory prior to any procedure that requires general anesthesia. Surgeons used the Baden-Walker grading system to characterize the severity of prolapse in each pelvic compartment.(10) In addition, the maximum defect in any compartment was used to assign an overall grade for prolapse.(11) For pelvic floor repair procedures after hysterectomy, the types and indications were documented.
Statistical Analysis
The marginal distributions for all clinical variables were summarized separately in cases and in controls. Univariable associations between risk factors and case-control status were evaluated by conditional logistic regression models. Although cases and controls were matched by age and year of registration, these variables were included as covariates in these models since matching was not precise. The year of registration was used to ensure a similar duration of medical history was available for review. A multivariable model was used to identify independent risk factors.
The risk factors for pelvic floor repair after hysterectomy may differ depending on the progression of pelvic organ prolapse between the hysterectomy and pelvic floor repair procedure. Among women in whom there was little or no change in pelvic organ prolapse severity over time (i.e., between the hysterectomy and pelvic floor repair procedure), we postulated that pelvic organ prolapse severity at hysterectomy would predict the need for a subsequent pelvic floor repair procedure. Conversely, among women in whom pelvic organ prolapse severity increased over time, we postulated that other putative risk factors (e.g., chronic pulmonary disease) for pelvic organ prolapse would predict the need for a subsequent pelvic floor repair procedure. Thus, in addition to the overall analysis comparing cases and controls, separate but similar models evaluated the risk factors in two subsets of cases versus their matched controls. In these 2 subsets, cases were defined by progression or lack thereof in overall pelvic organ prolapse severity between the hysterectomy and the pelvic floor repair procedure. In group A, cases were defined by little or no change (i.e., increased or decreased by ≤1 grade) in overall pelvic organ prolapse between the hysterectomy and pelvic floor repair procedure. In Group B, severity increased two grades or more.(10) The process of matching cases and controls for these analyses was identical to the overall analysis. Unconditional logistic regression models were used to identify factors associated with progression among the cases. The odd ratios (OR) and 95% confidence intervals (CI) were calculated using the model estimated coefficients and their standard errors for variables in which stable estimates were obtained. For variables in which the risk factor models did not converge, an analysis of the discordant pairs was examined (McNemar's Test). The number of cases available was limited by eligibility criteria. Nonetheless, with 144 cases and 144 controls, we had adequate power (approximately 70% or greater) to detect an odds ratio of 2.0 (or greater) assuming the exposure probability in controls (proportion of controls with the risk factors) were 30-50%, and the exposure rate correlations between matched cases and controls induced by the matching were modest (e.g., less than 0.25). The Statistical Analysis System (SAS Institute, Inc., Cary, NC) was used for all analyses, and a two-sided level of 0.05 was deemed significant.
Results
Because of matching, the mean age of cases (45.7 years) and controls (45.4 years) was nearly identical. (Table 1) The mean BMI was also similar in cases (24.9 kg/m2) and controls (25.8 kg/m2). Thirty-three cases (23%) and 34 controls (24%) were overweight while 18 cases (13%) and 25 controls (17%) were obese. Approximately 20% of cases and controls were postmenopausal at baseline.
Table 1. Clinical characteristics at hysterectomy among Olmsted County, Minnesota women.
| Clinical characteristics | Cases (n=144) | Controls (n=144) | OR (95% CI)* |
|---|---|---|---|
| Age†, mean +/- std | 45.7 ± 12.2 | 45.4 ± 11.5 | 1.0 (1.0,1.1) |
| Height (cm), mean +/- std | 162.2 ± 7.6 | 161.8 ± 5.9 | 1.0 (1.0,1.0) |
| Weight (kg), mean +/- std | 65.8 ± 12.1 | 67.6 ± 14.7 | 1.0 (1.0,1.0) |
| BMI, mean +/- std | 24.9 ± 4.3 | 25.8 ± 5.5 | 1.0 (0.9,1.0) |
| Menopause | 29 (20.1%) | 27 (18.8%) | 1.2 (0.3, 4.0) |
| Current smoker | 19 (13.2%) | 34 (23.6%) | 0.5 (0.3,0.9) |
| Diabetes | 4 (2.8%) | 1 (0.7%) | 4.0 (0.4,36) |
| Coronary artery disease | 2 (1.4%) | 2 (1.4%) | 1.0 (0.1,7.0) |
| Hypertension | 17 (11.8%) | 14 (9.7%) | 1.3 (0.5,3.1) |
| Chronic pulmonary disease‡ | 14 (9.7%) | 5 (3.5%) | 3.5 (1.1,11) |
| Constipation | 8 (5.6%) | 5 (3.5%) | 2.5 (0.6,13) |
| Prior hernia surgery | 7 (4.9%) | 0 | not estimable |
| Chronic steroid use | 3 (2.1%) | 0 | not estimable |
| HRT§ | 9 (6.2%) | 1 (0.7%) | not estimable |
Odds ratio (OR) adjusted for age at time of hysterectomy and year of first registration.
Cases and controls matched on this variable.
Includes asthma, bronchiectasis, chronic obstructive pulmonary disease, and interstitial lung disease.
HRT – hormone replacement therapy.
Case-Control Analysis in Overall Sample
With the exception of smoking history (OR 0.5; 95% CI 0.3 to 0.9) and chronic pulmonary disease (OR 3.5; 95% CI 1.1 to 11), medical conditions (Table 1), obstetric history (Table 2), indications for hysterectomy (Table 3), and concomitant surgical procedures (Table 4) were not associated with pelvic floor repair after hysterectomy by univariable analysis. Only chronic pulmonary disease was associated with an increased risk for subsequent pelvic floor repair in the multivariable analysis (OR 14.3; 95% CI 1.2 to 178) (Table 5). Only 53 women (19 cases and 34 controls) were current smokers at the time of hysterectomy. The inverse association between smoking and subsequent pelvic floor repair observed in the univariable analysis was not seen in multivariable analysis (OR 0.6; 95% CI 0.2 to 2.0 for < 20 pack years; OR 0.2; 95% CI 0.03 to 1.5 for ≥ 20 pack years).
Table 2. Obstetric history in Olmsted County, Minnesota women who did (cases) or did not (controls) undergo subsequent pelvic floor repair following hysterectomy.
| Obstetric history | Cases (n=144) | Controls (n=144) | OR (95% CI) † |
|---|---|---|---|
| Gravity | 3.5 ± 2.2 | 3.6 ± 2.3 | 1.0 (0.9,1.1) |
| Parity | 3.2 ± 1.8 | 3.2 ± 1.8 | 1.0 (0.9,1.2) |
| No. of vaginal deliveries | 3.2 ± 1.8 | 3.1 ± 1.9 | 1.0 (0.9,1.1) |
| Forceps deliveries, n (%) | 45 (31.2%) | 37 (25.7%) | 1.1 (0.5,2.6) |
| Cesarean deliveries, n (%) | 7 (4.9%) | 7 (4.9%) | 1.3 (0.4,4.1) |
| Largest infant weight (gm) | 3726 ± 578 | 3704 ± 461 | 1.0 (1.0,1.0) |
Unless stated otherwise, all values are Means ± SD.
Odds ratio (OR) adjusted for age at time of hysterectomy and year of first registration.
Table 3. Indications for hysterectomy among Olmsted County, Minnesota women who did (cases) or did not (controls) undergo subsequent pelvic floor repair.
| Diagnosis* | Cases (n=144) | Controls (n=144) | OR (95% CI) † |
|---|---|---|---|
| Prolapse (includes uterine prolapse, cystocele, rectocele) ‡ | 72 (50.0%) | 74 (51.4%) | 0.5 (0.1,2.2) |
| Uterine prolapse | 62 (43.1%) | 54 (37.5%) | 1.9 (0.8, 4.5) |
| Cystocele | 59 (41.0%) | 54 (37.5%) | 1.7 (0.7, 4.4) |
| Rectocele | 56 (38.9%) | 52 (36.1%) | 1.9 (0.6, 6.0) |
| Menstrual disorders | 45 (31.2%) | 57 (39.6%) | 0.7 (0.4,1.1) |
| Urinary incontinence | 38 (26.4%) | 36 (25.0%) | 1.2 (0.6,2.3) |
| Uterine fibroids | 29 (20.1%) | 36 (25.0%) | 0.7 (0.4,1.4) |
| Endometriosis or adenomyosis | 21 (14.6%) | 25 (17.4%) | 0.8 (0.4,1.5) |
| Pelvic pain | 19 (13.2%) | 12 (8.3%) | 1.9 (0.8,4.7) |
| Adnexal mass | 18 (12.5%) | 8 (5.6%) | 2.4 (1.0,5.8) |
| Premalignant conditions | 5 (3.5%) | 5 (3.5%) | 1.0 (0.3,3.5) |
| Menopausal disorders | 3 (2.1%) | 5 (3.5%) | 0.6 (0.2,2.7) |
| Inflammatory diseases | 3 (2.1%) | 1 (0.7%) | not estimable |
| Other§ | 12 (8.3%) | 15 (10.4%) | 0.7 (0.3,1.6) |
Based on the final post-operative diagnosis.
Odds ratio (OR) adjusted for age at time of hysterectomy and year of first registration.
Cases and controls matched on this variable.
Other category includes congenital uterine anomaly, abdominal or inguinal hernia, multiparity desiring sterilization, chronic anemia, and anal incontinence.
Table 4. Concomitant surgical procedures during hysterectomy among Olmsted County, Minnesota women who did (cases) or did not (controls) undergo subsequent pelvic floor repair.
| Procedures | Cases (n=144) | Controls (n=144) | OR (95% CI) * |
|---|---|---|---|
| Unilateral or bilateral oophorectomy | 60 (41.7%) | 60 (41.7%) | 0.9 (0.5,1.9) |
| Prophylactic culdoplasty | 115 (79.9%) | 122 (84.7%) | 0.7 (0.4,1.3) |
| Anterior compartment repair | 57 (39.6%) | 55 (38.2%) | 1.3 (0.5,3.0) |
| Posterior compartment repair | 56 ((38.9%) | 51 (35.4%) | 2.3 (0.7,7.4) |
| Apical repair | 20 (13.9%) | 17 (11.8%) | 1.2 (0.6,2.6) |
Odds ratio (OR) adjusted for age at time of hysterectomy and year of first registration.
Table 5. Multivariable model of clinical risk factors for pelvic floor repair after hysterectomy among Olmsted County, Minnesota women*.
| Risk Factor Assessment at Hysterectomy | OR (95% CI) † | p-value |
|---|---|---|
| Age | 1.0 (1.0,1.1) | 0.39 |
| Year of registration | 1.0 (0.8,1.3) | 0.75 |
| BMI at time of hysterectomy | 0.9 (0.8,1.0) | 0.06 |
| Chronic pulmonary disease‡ | 14 (1.2,178) | 0.04 |
| Current smoker, < 20 pack years | 0.6 (0.2,2.0) | 0.40 |
| Current smoker, >= 20 pack years | 0.2(0.0,1.5) | 0.12 |
| No. of vaginal deliveries | 0.8 (0.7,1.1) | 0.12 |
| Endometriosis or adenomyosis | 0.7(0.2,2.9) | 0.67 |
| Inflammatory disease or pelvic pain or adnexal mass | 3.5 (0.6,19) | 0.15 |
| Uterine prolapse | 2.6 (0.4,20) | 0.35 |
| Cystocele | 3.7 (0.3,41) | 0.28 |
| Rectocele | 14 (0.6,376) | 0.11 |
| Unilateral or bilateral oophorectomy | 0.8 (0.2, 3.2) | 0.69 |
| Other diagnoses | 0.3 (0.1, 1.8) | 0.19 |
This model based on 74 pairs with complete data (n=148).
Odds ratio (OR) adjusted for age at time of hysterectomy and year of first registration.
Includes asthma, bronchiectasis, chronic obstructive pulmonary disease, and interstitial lung disease.
Uterine prolapse, menstrual symptoms, urinary incontinence or fibroids were the commonest indications for the baseline hysterectomy in cases and in controls (Table 3). The adequacy of our matching procedure is demonstrated by a similar prevalence of any pelvic organ prolapse in cases (50%) and controls (51%). The location and severity of prolapse, other indications (Table 3) and concomitant surgical procedures at the time of hysterectomy (Table 4) were not matched between cases and controls. Sixty-two cases (43%) had uterine prolapse compared to 54 (37%) controls (OR 1.9; 95% CI 0.8 to 4.5). Fifty-nine cases (41%) and 54 (37%) controls (OR 1.7; 95% CI 0.7 to 4.4) had a cystocele, while 56 (39%) cases and 52 (36%) controls had a rectocele (OR 1.9; 95% CI 0.6 to 6.0). During the hysterectomy, 64 of 144 cases (45%) and 55 of 144 (38%) controls also had a pelvic floor repair procedure. A prophylactic culdoplasty was the most frequently performed adjunct procedure (Table 4). Approximately 40% of subjects each had an oophorectomy, an anterior compartment repair, or a posterior compartment repair during a hysterectomy. These adjunct surgical procedures also were not associated with a subsequent pelvic floor repair in the univariable analysis.
Case-Control Comparison in Subsets defined by Change in Pelvic Organ Prolapse between Hysterectomy and Pelvic Floor Repair
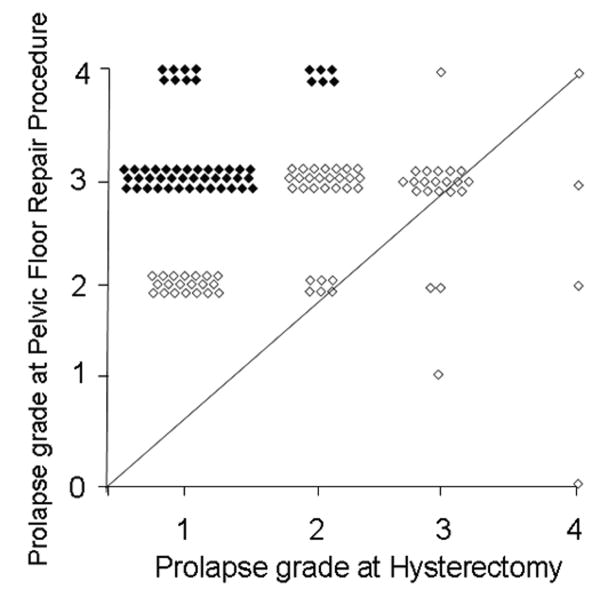
The median time interval between hysterectomy and pelvic floor repair surgery was 13 years (range, 9 months to 36 years) in the cases. A comparison of prolapse severity at hysterectomy and during a subsequent pelvic floor repair procedure indicates that the overall severity of prolapse was essentially unchanged (i.e., increased or decreased by 1 grade or less) in 54 cases (38%, Group A); in 72 of 144 cases (50%, Group B), severity increased by 2 or more grades (Figure 1). In the remaining 18 cases, the change in prolapse severity could not be ascertained from the records. The time interval from hysterectomy to pelvic floor repair was similar (i.e., median, 13.6 years) in Groups A and B. Uterine prolapse (OR 25; 95% CI 2.1 to 300) and chronic pulmonary disease (OR 22; 95% CI 1.5 to 328) at the time of hysterectomy were risk factors for a pelvic floor repair after hysterectomy in Group A. (Table 6). In contrast to Group A, no factors were significantly associated with pelvic floor repair after hysterectomy in Group B (Table 7).
Figure 1. Comparison of pelvic organ prolapse severity in cases at hysterectomy and pelvic floor repair surgery.
Observe that two-thirds of cases were to the left of the line of equality indicating more severe pelvic organ prolapse at pelvic floor repair surgery than at hysterectomy. Cases in Groups A and B are identified by open and filled symbols, respectively.
Table 6. Multivariable model of risk factors for PFR after hysterectomy in Olmsted County, Minnesota women who had minimal or no change in overall pelvic organ prolapse severity between the two procedures (Group A)*.
| Risk Factor Assessment at Hysterectomy | Cases (n = 43) | Controls (n =43) | OR (95% CI) † | p-value |
|---|---|---|---|---|
| Age, Mean ± SD | 49.9 ± 13.3 | 50.2 ± 12.8 | 1.1 (0.7,1.7) | 0.78 |
| BMI, Mean ± SD | 26.0 ± 4.3 | 25.7 ± 4.3 | 0.9 (0.8,1.1) | 0.22 |
| Chronic pulmonary disease‡ | 9 (20.9%) | 1 (2.3%) | 22 (1.5,328) | 0.02 |
| Uterine prolapse | 38 (88.4%) | 27 (62.8%) | 25 (2.1,300) | 0.01 |
| Cystocele | 37 (86.1%) | 33 (76.7%) | 1.6 (0.3,8.7) | 0.60 |
This model based on 43 pairs (n=86) with complete data out of 54 pairs in Group A.
Odds ratio (OR) adjusted for age at time of hysterectomy.
Includes asthma, bronchiectasis, chronic obstructive pulmonary disease, and interstitial lung disease.
Table 7. Multivariable model of risk factors for PFR after hysterectomy in Olmsted County, Minnesota women who had a substantial increase in overall pelvic organ prolapse severity between the two procedures (Group B)*.
| Risk Factor Assessment at Hysterectomy | Cases (n = 46) | Controls (n = 46) | OR (95% CI) † | p-value |
|---|---|---|---|---|
| Age, Mean ± SD | 44.8 ± 9.3 | 44.1 ± 9.3 | 1.0 (0.9, 1.1) | 0.73 |
| BMI, Mean ± SD | 24.2 ± 4.4 | 26.4 ± 6.5 | 1.0 (0.9, 1.0) | 0.26 |
| Uterine fibroids | 14 (30.4%) | 16 (34.9%) | 1.1 (0.3, 4.2) | 0.85 |
| Endometriosis or adenomyosis | 6 (13.0%) | 10 (21.7%) | 0.7 (0.2, 2.0) | 0.44 |
| Inflammatory disease or pelvic pain disorder or adnexal mass | 21 (45.6%) | 13 (28.3%) | 2.5 (0.6, 9.6) | 0.20 |
| Other diagnosis | 6 (13.0%) | 7 (15.2%) | 0.9 (0.2, 3.0) | 0.80 |
This model based on 46 pairs (n=92) with complete data out of 72 pairs in Group B.
Odds ratio (OR) adjusted for age at time of hysterectomy.
Comment
The cumulative risk of surgically treated prolapse after hysterectomy increases linearly over time, reaching up to 5% after 30 years.(4) Uterine prolapse at hysterectomy, advancing age, parity, and a history of vaginal delivery are known risk factors for pelvic floor repair after hysterectomy.(3-5) However, previous studies have not ascertained the role of other factors, particularly medical conditions such as obesity, constipation, and chronic pulmonary disease. The impact of these risk factors in the development of pelvic floor repair after hysterectomy is unclear. Appropriate identification of these potential risk factors has important clinical implications that may impact the counseling of thousands of women requiring hysterectomies every year. Therefore, this study employed a nested case-control design and matched for known risk factors (i.e., presence or absence of prolapse, type of hysterectomy, and need for concomitant pelvic floor repair during hysterectomy) in order to reduce the likelihood that known risk factors would overshadow the contribution of other poorly understood risk factors for pelvic floor repair after hysterectomy.
Chronic pulmonary disease was associated with an increased risk for future pelvic floor repair after a hysterectomy in this study. This observation reinforces the need to manage chronic pulmonary disease in women undergoing hysterectomy. Chronic coughing may induce repetitive increases in intra-abdominal pressure, which may weaken the pelvic floor muscles, causing pelvic prolapse.(12) Alternatively, or in addition, this association may also be explained by generalized skeletal muscle dysfunction and obesity, which are also associated with chronic pulmonary disease.(13, 14) However, chronic pulmonary disease remained a risk factor for pelvic floor repair even after correcting for body mass index (BMI) in the multivariable analysis. In contrast, other diseases (i.e., chronic constipation and diabetes mellitus) were not associated with the need for pelvic floor repair after hysterectomy. Although smoking was associated with a lower risk of pelvic floor repair in the univariable analysis, this protective effect disappeared in the multivariable analysis after adjusting for chronic pulmonary disease. The protective effect of smoking was also observed by Bradley et al, who concluded that the known inverse association between smoking and BMI could explain this finding, with smoking being a proxy for lower BMI rather than having a true protective effect.
Our observations also shed light on the long-term natural history of pelvic organ prolapse in women who have had a hysterectomy. Consistent with previous studies (15, 16) and contrary to traditional concepts, these observations demonstrate that it is not inevitable that pelvic organ prolapse will progress over time. Indeed, among women with an intact uterus, pelvic organ prolapse may even improve over time.(16, 17) In this study, the severity of prolapse increased substantially in two-thirds of women over a median 13 years after hysterectomy. Similar to the overall group, chronic pulmonary disease remained a risk factor for pelvic floor repair in Group A (i.e., small or no change in prolapse severity between hysterectomy and prolapse) but not in Group B, suggesting that the effects of chronic lung disease may be influenced by the overall prolapse severity. Uterine prolapse at the time of hysterectomy was also a significant risk factor in Group A, which is consistent with previous studies,(3-5) but not in Group B. Because overall prolapse severity increased substantially only in Group B, it is logical that baseline prolapse (i.e., at hysterectomy) predicted pelvic floor repair after hysterectomy in Group A but not in Group B.
Parity and one or more vaginal deliveries are risk factors for pelvic organ prolapse and surgery for pelvic organ prolapse after hysterectomy.(3, 18-20) In contrast, neither parity nor other putative obstetric risk factors (e.g., history of forceps deliveries) were associated with pelvic floor repair procedure after hysterectomy in our study, perhaps because cases and controls were matched for any pelvic floor disorder at baseline, hysterectomy type, and need for concomitant pelvic floor repair during hysterectomy. While obesity was a risk factor for pelvic organ prolapse in two large cross sectional studies,(20, 21) it was not associated with pelvic organ prolapse or with surgery for prolapse in this and other studies.(19, 22, 23)
Using the extensive database of the Rochester Epidemiology Project, we could identify a history of these conditions in cases and controls using comparably documented medical records of long duration (median observation period of 36 years prior to pelvic floor repair). Moreover, this information was recorded in the medical records prior to any knowledge of whether women would have a pelvic floor repair after hysterectomy. These features help reduce the potential for selection and measurement biases often associated with hospital-based, case-control studies. Institutional guidelines required that the medical conditions of interest herein be documented in the mandatory medical evaluation prior to any surgery. There are, however, corresponding limitations. These relate to our reliance on retrospective review of medical records written by diverse physicians over a long period of time, inability to ascertain the status of all risk factors in all subjects, and inability to evaluate the risk factors for pelvic floor repair after hysterectomy in minority populations due to the racial composition of the community (96% white in 1990). Although out-migration rates for this population are low, it is conceivable that some patients who had a hysterectomy in Olmsted County subsequently had pelvic floor repairs at another medical facility. Matching each case by 2 rather than 1 control would have provided only slightly better statistical power, but would have been more cumbersome, since we matched for several variables. In addition, further studies are necessary to ascertain whether other factors, including occupational history (e.g., heavy lifting), influence the risk of pelvic floor repair after hysterectomy. Nonetheless, the identification of modifiable risk factors at the time of hysterectomy, especially for women undergoing hysterectomy for prolapse, should prompt targeted therapies (e.g., aggressive management of chronic lung disease, weight loss, and smoking cessation).
Acknowledgments
Supported in part by research grants DK 78924 and AR 30582 from the National Institutes of Health, U.S. Public Health Service.
Footnotes
Financial Disclosure: The authors did not report any potential conflicts of interest.
References
- 1.Shah AD, Kohli N, Rajan SS, Hoyte L. The age distribution, rates, and types of surgery for pelvic organ prolapse in the USA. Int Urogynecol J Pelvic Floor Dysfunct. 2008;19:421–8. doi: 10.1007/s00192-007-0457-y. [DOI] [PubMed] [Google Scholar]
- 2.Olsen AL, Smith VJ, Bergstrom JO, Colling JC, Clark AL. Epidemiology of surgically managed pelvic organ prolapse and urinary incontinence. Obstet Gynecol. 1997;89:501–6. doi: 10.1016/S0029-7844(97)00058-6. [DOI] [PubMed] [Google Scholar]
- 3.Mant J, Painter R, Vessey M. Epidemiology of genital prolapse: observations from the Oxford Family Planning Association Study. Br J Obstet Gynaecol. 1997;104:579–85. doi: 10.1111/j.1471-0528.1997.tb11536.x. [DOI] [PubMed] [Google Scholar]
- 4.Blandon RE, Bharucha AE, Melton LJ, 3rd, Schleck CD, Babalola EO, Zinsmeister AR, et al. Incidence of pelvic floor repair after hysterectomy: A population-based cohort study. Am J Obstet Gynecol. 2007;197:664 e1–7. doi: 10.1016/j.ajog.2007.08.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dallenbach P, Kaelin-Gambirasio I, Dubuisson JB, Boulvain M. Risk factors for pelvic organ prolapse repair after hysterectomy. Obstet Gynecol. 2007;110:625–32. doi: 10.1097/01.AOG.0000278567.37925.4e. [DOI] [PubMed] [Google Scholar]
- 6.Jelovsek JE, Maher C, Barber MD. Pelvic organ prolapse. Lancet. 2007;369:1027–38. doi: 10.1016/S0140-6736(07)60462-0. [DOI] [PubMed] [Google Scholar]
- 7.Schaffer JI, Wai CY, Boreham MK. Etiology of pelvic organ prolapse. Clin Obstet Gynecol. 2005;48:639–47. doi: 10.1097/01.grf.0000170428.45819.4e. [DOI] [PubMed] [Google Scholar]
- 8.Babalola EO, Bharucha AE, Schleck CD, Gebhart JB, Zinsmeister AR, Melton LJ., 3rd Decreasing utilization of hysterectomy: a population-based study in Olmsted County, Minnesota, 1965-2002. Am J Obstet Gynecol. 2007;196:214 e1–7. doi: 10.1016/j.ajog.2006.10.390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Melton LJ., 3rd History of the Rochester Epidemiology Project. Mayo Clin Proc. 1996;71:266–74. doi: 10.4065/71.3.266. [DOI] [PubMed] [Google Scholar]
- 10.Baden WF, Walker TA. Genesis of the vaginal profile: a correlated classification of vaginal relaxation. Clin Obstet Gynecol. 1972;15:1048–54. doi: 10.1097/00003081-197212000-00020. [DOI] [PubMed] [Google Scholar]
- 11.Baden WF, Walker T. Surgical repair of vaginal defects. Philadelphia (PA): Lippincott Williams & Wilkins; 1992. [Google Scholar]
- 12.Mouritsen L, Hulbaek M, Brostrom S, Bogstad J. Vaginal pressure during daily activities before and after vaginal repair. Int Urogynecol J Pelvic Floor Dysfunct. 2007;18:943–8. doi: 10.1007/s00192-006-0267-7. [DOI] [PubMed] [Google Scholar]
- 13.Ford ES, Mannino DM. Time trends in obesity among adults with asthma in the United States: findings from three national surveys. J Asthma. 2005;42:91–5. [PubMed] [Google Scholar]
- 14.Wagner PD. Skeletal muscles in chronic obstructive pulmonary disease: deconditioning, or myopathy? Respirology. 2006;11:681–6. doi: 10.1111/j.1440-1843.2006.00939.x. [DOI] [PubMed] [Google Scholar]
- 15.Bump RC, Norton PA. Epidemiology and natural history of pelvic floor dysfunction. Obstet Gynecol Clin North Am. 1998;25:723–46. doi: 10.1016/s0889-8545(05)70039-5. [DOI] [PubMed] [Google Scholar]
- 16.Bradley CS, Zimmerman MB, Qi Y, Nygaard IE. Natural history of pelvic organ prolapse in postmenopausal women. Obstet Gynecol. 2007;109:848–54. doi: 10.1097/01.AOG.0000255977.91296.5d. [DOI] [PubMed] [Google Scholar]
- 17.Handa VL, Garrett E, Hendrix S, Gold E, Robbins J. Progression and remission of pelvic organ prolapse: a longitudinal study of menopausal women. Am J Obstet Gynecol. 2004;190:27–32. doi: 10.1016/j.ajog.2003.07.017. [DOI] [PubMed] [Google Scholar]
- 18.Samuelsson EC, Arne Victor FT, Tibblin G, Svardsudd KF. Signs of genital prolapse in a Swedish population of women 20 to 59 years of age and possible related factors. Am J Obstet Gynecol. 1999;180:299–305. doi: 10.1016/s0002-9378(99)70203-6. [DOI] [PubMed] [Google Scholar]
- 19.Swift SE, Pound T, Dias JK. Case-control study of etiologic factors in the development of severe pelvic organ prolapse. Int Urogynecol J Pelvic Floor Dysfunct. 2001;12:187–92. doi: 10.1007/s001920170062. [DOI] [PubMed] [Google Scholar]
- 20.Hendrix SL, Clark A, Nygaard I, Aragaki A, Barnabei V, McTiernan A. Pelvic organ prolapse in the Women's Health Initiative: gravity and gravidity. Am J Obstet Gynecol. 2002;186:1160–6. doi: 10.1067/mob.2002.123819. [DOI] [PubMed] [Google Scholar]
- 21.Swift S, Woodman P, O'Boyle A, Kahn M, Valley M, Bland D, et al. Pelvic Organ Support Study (POSST): the distribution, clinical definition, and epidemiologic condition of pelvic organ support defects. Am J Obstet Gynecol. 2005;192:795–806. doi: 10.1016/j.ajog.2004.10.602. [DOI] [PubMed] [Google Scholar]
- 22.Clark AL, Gregory T, Smith VJ, Edwards R. Epidemiologic evaluation of reoperation for surgically treated pelvic organ prolapse and urinary incontinence. Am J Obstet Gynecol. 2003;189:1261–7. doi: 10.1067/s0002-9378(03)00829-9. [DOI] [PubMed] [Google Scholar]
- 23.Rortveit G, Brown JS, Thom DH, Van Den Eeden SK, Creasman JM, Subak LL. Symptomatic pelvic organ prolapse: prevalence and risk factors in a population-based, racially diverse cohort. Obstet Gynecol. 2007;109:1396–403. doi: 10.1097/01.AOG.0000263469.68106.90. [DOI] [PubMed] [Google Scholar]