Abstract
Adult slow nicotine metabolizers have lower smoke exposure, carbon monoxide levels, and plasma nicotine levels than normal and fast metabolizers. Emerging evidence suggests nicotine metabolism influences smoking topography. This study investigated the association of nicotine metabolism (the ratio of plasma 3-hydroxycotinine to cotinine; 3OHCOT/COT) with smoking topography in adolescent smokers (N=85, 65% female, 68% European American, mean age 15.3 ± 1.2, mean cigarettes per day (CPD) 18.5 ± 8.5, mean Fagerström Test for Nicotine Dependence (FTND) 7.0 ± 1.2) presenting for a nicotine replacement therapy trial. Measures obtained included puff volume, inter-puff interval, number of puffs, puff duration, and puff velocity. Linear regression analysis controlling for hormonal contraception use showed that 3OHCOT/COT ratios predicted mean puff volume in the overall sample (t = 2.126, p = .037, adjusted R2 = .067). After gender stratification, faster metabolism predicted higher mean puff volume (t = 2.81, p = .009, adjusted R2 = .192) but fewer puffs (t = −3.160, p=0.004, adjusted R2 = .237) and lower mean puff duration (t = −2.06, p = .048, adjusted R2 = .101) among boys only, suggesting that as nicotine metabolism increases, puff volume increases but puffing frequency decreases. No significant relationships were found between nicotine metabolism and total puff volume, mean puff duration, inter-puff interval, or puff velocity. If confirmed in a broader sample of adolescent smokers, these findings suggest that, as among dependent adult smokers, rate of metabolism among adolescent boys is linked to select parameters of puffing behavior that may impact cessation ability.
Keywords: adolescent, metabolism, nicotine, smoking
Introduction
The rate of nicotine metabolism has been shown to influence tobacco smoking patterns [1, 2]. For example, adult slow nicotine metabolizers smoke fewer cigarettes daily and weekly [3–5] and have lower smoke exposure as measured by expired air carbon monoxide and plasma nicotine levels, than normal and fast metabolizers [4]. Consumption patterns, as well as smoking topography (i.e., how each cigarette is smoked), influence body nicotine and toxicant concentrations and have implications for tobacco addiction and health harm [6–8].
Preliminary evidence from adult smokers suggests that nicotine metabolism might bear a relationship to select parameters of smoking topography among adult smokers. CYP2A6 genotype, thought to be the primary determinant of rate of nicotine metabolism [2], was significantly associated with mean puff volume and total puff volume, but not number of puffs, such that slow metabolizers exhibited reduced intake compared to others [9]. Similarly to the number of cigarettes per day (CPD), smoking topography influences negative health outcomes. For example, greater maximum puff velocity has been linked to higher alveolar carbon monoxide concentrations [10], risk of lung damage [11], and mortality from lung cancer in women [12]. Because most smokers initiate tobacco use during childhood and adolescence, studying smoking topography among adolescents will likely provide insights on early stages of the tobacco addiction cycle and thus merits further investigation [13].
Among topography components, the mean puff volume reflects the most elementary unit of smoking reinforcement. Puff volume is an objective measure that may relate to the drive to smoke, especially among youths, in whom the combination of parental, social, and legal smoking prohibitions may cause youths to underreport tobacco consumption on subjective questionnaires. In a recent adolescent trial [14] mean puff volume and CPD inversely predicted successful abstinence at the end of treatment; however, only mean puff volume (not CPD) predicted continued abstinence from smoking at the 3-month follow-up after treatment. Those results argued for the potential superiority of puff volume, as compared to smoking rate, to index the level of tobacco dependence that reflects difficulty in achieving cessation.
While depth of inhalation is not directly measured by standard smoking topography, self-reported depth of inhalation was linked to the severity of tobacco-withdrawal symptoms in another study among adolescent smokers [15]. Thus, taken together, as bio-behavioral measures that represent both progression along the addictive cycle and potential correlates of the toxic effects of tobacco smoking constituents, puff topography parameters provide valuable data to predict successful cessation in young established smokers.
The aim of this study was to investigate the relationship between the rate of adolescent nicotine metabolism and the components of cigarette puffing patterns recorded during a smoking topography session among adolescent dependent smokers. In addition, because sex differences have been shown in some topography measures [16, 17], and because nicotine metabolism may differ by sex and hormonal contraceptive (HC) use [18, 19] and ethnicity [20], we examined the same relationships in stratified subsets of our overall sample.
Methods
Participants
Adolescent tobacco smokers were recruited through television, radio, and print advertisements for a randomized, double-blind, double-dummy, placebo-controlled smoking cessation trial examining the safety, efficacy, and tolerability of two forms of nicotine-replacement therapy (NRT), nicotine patch and nicotine gum [13]. The study was approved by the Institutional Review Board of the National Institute on Drug Abuse. All participants received cognitive-behavioral therapy. Eligible participants were 13–17 years of age, had smoked at least ten cigarettes per day (CPD) for the last six months, and scored at least 5 (max 10) on the Fagerström Test for Nicotine Dependence (FTND) [21]. Exclusion criteria included any untreated acute psychiatric disorder (including other drug or alcohol dependence), recent use of NRT, pregnancy, and lack of parental permission. All participants selected for this study received a medical examination and were in good physical health. Adolescents were accompanied by their parent or guardian on the first visit and written consent for parents and assent for adolescents were obtained.
Procedures
Sample collection
During the first visit, a registered nurse collected a blood sample from an antecubital vein to a 7 mL vacutainer tube with sodium heparinate. All samples were iced immediately and centrifuged for five minutes at 3000 rpm. The plasma was then removed and frozen at −20°C until time of assay. Measurements of plasma nicotine, and its metabolites cotinine and 3-hydroxycotinine, were performed by Labstat Inc. (Kitchner, Ontario, Canada). Nicotine and cotinine were measured by highresolution capillary-column gas chromatography [22, 23]. Plasma 3-hydroxycotinine was measured using capillary gas chromatography-mass spectrometry (GC-MS) [24].
Topography session
Smoking topography was assessed in a smoking chamber with a chimney vent. Participants were asked to smoke a cigarette of their own brand ad libitum. The cigarette is attached to a sterilized flow-meter mouthpiece that runs through a pressure transducer. The pressure transducer is connected to a computer that translates the puffing measures into stored data (Clinical Research Support System, Plowshare Inc., Baltimore, MD). Vital signs (heart rate, blood pressure and pulse oximetry) were measured prior to smoking and immediately after smoking. Smoking sessions averaged 25 minutes in duration. The 25 minutes included the requisite safety observation time, some acclimation to the smoking chamber to enhance the validity of the smoking measures, as well as a measure of vital signs before and after the smoking session.
Measures
Nicotine metabolism
The ratio of 3-hydroxycotinine to cotinine concentrations during a nondeprived, nontreated baseline was used as a non-invasive measure of nicotine metabolism.
Smoking topography
For each participant, topography measures were obtained by averaging the measures from five artifact-free puffs, as previously reported [25, 26]. Additionally, the first and last puffs of the cigarette were not included in the averages, nor were puffs whose volumes were below 15ml (considered artifacts) or above 400 mL (age-adjusted average tidal volume). Dependent variables included mean puff volume (mL), mean puff duration (msec), mean inter-puff interval (sec), mean puff velocity (mL/sec), and total puff volume (mL).
Data Analysis
Descriptive statistics were performed to characterize demographic variables, smoking patterns, and topography measures. The sample size was dictated by the power analysis for the clinical trial. Due to non-normal distribution, metabolism and topography data were square-root transformed.
Linear regression analysis was first used to assess the relationship between nicotine metabolism (3OHCOT/COT) and smoking-topography measures (mean puff volume, total puff volume, mean inter-puff interval, mean puff duration, and mean puff velocity) in the total sample. Previous research has shown that ethnicity and sex including contraceptive (HC) use among girls may influence nicotine metabolism among adolescent smokers [18, 19]. Therefore, analyses were then repeated within subsets stratified by sex and ethnicity. Statistical significance was defined as p< .05. SPSS (version 15) was used for all analyses.
Results
The overall sample was comprised of 85 adolescents (65% female, 68% European American). Smoking rates and mean FTND score indicated substantial dependence (see Table 1 for sample descriptives). Duration of smoking (mean 3.71 (SD 1.93) yrs) did not differ by sex.
Table 1.
Sample Demographic Characteristics (N=85)
| Age | CPD | FTND | Years Smoking |
Pre Topo CO |
Post Topo CO |
30HCOT/COT | mPuff-Vol | TotPuff-Vol | |
|---|---|---|---|---|---|---|---|---|---|
|
Gender |
|||||||||
| Male (n = 30) | 15.36(1.15) | 18.05(9.97) | 6.84(1.32) | 3.62(1.83) | 9.75(5.75) | 17.74(7.29) | .28(.08) | 40.32(19.82) | 647.95(206.5) |
| Female (n = 55) | 15.30(1.25) | 18.83(7.60) | 7.09(1.18) | 3.76(2.00) | 10.94(6.89) | 21.37(6.61)* | .35(.18)* | 37.47(12.83) | 571.03(196.12) |
| Ethnicity | |||||||||
| Euro. American (n = 58) | 15.45(1.28) | 18.84(8.40) | 7.18(1.22) | 3.83(2.09) | 11.21(6.69) | 21.10(7.06) | .35(.16)** | 40.87(16.63) | 580.82(207.28) |
| African American (n =24) | 15.17(0.97) | 18.32(9.00) | 6.67(1.17) | 3.33(1.52) | 9.44(6.10) | 18.63(6.67) | .26(.16) | 33.67(12.30) | 659.52(181.56) |
| Total (N = 85) | 15.32(1.21) | 18.55(8.46) | 7.00(1.23) | 3.71(1.93) | 10.54(6.50) | 20.09(7.04) | .33(.16) | 38.47(15.59) | 599.52(201.61) |
p < 01
p < .05
In our initial regression models, we controlled for variables known to be associated with metabolism (the predictor variable), including sex, race, and CPD. However, we opted to exclude these variables because they decreased the adjusted R2 values for the models. In addition, the analysis revealed that Body Mass Index (BMI) was negatively correlated with metabolism (r = −.229, p < .05); however, models controlling for BMI did not alter results. A significant relationship between hormonal contraception use and nicotine metabolism was found (r = −.345, p = .001). Thus we controlled for hormonal contraception use in subsequent relevant analyses. Therefore, we used bivariate linear regressions, with each topography measure as the dependent variable (mean puff volume, mean puff duration, mean puff interval, mean puff velocity, or total puff volume) and metabolism and hormonal contraception (HC) use as the predictor variables. Additional regression analyses were performed using the dependent variables: number of puffs and CO level.
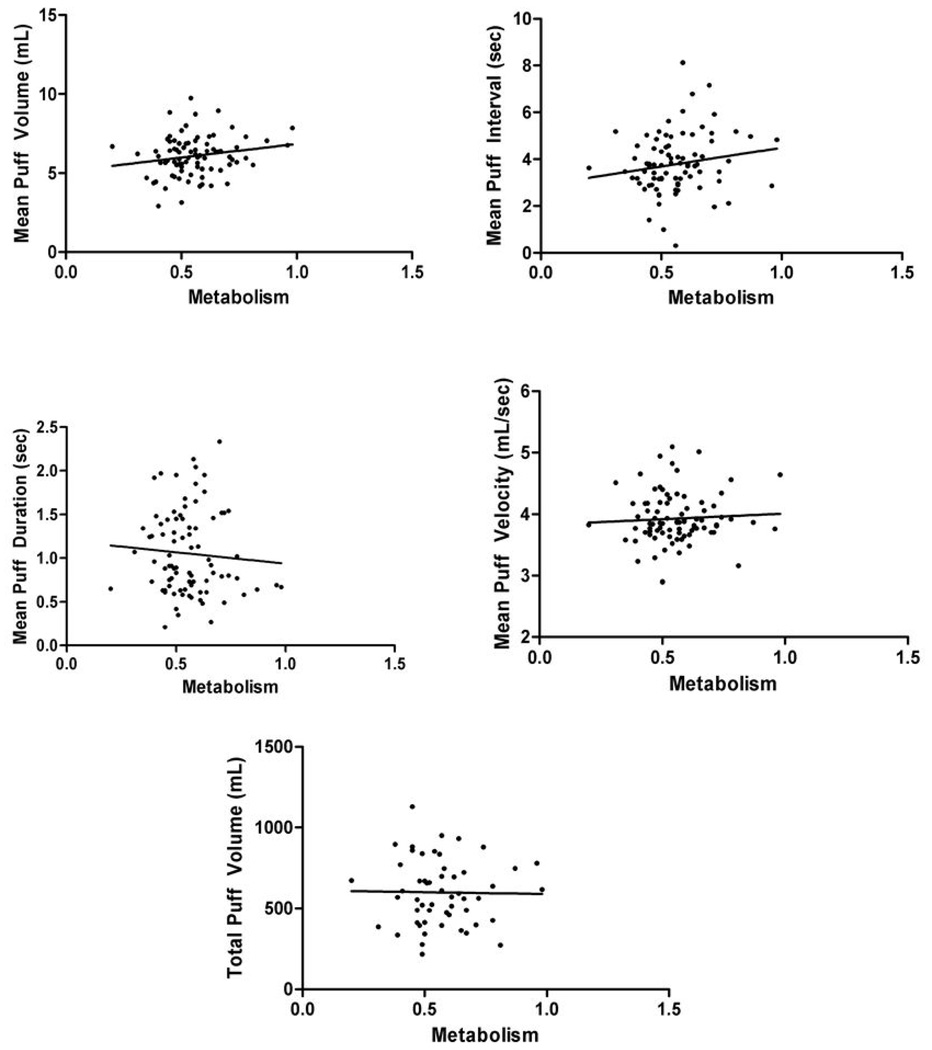
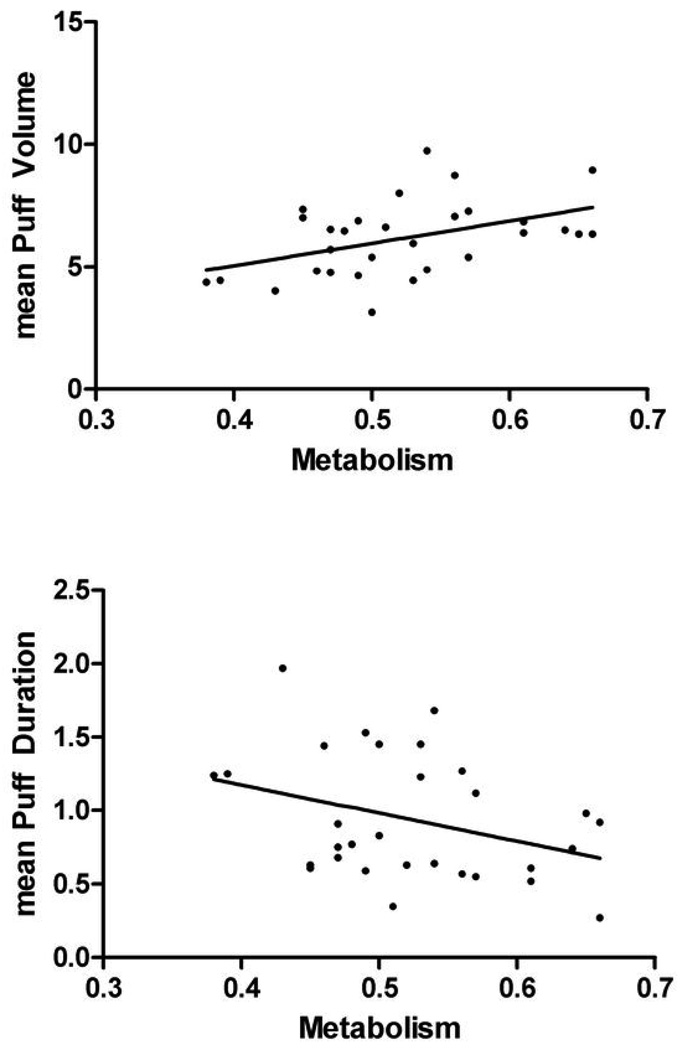
Results indicated a trend toward rate of metabolism predicting mean puff volume in the total sample, however, after controlling for HC use, this trend became significant (t = 2.126, p = .037, adjusted R2 = .067) with higher metabolism predicting higher mean puff volume. Metabolism also showed a trend toward inversely predicting number of puffs in the overall sample (t = −1.763, p = 0.82, adjusted R2 = .024). There were no significant relationships between metabolism and mean puff duration, interval, velocity, or total puff volume (Figure 1. displays scatter plots and regression lines for the total sample). When the sample was stratified by sex, significant findings occurred only among males: metabolism positively predicted mean puff volume (t = 2.812, p = .009, adjusted R2 = .192), and inversely predicted both number of puffs (t = −3.160, p=0.004, adjusted R2 = .237) and mean puff duration (t = −2.064, p = .048, adjusted R2 = .101). The regression scatter plots are shown in Figure 2. Among boys, metabolism accounted for 19.2% of the variability in mean puff volume; metabolism and mean puff duration and number of puffs were inversely related, with metabolism accounting for 10.1% of the variance in mean puff duration and 23.7% of the variance in number of puffs. No other relationships reached significance among sex-stratified or ethnicity-stratified samples and topography measures. Metabolism did not significantly predict CO levels in any sample (data not shown). Table 2 shows unstandardized regression coefficients (B), standard errors, and standardized regression coefficients (β) for topography analyses.
Figure 1.
Linear Regression showing the relationship between metabolism and topography parameters (square root transformed except for Total Puff Volume) among the total sample (N=85)
Figure 2.
Linear Regression showing the relationship between metabolism and mean puff volume (square root transformed values shown) among boys (n=30)
Table 2.
Unstandardized regression coefficient (B), standard error, and standardized regression coefficients (β) for the total sample (N=85) and stratified samples (controlling for hormonal contraception use when applicable)
| METABOLISM | ||||
|---|---|---|---|---|
| DEPENDENT VARIABLE | B | SE B | β | |
| Total | ||||
| mPuff-Vol | 2.188 | 1.029 | .237* | |
| mPuff-Dur | −.432 | .411 | −.119 | |
| mPuff-lnter | .993 | 1.079 | .102 | |
| mPuff-Vel | .319 | .361 | ..102 | |
| totPuff-Vol | 183.923 | 171.007 | .121 | |
| Male (n=30) | ||||
| mPuff-Vol | 9.572 | 3.404 | .469** | |
| mPuff-Dur | −2.049 | .992 | −.363* | |
| mPuff-lnter | −.436 | 2.644 | −.031 | |
| mPuff-Vel | 1.623 | 1.230 | .242 | |
| totPuff-Vol | −6.750 | 10.692 | −.147 | |
| Female (n=55) | ||||
| mPuff-Vol | 1.357 | .866 | .202 | |
| mPuff-Dur | −..309 | .443 | −.095 | |
| mPuff-lnter | .945 | 1.196 | .103 | |
| mPuff-Vel | .148 | .351 | .060 | |
| totPuff-Vol | 189.850 | 159.139 | .153 | |
| Euro. American | ||||
| mPuff-Vol | .1.579 | 1.376 | .156 | |
| mPuff-Dur | −.260 | .554 | −.066 | |
| mPuff-lnter | 1.648 | 1.564 | .142 | |
| mPuff-Vel | .295 | .461 | .090 | |
| totPuff-Vol | 105.294 | 217.406 | .066 | |
| African American | ||||
| mPuff-Vol | 2.215 | 1.697 | .268 | |
| mPuff-Dur | −.696 | .707 | −.205 | |
| mPuff-lnter | −1.503 | 1.240 | −.250 | |
| mPuff-Vel | −.342 | .616 | −.118 | |
| totPuff-Vol | 140.310 | 307.284 | .126 | |
p < .01
p < .05
Discussion
The main finding from this study is that among nicotine-dependent boys, faster nicotine metabolism predicted higher mean puff volume (a predictor of cessation-treatment failure) but lower puff duration and fewer puffs. These results are in partial agreement with those of a recent study of adult smokers [9]. Metabolic differences across sex and ethnicity found in this adolescent sample have been described elsewhere [18, 20] and are consistent with those demonstrated among adult smokers [19, 27].
A similar pattern of results, though slightly weaker, was observed in the total sample of boys and girls, but only after controlling for HC use. This may suggest a link between HC use and smoking topography measures. The stronger relationship found by Strasser [9] and colleagues with total puff volume might be due to both the use of genotype as predictor and the categorical nature of that analysis. In that study of adult smokers, the authors compared mean topography measures among 3 groups (slow, intermediate and normal rate metabolism) of CYP 2A6 genotypes. In the current study, metabolism predicted higher mean puff volume but lower puff duration and smaller number of puffs explaining the lack of relationship of metabolism with total puff volume (the product of mean puff volume by number of puffs). Important differences in smoking histories between adult [10] and adolescent smokers might also have contributed to the different relationships observed. While the samples in the two studies are quite different (i.e., adolescents versus adults), notably, indices of nicotine metabolism (data not shown) and puff volumes found in the current study were comparable to values reported in other adult smokers [20].
Sex differences in puff volume have been described among adult smokers [16]. In our sample, although the pattern of results differed across sex, there was no significant sex difference in any topography parameter in direct comparisons (data not shown). Differences have also been reported for adolescent nicotine metabolism between boys and girls using hormonal contraception [18]. Controlling for HC use accounted for a substantial amount of variance in our sample. Future research should examine the direct effects of HC use on smoking patterns in a larger sample. Controlling for the inverse relationship between BMI and metabolism did not affect results.
One limitation of the current study is that the sample consisted of highly tobacco dependent and treatment-seeking adolescents, and therefore was not representative of all teenage smokers. We did not control for degree of craving or time since last cigarette. Also, unexamined determinants of puff volume, such as nicotine and tar yield from cigarettes, may have affected our findings. Because of suboptimal data on usual brand smoked, our analyses did not control for nicotine and tar delivery which have been shown to impact volume of smoke inhaled [28]. Interestingly, the absence of a relationship between rate of metabolism and total puff volume suggests that any potential causal inference might be more attributable to pattern of puffing. Mechanisms for such relationships have been suggested (e.g., nicotine receptor activation, airway sensorial effect [29]) but remain speculative. Finally, the implications of our puff-duration findings are unclear, because in at least one study, puff duration did not affect nicotine concentrations [8].
Studies are now warranted to confirm the relationship found here within a broader sample of adolescent smokers. Research is also needed to elucidate, early in the use of tobacco, the mechanisms underlying the association between rate of nicotine metabolism and progression of use, addiction, and health harm [6, 20].
In conclusion, the current study indicates a link between the metabolic disposition of nicotine and puff topography measures of smoke intake among adolescent male smokers with possible implications for the development of dependence and ability to achieve cessation.
Acknowledgements
This research was supported by NIDA intramural research funds. The authors are grateful to David H. Epstein and Suzanne J. Lo for critical review of the manuscript.
References
- 1.Benowitz NL, Pomerleau OF, Pomerleau CS, Jacob P., 3rd Nicotine metabolite ratio as a predictor of cigarette consumption. Nicotine Tob Res. 2003;5:621–624. doi: 10.1080/1462220031000158717. [DOI] [PubMed] [Google Scholar]
- 2.Pianezza ML, Sellers EM, Tyndale RF. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 3.O’Loughlin J, Paradis G, Kim W, et al. Genetically decreased CYP2A6 and the risk of tobacco dependence: a prospective study of novice smokers. Tob Control. 2004;13:422–428. doi: 10.1136/tc.2003.007070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rao Y, Hoffman E, Zia M, et al. Duplications and defects in the CYP2A6 gene: identification, genotyping, and in vivo effects on smoking. Mol Pharmacol. 2000;58:747–755. doi: 10.1124/mol.58.4.747. [DOI] [PubMed] [Google Scholar]
- 5.Schoedel KA, Hoffmann EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasians. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 6.Audrain-McGovern J, Al Koudsi N, Rodriguez D, et al. The role of CYP2A6 in the emergence of nicotine dependence in adolescents. Pediatrics. 2007;119:e264–e274. doi: 10.1542/peds.2006-1583. [DOI] [PubMed] [Google Scholar]
- 7.Herning RI, Jones RT, Benowitz NL, Mines AH. How a cigarette is smoked determines blood nicotine levels. Clin Pharmacol Ther. 1983;33:84–90. doi: 10.1038/clpt.1983.12. [DOI] [PubMed] [Google Scholar]
- 8.Rieben FW. Smoking behaviour and increase in nicotine and carboxyhaemoglobin in venous blood. Clin Investig. 1992;70:335–342. doi: 10.1007/BF00184670. [DOI] [PubMed] [Google Scholar]
- 9.Strasser AA, Malaiyandi V, Hoffmann E, Tyndale RF, Lerman C. An association of CYP2A6 genotype and smoking topography. Nicotine Tob Res. 2007;9:511–518. doi: 10.1080/14622200701239605. [DOI] [PubMed] [Google Scholar]
- 10.Strasser AA, Pickworth WB, Patterson F, Lerman C. Smoking topography predicts abstinence following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2004;13:1800–1804. [PubMed] [Google Scholar]
- 11.Clark KD, Wardrobe-Wong N, Elliott JJ, Gill PT, Tait NP, Snashall PD. Cigarette smoke inhalation and lung damage in smoking volunteers. Eur Respir J. 1998;12:395–399. doi: 10.1183/09031936.98.12020395. [DOI] [PubMed] [Google Scholar]
- 12.Garfinkel L, Stellman SD. Smoking and lung cancer in women: findings in a prospective study. Cancer Res. 1988;48:6951–6955. [PubMed] [Google Scholar]
- 13.Moolchan ET, Ernst M, Henningfield JE. A review of tobacco smoking in adolescents: treatment implications. J Am Acad Child Adolesc Psychiatry. 2000;39:682–693. doi: 10.1097/00004583-200006000-00006. [DOI] [PubMed] [Google Scholar]
- 14.Franken FH, Pickworth WB, Epstein DH, Moolchan ET. Smoking rates and topography predict adolescent smoking cessation following treatment with nicotine replacement therapy. Cancer Epidemiol Biomarkers Prev. 2006;15:154–157. doi: 10.1158/1055-9965.EPI-05-0167. [DOI] [PubMed] [Google Scholar]
- 15.McNeill AD, West RJ, Jarvis M, Jackson P, Bryant A. Cigarette withdrawal symptoms in adolescent smokers. Psychopharmacology (Berl) 1986;90:533–536. doi: 10.1007/BF00174074. [DOI] [PubMed] [Google Scholar]
- 16.Eissenberg T, Adams C, Riggins EC, 3rd, Likness M. Smokers’ sex and the effects of tobacco cigarettes: subject-rated and physiological measures. Nicotine Tob Res. 1999;1:317–324. doi: 10.1080/14622299050011441. [DOI] [PubMed] [Google Scholar]
- 17.Wood T, Wewers ME, Groner J, Ahijevych K. Smoke constituent exposure and smoking topography of adolescent daily cigarette smokers. Nicotine Tob Res. 2004;6:853–862. doi: 10.1080/1462220042000282537. [DOI] [PubMed] [Google Scholar]
- 18.Berlin I, Gasior MJ, Moolchan ET. Sex-based and hormonal contraception effects on the metabolism of nicotine among adolescent tobacco-dependent smokers. Nicotine Tob Res. 2007;9:493–498. doi: 10.1080/14622200701243193. [DOI] [PubMed] [Google Scholar]
- 19.Benowitz NL, Lessov-Schlaggar CN, Swan GE, Jacob P., 3rd Female sex and oral contraceptive use accelerate nicotine metabolism. Clin Pharmacol Ther. 2006;9:480–488. doi: 10.1016/j.clpt.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 20.Moolchan ET, Franken FH, Jaszyna-Gasior M. Adolescent nicotine metabolism: ethnoracial differences among dependent smokers. Ethn Dis. 2006;16:239–243. [PubMed] [Google Scholar]
- 21.Heatherton TF, Kozlowski LT, Frecker RC, Fagerstrom KO. The Fagerstrom Test for Nicotine Dependence: a revision of the Fagerstrom Tolerance Questionnaire. Br J Addict. 1991;86:1119–1127. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- 22.Teeuwen HW, Aalders RJ, Van Rossum JM. Simultaneous estimation of nicotine and cotinine levels in biological fluids using high-resolution capillary-column gas chromatography combined with solid phase extraction work-up. Mol Biol Rep. 1988;13:165–175. doi: 10.1007/BF00444313. [DOI] [PubMed] [Google Scholar]
- 23.Feyerabend C, Russell MA. A rapid gas-liquid chromatographic method for the determination of cotinine and nicotine in biological fluids. J Pharm Pharmacol. 1990;42:450–452. doi: 10.1111/j.2042-7158.1990.tb06592.x. [DOI] [PubMed] [Google Scholar]
- 24.Jacob P, 3rd, Shulgin AT, Yu L, Benowitz NL. Determination of the nicotine metabolite trans-3′-hydroxycotinine in urine of smokers using gas chromatography with nitrogen-selective detection or selected ion monitoring. J Chromatogr. 1992;583:145–154. doi: 10.1016/0378-4347(92)80547-4. [DOI] [PubMed] [Google Scholar]
- 25.Aung AT, Pickworth WB, Moolchan ET. History of marijuana use and tobacco smoking topography in tobacco-dependent adolescents. Addict Behav. 2004;29:699–706. doi: 10.1016/j.addbeh.2004.02.012. [DOI] [PubMed] [Google Scholar]
- 26.Zimmerman DM, Sehnert SS, Epstein DH, Pickworth WB, Robinson ML, Moolchan ET. Smoking topography and trajectory of asthmatic adolescents requesting cessation treatment. Prev Med. 2004;39:940–942. doi: 10.1016/j.ypmed.2004.03.032. [DOI] [PubMed] [Google Scholar]
- 27.Benowitz NL, Perez-Stable EJ, Fong I, Modin G, Herrera B, Jacob P., 3rd Ethnic differences in N-glucuronidation of nicotine and cotinine. J Pharmacol Exp Ther. 1999;1:1196–1203. [PubMed] [Google Scholar]
- 28.Woodman G, Newman SP, Pavia D, Clarke SW. Inhaled smoke volume and puff indices with cigarettes of different tar and nicotine levels. Eur J Respir Dis. 1987;70:187–192. [PubMed] [Google Scholar]
- 29.Naqvi NH, Bechara A. The airway sensory impact of nicotine contributes to the conditioned reinforcing effects of individual puffs from cigarettes. Pharmacol Biochem Behav. 2005;81:821–829. doi: 10.1016/j.pbb.2005.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]