Abstract
Factors such as age and race/ethnicity might influence blood pressure (BP) response to drugs. Therapeutic response to the angiotensin-converting enzyme inhibitor trandolapril used as add-on therapy to stable calcium channel blocker therapy with verapamil sustained release 240 mg was addressed in a racially/ethnically diverse group of 1,832 hypertensive patients with coronary artery disease. Furthermore, the association with a polymorphism (1166A→C) in the angiotensin II type 1 receptor gene (AGTR1) was tested. BP response was compared between groups using analysis of covariance after adjustment for covariates associated with BP response. Genotyping was performed using polymerase chain reaction and pyrosequencing. Trandolapril decreased mean unadjusted systolic and diastolic BPs by −9.1 ± 17.3 (SD) and −4.1 ± 10.1 mm Hg, respectively. The percentage of patients with BP under control (<140/90 mm Hg) increased from 6.7% to 41.3% (p <0.0001). Adjusted BP response was significantly associated with age and baseline systolic and diastolic BP (p <0.0001). Whereas the decrease in systolic BP was more pronounced in younger patients, the opposite was observed for diastolic BP decrease. Diastolic BP response was also significantly associated with race. Specifically, the adjusted diastolic BP decrease was significantly smaller in Hispanics and blacks than whites (p = 0.0032 and p = 0.0069, respectively). However, Hispanics achieved a decrease in systolic BP and an increase in BP control similar to the other ethnic groups. There was no genetic association between AGTR1 1166A→C genotype and BP response. In conclusion, trandolapril add-on therapy was effective in increasing BP control, with age and baseline BP associated with both systolic and diastolic BP response. Race was associated with diastolic BP response, although the difference is likely not to be clinically significant and AGTR1 genotype was not associated with BP response.
We assessed factors associated with the response to add-on therapy with the angiotensin-converting enzyme (ACE) inhibitor trandolapril, including race/ethnicity, angiotensin II type 1 receptor (AGTR1) genotype, age, and clinical factors in hypertensive patients with coronary artery disease. Patients were selected from the INternational VErapamil SR/Trandolapril STudy (INVEST), an ethnically diverse multicenter trial that included white and black patients with hypertension and 1 of the largest Hispanic patient populations reported.
Methods
The INVEST rationale, design, inclusion and exclusion criteria, treatment strategies, and main results were published in detail elsewhere. Briefly, after an extensive cardiovascular history and physical examination, patients were randomly assigned to either a verapamil sustained release (SR)–trandolapril– or atenolol-hydrochlorothiazide– based antihypertensive strategy. Patients were evaluated every 6 weeks for the first 6 months and then biannually for ≥2 years to assess blood pressure (BP) and adverse outcomes. The detailed BP measurement method was published previously. 1,2 The INVEST on-line data system provided detailed tracking of study medication prescriptions.3 From 1997 to 2003, follow-up of 61,835 patient-years was accumulated, and each strategy provided excellent BP control (>70% of patients achieved BP <140/90 mm Hg) without differences in BPs between strategies. The strategies were equally effective in preventing the composite primary outcome of all-cause death, nonfatal myocardial infarction, or nonfatal stroke.1
Genetic samples were collected as part of the GENEtic Substudy of INVEST (INVEST-GENES) of 5,979 subjects from 184 sites in mainland United States and Puerto Rico to elucidate the contribution of genetic variants to interpatient and interdrug variations in response and outcome during antihypertensive drug therapy.4 Both studies were approved by appropriate institutional review boards and, in the case of INVEST-GENES, by the University of Florida, which served as the central institutional review board for all participating sites in the genetic substudy. Written informed consent for participation in both the main INVEST and the genetic substudy was provided by each patient.
A total of 1,832 INVEST patients randomly assigned to the verapamil SR strategy who received monotherapy with verapamil SR 240 mg and had trandolapril (1, 2, or 4 mg) added to their treatment because of failing to meet BP goals were included in this analysis. Response to ACE-inhibitor addition was assessed by comparing BP readings from 2 visits, the first reading obtained at the visit that led to prescription of trandolapril and the second reading obtained at the next follow-up visit. In other words, BP change was assessed before and after the addition of trandolapril. Only patients with BP readings at these 2 visits were included in the final analysis. BP control is defined as achieving BP <140/90 mm Hg at the follow-up visit.
Determination of race/ethnicity was self-identified using patient report with interaction by the site investigator, choosing all that were applicable of the options on the INVEST data collection form of white, black, Hispanic, Asian, and “other.” Patients of mixed race/ethnicity could choose >1 category. Hispanic was used as a race rather than ethnicity term. Due to comparably small sample sizes, Asians and the multiracial group were defined as other for analysis. Of these 1,832 INVEST patients, 551 also consented to participate in INVEST-GENES, provided genetic samples, and were subsequently genotyped for AGTR1 1166A→C.
Genomic DNA was isolated from buccal genetic samples using commercially available kits (PureGene, Gentra Systems Inc, Minneapolis, Minnesota) and normalized to 20 ng/µl. Genotyping for the AGTR1 1166A→C polymorphism was performed using polymerase chain reaction (PCR) followed by pyrosequencing (PSQ; Pyrosequencing, Uppsala, Sweden). Primers used for PCR reaction and PSQ were 5′- CCCCTCAGATAATGTAAGC-3′ (PCR forward), 5′-Bio-GTCGGTTCAGTCCACATAATG-3′ (PCR reverse), and sequencing primer 5′-ACTTCACTACCAAATGAGC-3′. The PCR mixture (12.50 µl) consisted of 6.25 µl of HotStarTaq Master Mix Kit (Qiagen Inc, Valencia, California), 1 µl of PCR primers (10 pmol/µl), 1 µl of dimethyl sulfoxide, 1.25 µl of water, and 40 ng of DNA. PCR was performed under the conditions of 95°C for 15 minutes; 40 cycles consisting of denaturation at 95°C for 30 seconds, annealing at 54°C for 30 seconds, and extension at 72°C for 30 seconds; and final extension for 7 minutes. PSQ was performed under standard conditions for sequence determination and allele designation in a Biotage PSQ HS 96 System (Biotage AB, Uppsala, Sweden), and data were captured using PSQ HS 96 SNP software.
Analysis of covariance was used to compare BP response to ACE inhibitor addition adjusting for systolic or diastolic BP before ACE inhibitor addition and covariates found to be associated with BP response using a stepwise selection method. Age was considered a continuous variable with 1 degree of freedom. For presentation purpose, patients were also categorized into four 10-year age groups. BP control before and after trandolapril treatment was compared using McNemar’s test for matched pairs. The t test was used for continuous variables and chi-square test was used for categorical variables. A p value <0.05 was considered statistically significant. Hardy-Weinberg equilibrium was tested using chi-square test with 1 degree of freedom. Due to the small number of patients homozygous for the C allele, A/C and C/C genotypes were collapsed for further analysis.
Results
Detailed demographic and baseline characteristics for the main study population and subgroup of patients with genotype data are listed in Table 1. Separated according to decade, 544 patients were aged 50 to 59 years, 590 were 60 to 69 years, 502 were 70 to 79 years, and 196 were >80 years. Mean time from trandolapril addition to BP response assessment was 74.5 ± 89.1 days. Median duration of verapamil monotherapy before the addition of trandolapril was 51 days (interquartile range 42 to 97 days). Most whites (84.0%), Hispanics (90.5%), and others (88.1%) were treated with 2 mg of trandolapril, whereas in 85.2% of African-American patients, trandolapril was initiated at 4 mg, in addition to verapamil SR 240 mg/d, as suggested by the INVEST protocol.
Table 1.
Patient baseline characteristics
| INVEST (n = 1,832) |
INVEST GENES (n = 551) |
|
|---|---|---|
| Age, mean (yrs) | 66.3 ± 10.0 | 65.8 ± 9.8 |
| Women | 975 (53.2%) | 317 (57.5%) |
| Ethnicity | ||
| White | 792 (43.2%) | 193 (35.0%) |
| Black | 332 (18.1%) | 80 (14.5%) |
| Hispanic | 641 (35.0%) | 242 (43.9%) |
| Other | 67 (3.7%) | 36 (6.5%) |
| Body mass index (kg/m2) | 29.6 ± 5.7 | 29.7 ± 5.8 |
| Baseline Systolic BP (mm Hg) | 150.5 ± 17.6 | 151.3 ± 17.4 |
| Baseline diastolic BP (mm Hg) | 86.7 ± 10.9 | 87.5 ± 10.3 |
| Baseline heart rate (beats/min) | 75.8 ± 9.5 | 75.1 ± 9.4 |
| Myocardial infarction | 542 (29.6%) | 125 (22.7%) |
| Angina pectoris | 1,225 (66.9%) | 410 (74.4%) |
| Stroke/transient ischemic attack | 110 (6.0%) | 38 (6.9%) |
| Peripheral vascular disease | 207 (11.3%) | 56 (10.2%) |
| Smoker | ||
| Past | 790 (43.1%) | 208 (37.8%) |
| With in last 30 d | 235 (12.8%) | 58 (10.5%) |
| Diabetes mellitus* | 525 (28.6%) | 159 (28.9%) |
| Hypercholesterolemia† | 940 (51.3%) | 278 (50.5%) |
| Aspirin or other antiplatelet drug | 859 (46.9%) | 228 (41.4%) |
| Other nonsteroidal anti-inflammatory drugs |
315 (17.2%) | 125 (22.7%) |
| Antidiabetic medication | 448 (24.5%) | 132 (24.0%) |
| Any lipid-lowering drug | 685 (37.4%) | 205 (37.2%) |
INVEST-GENES patients represent a subgroup of INVEST patients who were genotyped for AGTR1 1166A→C. Data presented as number (percentage of population) or mean ± SD.
History of diabetes or antidiabetic medication use.
History of hypercholesterolemia or lipid-lowering medication use.
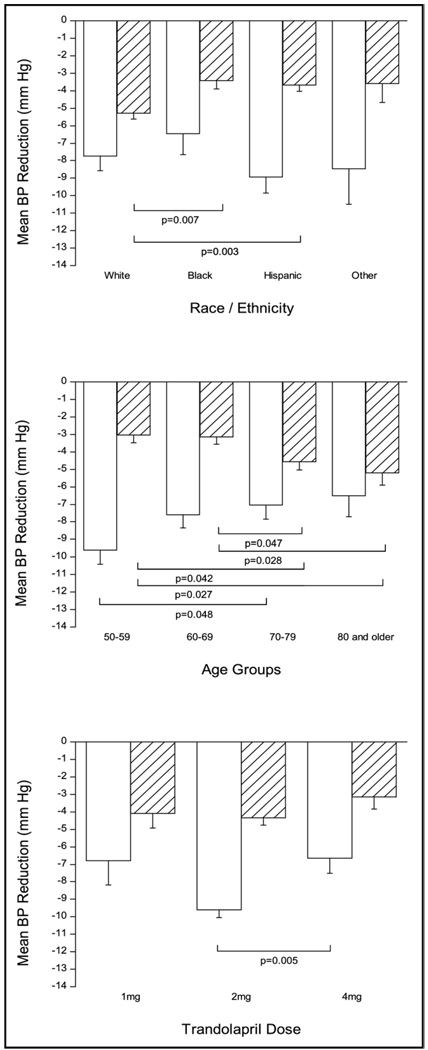
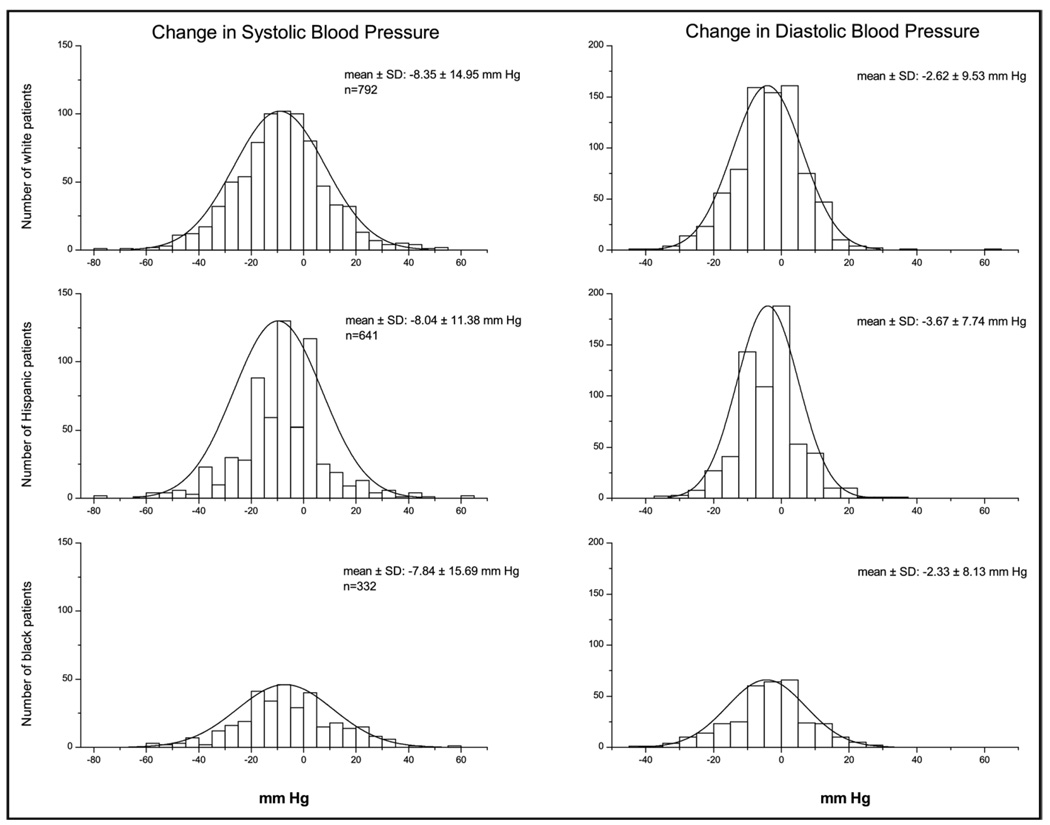
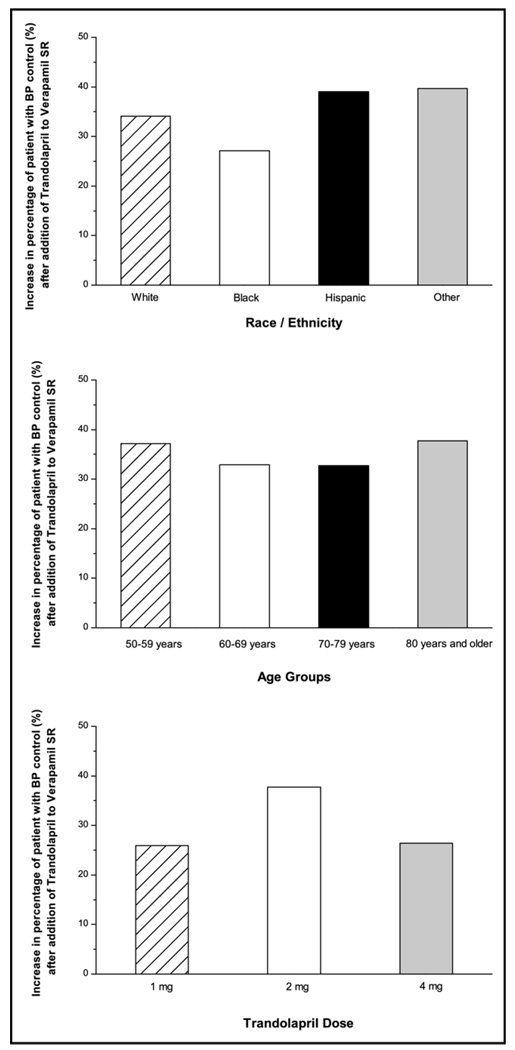
ACE inhibitor addition decreased mean unadjusted systolic and diastolic BPs by −9.1 ± 17.3 and −4.1 ± 10.1 mm Hg to mean unadjusted systolic and diastolic BPs of 139.7 ± 17.5 and 80.1 ± 10.3 mm Hg, respectively. After adjusting for covariates and multiple comparisons, no statistically significant differences were observed for systolic BP decreases in different races/ethnicities, whereas diastolic BP decrease was significantly smaller in Hispanics and blacks than whites (Figure 1). The adjusted systolic BP decrease was significantly greater in younger patients (50 to 59 years) compared with those aged 70 to 79 years (Figure 1). It is of interest to note that the effect of age is in opposite directions for systolic and diastolic BP, with younger patients having better systolic BP response and older patients having better diastolic BP response. Figure 2 shows ranges of systolic and diastolic BP responses after trandolapril addition in the different racial/ethnic groups and that both ranges and average responses are similar by race/ethnicity. It also highlights the wide range of responses around the mean. Overall, trandolapril therapy significantly increased the percentage of patients with BP under control from 6.7% before trandolapril addition to 41.3% after add-on therapy (p <0.0001). Figure 3 shows increases in percentages of patients with BP control according to race/ethnicity, age group, and trandolapril dose. There were no differences among these groups.
Figure 1.
Systolic (open bars) and diastolic (filled bars) BP decreases (mm Hg) after the addition of trandolapril to verapamil SR 240 mg monotherapy according to race/ethnicity (upper panel), age groups (middle panel), and trandolapril dose (lower panel) and adjusted for systolic and diastolic BP before trandolapril addition and other covariates found to be associated with BP response (age, gender, race, trandolapril dose, diabetes mellitus, and body mass index). Results presented as mean ± SE.
Figure 2.
Ranges of systolic (left column) and diastolic BP (right column) responses after the addition of trandolapril to verapamil SR 240 mg monotherapy in whites (upper row), Hispanics (middle row), and blacks (lower row). Histograms show changes in BP in equal-sized bins of 5 mm Hg for the respective number of patients. The superimposed curve shows the normal distribution of the data sets.
Figure 3.
Increase in BP control (<140/90 mm Hg) after the addition of trandolapril to verapamil SR 240 mg monotherapy according to race/ethnicity (upper panel), age groups (middle panel), and trandolapril dose (lower panel). Results given as percentage of the different subgroups.
In the cohort with DNA samples provided, minor allele frequencies for AGTR1 1166A→C were 0.30 for whites, 0.06 for blacks, 0.24 for Hispanics, and 0.28 for others. Genotype frequencies did not deviate from Hardy-Weinberg equilibrium. Adjusted mean systolic/diastolic BPs after the addition of trandolapril to verapamil SR monotherapy were 142.6 ± 1.3/82.0 ± 0.7 mm Hg for A/A homozygotes and 140.6 ± 1.4/81.4 ± 0.8 mm Hg for C-allele carriers (p = 0.17 and p = 0.48). The corresponding adjusted mean systolic BP decreases for A/A and C/C genotypes were −5.9 ± 1.3 and −7.9 ± 1.5 mm Hg (p = 0.17). Adjusted mean diastolic BP decreases were −2.9 ± 0.7 and −3.4 ± 0.8 mm Hg, respectively (p = 0.47). After adjusting for covariates, AGTR1 genotype was not significantly associated with either systolic or diastolic BP response (p = 0.17 and p = 0.48, respectively). In whites, the lowest adjusted systolic and diastolic BP values after trandolapril addition were seen in patients homozygous for the A allele (Table 2). In blacks and Hispanics, the opposite was observed (Table 2). Due to the small number of others, this patient group is not reported in the final analysis.
Table 2.
Adjusted mean systolic and diastolic blood pressure (BP) and systolic and diastolic BP decreases after the addition of trandolapril to verapamil sustained release monotherapy in different races/ethnicities according to AGTR1 1166 A→C genotype (n = 551)
| Adjusted Systolic/Diastolic BP (mm Hg) | |||
|---|---|---|---|
| A/A | C Allele Carriers | p Value (systolic/diastolic BP) |
|
| White | 139.9 ± 2.5/77.7 ± 1.4 | 141.0 ± 2.4/79.5 ± 1.4 | 0.63/0.12 |
| Black | 143.9 ± 6.3/82.8 ± 3.3 | 137.1 ± 7.8/80.1 ± 4.1 | 0.22/0.36 |
| Hispanic | 144.6 ± 2.7/85.3 ± 1.4 | 141.3 ± 3.0/83.9 ± 1.2 | 0.11/0.19 |
| Systolic/Diastolic BP Decrease (mm Hg) | |||
| A/A | C Allele Carriers | p Values (systolic/diastolic BP decrease) |
|
| White | −8.5 ± 2.5/−5.6 ± 1.4 | −7.4 ± 2.4/−3.7 ± 1.4 | 0.63/0.16 |
| Black | −7.0 ± 6.3/−3.1 ± 3.4 | −13.9 ± 7.8/−6.3 ± 4.2 | 0.22/0.29 |
| Hispanic | −3.3 ± 2.7/−0.5 ± 1.4 | −6.6 ± 3.0/−1.9 ± 1.5 | 0.11/0.12 |
C Allele carriers were combined in 1 group. p Values were adjusted for multiple comparisons. Results presented as mean ± SE.
Discussion
In the present study, we address the therapeutic response to an ACE inhibitor used as add-on therapy to a calcium channel blocker in a large ethnically diverse population. We show that diastolic BP response is modestly influenced by race/ethnicity and age, whereas race/ethnicity does not influence systolic BP response. AGTR1 1166 genotype also was not associated with BP response.
To our knowledge, these are the first data for the combination of trandolapril and verapamil SR in a Hispanic patient population with hypertension and coronary artery disease. These patients responded well to add-on therapy with 2 mg of trandolapril and achieved a decrease in systolic BP (Figure 1 and Figure 2) and an increase in BP control (Figure 3) similar to those of other racial/ethnic groups when analysis was adjusted for confounding factors. However, compared with whites, trandolapril addition led to a statistically smaller diastolic BP decrease, although this difference could be argued to be of limited clinical significance given the small absolute difference and that diastolic BP tends to be easier to control than systolic BP. That the diastolic BP response difference in Hispanics is of limited clinical significance is also supported because Hispanics had the numerically highest increase in percentage of BP control with trandolapril addition (39.0%) versus increases of 34.1% in whites and 27.1% in blacks. In a recent publication, the Hispanic cohort of INVEST also yielded better BP control compared with the non-Hispanic cohort after 24 months of treatment with a mean of 2.4 drugs required to achieve BP control.5 From the present analysis, one might conclude that a substantial proportion of BP control after 24 months can be attributed to trandolapril addition to verapamil SR, which justifies the administration of these drug classes in Hispanics. The Hispanic subpopulation of the present study was recruited from countries in North and Central America and the Caribbean, with a high percentage from Puerto Rico. Thus, our results may not be fully generalizable to Hispanics with other ancestry, which is emphasized by recent studies showing variable prevalences of hypertension among Hispanic patients from the United States, Mexico, and Europe.6
Although Hispanics have not been well represented in hypertension trials to date, there are many examples of differences between blacks and whites with respect to prevalence, pathophysiological characteristics, target-organ damage, and responses to hypertension treatment.7,8 For black patients with hypertension, for example, a decreased therapeutic response to ACE inhibitor treatment was reported,9 although more aggressive dosing compensated for the observed differences and led to similar BP decreases in blacks and whites.10 The present study is in line with these findings, showing no significant differences in systolic BP decreases and BP control in blacks compared with the other races/ethnicities when trandolapril was added to verapamil. However, black patients received a higher trandolapril dose (4 mg) according to the INVEST protocol,2 which increased the percentage of blacks with BP <140/90 mm Hg by approximately 30% (Figure 3). To further increase the percentage of patients with controlled BP, black patients are likely to require >2 drugs, shown in a previous subanalysis of INVEST that reported that 62.1% of black patients in the verapamil SR arm of the trial achieved BP control at 24 months, most of them with ≥3 antihypertensive drugs.11
Finally, to test whether genetic variants affect BP response to trandolapril addition, we assessed the contribution of the most frequently studied AGTR1 single-nucleotide polymorphism (rs5186), which shows an adenine (A) to cytosine (C) transversion (1166A→C). This was based on studies reporting associations between 1166A→C genotype, hypertension, and/or cardiovascular disease,12–15 although sometimes with conflicting results.16,17 Compared with other genetic studies that usually concentrated on white and black subjects/patients, the present study additionally provides genetic data for a comparatively large Hispanic population and shows that allele frequencies in Hispanics were similar to those reported for Caucasians, with the majority of patients homozygous for the wild-type A allele.13 Although we failed to show an association between AGTR1 1166A→C genotype and BP response, there were suggestions that the magnitude of BP decrease was influenced by genotype and race (Table 2). These findings are consistent with previous studies reporting higher systolic18 and diastolic BPs19 in white patients with the C/C genotype, but higher BPs in black carriers of the A allele.18 1166A→C single-nucleotide polymorphism is located in an untranslated region of the AGTR1 gene and might not be functional itself. The genotype-driven discordance of BP data in different populations suggests that 1166A→C might be in linkage disequilibrium with 1 or several mutations of functional importance, and the linkage disequilibrium might differ between whites, blacks, and Hispanics. Data from the International HapMap Project showed racial/ethnic differences in linkage disequilibrium structure for the AGTR1 gene.20 Furthermore, to overcome the limitations of candidate single-nucleotide polymorphism studies, race/ethnicity-specific linkage disequilibrium and haplotype patterns should be taken into account, for example, through a tag–single-nucleotide polymorphism approach.21 Ultimately, approaches that consider multiple genes together in a statistical model will be needed to help understand the role of genetics on variable antihypertensive response.22
Acknowledgments
This study was supported by Grants No. HL74730, HL68834, HL69758, and RR17568 from the National Institutes of Health, Bethesda, Maryland, and grants from Abbott Laboratories, Abbott Park, Illinois, and the University of Florida Opportunity Fund, Gainesville, Florida. Dr. Brunner was supported by the Erwin Schrödinger Fellowship Program of the Austrian Science Fund (J 2403-B11), Vienna, Austria. Mr. Karnes was supported by a grant from the American Heart Association (0515074B), St. Petersburg, Florida.
References
- 1.Pepine CJ, Handberg EM, Cooper-DeHoff RM, Marks RG, Kowey P, Messerli FH, Mancia G, Cangiano JL, Garcia-Barreto D, Keltai M, et al. for the INVEST Investigators. A calcium antagonist vs a non-calcium antagonist hypertension treatment strategy for patients with coronary artery disease. The International Verapamil-Trandolapril Study (INVEST): a randomized controlled trial. JAMA. 2003;290:2805–2816. doi: 10.1001/jama.290.21.2805. [DOI] [PubMed] [Google Scholar]
- 2.Pepine CJ, Handberg-Thurmond E, Marks RG, Conlon M, Cooper-DeHoff R, Volkers P, Zellig P. Rationale and design of the International Verapamil SR/Trandolapril Study (INVEST): an Internet-based randomized trial in coronary artery disease patients with hypertension. J Am Coll Cardiol. 1998;32:1228–1237. doi: 10.1016/s0735-1097(98)00423-9. [DOI] [PubMed] [Google Scholar]
- 3.Cooper-DeHoff R, Handberg E, Heissenberg C, Johnson K. Electronic prescribing via the Internet for a coronary artery disease and hypertension megatrial. Clin Cardiol. 2001;24 suppl:SV14–SV16. doi: 10.1002/clc.4960241706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Keltai M, Johnson JA, Kowey PR, Ried LD, Tueth M. INVEST substudies: design and patient characteristics. Clin Cardiol. 2001;24 suppl:SV9–SV11. doi: 10.1002/clc.4960241704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cooper-DeHoff RM, Aranda JM, Jr, Gaxiola E, Cangiano JL, Garcia-Barreto D, Conti CR, Hewkin A, Pepine CJ for the INVEST Investigators. Blood pressure control and cardiovascular outcomes in high-risk Hispanic patients—findings from the International Verapamil SR/Trandolapril Study (INVEST) Am Heart J. 2006;151:1079–1086. doi: 10.1016/j.ahj.2005.05.024. [DOI] [PubMed] [Google Scholar]
- 6.Lorenzo C, Serrano-Rios M, Martinez-Larrad MT, Gabriel R, Williams K, Gonzalez-Villalpando C, Stern MP, Hazuda HP, Haffner S. Prevalence of hypertension in Hispanic and non-Hispanic white populations. Hypertension. 2002;39:203–208. doi: 10.1161/hy0202.103439. [DOI] [PubMed] [Google Scholar]
- 7.Bosworth HB, Dudley T, Olsen MK, Voils CI, Powers B, Goldstein MK, Oddone EZ. Racial differences in blood pressure control: potential explanatory factors. Am J Med. 2006;119:70.e9–70.e15. doi: 10.1016/j.amjmed.2005.08.019. [DOI] [PubMed] [Google Scholar]
- 8.Gadegbeku CA, Lea JP, Jamerson KA. Update on disparities in the pathophysiology and management of hypertension: focus on African Americans. Med Clin North Am. 2005;89:921–933. doi: 10.1016/j.mcna.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 9.Wright JT, Jr, Dunn JK, Cutler JA, Davis BR, Cushman WC, Ford CE, Haywood LJ, Leenen FH, Margolis KL, Papademetriou V, et al. for the ALLHAT Collaborative Research Group. Outcomes in hypertensive black and nonblack patients treated with chlorthalidone, amlodipine, and lisinopril. JAMA. 2005;293:1595–1608. doi: 10.1001/jama.293.13.1595. [DOI] [PubMed] [Google Scholar]
- 10.Weir MR, Gray JM, Paster R, Saunders E The Trandolapril Multicenter Study Group. Related differing mechanisms of action of angiotensin-converting enzyme inhibition in black and white hypertensive patients. Hypertension. 1995;26:124–130. doi: 10.1161/01.hyp.26.1.124. [DOI] [PubMed] [Google Scholar]
- 11.Jamerson K, Champion A, Zhou Q, Pepine C. Verapamil- and atenolol based strategies are equally effective in black patients with hypertension and coronary artery disease: a sub-analysis of the International Verapamil SR-Trandolapril study (INVEST) (abstr) Am J Hypertens. 2005;18 suppl 1:S109A. [Google Scholar]
- 12.Baudin B. Polymorphism in angiotensin II receptor genes and hypertension. Exp Physiol. 2005;90:277–282. doi: 10.1113/expphysiol.2004.028456. [DOI] [PubMed] [Google Scholar]
- 13.Bonnardeaux A, Davies E, Jeunemaitre X, Fery I, Charru A, Clauser E, Tiret L, Cambien F, Corvol P, Soubrier F. Angiotensin II type 1 receptor gene polymorphisms in human essential hypertension. Hypertension. 1994;24:63–69. doi: 10.1161/01.hyp.24.1.63. [DOI] [PubMed] [Google Scholar]
- 14.Miller JA, Thai K, Scholey JW. Angiotensin II type 1 receptor gene polymorphism predicts response to losartan and angiotensin II. Kidney Int. 1999;56:2173–2180. doi: 10.1046/j.1523-1755.1999.00770.x. [DOI] [PubMed] [Google Scholar]
- 15.Rubattu S, Di Angelantonio E, Stanzione R, Zanda B, Evangelista A, Pirisi A, De Paolis P, Cota L, Brunetti E, Volpe M. Gene polymorphisms of the renin-angiotensin-aldosterone system and the risk of ischemic stroke: a role of the A1166C/AT1 gene variant. J Hypertens. 2004;22:2129–2134. doi: 10.1097/00004872-200411000-00015. [DOI] [PubMed] [Google Scholar]
- 16.Ono K, Mannami T, Baba S, Yasui N, Ogihara T, Iwai N. Lack of association between angiotensin II type 1 receptor gene polymorphism and hypertension in Japanese. Hypertens Res. 2003;26:131–134. doi: 10.1291/hypres.26.131. [DOI] [PubMed] [Google Scholar]
- 17.Ortlepp JR, Breithardt O, Ohme F, Hanrath P, Hoffmann R. Lack of association among five genetic polymorphisms of the renin-angiotensin system and cardiac hypertrophy in patients with aortic stenosis. Am Heart J. 2001;141:671–676. doi: 10.1067/mhj.2001.113394. [DOI] [PubMed] [Google Scholar]
- 18.Hindorff LA, Heckbert SR, Tracy R, Tang Z, Psaty BM, Edwards KL, Siscovick DS, Kronmal RA, Nazar-Stewart V. Angiotensin II type 1 receptor polymorphisms in the Cardiovascular Health Study: relation to blood pressure, ethnicity, and cardiovascular events. Am J Hypertens. 2002;15:1050–1056. doi: 10.1016/s0895-7061(02)03063-7. [DOI] [PubMed] [Google Scholar]
- 19.Barbeau P, Kulharya A, Harshfield G, Snieder H, Davis H, Treiber F. Association between angiotensin II type I receptor polymorphism and resting hemodynamics in black and white youth. Ethn Dis. 2002;12:68–71. [PubMed] [Google Scholar]
- 20.Tapper W, Collins A, Gibson J, Maniatis N, Ennis S, Morton NE. A map of the human genome in linkage disequilibrium units. Proc Natl Acad Sci U S A. 2005;102:11835–11839. doi: 10.1073/pnas.0505262102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Su S, Chen J, Zhao J, Huang J, Wang X, Chen R, Gu D for the Beijing Atherosclerosis Study. Angiotensin II type I receptor gene and myocardial infarction: tagging SNPs and haplotype based association study. The Beijing Atherosclerosis Study . Pharmacogenetics. 2004;14:673–681. doi: 10.1097/00008571-200410000-00005. [DOI] [PubMed] [Google Scholar]
- 22.Johnson JA, Turner ST. Hypertension pharmacogenomics: current status and future directions. Curr Opin Mol Ther. 2005;7:218–225. [PubMed] [Google Scholar]